Abstract
A malignant brain tumor diagnosis is often accompanied with intense feelings and can be associated with psychosocial conditions including depression, anxiety, and/or increased distress levels. Previous work has highlighted the impact of uncontrolled psychological distress among brain tumor patients. Given the negative impact of maladaptive psychosocial and biobehavioral factors on normal immune system functions, the question remains as to how psychological conditions potentially affect the brain tumor patient anti-tumor immune response. Since immunotherapy has yet to show efficacy at increasing malignant glioma patient survival in all randomized, phase III clinical trials to-date, this review provides new insights into the potential negative effects of chronic distress on brain tumor patient immune functions and outcomes.
Keywords: Psychosocial, biobehavioral, glioma, glioblastoma, immunosuppression
Challenges of immunotherapy for malignant glioma
Brain tumors are a relatively rare but potentially life-threatening diagnosis, accounting for approximately 1–2% of cancers in the United States [1]. Nearly 80% of primary malignant brain tumors are a form of glioma, with glioblastoma (GBM, WHO grade IV) representing the most common, deadly subtype, and accounting for over half of all malignant glioma cases [2]. Despite the current standard of care, GBM remains incurable with a median overall survival (OS) ranging from 9 to 21 months, and a five-year survival of only 5% to 14% [3–5]. Standard of care for GBM patients consists of surgical resection, radiation, chemotherapy, and tumor treating fields, which prolongs survival and may transiently reduce symptom burden. However, these treatments may impair quality of life in addition to a diagnosis that already induces significant psychological distress [6].
Recent research efforts aimed at enhancing treatment efficacy for GBM have focused on immunotherapy, an effective treatment strategy for some cancer patients diagnosed with melanoma [7], non-small cell lung [8], and renal cell [9] malignancies, as well as many others that originate outside of the central nervous system (CNS) [10–14]. In contrast, high-grade glioma patients treated with immunotherapy have not demonstrated an OS benefit in all phase III clinical trials to-date [15, 16]. Several immunotherapeutic strategies have been investigated, including: (i) vaccines (ie. rindopepimut, HSPPC96, or dendritic cells); (ii) checkpoint inhibitors - drugs that enhance immune system efficacy in destroying cancer cells [ie. nivolumab (anti- PD-1 mAb)]; and (iii) chimeric antigen receptor (CAR) T cells. However, despite the excitement from early phase trials, immunotherapy has yet to translate into an effective clinical solution for patients, as demonstrated in late phase, randomized, multi-site clinical evaluation [15, 17–19].
Additional challenges likely contributing to the effective application of immunotherapy for treating GBM include the: (i) highly infiltrating nature of the disease, which prevents surgical resection from completely removing all associated tumor burden; (ii) potently immunosuppressive microenvironment, characterized by a relatively low level of tumor-infiltrating effector T cells, combined with an accumulation of immunosuppressive regulatory T cells (Tregs; CD4+CD25+FoxP3+) and myeloid derived suppressor cells; (iii) older age of the patient; and (iv) anatomical location that impedes a routine use of tumor biopsies for monitoring the response to therapy. A critical factor that may be overlooked during immunotherapeutic treatment considerations is the impact of high psychosocial and biobehavioral levels of distress. The subject of distress, specifically its prevalence and influence on the immune system and outcomes in patients with a malignant brain tumor, forms the basis of our review. Research in the past two years will be emphasized, with attention to the associations between increased distress levels, the negative impact of systemic immune/neuroendocrine functions, and preclinical and clinical outcomes in the setting of primary brain cancer.
Psychological distress in patients with a brain tumor
Depression and anxiety are common in patients with a terminal illness [20].Meta-analyses have found that cancer patients in the palliative care setting have a rate of mood disorders, including depression, anxiety, and adjustment disorder, of 29% [20]. Notably, this rate of psychosocial conditions is likely underappreciated and/or underreported, as cancer patients experience chronic distress and demoralization without the presence of a psychiatric diagnosis [21–23].
Psychological distress, depression, and anxiety may be particularly enhanced in patients with primary brain tumors. A recent analysis found that 24% of a 4,000 mixed-cancer patient cohort possessed clinical signs of depression, significantly higher than the general population [24]. Strikingly, the rate among patients with primary brain cancer was markedly higher, with 36% showing signs of depression. In contrast, patients diagnosed with malignant melanoma and prostate cancer had reduced rates of depression, at 16% and 10% respectively. Additional meta-analyses and prospective studies have discovered an increased rate of psychological conditions and distress among brain tumor patients as compared to the general population [25, 26] and as compared to patients diagnosed with non-CNS tumors [27].
Furthermore, psychological distress in brain tumor patients is not always appropriately acknowledged or well-managed. Although attention to the mental health care of malignant brain tumor patients has increased [28–30], a recent review by the European Association of Neuro-Oncology emphasized the lack of evidence for appropriately treating depression and anxiety in glioma patients and, as such, was unable to provide strong recommendations on the subject [31]. The direct neurological involvement of primary brain tumors further complicates management guidance from a psychosocial standpoint compared to non-CNS cancers. Recent studies have found that psychological needs are highly unmet among brain tumor patients [32, 33]. A longitudinal investigation of mostly high-grade glioma patients found that despite increased distress in approximately half of the patients during various time points, only 14% of the distressed patients received psychological care as an in-patient, and less than half of the distressed patients received psychological care in an outpatient setting [34]. Collectively, the above studies indicate psychological distress and depression as common concerns for patients with primary brain cancer.
The etiology of distress among primary brain cancer patients may be related to the inherently high load of stressors accompanying their diagnosis. However, neurological insult, regardless of tumor burden, has been shown to be correlated with increased depression and distress. Patients who present with a stroke have an increased rate of depression as compared to those with a myocardial infarction [35], and the prevalence of depression is also higher for individuals diagnosed with the CNS autoimmune disease multiple sclerosis (MS), as well as for traumatic brain injury (TBI) patients [36, 37]. Although potentially resulting from the reduced quality of life suffered by patients with neurological insult(s), increased depression may be the direct result of enhanced inflammation. There is a well-established link between the development of depression and stressors that trigger inflammatory phenotypes and increased immune activity through the sympathetic nervous system (SNS) [38, 39]. Indeed, inflammation resulting from stroke, MS, and TBI is associated with depression, further supporting a role for generalized inflammation in the pathogenesis of depression [40–43]. The connection between inflammation and depression is not well-characterized for patients with primary brain cancer. However, levels of inflammatory cytokines are significantly increased in GBM patients and may play a role in outcomes dependent on levels of depression, anxiety, and distress [44–46] (Fig. 1). Additional work is necessary to comprehensively profile the incidence of psychosocial conditions in brain tumor patients and its effects on OS outcomes and responses to therapy.
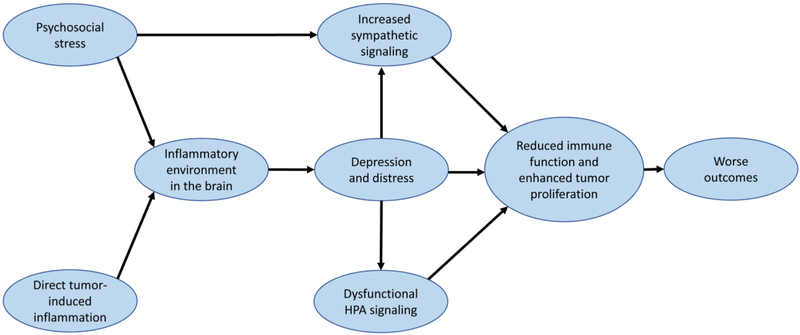
Figure 1. Proposed interactions between inflammation, depression, and immune cell dysfunction in subjects with brain cancer.
Brain tumors and psychosocial stress independently contribute to brain inflammation, which provide favorable conditions for the development of depression. High distress levels lead to a cycle of increased sympathetic signaling and dysfunctional HPA signaling. Together, signaling due to the sympathetic and HPA axes decrease immune effector control of tumor cell proliferation, resulting in worse outcomes for patients. Breaking these cycles and addressing increased distress / depression in brain cancer patients may improve survival outcomes.
Interactions between distress, immunosuppression, and cancer
Untreated distress may affect brain tumor patient outcomes through the interactions between psychosocial distress and the immune system. The relationship between excess adrenergic signaling and immune dysregulation, inflammation, and tumor growth is well-established [47–49]. Increased distress promotes the release of stress hormones, primarily in the form of catecholamines including epinephrine and norepinephrine via the sympathetic nervous system (SNS), as well as glucocorticoids via the hypothalamic-pituitary-adrenal axis. Chronic release of these molecules, in turn, increases immunosuppression that facilitates uncontrolled tumor growth by inhibiting the anti-tumor immune response as demonstrated in preclinical models of lymphoma and fibrosarcoma [50, 51]. Interestingly, beta blockers, which inhibit catecholamine-induced β-adrenergic receptor signaling, inhibit proliferation and tumor growth in models of liver cancer, early stage breast cancer, and melanoma [52–54], although this effect is not universal [55]. Additionally, recent studies found that the inhibition of β-adrenergic signaling with the pan-beta blocker propranolol enhances PD-1 checkpoint inhibitor efficacy in preclinical, syngeneic, mouse melanoma models [54, 56]. Notably, β-adrenergic blockade increased the frequency of CD8+ tumor-infiltrating lymphocytes, as well as the ratio of CD8+ effector T cells to Tregs in B16-OVA tumors [56]. Altogether, these studies suggest a potential role for targeting β-adrenergic signaling to decrease tumor cell proliferation and synergize with immunotherapy.
Although once thought to be an immuno-privileged site, it is now well-established that leukocytes infiltrate the brain upon appropriate activation signals [57–59]. However, potent immunosuppressive factors are normally present in the brain, including TGF-β, IL-10, and VEGF, which dampen cytotoxic T lymphocyte migration while enhancing Treg accumulation [60]. Parenchymal cells of the CNS also express Fas ligand (FasL), which can facilitate brain-infiltrating Fas+ T cell apoptosis through cell-to-cell signaling. Collectively, these characteristics contribute to the profoundly immunosuppressive environment under normal circumstances that likely enhances the poor immune response against malignant glioma. Increased β- adrenergic signaling in response to psychosocial distress would only exacerbate the profoundly immunosuppressive microenvironment and facilitate immune escape of GBM cells.
To counteract immunosuppressive β-adrenergic signaling, propranolol has been considered for adjuvant therapy in patients diagnosed with malignant glioma. However, this consideration is primarily based on the results observed in other cancers, or as direct evidence in GBM cells [61]. Accordingly, isoproterenol, a β-adrenergic receptor agonist, enhances GBM U251 cell proliferation and metalloproteinase expression – an effect inhibited by propranolol treatment [62], Sympathetic blockade with β-adrenergic receptors blockade with prazosin also inhibits glioblastoma tumor growth in an orthotopic, immunocompetent, syngeneic mouse model, highlighting the potential for an immunosuppressive contribution by β-adrenergic signaling mechanisms [63]. Beyond adult brain tumors, β-adrenergic receptors are expressed by malignant pediatric brain tumor cells [64], raising the possible generalizability of targeting the β-adrenergic signaling axis in both the pediatric and adult brain tumor settings.
Cortisol is a glucocorticoid released during periods of distress and may also play a dominant role in promoting potent immunosuppression [65]. The chronic release of glucocorticoids, as mediated by mechanisms associated with chronically high levels of distress, may promote tumor progression by suppressing the anti-GBM immune response [66, 67]. While the impact of endogenous glucocorticoid signaling has not been characterized among brain tumor patients and their associated outcomes, a large body of evidence suggests that the use of exogenously-administered dexamethasone (trade name, Decadron), is potently immunosuppressive [68]. Dexamethasone is a corticosteroid commonly used to relieve symptomatic effects and intracranial edema during therapy for high-grade glioma. Treatment with dexamethasone is associated with a worse prognosis of patients with GBM [69, 70]. In contrast, the fast tapering of dexamethasone is associated with significantly better outcomes [71]. Furthermore, phase I trial evaluation of GBM patients treated with the immune checkpoint inhibitor, atezolizumab, had a decreased level of circulating lymphocytes and a trend toward decreased OS when concurrently treated with corticosteroids [72]. Strikingly, long-term survivors in the trial were not treated with corticosteroids. While it is inappropriate to assume that the effects of synthetic steroids are directly comparable to endogenous glucocorticoid signaling, it is possible that the chronic release of cortisol due to high distress levels suppresses leukocyte functions and results in a worse patient prognosis, similar to those effects associated with dexamethasone treatment.
Impact of distress on outcomes in brain cancer patients
Psychological distress has been linked to a reduced quality of life among individuals diagnosed with glioma and in other brain tumor patients [24, 73]. A recent meta-analysis found a decrease in glioma patient OS when also diagnosed with depression [74]. Similarly, a retrospective analysis of 1,003 patients undergoing surgical management of high-grade glioma found that a clinical diagnosis of depression prior to surgical intervention was associated with a decreased landmark rate of survival [75]. Although there was significant heterogeneity within the studies, these collective results suggest a potential for depression to negatively impact the physical health of glioma patients. In contrast, demographic factors associated with the potential strengthening of a glioma patient’s support system, including marriage, are predictors for improved GBM patient OS [76]. Exercise also improves glioma outcomes [77, 78] potentially by decreasing the effects of inflammation and/or inflammatory mediator-induced depression [79, 80]. Therefore, interventions that decrease the level of psychological distress may improve future prognoses among patients with malignant glioma (Fig. 2).
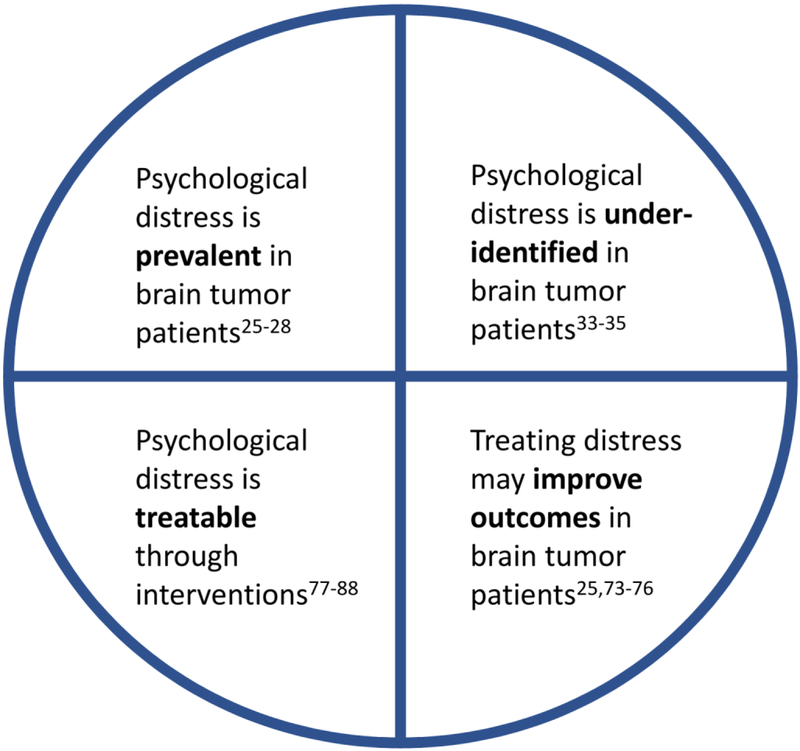
Figure 2. Psychological distress in patients with brain cancer.
The needs and actionable items of psychological distress are summarized for brain cancer patients. Addressing and acknowledging these challenges may help to improve the overall prognosis of brain cancer patients
Standardized surveys have been proposed as mechanisms to better assess psychological distress levels in patients with brain cancer [81–83]. Reducing stigma to increase participation in psychosocial assistance programs is also necessary to properly care for patients with elevated distress levels [84, 85]. After identification and connection with psychosocial services, proper treatment can be initiated. Currently, home-based psychosocial interventions and other therapies have been shown to address distress in brain cancer patients [86, 87].
Few studies have previously evaluated the role of medications in addressing psychosocial distress in malignant brain tumor patients. Although beta blockers may help improve survival in distressed glioma patients based on evidence from the treatment of patients diagnosed with non-CNS cancers, the use of selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants (TCAs) may be another beneficial option. Exposure of tumor cells to SSRIs causes anti-proliferative effects in vitro and in mouse models of GBM. Theoretically, SSRIs would also decrease distress levels in glioma patients [88– 90]. A study of 160 individuals diagnosed with glioma showed an OS benefit for 35 patients treated with an SSRI, while several reports have advocated for their use as potential adjunct therapy during standard of care [91, 92]. Ultimately, more investigation is needed to objectively evaluate the efficacy of psychosocial modifiers in patients with malignant brain cancer.
Conclusions
Malignant brain tumors are a profoundly devastating disease associated with significant increases in depression, anxiety, and distress among patients. We propose a direct biological link between psychosocial stressors and poor outcomes in malignant glioma patients. High adrenergic signaling and endogenous steroid activity may impair immune system functions, resulting in the uncontrolled proliferation of tumor cells, as observed in non-CNS cancer patients. Notably, high levels of distress are not always appropriately identified or managed among brain tumor patients. Therefore, additional work is required for understanding the role of distress on prognosis, immune suppression, tumor growth, and clinical care. Ultimately, this future work may substantially improve the quality, and potentially, quantity of life.
HIGHLIGHTS.
Depression and psychological distress are prevalent in patients with brain tumors.
Chronic distress leads to increased adrenergic signaling and immunosuppression.
Adrenergic signaling and immunotherapy failure is unexplored in brain tumors.
Increased distress is associated with poor brain cancer patient outcomes.
New methods to identify and treat distress are being developed.
Funding:
This work was supported by NIH grants R00 NS082381 (D.A.W.), R01 NS097851–01 (D.A.W.), P50 CA221747 Project 2 (D.A.W. and R.V.L.), and T32 CA070085 (E.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None
REFERENCES
- 1.United States Cancer Statistics: 1999–2011 Incidence, WONDER Online Database., in United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. 2015, Centers for Disease Control and Prevention National Center for Health Statistics. . [Google Scholar]
- 2.Ostrom QT, et al. , CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncology, 2017. 19(suppl_5): p. v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubrow R, et al. , Time trends in glioblastoma multiforme survival: the role of temozolomide. Neuro-Oncology, 2013. 15(12): p. 1750–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darefsky AS, King JT, and Dubrow R, Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer, 2012. 118(8): p. 2163–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stupp R, et al. , Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. Jama, 2017. 318(23): p. 2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu R, et al. , Quality of life in adults with brain tumors: current knowledge and future directions. Neuro Oncol, 2009. 11(3): p. 330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larkin J, et al. , Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med, 2015. 373(1): p. 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonia SJ, et al. , Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol, 2016. 17(7): p. 883–95. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, et al. , Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med, 2015. 373(19): p. 1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen R, et al. , Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J Clin Oncol, 2017. 35(19): p. 2125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zinzani PL, et al. , Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood, 2017. 130(3): p. 267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellmunt J, et al. , Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med, 2017. 376(11): p. 1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frenel JS, et al. , Safety and Efficacy of Pembrolizumab in Advanced, Programmed Death Ligand 1-Positive Cervical Cancer: Results From the Phase Ib KEYNOTE-028 Trial. J Clin Oncol, 2017. 35(36): p. 4035–4041. [DOI] [PubMed] [Google Scholar]
- 14.Muro K, et al. , Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol, 2016. 17(6): p. 717–726. [DOI] [PubMed] [Google Scholar]
- 15.Weller M, et al. , Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol, 2017. 18(10): p. 1373–1385. [DOI] [PubMed] [Google Scholar]
- 16.Reardon DA, et al. , OS10.3 Randomized Phase 3 Study Evaluating the Efficacy and Safety of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: CheckMate 143. Neuro-Oncology, 2017. 19(suppl_3): p. iii21–iii21. [Google Scholar]
- 17.Schuster J, et al. , A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro Oncol, 2015. 17(6): p. 854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampson JH, et al. , Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol, 2010. 28(31): p. 4722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloch O, et al. , ATIM-14. ALLIANCE A071101: A PHASE II RANDOMIZED TRIAL COMPARING THE EFFICACY OF HEAT SHOCK PROTEIN PEPTIDE COMPLEX-96 (HSPPC-96) VACCINE GIVEN WITH BEVACIZUMAB VERSUS BEVACIZUMAB ALONE IN THE TREATMENT OF SURGICALLY RESECTABLE RECURRENT GLIOBLASTOMA. Neuro-Oncology, 2017. 19(suppl_6): p. vi29–vi29. [Google Scholar]
- 20.Mitchell AJ, et al. , Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol, 2011. 12(2): p. 160–74. [DOI] [PubMed] [Google Scholar]
- 21.Vehling S, et al. , The association of demoralization with mental disorders and suicidal ideation in patients with cancer. Cancer, 2017. 123(17): p. 3394–3401. [DOI] [PubMed] [Google Scholar]
- 22.Sterckx W, et al. , Living with a high-grade glioma: A qualitative study of patients’ experiences and care needs. Eur J Oncol Nurs, 2015. 19(4): p. 383–90. [DOI] [PubMed] [Google Scholar]
- 23.Philip J, et al. , “I’m just waiting…”: an exploration of the experience of living and dying with primary malignant glioma. (1433–7339 (Electronic)). [DOI] [PubMed] [Google Scholar]
- 24.Hartung TJ, et al. , The risk of being depressed is significantly higher in cancer patients than in the general population: Prevalence and severity of depressive symptoms across major cancer types. Eur J Cancer, 2017. 72: p. 46–53. [DOI] [PubMed] [Google Scholar]
- 25.Liu F, et al. , Screening for distress in patients with primary brain tumor using distress thermometer: a systematic review and meta-analysis. BMC Cancer, 2018. 18(1): p. 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang J, et al. , Association between depression and brain tumor: a systematic review and meta-analysis. Oncotarget, 2017. 8(55): p. 94932–94943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edelstein K, et al. , Illness intrusiveness and subjective well-being in patients with glioblastoma. Neurooncol, 2016. 126(1): p. 127–35. [DOI] [PubMed] [Google Scholar]
- 28.Rooney AG, Carson A, and Grant R, Depression in Cerebral Glioma Patients: A Systematic Review of Observational Studies. JNCI: Journal of the National Cancer Institute, 2011. 103(1): p. 61–76. [DOI] [PubMed] [Google Scholar]
- 29.Catt S, Chalmers A, and Fallowfield L, Psychosocial and supportive-care needs in high-grade glioma. Lancet Oncol, 2008. 9(9): p. 884–91. [DOI] [PubMed] [Google Scholar]
- 30.Randazzo D and Peters KB, Psychosocial distress and its effects on the health-related quality of life of primary brain tumor patients. CNS Oncol, 2016. 5(4): p. 241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pace A, et al. , European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol, 2017. 18(6): p. e330–e340. [DOI] [PubMed] [Google Scholar]
- 32.Langbecker D and Yates P, Primary brain tumor patients’ supportive care needs and multidisciplinary rehabilitation, community and psychosocial support services: awareness, referral and utilization. J Neurooncol, 2016. 127(1): p. 91–102. [DOI] [PubMed] [Google Scholar]
- 33.Renovanz M, et al. , Factors associated with supportive care needs in glioma patients in the neuro-oncological outpatient setting. J Neurooncol, 2017. 133(3): p. 653–662. [DOI] [PubMed] [Google Scholar]
- 34.Singer S, et al. , Psychiatric co-morbidity, distress, and use of psycho-social services in adult glioma patients-a prospective study. Acta Neurochir (Wien), 2018. 160(6): p. 1187–1194. [DOI] [PubMed] [Google Scholar]
- 35.Ladwig S, et al. , Comparison of Treatment Rates of Depression After Stroke Versus Myocardial Infarction: A Systematic Review and Meta-Analysis of Observational Data. Psychosom Med, 2018. 80(8): p. 754–763. [DOI] [PubMed] [Google Scholar]
- 36.Feinstein A, et al. , The link between multiple sclerosis and depression. Nature Reviews Neurology, 2014. 10: p. 507. [DOI] [PubMed] [Google Scholar]
- 37.Osborn AJ, Mathias JL, and Fairweather-Schmidt AK, Depression following adult, non-penetrating traumatic brain injury: a meta-analysis examining methodological variables and sample characteristics. Neurosci Biobehav Rev, 2014. 47: p. 1–15. [DOI] [PubMed] [Google Scholar]
- 38.Miller AH and Raison CL, The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature reviews. Immunology, 2016. 16(1): p. 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slavich GM and Irwin MR, From Stress to Inflammation and Major Depressive Disorder: A Social Signal Transduction Theory of Depression. Psychological bulletin, 2014. 140(3): p. 774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bodnar CN, Morganti JM, and Bachstetter AD, Depression following a traumatic brain injury: uncovering cytokine dysregulation as a pathogenic mechanism. Neural Regen Res, 2018. 13(10): p. 1693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fenn AM, et al. , Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biol Psychiatry, 2014. 76(7): p. 575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levada OA and Troyan AS, Poststroke Depression Biomarkers: A Narrative Review. Front Neurol, 2018. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris G, et al. , Multiple Immune-Inflammatory and Oxidative and Nitrosative Stress Pathways Explain the Frequent Presence of Depression in Multiple Sclerosis. Mol Neurobiol, 2018. 55(8): p. 6282–6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeung YT, et al. , Interleukins in glioblastoma pathophysiology: implications for therapy. Br J Pharmacol, 2013. 168(3): p. 591–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kore RA and Abraham EC, Inflammatory cytokines, interleukin-1 beta and tumor necrosis factor-alpha, upregulated in glioblastoma multiforme, raise the levels of CRYAB in exosomes secreted by U373 glioma cells. Biochem Biophys Res Commun, 2014. 453(3): p. 326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiorean R, et al. , Quantitative expression of serum biomarkers involved in angiogenesis and inflammation, in patients with glioblastoma multiforme: correlations with clinical data. Cancer Biomark, 2014. 14(2–3): p. 185–94. [DOI] [PubMed] [Google Scholar]
- 47.Powell ND, Tarr AJ, and Sheridan JF, Psychosocial stress and inflammation in cancer. Brain Behav Immun, 2013. 30 Suppl: p. S41–7. [DOI] [PubMed] [Google Scholar]
- 48.Qiao G, et al. , Adrenergic Signaling: A Targetable Checkpoint Limiting Development of the Antitumor Immune Response. Front Immunol, 2018. 9: p. 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Partecke LI, et al. , Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonised by beta-adrenergic receptor blockade. Pancreatology, 2016. 16(3): p. 423–33. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt D, et al. , Induction of Suppressor Cells and Increased Tumor Growth following Chronic Psychosocial Stress in Male Mice. PLoS ONE, 2016. 11(7): p. e0159059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nissen MD, Sloan EK, and Mattarollo SR, beta-Adrenergic Signaling Impairs Antitumor CD8(+) T-cell Responses to B-cell Lymphoma Immunotherapy. Cancer Immunol Res, 2018. 6(1): p. 98–109. [DOI] [PubMed] [Google Scholar]
- 52.Wang F, et al. , Propranolol suppresses the proliferation and induces the apoptosis of liver cancer cells. Mol Med Rep, 2018. 17(4): p. 5213–5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montoya A, et al. , Use of non-selective beta-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget, 2017. 8(4): p. 6446–6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kokolus KM, et al. , Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology, 2018. 7(3): p. e1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cardwell CR, et al. , Propranolol and survival from breast cancer: a pooled analysis of European breast cancer cohorts. Breast Cancer Res, 2016. 18(1): p. 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bucsek MJ, et al. , beta-Adrenergic Signaling in Mice Housed at Standard Temperatures Suppresses an Effector Phenotype in CD8(+) T Cells and Undermines Checkpoint Inhibitor Therapy. Cancer Res, 2017. 77(20): p. 5639–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lohr J, et al. , Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res, 2011. 17(13): p. 4296–308. [DOI] [PubMed] [Google Scholar]
- 58.Kmiecik J, et al. , Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. J Neuroimmunol, 2013. 264(1–2): p. 71–83. [DOI] [PubMed] [Google Scholar]
- 59.Zhai L, et al. , Infiltrating T cells increase IDO1 expression in glioblastoma and contribute to decreased patient survival. Clinical Cancer Research, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dutoit V, et al. , Immunotherapy of Malignant Tumors in the Brain: How Different from Other Sites? Front Oncol, 2016. 6: p. 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rundle‐Thiele D, et al. , Repurposing some older drugs that cross the blood–brain barrier and have potential anticancer activity to provide new treatment options for glioblastoma. British Journal of Clinical Pharmacology, 2016. 81(2): p. 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He J-J, et al. , Activation of β-adrenergic receptor promotes cellular proliferation in human glioblastoma. Oncology Letters, 2017. 14(3): p. 3846–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Assad Kahn S, et al. , The anti-hypertensive drug prazosin inhibits glioblastoma growth via the PKCdelta-dependent inhibition of the AKT pathway. EMBO Mol Med, 2016. 8(5): p. 511–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sardi I, et al. , Expression of beta-adrenergic receptors in pediatric malignant brain tumors. Oncol Lett, 2013. 5(1): p. 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silverman MN and Sternberg EM, Glucocorticoid regulation of inflammation and its behavioral and metabolic correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci, 2012. 1261: p. 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dhabhar FS and McEwen BS, Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun, 1997. 11(4): p. 286–306. [DOI] [PubMed] [Google Scholar]
- 67.Eng JW, et al. , A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother, 2014. 63(11): p. 1115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giles AJ, et al. , Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J Immunother Cancer, 2018. 6(1): p. 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pitter KL, et al. , Corticosteroids compromise survival in glioblastoma. Brain, 2016. 139(Pt 5): p. 1458–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shields LB, et al. , Dexamethasone administration during definitive radiation and temozolomide renders a poor prognosis in a retrospective analysis of newly diagnosed glioblastoma patients. Radiat Oncol, 2015. 10: p. 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diez Valle R, et al. , Results of a Policy of Fast Tapering of Steroids After Resection Surgery in Glioblastoma. World Neurosurg, 2018. 109: p. e845–e852. [DOI] [PubMed] [Google Scholar]
- 72.Lukas RV, et al. , Clinical activity and safety of atezolizumab in patients with recurrent glioblastoma. Journal of Neuro-Oncology, 2018. [DOI] [PubMed] [Google Scholar]
- 73.Hoffmann K, et al. , Correlation of psychooncological distress- screening and quality of life assessment in neurosurgical patients. Oncotarget, 2017. 8(67): p. 111396–111404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi C, et al. , Depression and survival of glioma patients: A systematic review and meta-analysis. Clinical Neurology and Neurosurgery, 2018. 172: p. 8–19. [DOI] [PubMed] [Google Scholar]
- 75.Gathinji M, et al. , Association of preoperative depression and survival after resection of malignant brain astrocytoma. Surg Neurol, 2009. 71(3): p. 299–303, discussion 303. [DOI] [PubMed] [Google Scholar]
- 76.Xie JC, et al. , Effect of marital status on survival in glioblastoma multiforme by demographics, education, economic factors, and insurance status. Cancer Medicine, 2018. 7(8): p. 3722–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ruden E, et al. , Exercise Behavior, Functional Capacity, and Survival in Adults With Malignant Recurrent Glioma. Journal of Clinical Oncology, 2011. 29(21): p. 2918–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levin GT, et al. , Exercise Improves Physical Function and Mental Health of Brain Cancer Survivors: Two Exploratory Case Studies. Integr Cancer Ther, 2016. 15(2): p. 190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woods JA, et al. , Exercise, Inflammation and Aging. Aging Dis, 2012. 3(1): p. 130–40. [PMC free article] [PubMed] [Google Scholar]
- 80.Gourgouvelis J, et al. , Exercise Leads to Better Clinical Outcomes in Those Receiving Medication Plus Cognitive Behavioral Therapy for Major Depressive Disorder. Front Psychiatry, 2018. 9: p. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hickmann AK, et al. , Evaluating patients for psychosocial distress and supportive care needs based on health-related quality of life in primary brain tumors: a prospective multicenter analysis of patients with gliomas in an outpatient setting. J Neurooncol, 2017. 131(1): p. 135–151. [DOI] [PubMed] [Google Scholar]
- 82.Renovanz M, et al. , Assessing psychological and supportive care needs in glioma patients -feasibility study on the use of the Supportive Care Needs Survey Short Form (SCNS-SF34-G) and the Supportive Care Needs Survey Screening Tool (SCNS-ST9) in clinical practice. Eur J Cancer Care (Engl), 2018. 27(1). [DOI] [PubMed] [Google Scholar]
- 83.Renovanz M, et al. , Screening for distress in patients with intracranial tumors during the first 6 months after diagnosis using self-reporting instruments and an expert rating scale (the basic documentation for psycho-oncology short form - PO-Bado SF). Oncotarget, 2018. 9(57): p. 31133–31145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Langbecker D, Ekberg S, and Yates P, Don’t need help, don’t want help, can’t get help: How patients with brain tumors account for not using rehabilitation, psychosocial and community services. Patient Educ Couns, 2017. 100(9): p. 1744–1750. [DOI] [PubMed] [Google Scholar]
- 85.Tondorf T, et al. , Focusing on cancer patients’ intentions to use psychooncological support: A longitudinal, mixed-methods study. Psychooncology, 2018. 27(6): p. 1656–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ownsworth T, et al. , Evaluation of the making sense of brain tumor program: a randomized controlled trial of a home-based psychosocial intervention. Psychooncology, 2015. 24(5): p. 540–7. [DOI] [PubMed] [Google Scholar]
- 87.Amidei C, Symptom-based Interventions to Promote Quality Survivorship. Neuro Oncol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu K-H, et al. , Fluoxetine, an antidepressant, suppresses glioblastoma by evoking AMPAR-mediated calcium-dependent apoptosis. Oncotarget, 2015. 6(7): p. 5088–5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen VCH, et al. , Escitalopram oxalate induces apoptosis in U‐87MG cells and autophagy in GBM8401 cells. Journal of Cellular and Molecular Medicine, 2018. 22(2): p. 1167–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Then C-K, et al. , Antidepressants, sertraline and paroxetine, increase calcium influx and induce mitochondrial damage-mediated apoptosis of astrocytes. Oncotarget, 2017. 8(70): p. 115490–115502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caudill JS, et al. , Selective Serotonin Reuptake Inhibitors, Glioblastoma Multiforme, and Impact on Toxicities and Overall Survival: The Mayo Clinic Experience. American Journal of Clinical Oncology, 2011. 34(4). [DOI] [PubMed] [Google Scholar]
- 92.Tan SK, et al. , Drug Repositioning in Glioblastoma: A Pathway Perspective. Frontiers in Pharmacology, 2018. 9: p. 218. [DOI] [PMC free article] [PubMed] [Google Scholar]