Abstract
Biospecimen collection in the Adolescent Brain Cognitive Development (ABCD) study – of hair samples, shed deciduous (baby) teeth, and body fluids – will serve dual functions of screening for study eligibility, and providing measures of biological processes thought to predict or correlate with key study outcomes on brain and cognitive development. Biosamples are being collected annually to screen for recency of drug use prior to the neuroimaging or cognitive testing visit, and to store for the following future studies: (1) on the effects of exposure to illicit and recreational drugs (including alcohol and nicotine); (2) of pubertal hormones on brain and cognitive developmental trajectories; (3) on the contribution of genomics and epigenomics to child and adolescent development and behavioral outcomes; and (4) with pre- and post-natal exposure to environmental neurotoxicants and drugs of abuse measured from novel tooth analyses. The present manuscript describes the rationales for inclusion and selection of the specific biospecimens, methodological considerations for each measure, future plans for assessment of biospecimens during follow-up visits, and preliminary ABCD data to illustrate methodological considerations.
Keywords: Biospecimens, ABCD study, Substance use, Gonadal hormones, Genetics, Environmental exposures
1. Introduction
SU initiation typically begins in the teens. According to the 2016 Monitoring the Future Survey, 7.3% of 8th graders (13–14 years old) have used alcohol, 5.4% have used marijuana, and 2.6% have used tobacco within the last 30 days. Marijuana, alcohol and other substances of abuse are known to negatively impact neurodevelopment in adolescents suggesting that the adolescent brain may have heightened vulnerability to toxic substance effects (Gray and Squeglia, 2017). The Adolescent Brain Cognitive Development (ABCD) Study is a large-scale, prospective, longitudinal, multi-site project designed to study brain and cognitive development in youth, as they transition into adolescence and young adulthood, across the United States (see ABCDStudy.org). Participating families are recruited through school and community events at each respective ABCD study site, online and paper ads, and word of mouth. This is the first study of its kind in the US to focus on factors of critical importance to trajectories of developmental change in brain and cognition during a period of vulnerability to substance use (SU) and other mental health problems. Study outcomes will inform evidence-based standards for normal cognitive and brain development, as well as provide large repositories of data and bio-materials for the study of experiential and environmental influences on brain and cognitive development in youth. Focus on the contributions of pubertal hormones, genomic and epigenomic factors, and the interactions across these many influences, are serving as key biological measures for informing our understanding of developmental and behavioral outcomes in the ABCD study. To this end, along with brain imaging, neurocognitive, and other measures, including a comprehensive battery of mental and physical heath, history of SU, behavioral assessments and key bio-samples (see Table 1) are being obtained from participating youth (target sample: more than 10,000 children, initially 9–10 years of age at enrollment, followed for up to 10 years). The present manuscript describes the rationales for inclusion and selection of the specific biospecimens, methodological considerations for each measure, future plans for assessment of biospecimens during follow-up visits, and preliminary ABCD data for some example topics.
Table 1.
Biospecimen Samples.
| Baseline 9–10 yrs |
1 Year Follow-Up 10–11 yrs |
||
|---|---|---|---|
| Sample Type | Purpose | #/participant | #/participant |
| Screening for Alcohol & Drugs | |||
| Saliva (onsite Dräger)* | Recent Substance Use | 1 | 1 |
| Breath (onsite Breathalyzer)* | Recent Alcohol Use | 1 | 1 |
| Hair (Psychemedicsa)** | Substance Use | 1 | 1 |
| Urine (NicAlert)** | Recent Tobacco Use | 0 | 1 |
| Pubertal Hormones | |||
| Saliva (Salimetricsb) | Pubertal Hormones | 1 | 1 |
| Genomics/Epigenetics | |||
| Saliva (RUCDRc) | Genetics | 1 | 0 |
| Blood (RUCDRc) (saliva if refuse blood) | Genetics | 0*** | 0 |
| Timeline of Exposures | |||
| Baby teeth (Dr. Arora – Mt. Sinaid) | Environmental Exposures | 1 | 1 |
Samples sent to Psychemedics for analysis.
Samples sent to Salimetrics for analysis.
Samples stored at Rutgers University Cell and DNA Repository (RUCDR) for future analysis.
Samples stored at Mt. Sinai in laboratory of Dr. Arora for future analysis.
Collect on 10% of participants at each site.
Send 10 samples per site per year for analysis.
Future collection for twin sites only.
All procedures are approved by each site’s Institutional Review Board, and all participants undergo verbal and written consent/assent procedure. Participants are compensated for their time with cash and/or gift cards. A description of the rationales for inclusion and selection of the specific biospecimens, methodological considerations for each measure, future plans for assessment of biospecimens during follow-up visits, and preliminary ABCD data to illustrate methodological considerations (see below sections) for all biological samples under collection from youth. These include breath, saliva, urine, hair, blood and baby teeth, collected for purposes of: (1) screening for SU; (2) measurement of pubertal hormone levels; (3) characterization of genetic and epigenetic factors, and (4) analyses of environmental exposures during development from baby teeth. Biomaterial obtained from the ABCD Study are being stored in repositories, such as the Rutgers University Cell and DNA Repository (RUCDR), and baby teeth at the Icahn School of Medicine at Mount Sinai in the laboratory of Dr. Manish Arora. These stored biomaterials include measures from the assay of biospecimens, genotyping, and biosamples collected for future research. Preparation and processing of biosamples at the ABCD data collection sites is occurring during or just following baseline assessment of youth, and is planned for follow-up assessments for utilization by members of the scientific community. Results from analyses of all ABCD biospecimens will be made available through the ABCD Data Repository. Although we anticipate core specimens to be the same across the ten years of ABCD, the kinds and/or amounts of specimens to be collected in subsequent follow-up years may be adjusted to account for: 1. changes in technology; 2. shifts in the scientific questions being addressed (e.g, greater emphasis on environmental exposures); or additional funding for analyses. Given these considerations, future specimen collections may include measures of the microbiome, parental specimens, or other types of specimens in subsequent follow-up years.
2. Screening for exposure to alcohol and drugs
The ABCD study baseline visits occur at 9–10 years of age, prior to initiation of SU for most youth allowing for measures of brain, cognitive, environmental, and genetic variability that may precede SU or other negative developmental influences. The ABCD study uses a combination of biospecimens and self-report to evaluate consistency between biological testing, participant self-report and research assistant assessment of intoxication. Self-report alone (e.g., tobacco use) may lead to biases due to under-reporting related to individualized motivations, or errors in recall (Connor Gorber et al., 2009; Morales et al., 2013). Further, as seen with youth self-reporting risky sexual behaviors, inaccurate self-reporting may vary as a function of race, gender or age (Pflieger et al., 2013). However, biospecimen sampling itself is subject to experimental error, and therefore reporting biospecimen measures in a thorough and standardized manner across published ABCD studies is important for accurate and reproducible results (Moore et al., 2011).
Self- and parent/guardian-report of SU is conducted through interview and questionnaire survey. Biospecimens include the annual collection of hair samples to evaluate recent and repeated use of alcohol and other drugs during the 1–3 months prior to testing, and testing of body fluids (saliva, urine) and breath prior to onsite assessment. Because of the low levels of SU among 9–10 year olds, only a small subset (10%) of youth participants are tested in the first two years of the ABCD Study, with increasingly larger proportions of youth selected randomly for testing as the cohort ages into adolescence, when experimentation, regular, and problem use with substances becomes more prevalent (Table 1). Decisions regarding the portion of youth participants tested for recent SU is informed by national estimates of prevalence of use (Miech et al., 2017).
Youth must be naïve to alcohol and recreational drugs at in-person study enrollment, while prescription drug use is permitted in youth participants at the initial baseline visit (and at all annual follow-up visits). Biospecimens addressing recent, past 3-month and lifetime SU exposures are assessed. Tests of recent drug use (using specimens of body fluids and breath) will provide the opportunity to evaluate the reliability of self-reports of current drug use. Given potential alcohol or drug effects on test results (e.g., neuro-cognitive and/or neuro-imaging assessments), youth participants testing positive for recent drug exposure (i.e., past day) at any follow-up onsite visit, will be asked to reschedule, and to return for testing on another date, drug and alcohol free. Youth reporting a history of non-prescription drug use, or participants suspected of substance intoxication, once they arrive to the lab for study assessments, will be tested for recent drug use, even if they have not been selected randomly for testing before their arrival. Because the effects of drug exposure and misuse of prescribed drugs on brain and cognitive development in youth is a primary focus of the ABCD Study, a reported history of use is not exclusionary at follow-up annual visits, and the assessment of past 3-month and lifetime SU exposures are of high value. The ABCD research team is in the process of developing protocols for future biospecimen collection, but, have not yet formalized additional biospecimens. Given the protocol for future onsite visits beyond 1-year follow-up remains undetermined, it is not included in this report.
2.1. Recent use of drugs and alcohol
Oral fluid will be collected for toxicology testing of 7 drugs. In follow-up years, urine will be collected and tested at the beginning of each onsite visit, supplemented by breathalyzer testing for a random selection of subjects, to rule out recent (past 2-3 days, sometimes past few hours) non-prescription drug, nicotine, and alcohol use prior to neurocognitive assessment. Onsite drug testing will be completed for alcohol use and a broad range of commonly used substances (see Table 1) at 1-year follow-up. At baseline, a positive drug test (except for prescription drugs) is exclusionary for initial enrollment into ABCD, as are self or parent/guardian report of youth ingestion of more than 1 whole drink, more than one whole cigarette or the equivalent amount of another tobacco product, any marijuana, or misuse of any drug (including the misuse of prescribed medication).
2.2. Past 3-month use of alcohol and drugs
Hair samples will be collected from youth subjects during each annual onsite visit for future confirmation of the presence or absence of SU. Hair provides an extended window of drug metabolite detection (several months compared to days prior to collection), and therefore can help confirm drug use despite irregular and/or infrequent drug ingestion. While not without some disadvantages (e.g., expensive, hair availability), hair is relatively easy to handle and store, less susceptible to adulterants, and provides longer detection times compared to other biological matrices (e.g., oral fluid, urine) that have shorter detection windows (Curtis and Greenberg, 2008). By banking hair samples for future testing, results can be used in combination with oral fluid and self-report to ensure that a ‘clean’ baseline is confirmed. This is especially important for the subsample of individuals who escalate to substantial levels of drug use by an early age (e.g., by age 14), as these individuals will provide the most crucial test for neurocognitive and brain structure/function differences predating their escalation, or emerging after that escalation.
2.3. Methods of collection and analysis
2.3.1. Oral fluid
Oral fluid will be collected for toxicology testing using a Draeger 5000 Drug Test Unit, which provides a qualitative test for 7 drugs. Oral fluid drug screening has advantages over conventional methods (i.e., blood, urine) including reduced biohazard generation, ease of collection, and less susceptibility to adulteration (Bosker and Huestis, 2009). Oral fluid concentrations are also more tightly correlated to blood than urine concentrations (Choo and Huestis, 2004; Lee et al., 2013) allowing inferences of impairment and flexible detection windows. The Dräger DrugTest 5000 screening device is used to test oral fluid (i.e. saliva/oral cavity secretions) at baseline and each follow-up year to identify recent use of amphetamine, benzodiazepines, cannabis (D9-tetrahydrocannabinol), methamphetamine, cocaine, methadone, and 3,4-methylenedioxymethamphetamine (MDMA) (Niedbala et al., 2001a, Niedbala et al., 2001b; Verstraete, 2004) (Table 2). The Draeger unit was selected over other on-site testing devices because of the high sensitivity for THC, one of the most common substances of abuse. The Draeger provides a lower THC cut-off concentration (i.e., 5 ng/mL) compared to other testing devices (e.g., 25 ng/mL) and therefore higher sensitivity and detection accuracy. The Dräger DrugTest 5000 is increasingly utilized for roadside testing (Desrosiers et al., 2014).
Table 2.
The Dräger DrugTest 5000 screening test.
| Drug Type | Cut-off concentration | Detection time |
|---|---|---|
| Amphetamine | 50 ng/mL | ∼20–50 h |
| Benzodiazepines | 15 ng/mL | ∼12–24 h |
| Cannabis | 5 ng/mL | ∼4–16 h |
| Cocaine | 20 ng/mL | ∼5–12 h |
| MDMA | 75 ng/mL | ∼24 h |
| Methadone | 20 ng/mL | ∼15 h |
| Methamphetamine | 35 ng/mL | ∼24 h |
The Dräger system consists of an analyzer, test cassette oral fluid collector, and buffer cartridge. To perform a screening test, the participant refrains from eating or drinking for 10 min. The test cassette contains a cellulose pad that is moved from one side of the mouth to the other for approximately one minute until the volume adequacy indicator turns blue to confirm a sufficient volume. The sample is then inserted into the Dräger analyzer with 3 mL buffer for drug stabilization. The cassette and buffer are placed in the chamber of the analyzer for lateral flow immunoassay. All drug results are displayed on the analyzer device within 5–8 min as “positive” or “negative.” The test is repeated for cases in which the results are unexpected based on self-report and/or clinical observation; repeat test results, and whether results are in-line with self-report and clinical observation are coded. Oral fluid drug testing can be influenced by several factors, including frequency of SU, body fat, and method of ingestion, however work by Huestis and colleagues suggest that 5 ng/mL concentration cut-off provides high diagnostic sensitivity, specificity, and efficiency (generally >80%) for oral fluid cannabinoid detection (Desrosiers et al., 2014; Newmeyer et al., 2017).
2.3.2. Breath
The Dräger Alcotest 5510 is used for breath alcohol detection and confirmation of sobriety from alcohol. Detection times vary based on blood alcohol concentration and hours since last drink (<24 h following consumption at maximum) (Jones, 1996). Participants are instructed to take a deep breath and blow into the mouthpiece as if blowing out “12 birthday candles.” The electrochemical fuel cell draws in breath for breath alcohol concentrations analysis and breath alcohol results are displayed immediately. The test is repeated for cases in which the results are unexpected based on self-report and/or clinical observation; all positive results (≥0.001 mg/L) are exclusionary at baseline. Follow-up appointments are rescheduled to ensure no youth is tested while under the influence of alcohol.
2.3.3. Hair
In addition to the screening measures for SU at the time of lab visits, the ABCD consortium is also collecting hair samples (100 mg) from all participants at each on-site lab visit for a wider detection window (∼3 months prior to hair collection) to provide longer-term information on history of drug consumption. Approximately 10 percent of participants will have hair analysis conducted at baseline, as the cost would be prohibitive to analyze every sample at every time-point. However, all hair samples are archived for each participant at each site, and future analyses can be done on hair samples for participants who endorse drug use as it becomes more prevalent as the ABCD cohort progresses through adolescence.
Participants are instructed that we will cut a sample that is ½ inch wide by two strands deep (about the same size as the tip of a shoelace) from the back of the head below the crown. After collection, the sample is placed in foil tightly and sent to Psychemedics. Gas chromatography-mass spectrometry (GC/MS/MS) and liquid chromatography-mass spectrometry (LC/MS/MS) procedures are used to test for the following parent drugs and metabolites: alcohol ethyl glucurolide (ETC), cannabis (11-Nor-9-carboxy-THC (THCCOOH) and cannabidiol (CBD)), methamphetamine and methylenedioxy-methamphetamine (MDMA), amphetamine, opiates (codeine morphone, hyrdomorph, oxycodone, hydrocodone), and cocaine/benzoylecgonine (BE). Commonly used hair procedures (e.g., shampoo, dyeing) do not impact quantitative results, and samples are relatively easy to collect and store compared to other biological matrices. Limitations include hair that is too short (e.g., crew cuts). This is particularly a challenge in testing pre-pubertal children, where hair on other parts of the body (e.g., legs and/or underarms) are less available than in older youth and young adults.
2.3.4. Urine
Starting at the first follow-up onsite visit, urine will also be collected from 10% of the sample for a semi-quantitative test (NicAlert) for cotinine, the principal metabolite of nicotine. The presence of cotinine suggests the subject has been exposed to, or used smoked tobacco products within the past several days (Benowitz et al., 2009; Raja et al., 2016). The NicAlert urine test system includes the specimen collection cup and NicAlert test strip that uses immunochromatographic assay to identify cotinine concentrations (0- > 1000 ng/mL) within 10–15 min. The sample is coded as positive when the concentration is >100 ng/mL (NicAlert level 3 or higher), which suggests a high likelihood the participant is a user of tobacco products. Published literature suggests that the NicAlert system provides the most sensitive and reliable method of cotinine detection, and this is especially true with urine, which contains more cotinine than oral fluid, which translates into higher levels of sensitivity at lower levels of exposure to nicotine (Acosta et al., 2004; Marrone et al., 2011).
3. Pubertal hormones
A hallmark of adolescence is reproductive maturation, known as puberty. Puberty heralds the onset of adolescence, and the hormonal surges that occur during this period of time impact the ‘environment’ of the developing brain. Pubertal maturation influences trajectories of, and sex differences in, brain development and behavior (Herting et al., 2014, Herting et al., 2012; Neufang et al., 2009; Nguyen et al., 2013; Paus et al., 2010; Peper et al., 2009, Peper et al., 2011; Perrin et al., 2008; Spielberg et al., 2015), including SU during adolescence (Andersen, 2016a; La Grange et al., 1995; Tschann et al., 1994; Witt, 2007). While much has been discovered in the last decade about the impact of pubertal hormones on adolescent brain and cognitive development (for a review, see (Herting and Sowell, 2017)), much is yet to be learned, particularly in connection with resilience or risk for SU during adolescence, and related mental health problems. To the best of our knowledge, the ABCD study is the largest most comprehensive study collecting pubertal hormone data longitudinally across adolescence, and the ability to connect them to brain development, cognition, behavior, genetics and SU.
Differences in onset of pubertal timing and maturation, and associations with SU and mental health vary as a function of sex, race and region (Leonard et al., 2010). Thus, investigating these important hormone associations with neurodevelopment across the United States in a representative sample, both racially and regionally, is essential. Important to the ABCD project, behavioral risk factors begin to emerge during pubertal onset and do so in a sex-specific fashion, with an increased prevalence of SU and externalizing disorders in boys compared to girls (Federal Interagency Forum on Child and Family Statistics, 2009). Adolescence is a period of prolonged sensitivity to environmental factors, when the maturing central nervous system is particularly sensitive to insult (Andersen, 2016b). How SU impacts the relationships between pubertal onset, mental health, and neurodevelopment remain unclear, and is therefore an objective of the ABCD Study. DHEA, testosterone and estradiol (girls only) is being assessed each year spanning across both pre- (e.g., 9–10 yrs) and well past post- (e.g., 19–20 yrs) pubertal stages of development. It is important to note that pubertal maturation is assessed independently of gonadal hormone measures (e.g., presence of physical markers of advanced pubertal maturation), and is a key interacting factor for understanding relationships between hormone levels, brain development and SU.
3.1. Methods and analysis for salivary hormones
Pubertal hormones are assessed in participating adolescents through the collection of a single salivary biospecimen (e.g., passive drool method) each year throughout the 10-year duration of ABCD. This method provides a quick, accurate and reliable method for measuring numerous key gonadal hormones from a single sample. The source of pubertal hormones found in saliva come from several glands in or near the mouth (Voegtline and Granger, 2014). Compared to collecting blood, the non-invasive nature of the salivary method requires less training for the administer, and eliminates the need for coordination with a phlebotomist. Saliva contains lower levels of pubertal hormones compared to levels found in blood, yet saliva levels are highly correlated with the free blood serum levels that typically exert biophysiological effects (Gröschl, 2008).
3.2. Limitations of salivary hormones
3.2.1. The participant
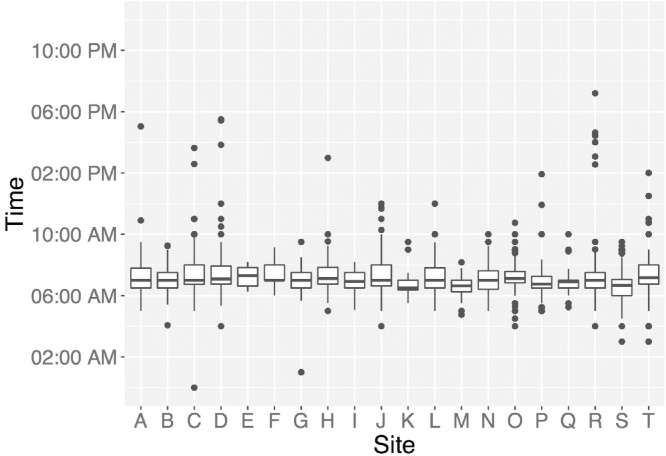
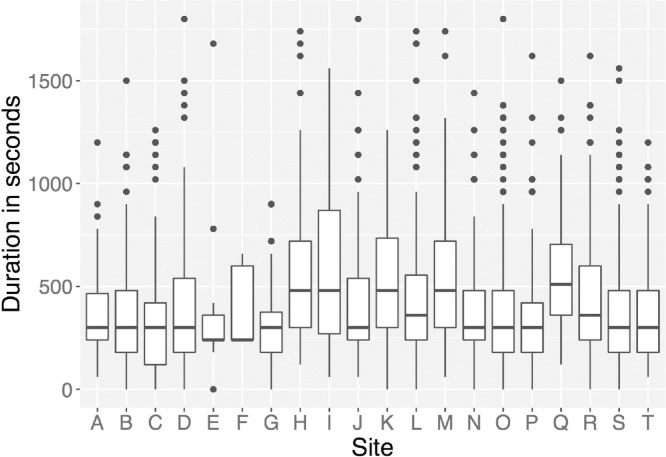
Like serum hormone levels, salivary hormone levels exhibit circadian patterns, making participant waking time (Fig. 1), and time of day of saliva collection important variables for statistical analyses and interpretation of results. Oral hygiene, injury resulting in bleeding or inflammation, and food particles can alter levels of hormones in the sample, and/or interfere with accuracy of the assays to assess hormone levels. To adjust for these confounds, notes on the color of the sample (e.g., saliva should be clear) should be taken into consideration when running statistical analyses with salivary hormone data (e.g., blood often results in yellow-brown hue, and particles of food are often visible). The amount of time it takes a participant to produce a saliva sample (duration of time from first to last saliva drop) can influence the concentrations of hormone levels. The duration of collection time can be impacted by the flow rate of saliva production, which may vary as a function of certain medications, making duration of sample collection an important factor to consider for analyses and interpretation of results. The average sample collection time for the ABCD cohort of 9–10 year olds is approximately 5.25 min (Fig. 2). For girls, additional fluctuations in gonadal hormones occurring across the menstrual cycle are captured by collecting key factors, such as: age of onset of menstruation, type of contraceptives used, regular or irregular cycles, length of cycles, and date of last menstrual cycle. Given the cyclic nature of estradiol, factors relating to menstruation are key for understanding possible (and unexpected) decreases in estradiol levels across years.
Fig. 1.
Variations in waking time across ABCD participants and sites. Salivary hormone levels are influenced by circadian patterns in hormone secretion, which is largely impacted by time of waking.
Fig. 2.
Variations in duration of saliva collection times across ABCD participants and sites. The amount of time it takes a participant to complete the passive drool sampling process can influence the concentration of pubertal hormone levels.
3.2.2. Experimental error
Researchers examining ABCD hormone data should be aware that approximately 4% of the currently existing hormone data is affected by some type of experimental error. Many of these errors can be found within the ABCD data set under the research associates’ notes, or become obvious upon close inspection of the data entered relating to factors described in above sections. Bacterial growth in the saliva sample is blocked upon freezing of the sample. However, logistical challenges can sometimes prevent immediate freezing (e.g., malfunctioning freezer, not enough time to place sample in freezer during a testing day, or forgetfulness). Errors in data entry occur. As can be seen in Fig. 3, some participants are listed as waking up after 15:00 (e.g., 3pm); however participants typically arrive much earlier in the day for testing/scanning, making this a data entry error. All saliva samples are stored at −20 to −80 °C before shipping on dry ice for analyses. The deep freezers are subject to malfunctioning (e.g., 2 ABCD freezers have lost power sources with samples inside); thus notes about samples thawing, which can significantly impact hormone levels, need to be considered. Upon receipt of frozen samples shipped from each ABCD site, Salimetrics completes all notes on sample quality and thawing, conducts assays, and the initial data entry of hormone levels. Samples are run in replicates, and key details of each hormone assay can be found on their website (https://www.salimetrics.com). Given the sheer quantity, saliva samples shipped from each site every 2 months, and subsequently analyzed in batches at Salimetrics; thus initial assessment for possible batch effects should be conducted before moving on with further analyses within a selected ABCD sub-sample.
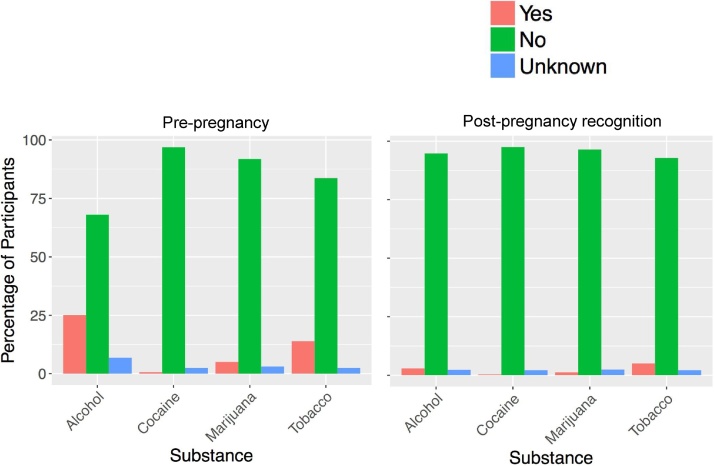
Fig. 3.
Percentage of parents/guardian of over 2000 ABCD participants studied so far who endorse use of Alcohol, Cocaine, Marijuana and Tobacco both before and after pregnancy recognition.
4. Genomics/Epigenetics
Genetics plays a crucial role in personality traits (Lo et al., 2017), psychiatric illness, including substance abuse disorders (e.g., (Hagenaars et al., 2016; Heath et al., 1997; Kendler et al., 2003a; Kendler and Prescott, 1998; Kendler et al., 2003b; Schizophrenia Working Group of the Psychiatric Genomics, 2014)), and cognition (Plomin et al., 2013). While estimates of heritability have often been derived from familial studies, work over the past 5–10 years has increasingly demonstrated that a substantial proportion of heritability is instantiated in common genetic variation captured by whole-genome genotyping arrays across a broad swath of anthropomorphic and neuropsychiatric traits (Boyle et al., 2017; Hibar et al., 2015; Rietveld et al., 2013; Schork et al., 2016; Vogler et al., 2014; Yang et al., 2010). Thus, a comprehensive understanding of both normative brain and cognitive development and their relation to early SU and abuse requires genetically-informed approaches, including both familial studies of heritability and molecular genetic studies. The ABCD study will take both approaches. Another paper in this issue describes the twin component to ABCD.
4.1. Assessing saliva and blood samples
Briefly, as shown in Table 1, a saliva sample is collected at the baseline visit and shipped from the collection site to RUCDR, where the sample is stored and the DNA is isolated. Blood sample will also be requested of twin pairs at baseline assessment. Saliva is being collected from twin pairs regardless of provision of blood samples at baseline. Genotyping to be conducted on saliva and blood DNA sources will be used to provide zygosity information on pairs of twins. On all subjects the Smokescreen™ Genoyping array (Baurley et al., 2016) will be assayed (https://grants.nih.gov/grants/guide/notice-files/NOT-DA-16-013.html), consisting of over 300,000 SNPs. SNPs will be QC’d, phased, and imputed using state-of-the field methods and software, e.g., SHAPEIT3 (https://jmarchini.org/shapeit3/) and impute2 (http://mathgen.stats.ox.ac.uk/impute/impute_v2.html). Resulting genotyped and imputed SNPs will then be available for genome-wide associations studies (GWASs), as well as a host of other methods recently developed for assessing associations, heritability and co-heritability, polygenic risk scores, and functional assessment and pathway analysis (Fan et al., 2015; Schork et al., 2016; Thompson et al., 2017, Thompson et al., 2015; Yang et al., 2010). Epigenetic studies such as those involving DNA methylation and histone modification data will be pursued in the future using stored samples at RUCDR from both baseline and longitudinal samples.
5. Assessing neurotoxin exposure with deciduous “Baby” teeth
Human and animal studies document that early life exposures to environmental neurotoxicants including heavy metals (e.g., lead, manganese, and cadmium), and prenatal exposure to drugs of abuse (e.g., alcohol, tobacco smoke, cocaine, and marijuana) can negatively impact brain development, leading to maladaptive and persistent alterations in brain structure and function and cognitive and behavioral development. Despite numerous studies describing the neurodevelopmental toxicity of early life environmental exposures, documenting associations between in utero and early life exposures with adverse health effects is hindered by the absence of direct fetal biomarkers that can be used safely to measure exposure in large study populations. Most associations between prenatal chemical exposure and neurodevelopmental outcomes are based on the analyses of maternal samples (e.g., maternal blood and/or urine) obtained at the time of birth, and perhaps at one to two time points during pregnancy. This timing may be months after the exposure occurred and the pharmacokinetic and tissue distribution of various chemicals may be quite different at different stages of fetal and child development (Makri et al., 2004). Documenting prenatal exposure to drugs of abuse is further compounded by social stigma associated with reporting licit and illicit drug use during pregnancy (Charness et al., 2016). To address this gap in our understanding of fetal and early life exposures, Dr. Manish Arora and colleagues have recently developed a novel methodology to retrospectively and objectively quantify the dose and timing of environmental exposures throughout pregnancy and early childhood using naturally shed deciduous “baby” teeth (Arora and Austin, 2013). In the following sections we briefly summarize the literature demonstrating associations between early life exposure to environmental toxicants (e.g., metals) and drugs of abuse on developmental outcomes, describe the novel approach to detecting exposures to some of these toxicants in shed deciduous “baby” teeth, and present the protocol for baby tooth processing and archival. By collecting teeth and leveraging the novel tooth biomarker described below, the ABCD Study is building a valuable repository that provides a unique, exciting and valuable opportunity to study the individual, interactive and/or additive effects of early life environmental exposures on childhood neurodevelopmental outcomes.
5.1. Environmental toxicants
A growing population of children around the world is exposed to various neurotoxicants present in urban and rural environments, which may damage their developing brains. Within the last several decades, strong evidence suggests that infants and children are uniquely vulnerable to environmental toxicants due to disproportionally higher exposures, immature metabolic pathways, and rapid growth and development (i.e., brain development) (Landrigan, 2004). It is now well accepted that low-level chronic exposure to environmental chemicals may contribute to the growing epidemic of childhood neurodevelopmental disorders worldwide (Grandjean and Landrigan, 2014). In adults, exposure to metals has been shown to induce psychotic behaviors or depressive symptoms and emotional instability in adults (reviewed in (Orisakwe, 2014)). In children, epidemiologic studies demonstrate associations between early life exposure to metals with poor cognitive, emotional and behavioral functioning in children (reviewed in (Sanders et al., 2015; Wright and Baccarelli, 2007)). Notably, current knowledge of the neurodevelopmental health risks associated with environmental chemical exposure has been derived mainly from the study of single agents; however, no human is exposed to just one chemical at a time. Evidence suggest combined effects of multiple chemicals might occur at levels far below those observable for any one component (reviewed in (Claus Henn et al., 2012; von Stackelberg et al., 2015)).
Notably, an individual’s risk of exposure to neurotoxicants, as well as the risk of adverse outcomes associated with exposure, may vary based on socio-economic status (SES) (reviewed in (Rauh and Margolis, 2016)). Childhood socioeconomic status (SES) is characterized by a combination of factors, including family income, parental educational attainment and occupational status (McLoyd, 1998), and is known to be an influential factor for brain development and cognitive function (Noble et al., 2012, Noble et al., 2005). Such associations could stem from ongoing disparities in postnatal experience or exposures, such as family stress, cognitive stimulation, environmental toxicants, or nutrition, or from corresponding differences in the prenatal environment. Given SES-related differences in brain development (Chaddock et al., 2010), and, observed relationships between brain structure and function and environmental toxicants (Peterson et al., 2015; Pujol et al., 2016), low SES youth may be at increased risk for negative outcomes from a multitude of environmental factors. Interactive and/or additive effects of various neurotoxicants and other environmental factors can be examined in the baby tooth biomarker as part of the ABCD study.
5.2. Prenatal exposure to drugs of abuse
Human studies of prenatal exposure to drugs of abuse such as alcohol (Donald et al., 2015; Gautam et al., 2015a), tobacco smoke (Gautam et al., 2015b; Tiesler and Heinrich, 2014), cocaine (McCarthy et al., 2014), and marijuana (Alpár et al., 2016) have shown brain and cognitive abnormalities among offspring of mothers who reported use during pregnancy. Most human studies on the impact of prenatal drug exposure on brain and cognitive development utilize retrospective samples and rely on mothers’ recollection of drug consumption patterns years after pregnancy (and, likely under-reported given stigma) (Moore et al., 2014), and/or select prospective samples of children with “heavy” exposure vs. low or no exposure. Validity of retrospective reports on maternal life style during pregnancy 10–12 years post-partum, including SU, has been shown to be sub-optimal (Cohen’s kappas = 0.03–0.11) (Jaspers et al., 2010).
The ABCD protocol includes a developmental history where parents/guardians are asked to recall SU patterns both before pregnancy, and after pregnancy recognition. While data collection is on-going in the ABCD study, maternal self-report in a sample of over 2000 participants studied as of the end of May 2017, when questioned about SU prior to pregnancy most report no SU, but, approximately 25% report use of alcohol, 0.6% cocaine, 5% marijuana, and 13.6% tobacco (Fig. 3). These percentages substantially decreased when parents/guardians were asked about their post-pregnancy recognition SU, but some continued after pregnancy recognition. Of course, we do not know how many, if any, parent/guardians of ABCD participants denied SU when there actually was use, but, given social stigma in many communities, it is unlikely that individuals would report drug use during pregnancy if there was none. However, as described below, some of these substances can be measured using novel assays of baby teeth.
5.3. Deciduous (Baby) teeth analyses
As discussed above, determining exposure to environmental toxicants during the prenatal period has been hampered by the lack of appropriate biomarkers to measure direct fetal exposure. Further, until recently, no single biomarker could provide continuous, time-resolved documentation of exposure throughout the fetal and early childhood period. Common biomarkers used for environmental assessment including maternal blood and urine are often not optimal matrixes for determining prenatal and early life exposure due to the timing and invasiveness of collection. Further, maternal biomarkers are not always a reliable measure for fetal exposure (Arora et al., 2014). For prenatal exposure to drugs of abuse, there are additional complications with parent self-report of licit and illicit drug use during pregnancy due to social stigma and length of time since pregnancy in remembering use patterns ∼9 to 10 years prior as will be the case in the ABCD cohort.
Establishing timing of exposures, especially over the prenatal period, is a major challenge in environmental epidemiologic studies. Teeth have long been used to estimate long-term cumulative exposure, including prenatal exposure, to environmental and other substances (Needleman et al., 1979). Notably, much of our knowledge of the impact of early life lead exposure on cognition was gained by examining associations between higher lead levels in children’s teeth and reduced IQ (Gulson and Wilson, 1994; McMichael et al., 1994; Needleman et al., 1972; Rabinowitz, 1995). However, previous tooth biomarker methods examined lead (or other bone-seeking toxicants) in the whole tooth providing a cumulative exposure of lifetime measure. Dr. Arora’s method incorporates laser ablation and micro-dissection techniques that leverages the physiology of tooth development to provide finely time-resolved assessments of exposure from the beginning of the 2nd trimester through the time the tooth is lost. Deciduous “baby” teeth growth proceeds in an incremental pattern, forming rings and layers similar to the rings of a tree. Toxicants circulating in the fetal blood stream (i.e., metals) are captured in the layers and measuring the amount of toxicant in the layers provides information about exposures dose and timing. These newly developed high-dimensional analytical methods combine sophisticated histological and chemical analyses to precisely sample tooth layers and have the potential to reconstruct a timeline of exposures during early development (Andra et al., 2015; Arora and Austin, 2013). These methods have been tested extensively in prior research (Arora et al., 2012, Arora et al., 2006; Austin et al., 2013; Hare et al., 2011), and hold promise for establishing timelines of exposures to environmental toxicants and drugs of abuse in the ABCD sample. In addition to fine-grained timelines of exposure spanning the pre- to postnatal periods, advantages to using baby teeth as biomarkers of toxic exposures is that shed teeth can be stored relatively easily at room temperature, and does not require any invasive procedures such as blood draw.
5.3.1. Tooth development and methodology
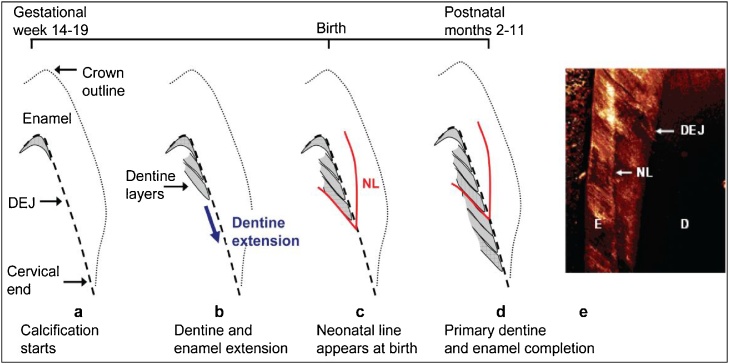
During development, enamel and dentine deposition occurs in a rhythmic manner, forming incremental lines akin to growth rings in both enamel and dentine (Fig. 4) (Berkovitz et al., 2009). At birth, an accentuated incremental line, the neonatal line, is formed due to disturbances in the secretory cells during protein matrix deposition (Sabel et al., 2008). This line forms a clear histological landmark that demarcates pre- and postnatally formed parts of teeth. Beyond the neonatal line teeth manifest daily growth lines, which allow chronological ages to be determined at various positions within tooth crowns and roots. The analytical approach involves sampling the growth rings of teeth using laser and other microdissection methods. Analyzing the sampled layers using mass spectrometers can yield time resolved information on organic and inorganic environmental compounds and their metabolites (Andra et al., 2016). Dr. Arora and colleagues have previously validated this biomarker for certain metals (Mn, Pb, Ba, Sr) (Arora et al., 2014, Arora et al., 2012, Arora et al., 2004, Arora et al., 2011; Austin et al., 2013) and validation for a range of organic targets is also underway in that laboratory.
Fig. 4.
Schematic of tooth development (Arora et al., 2012). (a) Earliest deposition of dentine (grey area) at DEJ at cusp tip (b) Continued extension of dentine (and enamel) towards the tooth cervix. (c) Neonatal line (NL), a histological feature, formed at the time of birth (d) Completion of enamel and primary dentine formation between 2–11 postnatal months depending on tooth type. Secondary dentine continues forming at pulpal margin (not shown). (e) Confocal laser scanning micrograph of NL in enamel.
By collecting multiple baby teeth from individuals enrolled in the ABCD cohort, we are building a repository that may be leveraged in the future to measure not only the validated metals but also early life exposure to organics, maternal stress, (Austin et al., 2013) and licit and illicit substances. Many other substances should be possible to measure on a detailed timeline during pre and post-natal development using baby teeth. Previous research has shown that metabolites of licit substances, such as alcohol and tobacco, and illicit substances, such as cocaine and marijuana have been measured in the teeth of adults, though, most of these studies have been done with ground adult teeth using material from dental extractions, and do not allow for timeline of exposure in earlier development (Andra et al., 2016). To our knowledge, these biomarkers have not yet been validated using shed deciduous teeth, which would require contemporary measurements of more conventional biomarkers, such as maternal, newborn, and childhood urine/blood samples at various points during pregnancy and childhood. Nonetheless, there is potential for measurements of these substances in deciduous teeth by adapting existing assays, but using methods which allow timelines of exposure during development (reviewed in (Andra et al., 2016)).
5.3.2. Tooth biomarker collection
Participants’ parents are asked for 5 baby teeth shed between the ages of 6–13 years old. Parents either bring the teeth in during a lab visit, or mail shed teeth into the lab with provided kits (5 plastic protective vials for each individual tooth inside a padded envelope to prevent damage to the teeth). Teeth may become brittle in very cold or hot temperatures, thus shipping and storage of shed teeth occurs at room temperature. Data collection sheets completed by the parents include information about how each tooth was shed (e.g., naturally, accident, removed), and how it was stored (e.g., dry, in liquid).
5.3.3. ABCD tooth collection status
As of November 21, 2017, 4524 ABCD participants have been recruited and enrolled across all data collection sites, and the overall goal is to recruit 11,500 participants by September 2018. Of those 4524 participants, 833 have already donated baby teeth, and an additional 225 families have said that they have saved baby teeth at home and will donate them (by mail or at 1 year ABCD in-person follow-up visit), and over 1200 families have stated that they have not saved previous teeth, but will save the next to fall out and donate to ABCD. Of participants tested thus far, only 7% have refused to donate a tooth.
6. Overall summary
The ABCD Study is collecting a plethora of biospecimens for assessment of SU, hormones, genetic/epigenetic markers, and developmental exposures. Some biospecimens will be analyzed in real time (on-site SU assessment), shortly afterward (hormones), or biobanked for future analysis with additional focused funding (DNA, baby teeth, stored saliva/blood/hair). This variety of stored biological samples allows interested investigators to select specific subgroups of ABCD participants as a function of their factor of interest (e.g., high and/or early onset of adolescent SU, early/late puberty, high/low hormone levels, high/low-SES, positive maternal endorsement of prenatal SU, positive adolescent endorsement of mental health problems, twins, etc.). Given the complex convergence of key developmental factors that shape an individual’s development, a large cohort, such as the ABCD cohort, is well suited for allowing investigators to perform highly focused analyses with biomarkers that are difficult to execute with smaller studies that are often statistically underpowered. For example, investigators will be able to include multiple covariates, multiple group comparisons and within-subject measures within a single analysis across the whole ABCD cohort, or vice-versa, select a highly specific subsample of ABCD across sites that is generally difficult to collect within a single geographical region. Discussions for biospecimen collection beyond the 1-year follow-up visit are ongoing, and have included parent DNA from blood, microbiome assessments from participant stool samples, and other measures that could include exposure to toxic substances such as air pollution and toxic metals from urine.
Conflict of Interest
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.dcn.2018.03.005.
Contributor Information
Pamela A.F. Madden, Email: pmadden@wustl.edu.
Elizabeth R. Sowell, Email: esowell@chla.usc.edu.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Acosta M.C., Buchhalter A.R., Breland A.B., Hamilton D.C.P., Eissenberg T. Urine cotinine as an index of smoking status in smokers during 96-hour abstinence: comparison between gas chromatography/mass spectrometry and immunoassay test strips. Nicotine Tob. Res. 2004;6(4):615–620. doi: 10.1080/14622200410001727867. [DOI] [PubMed] [Google Scholar]
- Alpár A., Di Marzo V., Harkany T. At the tip of an iceberg: prenatal marijuana and its possible relation to neuropsychiatric outcome in the offspring. Biol. Psychiatry. 2016;79(7):e33–45. doi: 10.1016/j.biopsych.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Andersen S.L. Commentary on the special issue on the adolescent brain: adolescence, trajectories, and the importance of prevention. Neurosci. Biobehav. Rev. 2016;70:329–333. doi: 10.1016/j.neubiorev.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.L. Commentary on the special issue on the adolescent brain: adolescence, trajectories, and the importance of prevention. Neurosci. Biobehav. Rev. 2016;70:329–333. doi: 10.1016/j.neubiorev.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andra S.S., Austin C., Wright R.O., Arora M. Reconstructing pre-natal and early childhood exposure to multi-class organic chemicals using teeth: towards a retrospective temporal exposome. Environ. Int. 2015;83:137–145. doi: 10.1016/j.envint.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andra S.S., Austin C., Arora M. The tooth exposome in children's health research. Curr. Opin. Pediatr. 2016;28(2):221–227. doi: 10.1097/MOP.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora M., Austin C. Teeth as a biomarker of past chemical exposure. Curr. Opin. Pediatr. 2013;25(2):261–267. doi: 10.1097/MOP.0b013e32835e9084. [DOI] [PubMed] [Google Scholar]
- Arora M., Chan S.W., Kennedy B.J., Sharma A., Crisante D., Walker D.M. Spatial distribution of lead in the roots of human primary teeth. J. Trace Elem. Med. Biol. 2004;18(2):135–139. doi: 10.1016/j.jtemb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Arora M., Kennedy B.J., Elhlou S., Pearson N.J., Walker D.M., Bayl P., Chan S.W. Spatial distribution of lead in human primary teeth as a biomarker of pre- and neonatal lead exposure. Sci. Total Environ. 2006;371(1–3):55–62. doi: 10.1016/j.scitotenv.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Arora M., Hare D., Austin C., Smith D.R., Doble P. Spatial distribution of manganese in enamel and coronal dentine of human primary teeth. Sci. Total Environ. 2011;409(7):1315–1319. doi: 10.1016/j.scitotenv.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Arora M., Bradman A., Austin C., Vedar M., Holland N., Eskenazi B., Smith D.R. Determining fetal manganese exposure from mantle dentine of deciduous teeth. Environ. Sci. Technol. 2012;46(9):5118–5125. doi: 10.1021/es203569f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora M., Austin C., Sarrafpour B., Hernández-Ávila M., Hu H., Wright R.O., Tellez-Rojo M.M. Determining prenatal, early childhood and cumulative long-term lead exposure using micro-spatial deciduous dentine levels. PLoS One. 2014;9(5):e97805. doi: 10.1371/journal.pone.0097805. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin C., Smith T.M., Bradman A., Hinde K., Joannes-Boyau R., Bishop D.…Arora M. Barium distributions in teeth reveal early-life dietary transitions in primates. Nature. 2013;498(7453):216–219. doi: 10.1038/nature12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baurley J.W., Edlund C.K., Pardamena C.I., Conti D.V., Bergen A.W. Smokescreen: a targeted genotyping array for addiction research. BMC Genomics. 2016;17:145. doi: 10.1186/s12864-016-2495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N.L., Bernert J.T., Caraballo R.S., Holiday D.B., Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the united statesbetween 1999 and 2004. Am. J. Epidemiol. 2009;169:236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- Berkovitz B.K.B., Holland G.R., Moxham B.J. fourth ed. Mosby Elsevier; Kidlington, Oxford, UK: 2009. Oral Anatomy, Histology and Embryology. [Google Scholar]
- Bosker W.M., Huestis M.A. Oral fluid testing for drugs of abuse. Clin. Chem. 2009;55(11):1910–1931. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle E.A., Li Y.I., Pritchard J.K. An expanded view of complex traits: from polygenic to omnigenic. Cell. 2017;169(7):1177–1186. doi: 10.1016/j.cell.2017.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L., Erickson K.I., Prakash R.S., VanPatter M., Voss M.W., Pontifex M.B.…Kramer A.F. Basal ganglia volume is associated with aerobic fitness in preadolescent children. Dev. Neurosci. 2010;32(3):249–256. doi: 10.1159/000316648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charness M.E., Riley E.P., Sowell E.R. Drinking during pregnancy and the developing brain: is any amount safe? Trends Cognit. Sci. 2016;20(2):80–82. doi: 10.1016/j.tics.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo R.E., Huestis M.A. Oral fluid as a diagnostic tool. Clin. Chem. Lab. Med. 2004;42(11):1273–1287. doi: 10.1515/CCLM.2004.248. [DOI] [PubMed] [Google Scholar]
- Claus Henn B., Schnaas L., Ettinger A.S., Schwartz J., Lamadrid-Figueroa H., Hernandez-Avila M.…Tellez-Rojo M.M. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ. Health Perspect. 2012;120(1):126–131. doi: 10.1289/ehp.1003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor Gorber S., Schofield-Hurwitz S., Hardt J., Levasseur G., Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob. Res. 2009;11(1):12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
- Curtis J., Greenberg M. Screening for drugs of abuse: hair as an alternative matrix: a review for the medical toxicologist. Clin. Toxicol. 2008;46(1):22–34. doi: 10.1080/15563650701261462. [DOI] [PubMed] [Google Scholar]
- Desrosiers N.A., Milman G., Mendu D.R., Lee D., Barnes A.J., Gorelick D.A., Huestis M.A. Cannabinoids in oral fluid by on-site immunoassay and by GC–MS using two different oral fluid collection devices. Anal. Bioanal. Chem. 2014;406(17):4117–4128. doi: 10.1007/s00216-014-7813-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald K.A., Eastman E., Howells F.M., Adnams C., Riley E.P., Woods R.P.…Stein D.J. Neuroimaging effects of prenatal alcohol exposure on the developing human brain: a magnetic resonance imaging review. Acta Neuropsychiatr. 2015;27(5):251–269. doi: 10.1017/neu.2015.12. [DOI] [PubMed] [Google Scholar]
- Fan C.C., Bartsch H., Schork A.J., Chen C.H., Wang Y.P., Lo M.T.…Neurocognition P.I. Modeling the 3D geometry of the cortical surface with genetic ancestry. Curr. Biol. 2015;25(15):1988–1992. doi: 10.1016/j.cub.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Interagency Forum on Child and Family Statistics . 2009. American's Children: Key National Indicators of Well-Being. Retrieved from Washington, D.C.: https://www.childstats.gov/pdf/ac2009/ac_09.pdf. (Accessed 2 June 2017) [Google Scholar]
- Gautam P., Nuñez S.C., Narr K.L., Mattson S.N., May P.A., Adnams C.M.…Sowell E.R. Developmental trajectories for visuo-spatial attention are altered by prenatal alcohol exposure: a longitudinal FMRI study. Cereb. Cortex. 2015;25(12):4761–4771. doi: 10.1093/cercor/bhu162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P., Warner T.D., Kan E.C., Sowell E.R. Executive function and cortical thickness in youths prenatally exposed to cocaine, alcohol and tobacco. Dev. Cognit. Neurosci. 2015;16:155–165. doi: 10.1016/j.dcn.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröschl M. Current status of salivary hormone analysis. Clin. Chem. 2008;54(11):1759–1769. doi: 10.1373/clinchem.2008.108910. [DOI] [PubMed] [Google Scholar]
- Grandjean P., Landrigan P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray K.M., Squeglia L.M. Research Review: what have we learned about adolescent substance use? J. Child Psychol. Psychiatry. 2017 doi: 10.1111/jcpp.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulson B., Wilson D. History of lead exposure in children revealed from isotopic analyses of teeth. Arch. Environ. Health. 1994;49(4):279–283. doi: 10.1080/00039896.1994.9937480. [DOI] [PubMed] [Google Scholar]
- Hagenaars S.P., Harris S.E., Davies G., Hill W.D., Liewald D.C.M., Ritchie S.J.…Longe C.C.A. Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N = 112151) and 24 GWAS consortia. Mol. Psychiatry. 2016;21(11):1624–1632. doi: 10.1038/mp.2015.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare D., Austin C., Doble P., Arora M. Elemental bio-imaging of trace elements in teeth using laser ablation-inductively coupled plasma-mass spectrometry. J. Dent. 2011;39(5):397–403. doi: 10.1016/j.jdent.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Heath A.C., Bucholz K.K., Madden P.A., Dinwiddie S.H., Slutske W.S., Bierut L.J., Martin N.G. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol. Med. 1997;27(6):1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Herting M.M., Sowell E.R. Puberty and structural brain development in humans. Front. Neuroendocrinol. 2017;44:122–137. doi: 10.1016/j.yfrne.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M., Maxwell E.C., Irvine C., Nagel B.J. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb. Cortex. 2012;22(9):1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M., Gautam P., Spielberg J.M., Kan E., Dahl R.E., Sowell E.R. The role of testosterone and estradiol in brain volume changes across adolescence: a longitudinal structural MRI study. Hum. Brain Mapp. 2014;35(11):5633–5645. doi: 10.1002/hbm.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar D.P., Stein J.L., Renteria M.E., Arias-Vasquez A., Desrivieres S., Jahanshad N.…S.Y.S Common genetic variants influence human subcortical brain structures. Nature. 2015;520(7546) doi: 10.1038/nature14101. 224–U216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspers M., de Meer G., Verhulst F.C., Ormel J., Reijneveld S.A. Limited validity of parental recall on pregnancy, birth, and early childhood at child age 10 years. J. Clin. Epidemiol. 2010;63(2):185–191. doi: 10.1016/j.jclinepi.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Jones A.W. Measuring alcohol in blood and breath for forensic purposes- A historical review. For. Sci. Rev. 1996;8(1):13–44. [PubMed] [Google Scholar]
- Kendler K.S., Prescott C.A. Cannabis use, abuse, and dependence in a population-based sample of female twins. Am. J. Psychiatry. 1998;155(8):1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Jacobson K.C., Prescott C.A., Neale M.C. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am. J. Psychiatry. 2003;160(4):687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Prescott C.A., Myers J., Neale M.C. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch. Gen. Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- La Grange L., Jones T.D., Erb L., Reyes E. Alcohol consumption: biochemical and personality correlates in a college student population. Addict. Behav. 1995;20(1):93–103. doi: 10.1016/0306-4603(94)00049-5. [DOI] [PubMed] [Google Scholar]
- Landrigan P.J. Children as a vulnerable population. Int. J. Occup. Med. Environ. Health. 2004;17(1):175–177. [PubMed] [Google Scholar]
- Lee D., Vandrey R., Milman G., Bergamaschi M., Mendu D.R., Murray J.A.…Huestis M.A. Oral fluid/plasma cannabinoid ratios following controlled oral THC and smoked cannabis administration. Anal. Bioanal. Chem. 2013;405(23):7269–7279. doi: 10.1007/s00216-013-7159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard M.B., Elmi A., Mostoufi-Moab S., Shults J., Burnham J.M., Thayu M.…Zemel B.S. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J. Clin. Endocrinol. Metab. 2010;95(4):1681–1689. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M.T., Hinds D.A., Tung J.Y., Franz C., Fan C.C., Wang Y.…Chen C.H. Genome-wide analyses for personality traits identify six genomic loci and show correlations with psychiatric disorders. Nat. Genet. 2017;49(1):152–156. doi: 10.1038/ng.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makri A., Goveia M., Balbus J., Parkin R. Children's susceptibility to chemicals: a review by developmental stage. J. Toxicol. Environ. Health B Crit. Rev. 2004;7(6):417–435. doi: 10.1080/10937400490512465. [DOI] [PubMed] [Google Scholar]
- Marrone G.F., Shakleya D.M., Scheidweiler K.B., Singleton E.G., Huestis M.A., Heishman S.J. Relative performance of common biochemical indicators in detecting cigarette smoking. Addiction. 2011;106(7):1325–1334. doi: 10.1111/j.1360-0443.2011.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D.M., Kabir Z.D., Bhide P.G., Kosofsky B.E. Effects of prenatal exposure to cocaine on brain structure and function. Prog. Brain Res. 2014;211:277–289. doi: 10.1016/B978-0-444-63425-2.00012-X. [DOI] [PubMed] [Google Scholar]
- McLoyd V.C. Socioeconomic disadvantage and child development. Am. Psychol. 1998;53(2):185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- McMichael A.J., Baghurst P.A., Vimpani G.V., Wigg N.R., Robertson E.F., Tong S. Tooth lead levels and IQ in school-age children: the Port Pirie Cohort Study. Am. J. Epidemiol. 1994;140(6):489–499. doi: 10.1093/oxfordjournals.aje.a117275. [DOI] [PubMed] [Google Scholar]
- Miech R.A., Johnston L.D., O'Malley P.M., Bachman J.G., Schulenberg J.E., Patrick M.E. Institute for Social Research; Ann Arbor: 2017. Monitoring the Future National Survey Results on Drug Use, 1975–2016; Volume I, Secondary School Students. Retrieved from. [Google Scholar]
- Moore H.M., Kelly A., Jewell S.D., McShane L.M., Clark D.P., Greenspan R.…Vaught J. Biospecimen reporting for improved study quality. Biopreserv Biobank. 2011;9(1):57–70. doi: 10.1089/bio.2010.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E.M., Migliorini R., Infante M.A., Riley E.P. Fetal alcohol spectrum disorders: recent neuroimaging findings. Curr. Dev. Disord. Rep. 2014;1(3):161–172. doi: 10.1007/s40474-014-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales N.A., Romano M.A., Michael Cummings K., Marshall J.R., Hyland A.J., Hutson A., Warren G.W. Accuracy of self-reported tobacco use in newly diagnosed cancer patients. Cancer Causes Control. 2013;24(6):1223–1230. doi: 10.1007/s10552-013-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman H.L., Tuncay O.C., Shapiro I.M. Lead levels in deciduous teeth of urban and suburban American children. Nature. 1972;235(5333):111–112. doi: 10.1038/235111a0. [DOI] [PubMed] [Google Scholar]
- Needleman H.L., Gunnoe C., Leviton A., Reed R., Peresie H., Maher C., Barrett P. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N. Engl. J. Med. 1979;300(13):689–695. doi: 10.1056/NEJM197903293001301. [DOI] [PubMed] [Google Scholar]
- Neufang S., Specht K., Hausmann M., Gunturkun O., Herpertz-Dahlmann B., Fink G.R., Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cereb. Cortex. 2009;19(2):464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Newmeyer M.N., Swortwood M.J., Andersson M., Abulseoud O.A., Scheidweiler K.B., Huestis M.A. Cannabis edibles: blood and oral fluid cannabinoid pharmacokinetics and evaluation of oral fluid screening devices for predicting delta9-Tetrahydrocannabinol in blood and oral fluid following cannabis brownie administration. Clin. Chem. 2017;63(3):647–662. doi: 10.1373/clinchem.2016.265371. [DOI] [PubMed] [Google Scholar]
- Nguyen T.V., McCracken J.T., Ducharme S., Cropp B.F., Botteron K.N., Evans A.C., Karama S. Interactive effects of dehydroepiandrosterone and testosterone on cortical thickness during early brain development. J. Neurosci. 2013;33(26):10840–10848. doi: 10.1523/JNEUROSCI.5747-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedbala R.S., Kardos K., Waga J., Fritch D., Yeager L., Doddamane S., Schoener E. Laboratory analysis of remotely collected oral fluid specimens for opiates by immunoassay. J. Anal. Toxicol. 2001;25(5):310–315. doi: 10.1093/jat/25.5.310. [DOI] [PubMed] [Google Scholar]
- Niedbala R.S., Kardos K.W., Fritch D.F., Kardos S., Fries T., Waga J.…Cone E.J. Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. J Anal Toxicol. 2001;25(5):289–303. doi: 10.1093/jat/25.5.289. [DOI] [PubMed] [Google Scholar]
- Noble K.G., Norman M.F., Farah M.J. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev. Sci. 2005;8(1):74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Kan E., Sowell E.R. Neural correlates of socioeconomic status in the developing human brain. Dev. Sci. 2012;15(4):516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orisakwe O.E. The role of lead and cadmium in psychiatry. N. Am. J. Med. Sci. 2014;6(8):370–376. doi: 10.4103/1947-2714.139283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Nawaz-Khan I., Leonard G., Perron M., Pike G.B., Pitiot A.…Pausova Z. Sexual dimorphism in the adolescent brain: role of testosterone and androgen receptor in global and local volumes of grey and white matter. Horm. Behav. 2010;57(1):63–75. doi: 10.1016/j.yhbeh.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Peper J.S., Brouwer R.M., Schnack H.G., van Baal G.C., van Leeuwen M.…van den Berg S.M., Hulshoff Pol H.E. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34(3):332–342. doi: 10.1016/j.psyneuen.2008.09.012. S0306-4530(08)00253-9 [pii] [DOI] [PubMed] [Google Scholar]
- Peper J.S., Hulshoff Pol H.E., Crone E.A., van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Perrin J.S., Herve P.Y., Leonard G., Perron M., Pike G.B., Pitiot A.…Paus T. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J. Neurosci. 2008;28(38):9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B.S., Rauh V.A., Bansal R., Hao X., Toth Z., Nati G.…Perera F. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry. 2015;72(6):531–540. doi: 10.1001/jamapsychiatry.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflieger J.C., Cook E.C., Niccolai L.M., Connell C.M. Racial/ethnic differences in patterns of sexual risk behavior and rates of sexually transmitted infections among female young adults. Am. J. Public Health. 2013;103(5):903–909. doi: 10.2105/AJPH.2012.301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R., Haworth C.M.A., Meaburn E.L., Price T.S., Davis O.S.P., Control W.T.C. Common DNA markers can account for more than half of the genetic influence on cognitive abilities. Psychol. Sci. 2013;24(4):562–568. doi: 10.1177/0956797612457952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J., Martínez-Vilavella G., Macià D., Fenoll R., Alvarez-Pedrerol M., Rivas I.…Sunyer J. Traffic pollution exposure is associated with altered brain connectivity in school children. Neuroimage. 2016;129:175–184. doi: 10.1016/j.neuroimage.2016.01.036. [DOI] [PubMed] [Google Scholar]
- Rabinowitz M.B. Relating tooth and blood lead levels in children. Bull. Environ. Contam. Toxicol. 1995;55(6):853–857. doi: 10.1007/BF00209464. [DOI] [PubMed] [Google Scholar]
- Raja M., Garg A., Yadav P., Jha K., Handa S. Diagnostic methods for detection of cotinine level of tobacco users: a review. J. Clin. Diagn. Res. 2016;10:ZE04–ZE06. doi: 10.7860/JCDR/2016/17360.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh V.A., Margolis A.E. Research Review: environmental exposures, neurodevelopment, and child mental health – new paradigms for the study of brain and behavioral effects. J. Child Psychol. Psychiatry. 2016;57(7):775–793. doi: 10.1111/jcpp.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietveld C.A., Medland S.E., Derringer J., Yang J., Esko T., Martin N.W.…Study L.C. GWAS of 126, 559 individuals identifies genetic variants associated with educational attainment. Science. 2013;340(6139):1467–1471. doi: 10.1126/science.1235488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabel N., Johansson C., Kuhnisch J., Robertson A., Steiniger F., Noren J.G.…Nietzsche S. Neonatal lines in the enamel of primary teeth-a morphological and scanning electron microscopic investigation. Arch. Oral Biol. 2008;53(10):954–963. doi: 10.1016/j.archoralbio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Sanders A.P., Claus Henn B., Wright R.O. Perinatal and childhood exposure to cadmium, manganese, and metal mixtures and effects on cognition and behavior: a review of recent literature. Curr. Environ. Health Rep. 2015;2(3):284–294. doi: 10.1007/s40572-015-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schork A.J., Wang Y.P., Thompson W.K., Dale A.M., Andreassen O.A. New statistical approaches exploit the polygenic architecture of schizophrenia – implications for the underlying neurobiology. Curr. Opin. Neurobiol. 2016;36:89–98. doi: 10.1016/j.conb.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg J.M., Forbes E.E., Ladouceur C.D., Worthman C.M., Olino T.M., Ryan N.D., Dahl R.E. Pubertal testosterone influences threat-related amygdala-orbitofrontal cortex coupling. Soc. Cogn. Affect Neurosci. 2015;10(3):408–415. doi: 10.1093/scan/nsu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W.K., Wang Y.P., Schork A.J., Witoelar A., Zuber V., Xu S.J., Psychiat S.W.G. An empirical bayes mixture model for effect size distributions in genome-wide association studies. PLoS Genet. 2015;11(12):e1005717. doi: 10.1371/journal.pgen.1005717. ARTN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P.M., Andreassen O.A., Arias-Vasquez A., Bearden C.E., Boedhoe P.S., Brouwer R.M., Consortium E. ENIGMA and the individual: predicting factors that affect the brain in 35 countries worldwide. Neuroimage. 2017;145:389–408. doi: 10.1016/j.neuroimage.2015.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiesler C.M., Heinrich J. Prenatal nicotine exposure and child behavioural problems. Eur. Child Adolesc. Psychiatry. 2014;23(10):913–929. doi: 10.1007/s00787-014-0615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschann J.M., Adler N.E., Irwin C.E., Jr., Millstein S.G., Turner R.A., Kegeles S.M. Initiation of substance use in early adolescence: the roles of pubertal timing and emotional distress. Health Psychol. 1994;13(4):326–333. doi: 10.1037//0278-6133.13.4.326. [DOI] [PubMed] [Google Scholar]
- Verstraete A.G. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther. Drug Monit. 2004;26(2):200–205. doi: 10.1097/00007691-200404000-00020. [DOI] [PubMed] [Google Scholar]
- Voegtline K.M., Granger D.A. Dispatches from the interface of salivary bioscience and neonatal research. Front. Endocrinol. (Lausanne) 2014;5:e25. doi: 10.3389/fendo.2014.00025. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler C., Gschwind L., Coynel D., Freytag V., Milnik A., Egli T., Papassotiropoulos A. Substantial SNP-based heritability estimates for working memory performance. Transl. Psychiatry. 2014;4:e438. doi: 10.1038/tp.2014.81. ARTN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stackelberg K., Guzy E., Chu T., Claus Henn B. Exposure to mixtures of metals and neurodevelopmental outcomes: a multidisciplinary review using an adverse outcome pathway framework. Risk Anal. 2015;35(6):971–1016. doi: 10.1111/risa.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt E.D. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol. Teratol. 2007;29(1):81–95. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Wright R.O., Baccarelli A. Metals and neurotoxicology. J. Nutr. 2007;137(12):2809–2813. doi: 10.1093/jn/137.12.2809. [DOI] [PubMed] [Google Scholar]
- Yang J.A., Benyamin B., McEvoy B.P., Gordon S., Henders A.K., Nyholt D.R.…Visscher P.M. Common SNPs explain a large proportion of the heritability for human height. Nat. Genet. 2010;42(7) doi: 10.1038/ng.608. 565-U131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.