Abstract
Purpose
Given that more cancers are being diagnosed earlier and that treatment of cancer is improving, health issues of cancer survivors are becoming more common and apparent. Pelvic radiation therapy for the treatment of gynecological cancers can lead to long-term collateral damage to the bladder, a condition termed radiation cystitis (RC). Late sequelae may take many years to develop and include incontinence and pain as well as hematuria. RC is a rare but potentially life-threatening condition for which there are few management and treatment options.
Methods
There are limited data in the literature regarding the effects of radiation on the bladder after gynecological cancer therapy and we hereby review the literature on cancer survivorship issues of pelvic radiation for gynecology literature.
Results
Treatment options are available for patients with radiation-induced hemorrhagic cystitis. However, most treatments are risky or only effective for a short timeframe and no therapy is currently available to reverse the disease progress. Furthermore, no standardized guidelines exist describing preferred management options. Common therapies include hyperbaric oxygen therapy, clot evacuation, fulguration, intravesical instillation of astringent agents, and surgery. Novel developing strategies include Botulinum Toxin injections and liposomal-tacrolimus instillations. These treatments and strategies are discussed.
Conclusions
In this review, we will present current and advanced therapeutic strategies for RC to help cancer survivors deal with long-term bladder health issues.
Keywords: Radiation cystitis, Hemorrhagic cystitis, Gynecology cancer, Cancer survivorship, Inflammation, Bladder
Introduction
Gynecological cancer treatment is improving in developed countries. In 2018, an estimated 110,070 women will be diagnosed with a genital cancer. In this review, genital cancers will refer to those associated with the female reproductive tract excluding breast cancer. However, early diagnosis, HPV vaccination, screening, increased awareness, and advanced treatment continue to improve survival rates. As of January 2016, 1.3 million genital cancer survivors were living in the United States alone. This number is expected to rise with 15% by 2026 [1]. With an increase in cancer survivorship, new medical and social issues due to cancer therapy are becoming apparent. Radiation therapy (RT) and chemotherapy represent an important therapeutic component in the management of gynecologic malignancies. Both can be employed as primary or adjuvant treatment.
One rare but severe disease affecting cancer survivors is hemorrhagic cystitis (HC). HC is a chronic inflammatory condition of the bladder with bladder hemorrhaging that in cancer survivors can be caused by chemotherapy or radiation therapy; when caused by the latter, it is also referred to as radiation cystitis (RC). As this review focusses on radiation-induced HC, the terms HC and RC will be used intermittently.
The bladder may suffer short- and long-term damage after therapy. This includes the presence of sustained hematuria and lower urinary tract symptoms in the absence of active tumor and other conditions, such as vaginal bleeding, general bleeding diathesis, and bacterial or fungal urinary tract infections. Chemotherapy-induced HC is caused by the cytotoxic agents cyclophosphamide (Cytoxan®, Neosar®) and ifosfamide (Ifex®) [2]. 20–25% of patients who receive high dose cyclophosphamide treatment develop HC within several weeks to months after treatment [3]. The metabolism of cyclophosphamide and ifosfamide generates acrolein, a compound that is secreted through the urine and can cause urothelial damage upon storage in the bladder [3]. Concurrent treatment with Mesna, or other uroprotective agents, can minimize acrolein-induced damage to the bladder [4]. Cyclophosphamide and ifosfamide are not commonly used for the treatment of genital cancers, thus little is known about long-term side effects of these drugs in genital cancer survivors [5]. However, these cytotoxic agents are being tested in patients who have an intolerance for taxane or platinum based chemotherapies [5]. In one case report, low-dose metronomic cyclophosphamide treatment was described to have no urological side effects in a patient with recurrent high-grade serous ovarian cancer [6].
Radiation therapy to the pelvis can lead to the development of RC, or radiation-induced HC. Radiation directly delivers high-energy particles to the tumor; however, some collateral damage to the normal neighboring tissue such as the bladder or gastrointestinal tract is inevitable. The appearance of RC can occur as early as 6 months and up to 20 years post RT.
Symptoms of RC include frequency, dysuria, urgency, nocturia, suprapubic pain, bladder infection, fatigue, and both microscopic and gross hematuria. Bleeding from HC ranges from mild, non-visible (or microscopic) hematuria to moderate or even severe gross hematuria with clots [3, 7, 8]. Severe HC is a challenging condition to treat and may give rise to disabling symptoms (e.g., frequency, urgency, and pelvic pain) or serious complications that can lead to prolonged hospitalization and/or mortality [9].
Radiation therapy for genital malignancies
RT is indicated in up to 60% of cervical, 45% of endometrial, 35% of vulvar, 100% of vaginal, and 5% of ovarian cancer patients [10, 11]. The incidence and severity of RT side effects depends upon the site, volume of tissue exposed, and treatment schedule including total dose, dose per fraction, and type of radiation. Higher risk for RT-related toxicity is observed among patients with advanced age, concomitant chemotherapy, tobacco use, active vascular disease, inflammatory bowel disease, diabetes, or hypertension. Higher total doses of radiation can be associated with focal injury and lead to a risk of hematuria or ulceration. Although HC has been reported to occur in up to 9% of patients who have received full dose RT, it is a severe and potentially life-threatening complication in less than 5%. The onset is typically 1–3 years after treatment; however, patients with a history of pelvic radiation can develop RC many years after RT [12].
Regardless of therapeutic approaches in the treatment of genital malignancies, the classification of them must encompass all possible complications. In the classification system developed by Chassagne and colleagues for reporting complications of treatment in gynecological cancers, complications related to the urinary bladder and urethra are detailed in section 2.2.1 and are scored from grade G1 to G4 which corresponds to increasing severity of symptoms. Grade G1 covers mild or occasional hematuria or lower urinary tract symptoms. Grade G2 includes hematuria requiring blood transfusion and/or hospitalization and/or intravesical therapy. Grade G3 encompasses hematuria requiring major surgery or embolization [13]. Lastly, G4 is for death directly due to hemorrhaging. Hematuria in patients with a history of pelvic radiation therapy can also be caused by secondary primary bladder cancer, e.g., bladder cancer caused by exposure to radiation. In fact, bladder cancer can be found in association with RC. Radiation-induced bladder cancer is not common, but needs to be taken into consideration when examining a cancer survivor with hematuria [14].
The reviews of several gynecological cancers with respect to bladder complications are included below.
Cervical Cancer is the second most commonly diagnosed cancer and the third leading cause of cancer mortality among females in underdeveloped countries. Cervical carcinoma can be treated with primary or adjuvant radiotherapy; the mode of treatment depends on size and extension of the tumor. Exclusive chemo-radiation (CT-RT) is the standard treatment for advanced and locally advanced cervical cancer. Despite survival advantages with respect to radiotherapy, the 5-year overall survival in this subset of patients still remains around 70%. RT and surgery are equally effective in early stages, though surgery is only considered in patients with earlier stages (up to Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) IIA) [15]. Common long-term side effects of brachy- or external beam radiation therapy include vaginal dryness, lymphedema, fragile pelvic bones, and RC. The reported incidence of RC in cervical cancer survivors 1-year post treatment is 3–6.7% [16].
Endometrial cancer is the sixth most common malignancy among females worldwide with an incidence of 320,000 new cases in 2012 according to the World Cancer Research Fund International. In the United States, uterine corpus cancer, of which 92% are endometrial cancers, is the second most common cancer in women with a predicted incidence of 63,230 new cases in 2018 [1]. More than 90% of cases of endome-trial cancer occur in women older than 50 years of age, with a median age of 63 years. Up to 5% of endometrial cancers are associated with Lynch syndrome type II (known as hereditary non-polyposis colorectal carcinoma syndrome); 30–60% of those with Lynch syndrome type II have a lifetime risk of developing endometrial cancers. The classical treatment of endometrial cancer is based on surgical approach. However, 5–10% of patients are not recommended for surgery due to medical contraindications or because of unrespectable disease. In these cases, external RT with or without intracavitary brachytherapy to the uterus and vagina is suitable for individual clinical use.
RT can be administered as vaginal brachytherapy (VBT), pelvic RT, or intensity-modulated radiation therapy (IMRT). While pelvic RT decreases the risk of a local recurrence, it is associated with more toxicity, including long-term urinary and bowel symptoms [17]. In the post-operative radiation therapy in endometrial cancer (PORTEC)-2 trial, 427 women with high/intermediate risk of disease were randomly assigned treatment with VBT or pelvic RT. VBT was associated with a significantly lower rate of treatment-related diarrhea and other bowel symptoms (13% vs. 54%, respectively). In a longer-term 7-year follow-up study, patients receiving pelvic RT reported more bowel symptoms with impact on daily activities, and a trend for more urinary symptoms [18].
Primary vaginal carcinoma is one of the most uncommon malignancies of the female genital tract. It accounts for 2% of all female genital tumors, and has an incidence of 7 per 1,000,000 women. The mean age at diagnosis of squamous cell carcinoma, the most common histologic type of vaginal cancer, is approximately 60 years. Most cases of vaginal cancer are likely mediated by human papillomavirus (HPV) infection, as with cervical cancer. The majority of vaginal malignancies are metastatic, often arising from the endometrium, cervix, vulva, ovary, breast, rectum, and kidney.
Treatment strategy should be individualized depending upon the location, size, and clinical stage of the tumor. In many cases, either surgery or radiation may be adequate treatment. A total radiation dose of 70–75 Gy is generally recommended, with 45–50 Gy being delivered with external beam radiation and the additional radiation being delivered with intracavitary or interstitial brachytherapy radiation. 10–15% of patients treated for vaginal cancer will develop treatment-related complications, including RC.
Radiation cystitis pathophysiology
Inflammation associated with RC consists of three phases. The first short phase occurs several weeks post RT. This is followed by a longer, symptom-free, phase lasting months to years that is largely dose dependent. The last phase is a chronic irreversible late-response phase [7, 8]. In 2014, approximately 22% of female cancer survivors were treated with pelvic radiation. At least 5% of those treated are expected to develop RC [7, 8].
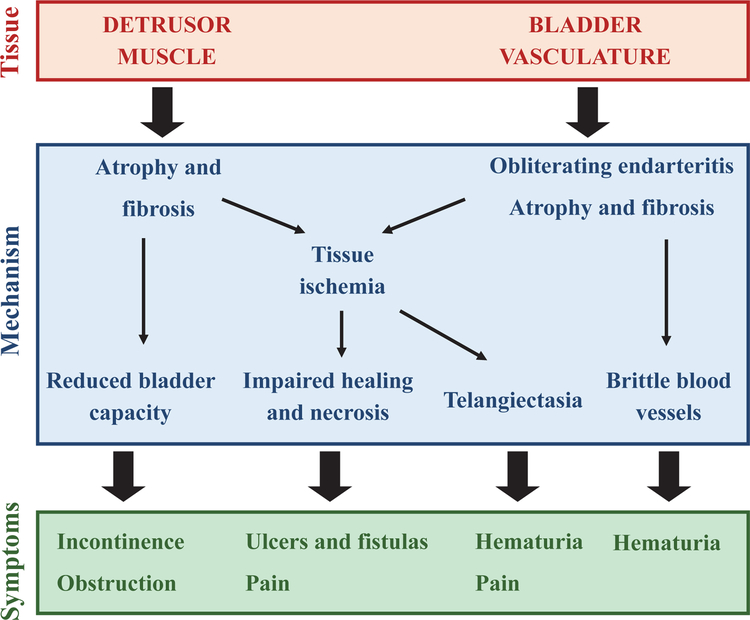
High-energy radiation can cause cell death through DNA damage or increasing permeability of cell membranes [2]. The bladder urothelium has a low cell turnover rate compared to other tissues and may therefore be more susceptible to radiation damage, resulting in the early symptoms of RC [2]. Urine is a strong irritant and any exposure through bladder barrier function can lead to additional irritability and inflammation of the bladder wall. Possible long-term consequences of radiation on the urinary system are listed in Table 1. Late radiation tissue injury includes obliterating endarteritis (inflammation of the inner lining of an artery) resulting in atrophy and fibrosis, and subsequently hematuria [19]. Tissue ischemia also results in impaired healing and necrosis of the bladder mucosa, and can eventually lead to ulcer and fistula formation. Telangiectasia (permanent dilation of blood vessels, usually in the mucous membranes) develops and can also cause bleeding and pain. Fibrosis, if severe, can lead to reduced bladder capacity (Fig. 1).
Table 1.
Radiation damaging effects on upper and lower urinary tract
| Bladder | Ureter | Urethra | |
|---|---|---|---|
| Inflammation | x | x | x |
| Fibrosis | x | x | x |
| Stricture | x | x | |
| Bleeding | x | x | x |
| Clot retention | x | ||
| Fistula | Vesico-cutaneous, GYN, and rectal | Uretero-enteric, vascular, and vaginal | Urethro-cutaneous, GYN and rectal |
| Reflux | x | ||
| Incontinence | Urge | stress | |
| Obstructive symptoms | x | ||
| Irritable symptoms | x | ||
| Pain | x | x | x |
| Retention | x | ||
| Cancer | x |
Fig. 1.
Late symptoms of radiation cystitis in pelvic cancer survivors
Urinary incontinence can be stress urinary incontinence due to intrinsic sphincter deficiency or urgency incontinence as a result of small bladder capacity and impaired detrusor compliance. Depending on the etiology, patients may present with obstructive lower urinary tract symptoms including urinary retention secondary to detrusor dysfunction and urethral stricture, the latter being common in men but rare in women (Fig. 1).
Evaluation for hemorrhagic cystitis
Hematuria is the sine qua non of HC. Hematuria can vary from mild to life-threatening and clot formation can lead to urinary retention. The patient may also present with other lower urinary tract symptoms such as pain, increased frequency, incontinence, and urgency (Table 1).
The most important diagnostic rule of HC is exclusion of other causes of hematuria. Microscopic hematuria or urinary tract infection can be detected with urinalysis and urine culture. Urine cytology may be considered to detect high-grade malignancies. Physical examination should focus on evaluating the presence of possible vaginal fistulas. Post-void residual volume can diagnose underactive bladder and a bladder diary provides an objective assessment of the patient’s symptoms. Radiographic imaging and cystoscopy can be performed to rule out upper uri-nary track pathology, fistula, and cancer. Under suspicion of a tumor, a bladder biopsy may be performed but must be done with great care to avoid perforation of the irradiated bladder wall or cause further bleeding. Urodynamic tests may help to assess cystometric capacity, detrusor compliance, sphincter function, and vesicoureteral reflux [3].
Treatment options for radiation‑induced hemorrhagic cystitis
Various treatment options are available for patients with radiation-induced HC. However, most treatments are risky or only effective for a short timeframe and no therapy is currently available to reverse the disease progress. Furthermore, no standardized guidelines exist describing preferred management options. Common therapies include hyperbaric oxygen therapy (HBOT), clot evacuation, fulguration, intravesical instillation of astringent agents, and surgery. Novel developing strategies include Botulinum Toxin injections and liposomal-tacrolimus instillations. These treatments and strategies are explained in detail below.
Hyperbaric oxygen
HBOT has been used for patients with ischemic diseases that are unresponsive to conventional treatment as it increases oxygen saturation of hemoglobin and may increase angio-genesis and fibroblast activity in damaged tissues [20]. A typical HBOT regimen is 100% oxygen at 1.5–2.5 atmospheric pressure for 45–120 min with additional time for compression and decompression. Sessions are once daily and usually involves 20–40 sessions [21]. HBOT in HC patients offers the advantage that it has no adverse effects on the urinary bladder. However, HBOT does have numerous side-effects, including: claustrophobia, ear pain due to high atmospheric pressure, rare oxygen toxicity (seizures and alveolar membrane damage), and digitalis toxicity (digoxin or digitoxin). HBOT is a time-consuming treatment with variable reported response rates (27–92%) and relatively high recurrence rates (8–63%) [22, 23]. HBOT is not recommended during pregnancy and for patients with a fever of which the cause is unknown.
Clot evacuation
In the presence of clots, clot evacuation is an important first line of treatment for HC as clots can block the urethra and subsequently cause sepsis and bladder rupture [24]. Clot evacuation involves irrigation of the bladder with water or sodium chloride through a wide-lumen catheter [3]. Hydration through intravenous fluids is key to prevent reoccurrence of clots. Persistent hematuria can be treated with continuous bladder irrigation through a 3-way catheter [24]. However, to avoid complications, continuous irrigation can only be performed when all clots have been cleared [2]. Persistent clots can be removed through cystoscopy.
Endoscopic fulguration
If bladder irrigation does not suffice, cystoscopy with fulguration of bleeding sites is recommended. Although it is more invasive and generally uses local or general anesthesia, this procedure is relatively effective in treating moderate cases of HC [25]. Fulguration can be performed using electro coagulation, diathermy, and several types of lasers. Severe complications of endoscopic fulguration include bladder perforation and/or fistula formation.
Intravesical instillation
Persistent hematuria can be treated with several astringent agents [3]. The most commonly used agents are alum and formalin.
Aluminum sulfate (Alum) Alum is an agent typically given in a 1% solution through irrigation. It causes vasoconstriction, decreases vascular permeability, and precipitates proteins. A risk for clot formation exists in severe cases, resulting in distension and recurrence of hematuria. Patients can experience suprapubic pain and incomplete bladder emptying, which are treatable with antispasmodic and/or analgesic drugs [26]. High levels of alum can cause encephalopathy and acidosis in patients with decreased kidney function [27]. Following Alum instillation, serum Alum levels should be closely monitored to help reduce potential toxicity from this treatment.
Aminocaproic acid Epsilon aminocaproic acid (Amicar®) is a drug used to treat excessive post-operative bleeding [28]. Due to the risk of large hard clots forming that are difficult to remove and can cause acute renal failure, it is recommended that Amicar is administered intravesically through continuous bladder irrigation [28]. In HC, Amicar is typically given at an initial dose of 5 g, followed by 1 g/h with a maximum dose of 30 g in 24 h.
Prostaglandins Prostaglandins are instilled at a starting dose of 0.8–1.0 mg/dl. They act by suppressing inflammation and arresting bleeding by contracting smooth muscle cells and causing platelet aggregation. Additionally, they are cytoprotective by inducing mucus production. As a side-effect, prostaglandins commonly cause flushing or severe bladder spasms [29].
Formaldehyde (Formalin) Formalin instillation arrests hemorrhaging by precipitating cellular proteins, and occluding and fixing telangiectasia tissue and small capillaries. Additionally, it hydrolyzes protein and coagulates superficial bladder mucosa tissue. Formalin is usually only considered as a last resort treatment [30]. Intravesical formalin instillations need to be done under anesthesia. In patients with vesicoureteral reflux, an occlusive ureteral balloon catheter needs to be placed before instillation [30]. Patients that have not previously been evaluated for possible reflux, pretreatment cystography is clinically indicated. Highly diluted formalin (1–4%) is preferred as higher doses are associated with increased morbidity and mortality. There is a heavy price to pay for using formalin as fixation of the bladder musculature may result in a small, contracted bladder, and fixation of the intramural ureter can also lead to obstruction with subsequent hydronephrosis and renal failure [30].
Surgery
Surgery is needed for unresponsive HC cases. However, surgery should be a last resort treatment as it is associated with high morbidity and mortality rates, especially in older individuals with co-morbidities. Selective embolization or ligation of the iliac or bladder arteries is an aggressive method to arrest bleeding [31]. Several urinary diversion methods have been used to decommission the bladder. The preferred method for urinary diversion is a transverse colon conduit, as, unlike the small bowel, the transverse colon generally has been outside the pelvic radiation field and thus has not experienced radiation damage. Urinary diversion can also be achieved through percutaneous nephrostomy and cutaneous ureterostomy [32]. Cystectomy is generally performed in conjunction with the diversion surgery as the decommissioned bladder can cause severe complications, such as pyocystitis, pain, bleeding, and neoplastic transformation [33]. A history of high dose radiation therapy is a risk factor for post-surgery complications.
Botulinum toxin (BoNT)
BoNT is used in many disciplines to induce local muscular paralysis. It acts by blocking the release of the neurotransmitter acetylcholine at neuromuscular junctions [34]. BoNT may also act as an anti-inflammatory drug by suppressing EP4 receptors and cyclooxygenase-2 expression [35, 36]. In a recent study, BoNT injections increased bladder capacity and moderately decreased frequency in 5 out of 6 enrolled patients refractory to conventional therapies [37]. No cases of urinary retention developed. While further studies are needed, BoNT injections may be a promising therapy to treat the lower urinary tract symptoms in patients with HC [37].
Tacrolimus
Tacrolimus is used as a systemic therapy for inhibiting transplant rejection and as topical ointment for moderate to severe atopic dermatitis, due to its immunosuppressive properties. Tacrolimus is an immunosuppressant agent by suppressing IL-2-dependent T-cell activation. In addition, Tacrolimus has vasoconstrive properties that can cause severe side-effects when administered systemically. Intravesical instillation of Tacrolimus in HC can thus have a dual effect: inhibition of chronic inflammation and arresting hemorrhaging without causing systemic complications.
Tacrolimus was used in a compassionate care case of a frail 81 year-old man with multiple co-morbidities who had received external beam radiation therapy for the treatment of prostate cancer approximately 10 years earlier [38]. The patient had a history of HC and had previously undergone HBOT. The patient was in and out of the hospital for nearly a month for HC requiring catheterization, bladder irrigation, blood transfusions, and two fulguration surgeries. Before considering formalin instillation and emergency cystectomy, the patient received two instillations of intravesical tacrolimus that were well tolerated. Blood tacrolimus levels remained well below reference range, gross hematuria diminished, and the patient was discharged without gross hematuria after an additional 48 h of observation. During the next 6 months, the patient was able to stay at home without further hematuria.
Conclusions
Despite a growing interest in cancer survivorship, radiation cystitis, and hemorrhagic cystitis in general, is under-recognized and lacks effective treatment options. Support groups for cancer survivors with radiation damage are being formed to bring attention to these diseases, including The Radiation Cystitis Foundation (http://www.radiationcystitis.org/). RC is a progressive and, currently, irreversible condition. Thus means to early diagnose patients and new fast, safe, and reliable therapies are needed to prevent the progression to more severe symptoms.
Acknowledgements
The authors gratefully acknowledge the funding support of the National Institute of Health, K01DK114334, and the U Can-Cer Vive Foundation.
Footnotes
Conflict of interest Dr. Chancellor is the founder and Chief Scientific Officer of Lipella Pharmaceuticals, Inc. The remaining authors declare that they have no relevant conflicts of interest.
References
- 1.American Cancer Society (2018) Cancer facts & figs. American Cancer Society, Atlanta [Google Scholar]
- 2.Basler JS (2009) Hemorrhagic cystitis eMedicine. Medscape, Santa Fe [Google Scholar]
- 3.Payne H, Adamson A, Bahl A, Borwell J, Dodds D, Heath C, Hud-dart R, McMenemin R, Patel P, Peters JL, Thompson A (2013) Chemical- and radiation-induced haemorrhagic cystitis: current treatments and challenges. BJU Int 112(7):885–897. 10.1111/bju.12291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matz EL, Hsieh MH (2017) Review of advances in uroprotective agents for cyclophosphamide- and ifosfamide-induced hemorrhagic cystitis. Urology 100:16–19. 10.1016/j.urology.2016.07.030 [DOI] [PubMed] [Google Scholar]
- 5.Isono-Nakata R, Tsubamoto H, Ueda T, Inoue K, Shibahara H (2018) Bevacizumab with metronomic chemotherapy of low-dose oral cyclophosphamide in recurrent cervical cancer: four cases. Gynecol Oncol Rep 24:57–60. 10.1016/j.gore.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boo LW, Vulink AJE, Bos M (2017) Metronomic cyclophosphamide-induced long-term remission after recurrent high-grade serous ovarian cancer: a case study. Mol Clin Oncol 7(6):1130–1134. 10.3892/mco.2017.1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zwaans BM, Chancellor MB, Lamb LE (2016) Modeling and treatment of radiation cystitis. Urology 88:14–21. 10.1016/j.urology.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 8.Zwaans BM, Nicolai HG, Chancellor MB, Lamb LE (2016) Challenges and opportunities in radiation-induced hemorrhagic cystitis. Rev Urol 18(2):57–65. 10.3909/riu0700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decker DB, Karam JA, Wilcox DT (2009) Pediatric hemorrhagic cystitis. J Pediatr Urol 5(4):254–264. 10.1016/j.jpurol.2009.02.199 [DOI] [PubMed] [Google Scholar]
- 10.Delaney G, Jacob S, Barton M (2004) Estimation of an optimal radiotherapy utilization rate for gynecologic carcinoma: part I–malignancies of the cervix, ovary, vagina and vulva. Cancer 101(4):671–681. 10.1002/cncr.20444 [DOI] [PubMed] [Google Scholar]
- 11.Delaney G, Jacob S, Barton M (2004) Estimation of an optimal radiotherapy utilization rate for gynecologic carcinoma: part II–carcinoma of the endometrium. Cancer 101(4):682–692. 10.1002/cncr.20445 [DOI] [PubMed] [Google Scholar]
- 12.Mendenhall WM, Henderson RH, Costa JA, Hoppe BS, Dagan R, Bryant CM, Nichols RC, Williams CR, Harris SE, Menden-hall NP (2015) Hemorrhagic radiation cystitis. Am J Clin Oncol 38(3):331–336. 10.1097/COC.0000000000000016 [DOI] [PubMed] [Google Scholar]
- 13.Chassagne D, Sismondi P, Horiot JC, Sinistrero G, Bey P, Zola P, Pernot M, Gerbaulet A, Kunkler I, Michel G (1993) A glossary for reporting complications of treatment in gynecological cancers. Radiother Oncol 26(3):195–202 [DOI] [PubMed] [Google Scholar]
- 14.Suriano F, Altobelli E, Sergi F, Buscarini M (2013) Bladder cancer after radiotherapy for prostate cancer. Rev Urol 15(3):108–112 [PMC free article] [PubMed] [Google Scholar]
- 15.Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N (2017) Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28(suppl_4):iv72–iv83. 10.1093/annonc/mdx220 [DOI] [PubMed] [Google Scholar]
- 16.Perez CA, Grigsby PW, Lockett MA, Chao KS, Williamson J (1999) Radiation therapy morbidity in carcinoma of the uterine cervix: dosimetric and clinical correlation. Int J Radiat Oncol Biol Phys 44(4):855–866 [DOI] [PubMed] [Google Scholar]
- 17.Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, Sessa C (2013) Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6):633–638. 10.1093/annonc/mdt353 [DOI] [PubMed] [Google Scholar]
- 18.Nout RA, Smit VT, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, Mens JW, Slot A, Kroese MC, van Bunningen BN, Ansink AC, van Putten WL, Creutzberg CL (2010) Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet 375(9717):816–823. 10.1016/s0140-6736(09)62163-2 [DOI] [PubMed] [Google Scholar]
- 19.Denton AS, Clarke NW, Maher EJ (2002) Non-surgical interventions for late radiation cystitis in patients who have received radical radiotherapy to the pelvis. Cochrane Database Syst Rev. 10.1002/14651858.CD001773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capelli-Schellpfeffer M, Gerber GS (1999) The use of hyperbaric oxygen in urology. J Urol 162(3 Pt 1):647–654 [DOI] [PubMed] [Google Scholar]
- 21.Zwaans BM, Chancellor MB, Lamb LE (2015) Modeling and treatment of radiation cystitis. Urology. 10.1016/j.urology.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 22.Chong KT, Hampson NB, Corman JM (2005) Early hyperbaric oxygen therapy improves outcome for radiation-induced hemorrhagic cystitis. Urology 65(4):649–653. 10.1016/j.urology.2004.10.050 [DOI] [PubMed] [Google Scholar]
- 23.Oliai C, Fisher B, Jani A, Wong M, Poli J, Brady LW, Komarnicky LT (2012) Hyperbaric oxygen therapy for radiation-induced cystitis and proctitis. Int J Radiat Oncol Biol Phys 84(3):733–740. 10.1016/j.ijrobp.2011.12.056 [DOI] [PubMed] [Google Scholar]
- 24.Choong SK, Walkden M, Kirby R (2000) The management of intractable haematuria. BJU Int 86(9):951–959 [DOI] [PubMed] [Google Scholar]
- 25.Levenback C, Eifel PJ, Burke TW, Morris M, Gershenson DM (1994) Hemorrhagic cystitis following radiotherapy for stage Ib cancer of the cervix. Gynecol Oncol 55(2):206–210. 10.1006/gyno.1994.1278 [DOI] [PubMed] [Google Scholar]
- 26.Goswami AK, Mahajan RK, Nath R, Sharma SK (1993) How safe is 1% alum irrigation in controlling intractable vesical hemorrhage? J Urol 149(2):264–267 [DOI] [PubMed] [Google Scholar]
- 27.Phelps KR, Naylor K, Brien TP, Wilbur H, Haqqie SS (1999) Encephalopathy after bladder irrigation with alum: case report and literature review. Am J Med Sci 318(3):181–185 [DOI] [PubMed] [Google Scholar]
- 28.Singh I, Laungani GB (1992) Intravesical epsilon aminocaproic acid in management of intractable bladder hemorrhage. Urology 40(3):227–229 [DOI] [PubMed] [Google Scholar]
- 29.Laszlo D, Bosi A, Guidi S, Saccardi R, Vannucchi AM, Lombardini L, Longo G, Fanci R, Azzi A, De Santis R et al. (1995) Prostaglandin E2 bladder instillation for the treatment of hemorrhagic cystitis after allogeneic bone marrow transplantation. Haematologica 80(5):421–425 [PubMed] [Google Scholar]
- 30.Dewan AK, Mohan GM, Ravi R (1993) Intravesical formalin for hemorrhagic cystitis following irradiation of cancer of the cervix. Int J Gynaecol Obstet 42(2):131–135 [DOI] [PubMed] [Google Scholar]
- 31.De Berardinis E, Vicini P, Salvatori F, Sciarra A, Gentile V, Di Silverio F (2005) Superselective embolization of bladder arteries in the treatment of intractable bladder haemorrhage. Int J Urol 12(5):503–505. 10.1111/j.1442-2042.2005.01074.x [DOI] [PubMed] [Google Scholar]
- 32.Bondavalli C, Dall’Oglio B, Schiavon L, Luciano M, Guatelli S, Parma P, Galletta V (2003) Complications of urinary diversion after radiotherapy. Arch Ital Urol Androl 75(1):10–13 [PubMed] [Google Scholar]
- 33.Fazili T, Bhat TR, Masood S, Palmer JH, Mufti GR (2006) Fate of the leftover bladder after supravesical urinary diversion for benign disease. J Urol 176(2):620–621. 10.1016/j.juro.2006.03.056 [DOI] [PubMed] [Google Scholar]
- 34.Duthie JB, Vincent M, Herbison GP, Wilson DI, Wilson D (2011) Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Database Syst Rev. 10.1002/14651858.CD005493.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chuang YC, Yoshimura N, Huang CC, Wu M, Chiang PH, Chancellor MB (2009) Intravesical botulinum toxin A administration inhibits COX-2 and EP4 expression and suppresses bladder hyper-activity in cyclophosphamide-induced cystitis in rats. Eur Urol 56(1):159–166. 10.1016/j.eururo.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 36.Ha US, Park EY, Kim JC (2011) Effect of botulinum toxin on expression of nerve growth factor and transient receptor potential vanilloid 1 in urothelium and detrusor muscle of rats with bladder outlet obstruction-induced detrusor overactivity. Urology 78(3):721 e721–721 e726. 10.1016/j.urology.2011.03.070 [DOI] [PubMed] [Google Scholar]
- 37.Chuang YC, Kim DK, Chiang PH, Chancellor MB (2008) Bladder botulinum toxin A injection can benefit patients with radiation and chemical cystitis. BJU Int 102(6):704–706. 10.1111/j.1464-410X.2008.07740.x [DOI] [PubMed] [Google Scholar]
- 38.Dave CN, Chaus F, Chancellor MB, Lajness M, Peters KM (2015) Innovative use of intravesical tacrolimus for hemorrhagic radiation cystitis. Int Urol Nephrol 47(10):1679–1681. 10.1007/s11255-015-1098-6 [DOI] [PubMed] [Google Scholar]