Abstract
Compromised oxygen supply to cerebral tissue could be an important mechanism contributing to age-related cognition decline. We recently showed in awake mice that resting cerebral tissue pO2 decreases with age, a phenomenon that manifests mainly after middle-age. To extend these findings, here we aimed to study how tissue pO2 response to neuronal stimulation is affected by aging. We used two-photon phosphorescence lifetime microscopy to directly measure the brain tissue pO2 response to whisker stimulation in healthy awake young, middle-aged and old mice. We show that despite a decrease in baseline tissue pO2, the amplitude of the tissue pO2 response to stimulation is well preserved with age. However, the response dynamics are altered towards a slower response with reduced post-stimulus undershoot in older ages, possibly due to stiffer vessel wall among other factors. An estimation of the net oxygen consumption rate using a modified Krogh model suggests that the O2 overshoot during stimulation may be necessary to secure a higher capillary O2 delivery to the tissue proportional to increased CMRO2 to maintain the capillary tissue pO2. It was observed that the coupling between the CMRO2 and capillary O2 delivery is preserved with age.
Keywords: Cerebral tissue oxygenation, Neural stimulation, Two-photon microscopy, Aging, Awake mice
1. Introduction
It has been established that cognitive function declines even with healthy aging [1–4]. With clinical observations showing a correlation between cognitive impairments and vascular disorders [5–7] and with cerebral blood flow (CBF) [3,8], it is hypothesized that impaired cerebral tissue oxygenation in old age may contribute to cognitive impairment.
In a previous study in awake mice, we revealed an age-related decrease in resting cerebral tissue pO2 which manifested itself mainly after 15 months of age. In 27-month old mice, the substantial decrease in brain tissue pO2 was accompanied by the presence of pockets of hypoxia in some regions of the cortex [9]. We anticipate that these tissue regions, with low pO2, are more susceptible to demand-induced hypoxia. Given the reductions in cerebral tissue pO2 with aging, it is thus important to understand if the pO2 response to neuronal stimulation is affected by aging. Most previous works studying oxygenation changes during neural stimulation were performed mainly with functional magnetic resonance imaging (fMRI) and near-infrared spectroscopy (NIRS), methods that measure the changes in blood oxygenation during neural activation. There are a few studies investigating tissue pO2 response to stimulation, but none of these studies addressed potential aging effects and furthermore were done in anesthetized conditions [10–13]. Although fMRI and NIRS studies have described an altered hemodynamic and blood oxygenation response with age [14–18], interpretation of these observations to make inferences about the tissue pO2 response is challenging, because the latter is affected by a complex interaction between vascular hemoglobin concentration, oxygen saturation of hemoglobin (SO2), CBF, the geometry and morphology of the vascular network, capillary density, and the cerebral metabolic rate of oxygen consumption (CMRO2); all potentially modulated by age.
Here, using two-photon phosphorescence lifetime microscopy [19] and the O2-sensitive two-photon enhanced phosphorescent dye PtP-C343 [20], we aimed to directly measure the brain tissue pO2 response to neuronal activation in awake mice and investigate its change with aging.
2. Methods
2.1. Synthesis of the PtP-C343 probe
The O2-sensitive phosphorescent molecule PtP-C343 [20] was synthetized as described in Moeini et al. (2018) [9] by adaptation and modification of a procedure described in the literature [20]. It has been reported that the brain interstitial fluid is slightly more acidic than blood, with a pH of ˜7.3 or even lower [21,22]. The dye was then calibrated at pH 7.2 and 37 °C, following previous protocols [19,23]. However, it should be noted that the pO2 dye PtP-C343 does not exhibit pH dependence between pH 6 and 8.5 [20]. Therefore, a slight deviation of the brain tissue pH from 7.2 will not affect our pO2 measurement.
2.2. Animal preparation
Animal handling and surgical procedures were approved by the ethics committee of the research center of the Montreal Heart Institute. All experiments were performed in accordance with the ARRIVE guidelines and the recommendations of the Canadian Council on Animal Care. Mice were randomly assigned to experimental groups. Young adult (n = 7), middle-aged (n = 6) and old (n = 7) C57BL/6 J healthy male mice were obtained from the colony of aged mice of the Quebec Network for Aging Research (RQRV) and housed in 12-hr lightdark cycle until imaging. In our study, in addition to the young and old groups, we also included a middle-age group to determine if potential age-related changes are progressive or they only happen in old ages. Indeed, in a previous study [9] we observed that the changes in resting cerebral tissue oxygenation are exacerbated after middle-age. Young adult mice were 8–9 months old, which ensured the absence of maturational growth and related anatomical and biological changes observed in younger ages [24]. Middle-aged mice were 15–16 months old, according to the recommended age range of 10–15 months for the middle-aged groups [24]. Old mice were 26–28 months old, well above 18 months after which almost all aging biomarkers show age-related changes [24]. No reduction in mice survival was observed until this age. 8–10 days before measurements, a thinned skull window was created over the left barrel cortex under isoflurane anesthesia (2.0% in pure oxygen) as described in Shih et al. [25]. Briefly, the scalp was removed and the exposed skull was covered with a thin layer of tissue adhesive (Vetbond). A custom head-plate made from titanium was fixed on the skull using dental cement. The skull was then slowly thinned to translucency with a micro-drill (OmniDrill 35, World Precision, USA). A 150 μm-thick cover glass was glued to the dried window using cyanoacrylate glue and the edges were sealed with dental cement to form a 3 mm diameter cranial window. A small thinned region at the edge of the cover glass (˜0.5 mm) was left uncovered with dental cement to allow the injection of the PtP-C343 dye into tissue through the soft thinned membrane. During the surgery, animals were fixed on a controlled physiological monitoring system (LabeoTech, Canada) which enabled continuous monitoring of the rectal temperature, respiration and heart rate. Ketoprofen (s.c., 5 mg/Kg, Merial, Canada) and buprenorphine (s.c., 0.05 mg/Kg, Reckitt Benckiser Healthcare, UK) were injected before the surgery and baytril (i.p., 5 mg/Kg, Bayer, Germany) was injected after the surgery. Animals were returned to the cages and injections were repeated 24 h after the surgery. Although this study was not blinded, the analysis of microscopic data was automated and the same parameters and algorithms were used to analyze all images limiting investigator bias. In addition, no data was discarded and care was taken to treat all groups equally during surgery, handling, training and imaging.
2.3. Awake imaging
All measurements were performed in awake animals to avoid potential age-related anesthesia confounds. During the imaging sessions, animals were fixed on an angled treadmill wheel which allowed free movement of the limbs while the head was restrained by a custom titanium bar, as described in Moeini et al. [9]. They were trained on the wheel over four fixation training sessions (starting after 3 days recovery following surgery) to habituate to head restraint and minimize stress during the imaging sessions. The duration of the restraint was gradually increased from 10 min to 45 min over 4 sessions.
2.4. Whisker stimulation
Air puffs, generated from pulses of compressed air (pressure: 25 psi, frequency: 5 Hz, pulse width: 100 ms) from a pressure microinjection system (PICOSPRITZER III, Parker Hannifin, USA) were used to stimulate the right whiskers (nozzle to whisker distance: 150–200 mm). We used stimulus duration of 5 s with interstimulus intervals of 30–35 s.
2.5. Tissue pO2 response to whisker stimulation measured by two-photon phosphorescence lifetime microscopy
Two-photon imaging was performed using a custom-built laser scanning microscope, as described by Moeini et al. [9]. For tissue pO2 imaging, the PtP-C343 dye solution (˜150 μM in ACSF) was slowly injected into the brain tissue ˜300 μm below the surface with a glass micropipette using a microsyringe injector (UMP3, World Precision Instruments, USA). The injection was done through the thinned skull next to the cover glass. The diffusion of the injected dye to the surrounding tissue allowed imaging of cerebral tissue beneath the cover glass. It is assumed that the dye is mainly distributed in the interstitial and perivascular space. Since we measured the phosphorescence lifetime rather than intensity (see below), our pO2 measurements are not affected even if the injected dye slightly concentrates near the surface or in the perivascular space. ˜200 μl 2 M Da FITC-Dextran (50 mg/ml in saline, Sigma) was also injected through the tail vein to visualize the vasculature. Each measurement contained 10 points around diving arterioles, up to 200 μm from the arteriole. At each point 150 decays were recorded before moving to the next point. This was repeated 600 times, covering 13 stimulus blocks, to increase the signal to noise ratio. Each excitation cycle consisted of 25 μs excitation period in which the laser pulse was “on” followed by 275 μs “off” period in which the phosphorescence emission was allowed to decay. The number of the points and decays were adjusted in a way to allow a temporal resolution below 1 s. For each point, all decays (from all 13 stimulation blocks and interstimulus periods) were sorted based on their time from the stimulation onset and were averaged in 0.9 s intervals to obtain the pO2 response for individual points. Averaged phosphorescence decay at each point/time was fitted with a single-exponential curve to determine the phosphorescence lifetime. The lifetimes were then converted to pO2 using a calibration curve.
For each measurement point, the time course of obtained pO2 values yielded the tissue pO2 response to stimulation. From this response, the baseline was estimated as the averaged pO2 within a 4 s window before stimulus onset. The post-stimulus pO2 was estimated as the average of values between 8–25 s following the stimulus onset while the peak response was estimated as the average response between 2–5 s following the stimulation onset. All sampled points in each age group were pooled for age comparisons.
Finally, tissue pO2 responses for all point measurements in each age group were averaged. The average responses were then used to parameterize the dynamics of tissue pO2 response to whisker stimulation. Two temporal parameters (Trise and Tfall) were obtained from the average response curves of age groups. Trise and Tfall were defined as the response times during the rise or fall periods in which tissue pO2 was at half maximum. It should be noted that since two-photon measures are very localized (compared to confocal microscopy, for example), the response curves for individual sampled points were more noisy which made it difficult to extract reliable Trise and Tfall for individual curves.
2.6. Estimation of net oxygen consumption rate (OC) in cerebral tissue
Previous studies on rats have observed a marked absence of capillaries in the vicinity of penetrating arterioles, forming a capillary depleted cylindrical region with a radius of ˜50 μm [26]. This has formed the basis of some efforts [27] to calculate the CMRO2 by fitting the tissue pO2 gradients around arterioles with the Krogh cylinder model [28] of O2 diffusion from a vessel to surrounding tissue. In our experiments on mice, however, we found that the use of the ideal Krogh model is not accurate because in most cases either tissue pO2 profiles from arterioles do not plateau within the capillary depleted region or the capillary depleted regions are absent. Therefore, for mice the oxygen consumption term in the Krogh model includes both CMRO2 and capillary O2 supply [9]. Assuming a uniform capillary O2 supply in tissue (for simplicity), a modification of the Krogh model was used to estimate the net oxygen consumption rate in the tissue (OC), defined as CMRO2 minus capillary O2 supply per unit volume of the tissue (Please note the difference between the CMRO2 and OC: CMRO2 is the oxygen consumption rate in tissue by cells, while OC is the same but after subtracting the rate of oxygen supply by cerebral capillaries. Therefore, OC represents the balance between the oxygen consumption and supply in the tissue.) [9]:
| (1) |
In Eq. (1) pO2 art is the arterial pO2, Rart is the mean arterial radius, Rt is the radius of the Krogh cylinder, α is oxygen solubility (1.27 × 10−3 μmoleO2/ml/mmHg [27]), and D is oxygen diffusivity in tissue (assumed to be ˜4000 μm2/s [27]). Tissue pO2 profiles around arterioles, obtained from tissue pO2 maps (at baseline and during stimulation or post-stimulation periods), were fitted with the Krogh model to find OC and pO2,art. Rt was determined as the distance at which the curve reached a plateau.
2.7. Non-capillary blood flow measurements with Doppler-OCT
We also measured blood flow in non-capillary vessels (diameter > 10 μm) at rest using a custom optical coherence tomography (OCT) setup, as described by Moeini et al. (2018) [9]. For each mouse, an OCT angiogram and a 3D Doppler OCT volume were acquired over a cortical surface of ˜700 μm × 700 μm with the procedure explained in Moeini et al. (2018) [9]. The 3D Doppler OCT datasets consisted of en face slices of z-projection blood velocity maps at cortical depth intervals of ˜3.8 μm. Pial vessels generated both positive and negative Doppler shifts because of their undulating shape, but diving arterioles and ascending venules exhibited pure positive or negative Doppler shifts. To avoid the pial vessels and focus only on the penetrating vessels, we excluded the top 100 μm of the OCT datasets in our analyses. Arterioles and venules were labeled on en face slices over the depth of 100–650 μm and their diameter, blood velocity, and mean blood flow were obtained. The relationship between the baseline flow and vessel diameter was then calculated by linear regression analysis between the arterial/venular velocity and the vessel diameter and also between the logarithmic arterial/venular flow and the logarithmic vessel diameter.
2.8. Statistical analysis
The results are presented as either box-and-whiskers plots (in which the box represents the 75th and 25th percentiles, the central line indicates the median, the whiskers represent the most extreme data not considered outliers (> ± 2.7SD), and the ‘+’ symbols represent the outliers) or bar plots (representing mean ± 95% confidence intervals, c.i.). Statistical significance was calculated using ANOVA followed by Tukey HSD post hoc test. Statistical significance was assigned at *p < 0.05, **p < 0.01, ***p < 0.001, **** p < 0.0001 and *****p < 0.00001. The sample sizes are described in the corresponding figure legends. The sample sizes were chosen empirically based on our previous experience.
3. Results
3.1. Preserved magnitude, but altered dynamics of tissue pO2 response to whisker stimulation with aging
Tissue pO2 responses to whisker stimulation for young, middle-aged and old mice are shown in Fig. 1a. We observed a substantial decrease in baseline tissue pO2 after middle-age, which is consistent with our previous observations at rest [9]. When converting the tissue pO2 responses to ΔpO2 (pO2 – baseline pO2), we found no significant difference in the response delay (˜1 s) (Fig. 1b) and the peak tissue ΔpO2 response between age groups (Fig. 1b and c). However, the response in young animals had faster dynamics (faster rise and fall, Table 1) and showed a clear post-stimulus undershoot (Fig. 1b and c), in agreement with previous BOLD fMRI [29–31] and NIRS [16] studies. On the other hand, no undershoot was observed in middle-age and old animals (Fig. 1b and c).
Fig. 1.
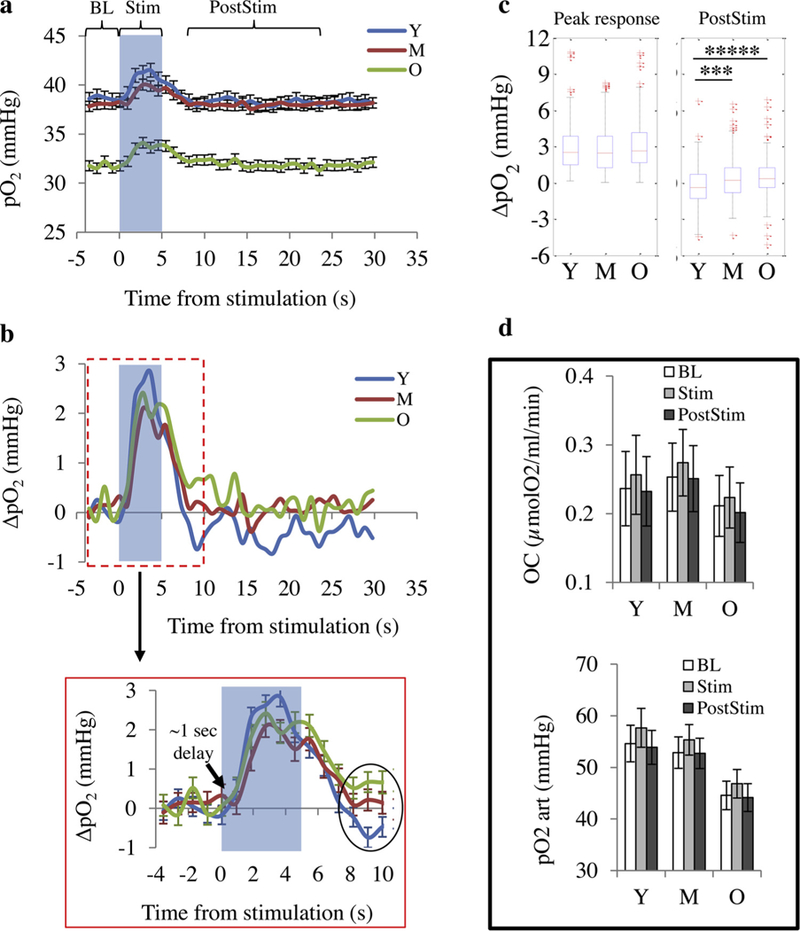
Tissue pO2 response to whisker stimulation. (a) Averaged time courses (mean ± s.e.m.) of absolute tissue pO2 changes in response to 5 s whisker stimulation (dark area). (b) The same data as in (a) but plotted as ΔpO2 (pO2 – baseline pO2). (c) Peak response magnitude (average response through 2–5 s from the stimulation onset) and average response during post-stimulation period (8–25 s from the stimulation onset, PostStim). The box represents interquartile range, the central line indicates the median and the whiskers extend to the most extreme data points not considered outliers (> ± 2.7SD); the outliers are plotted individually using the ‘+’ symbol. (d) Each stimulation test consisted of a gridline point measurement from an arteriole (up to 200 μm from the arteriole). Average pO2 values at baseline (BL) and during the stimulation (Stim) and pos-stimulation (PostStim) periods were found for all sampled points and were plotted against the distance from the arteriole. Net O2 consumption rates (OC) (top) and pO2 at arterial wall (pO2,art) (bottom) were obtained by fitting the data to the Krogh model, Eq. (1). Bar plots represent mean ± c.i.(95%). Statistical significance was calculated using ANOVA followed by Tukey HSD post hoc test. *****: p < 0.00001 ***: p < 0.001. Y: young (n = 195 points); M: middle-aged (n = 188 points); O: old (n = 222 points).
Table 1.
Parameterization of tissue pO2 response to whisker stimulation (obtained from the average response curves of age groups). Trise and Tfall were defined as the response times during the rise or fall periods in which tissue pO2 was half maximum.
| Trise (s) | Tfall (s) | |
|---|---|---|
| Young | 1.43 | 5.75 |
| Middle-aged | 1.70 | 6.41 |
| Old | 1.46 | 6.72 |
3.2. The balance between the CMRO2 increase and capillary O2 supply increase during stimulation is preserved with aging
Estimation of OC and arterial pO2 at baseline and during stimulation and post-stimulation periods (by fitting the tissue pO2 profiles from arterioles with Krogh model) showed a slight increase in arterial pO2 and OC during stimulation which returned to baseline values after the stimulation, but differences were subtle and did not reach statistical significance (Fig. 1d). This trend and the magnitude of changes were not affected by age. This shows that for all age groups, both CMRO2 and capillary O2 supply increase proportionally during stimulation such that the net oxygen consumption rate (OC = CMRO2 – capillary O2 supply) does not significantly change during stimulation.
3.3. Age-related increases in the slope of flow-diameter and speed-diameter correlations for venules
To investigate whether the change in response undershoot could have a biomechanical component, we performed a linear regression analysis of baseline flow-diameter (on a logarithmic scale) and baseline speed-diameter from Doppler OCT data (at rest). Data showed an agerelated increase in the slope of linear fits for venules, while the slopes for arterioles were unchanged with age (Fig. 2). This could indicate a reduced ability of venules and veins for passive dilation due to decreased compliance with aging (see below).
Fig. 2.
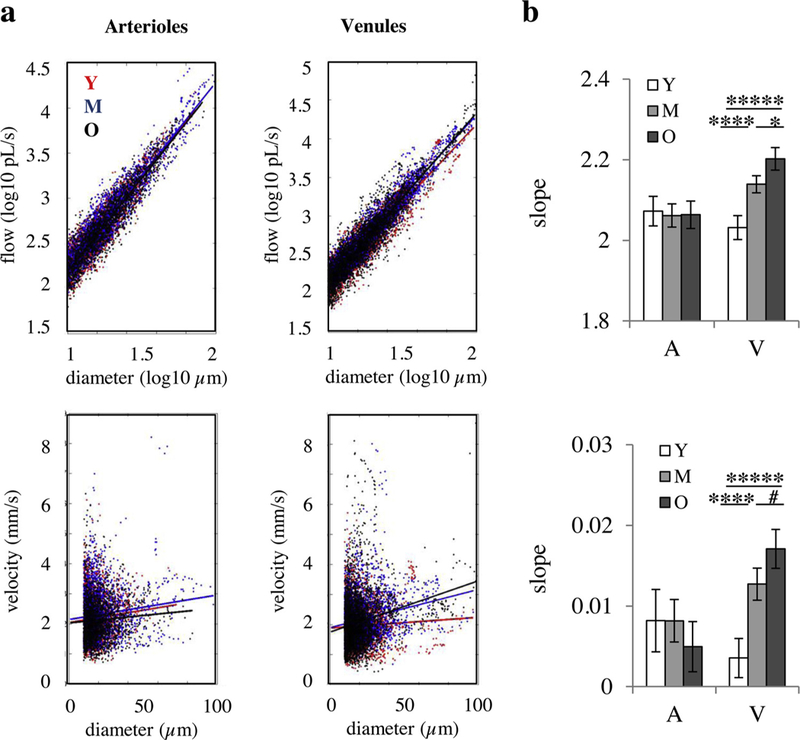
Flow-diameter correlations. (a) Correlation between the blood flow and diameter in a log-log scale (top) and linear correlation between the blood velocity and diameter (bottom) for arterioles (left) and venules (right). The points show the experimental data and the solid lines show the best linear fits. (b) The slop of the linear fits in (a) for arterioles and venues (V). Top: flow-diameter correlation in log-log scale. Bottom: velocity-diameter correlation. Bar plots represent mean ± c.i.(95%). *****: p < 0.00001 ****: p < 0.0001. *: p < 0.05, #: p-value approaches significance; statistical significance was calculated using ANOVA followed by Tukey HSD post hoc test. Y: young (n = 7 mice); M: middle-aged (n = 6 mice); O: old (n = 7 mice).
4. Discussion
In this study, we found a substantial decrease in average baseline tissue pO2 in the cortex of old mice, compared with young and middleaged groups (Fig. 1a). This is in agreement with our previous observations in awake mice at rest, indicating impaired resting cerebral tissue oxygenation in old mice [9]. Regarding the response to whisker stimulation, we observed that the peak magnitude of the tissue pO2 response was not changed significantly with age, but the response dynamics were altered towards a slower response with reduced post-stimulus undershoot in older ages (Fig. 1b and c).
All measurements were performed in awake behaving mice, removing the confounding effects of anesthesia. This is a very important aspect of this study since anesthetized experiments often need mechanical ventilation and/or adjustment of physiological parameters which confounds the results as many of these parameters normally change with aging. In addition, anesthesia may affect neuronal activation and corresponding flow and pO2 responses. To minimize animal stress during imaging sessions, a custom-built treadmill wheel was used in which the animal was able to freely walk or run, while the head was fixed, similar to that of Lyons et al. [32], and animals were trained on the wheel for habituation before the main experiments. To our knowledge this is the first study on direct measurement of the tissue pO2 response to neural stimulation under awake conditions and the changes in the response with aging.
The observed changes in the dynamics of tissue pO2 response to whisker stimulation are in agreement with previous studies. A slower hemodynamic response and a reduced post-stimulus undershoot in older subjects have also been described using BOLD fMRI [14,15,17] and NIRS [16] studies. Hemodynamic response modeling using the arteriolar compliance model coupled with the passive Balloon model [33] also predicted a slower response and no undershoot in aged subjects, mainly due to reduced arteriolar wall elasticity [34,35]. The Balloon model itself [29] also predicts a slower response for stiffer vessel wall. One observation in our study in support of these findings is that flow-diameter and speed-diameter correlations for venules from our Doppler OCT data showed age-related increase in the slope of linear correlations (Fig. 2). Considering the general relationship between flow and the pressure drop (ΔP) across a vessel section (flow=ΔP/resistance), and since resistance varies with diameter and vessel length, the slope of the flow-diameter correlation (across different vessels) quantifies either changes in ΔP across vessel segments of similar size with age or changes in vascular segment length. Changes in upstream network with age (for example, reduced capillary density [36]) will increase the upstream resistance, and reduce the input pressure to the venule (assuming no change of systemic pressure with age and that pressure goes to zero on the other end of the venule, and keeping all other variables constant). Our results then rather support another possibility of reduced venule compliance associated with a higher pressure drop. The latter is in agreement with previous reports of decreased elasticity of vessels with age [36,37], which reduces the ability of venules and veins for passive dilation. Other mechanisms have also been proposed for the post-stimulus undershoot, including prolonged elevation in CMRO2 and post-stimulus CBF undershoots [30] associated with a post-stimulus decrease in neuronal activity [38]. In addition, CO2 washout during the stimulation period has also been suggested to play a role by producing a brief post-stimulus CBF undershoot by hypocapnia induced vasoconstriction [39]. Further studies need to be performed to investigate the role of these proposed mechanisms in the observed age-related changes in the post-stimulation response. For example, possible age-related changes in the baseline pCO2 or post-stimulus neuronal inhibition may play a role.
There has been considerable debate over the underlying mechanisms for the O2 overshoot observed during the hemodynamic response and some investigators have perceived it as a paradox or uncoupling [40,41]. Prior measurements of tissue pO2 changes during brain activation suggest that the O2 overshoot occurs to prevent tissue pO2 decrease in the capillary bed during stimulation [10]. Having an O2 overshoot with constant tissue pO2 would result in a greater O2 concentration gradient from capillaries to tissue, which supports greater oxygen extraction from the capillaries and oxygen consumption by tissue. Indeed, our observation that OC (net oxygen consumption rate in tissue = CMRO2 – capillary O2 supply) remains unchanged or tends to increase slightly during neuronal activation, means that despite an O2 overshoot, the increase in capillary O2 supply during stimulation is still less than or at most equal to the CMRO2 increase. Thus, the O2 overshoot may be necessary to secure a higher capillary O2 delivery to the tissue proportional to increased CMRO2 to maintain the capillary tissue pO2. Our data shows that the coupling between the CMRO2 and capillary O2 delivery during neuronal activation is well preserved with age (Fig. 1d).
Given the reductions in baseline cerebral tissue pO2 with aging and the presence of hypoxic regions in the cortex of old mice [9], it is important to understand if neuronal stimulation can also dynamically create regions of tissue hypoxia and further compromise the cerebral tissue oxygenation in old ages. However, the limitations of the present study did not allow the investigation of the presence of hypoxic regions during stimulation. To reach a high temporal resolution as a requirement accurate measurement of tissue pO2 response, we had to measure limited number of sampling points which does not allow the spatial identification of sparse hypoxic pockets. With the development of brighter pO2 probes [42] which allow faster measurements, future studies may be able to investigate the hypoxia potential during stimulation in aged animals. One other limitation of the present study is that the OCT data (Fig. 2) were obtained only at rest. Although the findings at rest could also help in better understanding of the mechanisms behind altered pO2 response dynamics with age (as explained above), in future, fast repetitive OCT scans on single slices crossing the arterioles and venules, similar to the work done by Baraghis et al. [43], could be performed to estimate the vascular compliance by measuring the changes in the vessel diameter and blood flow during the stimulation. Specifically, the OCT and two-photon setups could be integrated to allow the measurement of vascular reactivity and tissue pO2 response for the same vessels.
Overall, our study reveals that while baseline cerebral tissue pO2 decreases with age in conscious mice, tissue pO2 response to stimulation is well preserved with age. However, the response dynamics are altered towards a slower response with reduced post-stimulus undershoot in older ages. In addition, our data suggests that the O2 overshoot during stimulation may be necessary to sustain capillary O2 supply, and that the coupling between the CMRO2 and capillary O2 delivery is preserved with age.
Acknowledgments
The authors thank Marc-Antoine Gillis and Natacha Duquette (Montreal Heart Institute) for their help with animal preparations. The authors are grateful to Sergei Vinogradov (University of Pennsylvania) for useful comments on PtP-C343 probe synthesis and calibrating the probe for us, Tina Lam, Nadim Saade, Anjali Sharma, William Curtis, Yu-Chen Wang (McGill University), Emmanuel Roussakis (Harvard Medical School), and Tatiana Esipova (University of Pennsylvania) for assistance with PtP-C343 probe synthesis and characterization. We thank the RQRV (Réseau Québécois de Recherche sur le Vieillissement) Network’s Colony of aged mice (in part funded by the Fonds de Recherche du Québec - Santé) for its valued contribution to the present study. This study was funded by a Canadian Institutes of Health Research (CIHR, 299166) operating grant and a Natural Sciences and Engineering Research Council of Canada (NSERC, 239876–2011) discovery grant to F. Lesage. M. Moeini was supported by the MÉDITIS Program (École Polytechnique) and Fonds de Recherche du Québec– Nature et Technologies (FRQNT).
The sources of support
Canadian Institutes of Health Research (CIHR, 299166) operating grant and Natural Sciences and Engineering Research Council of Canada (NSERC, 239876–2011) discovery grant to F. Lesage.
Footnotes
Conflict of interest
Dr. Lesage reports a minority ownership in LabeoTech Inc.
References
- [1].Hasher L, Zacks RT, Working memory, comprehension, and aging: a review and a new view, in: Bower Gordon H. (Ed.), Psychol. Learn. Motiv Academic Press, 1988, pp. 193–1988225 http://www.sciencedirect.com/science/article/pii/S0079742108600419. [Google Scholar]
- [2].Salthouse T, The processing-speed theory of adult age differences in cognition, Psychol. Rev 103 (1996) 403–428. [DOI] [PubMed] [Google Scholar]
- [3].Bertsch K, Hagemann D, Hermes M, Walter C, Khan R, Naumann E, Resting cerebral blood flow, attention, and aging, Brain Res 1267 (2009) 77–88, 10.1016/j.brainres.2009.02.053. [DOI] [PubMed] [Google Scholar]
- [4].Eustache F, Rioux P, Desgranges B, Marchal G, Petit-Taboué M-C, Dary M, Lechevalier B, Baron J-C, Healthy aging, memory subsystems and regional cerebral oxygen consumption, Neuropsychologia 33 (1995) 867–887, 10.1016/0028-3932(95)00021-T. [DOI] [PubMed] [Google Scholar]
- [5].Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, van Duijn CN, Van Broeckhoven C, Grobbee DE, Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study, Lancet 349 (1997) 151–154, 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- [6].Dickstein DL, Walsh J, Brautigam H, Stockton SD, Gandy S, Hof PR, Role of vascular risk factors and vascular dysfunction in Alzheimer’s disease, Mt. Sinai J. Med. J. Transl. Pers. Med 77 (2010) 82–102, 10.1002/msj.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer RA, Coker LH, Sidney S, Early adult to midlife cardiovascular risk factors and cognitive function, Circulation 129 (2014) 1560–1567, 10.1161/CIRCULATIONAHA.113.004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Berman RF, Goldman H, Altman HJ, Age-related changes in regional cerebral blood flow and behavior in Sprague-Dawley rats, Neurobiol. Aging 9 (1988) 691–696, 10.1016/S0197-4580(88)80134-9. [DOI] [PubMed] [Google Scholar]
- [9].Moeini M, Lu X, Avti PK, Damseh R, Bélanger S, Picard F, Boas D, Kakkar A, Lesage F, Compromised microvascular oxygen delivery increases brain tissue vulnerability with age, Sci. Rep 8 (2018) 8219, 10.1038/s41598-018-26543-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Devor A, Sakadžić S, Saisan PA, Yaseen MA, Roussakis E, Srinivasan VJ, Vinogradov SA, Rosen BR, Buxton RB, Dale AM, Boas DA, “Overshoot” of O2 is required to maintain baseline tissue oxygenation at locations distal to blood vessels, J. Neurosci 31 (2011) 13676–13681, 10.1523/JNEUROSCI.1968-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vazquez AL, Fukuda M, Tasker ML, Masamoto K, Kim S-G, Changes in cerebral arterial, tissue and venous oxygenation with evoked neural stimulation: implications for hemoglobin-based functional neuroimaging, J. Cereb. Blood Flow Metab 30 (2010) 428–439, 10.1038/jcbfm.2009.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ances B, Buerk D, Greenberg J, Detre J, Temporal dynamics of the partial pressure of brain tissue oxygen during functional forepaw stimulation in rats, Neurosci. Lett 306 (2001) 106–110. [DOI] [PubMed] [Google Scholar]
- [13].Ances B, Buerk D, Greenberg J, Detre J, Simultaneous measurements of brain tissue pO2 and cerebral blood flow during functional stimulation, Int. Congr. Ser 1235 (2002) 155–163. [Google Scholar]
- [14].Mehagnoul-Schipper DJ, van der Kallen BFW, Colier WNJM, van der Sluijs MC, van Erning LJTO, Thijssen HOM, Oeseburg B, Hoefnagels WHL, Jansen RWMM, Simultaneous measurements of cerebral oxygenation changes during brain activation by near-infrared spectroscopy and functional magnetic resonance imaging in healthy young and elderly subjects, Hum. Brain Mapp 16 (2002) 14–23, 10.1002/hbm.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Richter W, Richter M, The shape of the fMRI BOLD response in children and adults changes systematically with age, NeuroImage 20 (2003) 1122–1131, 10.1016/S1053-8119(03)00347-1. [DOI] [PubMed] [Google Scholar]
- [16].Schroeter ML, Zysset S, Kruggel F, von Cramon DY, Age dependency of the hemodynamic response as measured by functional near-infrared spectroscopy, NeuroImage 19 (2003) 555–564, 10.1016/S1053-8119(03)00155-1. [DOI] [PubMed] [Google Scholar]
- [17].Taoka T, Iwasaki S, Uchida H, Fukusumi A, Nakagawa H, Kichikawa K, Takayama K, Yoshioka T, Takewa M, Ohishi H, Age correlation of the time lag in signal change on EPI-fMRI, J. Comput. Assist. Tomogr 22 (1998), http://journals.lww.com/jcat/Fulltext/1998/07000/Age_Correlation_of_the_Time_Lag_in_Signal_Change.2.aspx. [DOI] [PubMed] [Google Scholar]
- [18].Dubeau S, Ferland G, Gaudreau P, Beaumont E, Lesage F, Cerebrovascular hemodynamic correlates of aging in the Lou/c rat: a model of healthy aging, NeuroImage 56 (2011) 1892–1901, 10.1016/j.neuroimage.2011.03.076. [DOI] [PubMed] [Google Scholar]
- [19].Sakadzic S, Roussakis E, Yaseen MA, Mandeville ET, Srinivasan VJ, Arai K, Ruvinskaya S, Devor A, Lo EH, Vinogradov SA, Boas DA, Two-photon highresolution measurement of partial pressure of oxygen in cerebral vasculature and tissue, Nat. Methods 7 (2010) 755–759, 10.1038/nmeth.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Finikova OS, Lebedev AY, Aprelev A, Troxler T, Gao F, Garnacho C, Muro S, Hochstrasser RM, Vinogradov SA, Oxygen microscopy by two-photon-excited phosphorescence, ChemPhysChem 9 (2008) 1673–1679, 10.1002/cphc.200800296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chesler M, Regulation and modulation of pH in the brain, Physiol. Rev 83 (2003) 1183–1221. [DOI] [PubMed] [Google Scholar]
- [22].Portman MA, Lassen NA, Cooper TG, Sills A, Potchen EJ, Intra-and extracellular pH of the brain in vivo studied by 31P-NMR during hyper-and hypocapnia, J. Appl. Physiol 71 (1991) 2168–2172. [DOI] [PubMed] [Google Scholar]
- [23].Rozhkov V, Wilson D, Vinogradov S, Phosphorescent Pd porphyrin−dendrimers: tuning core accessibility by varying the hydrophobicity of the dendritic matrix, Macromolecules 35 (2002) 1991–1993, 10.1021/ma0121161. [DOI] [Google Scholar]
- [24].Flurkey K, Currer JM, Harrison D, Mouse models in aging research, Mouse Biomed. Res second ed., Elsevier, 2007, pp. 637–672. [Google Scholar]
- [25].Shih AY, Mateo C, Drew PJ, Tsai PS, Kleinfeld D, A polished and reinforced thinned-skull window for long-term imaging of the mouse brain, J. Vis. Exp. JoVE (2012) 3742, 10.3791/3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kasischke KA, Lambert EM, Panepento B, Sun A, Gelbard HA, Burgess RW, Foster TH, Nedergaard M, Two-photon NADH imaging exposes boundaries of oxygen diffusion in cortical vascular supply regions, J. Cereb. Blood Flow Metab 31 (2011) 68–81, 10.1038/jcbfm.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sakadžić S, Yaseen MA, Jaswal R, Roussakis E, Dale AM, Buxton RB, Vinogradov SA, Boas DA, Devor A, Two-photon microscopy measurement of cerebral metabolic rate of oxygen using periarteriolar oxygen concentration gradients, Neurophotonics 3 (2016) 045005–3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Krogh A, The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue, J. Physiol 52 (1919) 409–415, 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Buxton RB, Wong EC, Frank LR, Dynamics of blood flow and oxygenation changes during brain activation: the balloon model, Magn. Reson. Med 39 (1998) 855–864, 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- [30].Chen JJ, Pike GB, Origins of the BOLD post-stimulus undershoot, NeuroImage 46 (2009) 559–568, 10.1016/j.neuroimage.2009.03.015. [DOI] [PubMed] [Google Scholar]
- [31].Mullinger KJ, Mayhew SD, Bagshaw AP, Bowtell R, Francis ST, Poststimulus undershoots in cerebral blood flow and BOLD fMRI responses are modulated by poststimulus neuronal activity, Proc. Natl. Acad. Sci 110 (2013) 13636–13641, 10.1073/pnas.1221287110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lyons DG, Parpaleix A, Roche M, Charpak S, Mapping oxygen concentration in the awake mouse brain, eLife 5 (2016) e12024, , 10.7554/eLife.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Behzadi Y, Liu TT, An arteriolar compliance model of the cerebral blood flow response to neural stimulus, NeuroImage 25 (2005) 1100–1111, 10.1016/j.neuroimage.2004.12.057. [DOI] [PubMed] [Google Scholar]
- [34].Hajdu MA, Heistad DD, Siems JE, Baumbach GL, Effects of aging on mechanics and composition of cerebral arterioles in rats, Circ. Res 66 (1990) 1747–1754, 10.1161/01.RES.66.6.1747. [DOI] [PubMed] [Google Scholar]
- [35].Riddle DR, Sonntag WE, Lichtenwalner RJ, Microvascular plasticity in aging, Ageing Res. Rev 2 (2003) 149–168, 10.1016/S1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- [36].Brown WR, Thore CR, Review: cerebral microvascular pathology in aging and neurodegeneration, Neuropathol. Appl. Neurobiol 37 (2011) 56–74, 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Steppan J, Barodka V, Berkowitz DE, Nyhan D, Vascular stiffness and increased pulse pressure in the aging cardiovascular system, Cardiol. Res. Pract 2011 (2011) 263585, 10.4061/2011/263585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shmuel A, Augath M, Oeltermann A, Logothetis NK, Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1, Nat. Neurosci 9 (2006) 569–577, 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- [39].Yücel MA, Devor A, Akin A, Boas DA, The possible role of CO(2) in producing a post-stimulus CBF and BOLD undershoot, Front. Neuroenergetics 1 (2009) 7, 10.3389/neuro.14.007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Leithner C, Royl G, The oxygen paradox of neurovascular coupling, J. Cereb. Blood Flow Metab 34 (2014) 19–29, 10.1038/jcbfm.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Paulson OB, Hasselbalch SG, Rostrup E, Knudsen GM, Pelligrino D, Cerebral blood flow response to functional activation, J. Cereb. Blood Flow Metab 30 (2010) 2–14, 10.1038/jcbfm.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Esipova TV, Rivera-Jacquez HJ, Weber B, Masunov AE, Vinogradov SA, Stabilizing g-states in centrosymmetric tetrapyrroles: two-photon-absorbing porphyrins with bright phosphorescence, J. Phys. Chem. A 121 (2017) 6243–6255, 10.1021/acs.jpca.7b04333. [DOI] [PubMed] [Google Scholar]
- [43].Baraghis E, Bolduc V, Lefebvre J, Srinivasan VJ, Boudoux C, Thorin E, Lesage F, Measurement of cerebral microvascular compliance in a model of atherosclerosis with optical coherence tomography, Biomed. Opt. Express 2 (2011) 3079–3093, 10.1364/BOE.2.003079. [DOI] [PMC free article] [PubMed] [Google Scholar]


