Abstract
Objective
To assess human papillomavirus (HPV) vaccination coverage among adolescents by provider recommendation status.
Study design
The 2011-2016 National Immunization Survey-Teen data were used to assess HPV vaccination coverage among male adolescents by provider recommendation status. Multivariable logistic analyses were conducted to evaluate associations between HPV vaccination and provider recommendation status.
Results
HPV vaccination coverage among male adolescents increased from 8.3% in 2011 to 57.3% in 2016. Likewise, the prevalence of provider recommendation increased from 14.2% in 2011 to 65.5% in 2016. In 2016, HPV coverage was higher in male adolescents with a provider recommendation than in those without a provider recommendation (68.8% vs 35.4%). In multivariable logistic regression, characteristics independently associated with a higher likelihood of HPV vaccination included receipt of a provider recommendation, age 16-17 years, black or Hispanic race/ethnicity, any Medicaid insurance, ≥2 physician contacts in the previous 12 months, and urban or suburban residence. Participants with a mother with some college or a college degree, those with a mother aged 35-44 years, and those who did not have a well-child visit at age 11-12 years had a lower likelihood of HPV vaccination.
Conclusions
Receiving a provider recommendation for vaccination was significantly associated with receipt of HPV vaccine among male adolescents, indicating that a provider recommendation for vaccination is an important approach to increase vaccination coverage. Evidence-based strategies, such as standing orders and provider reminders, alone or in combination with health system interventions, are useful for increasing provider recommendations and HPV vaccination coverage among male adolescents.
Human papillomavirus (HPV) is the most common sexually transmitted infection in men and women in the US.1–5 Based on data for 2011-2015, approximately 18 300 new cases of HPV-associated cancers occur among US males each year.6 Vaccination is an important tool to prevent and control HPV infection and its complications, including genital warts, precancerous lesions, and cancer.1 In 2006, the quadrivalent HPV vaccine (4vHPV) was licensed by the Food and Drug Administration for use in females.1 In 2009, the Advisory Committee on Immunization Practices (ACIP) voted and provided guidance for use of the 4vHPV vaccine may in males aged 9-26 years (a permissive recommendation).7,8 In 2011, the ACIP recommended the routine use of 4vHPV in males aged 11-12 years and recommended 4vHPV in males aged 13-21 years who had not been vaccinated previously or had not completed the 3-dose series.5 Males aged 22-26 may be vaccinated.5 Overall, HPV vaccination coverage of male adolescents have increased substantially in recent years.9,10
To evaluate trends in vaccination and factors associated with HPV vaccination and provider recommendations for HPV vaccination among adolescents, we analyzed data for male adolescents aged 13-17 years from the 2011-2016 National Immunization Survey-Teen (NIS-Teen) to assess HPV vaccination coverage among male adolescents, trends in HPV vaccination and provider recommendations for male adolescents, provider recommendations and other factors associated with HPV vaccination, and coverage disparities among male adolescents with and without a provider recommendation at the national and state levels.
Methods
The 2011-2016 NIS-Teen data were analyzed. The 2016 data were used to conduct major bivariable analyses and multivariable models, and the 2011-2016 data were used to evaluate trends in vaccination and the prevalence of provider recommendations. The NIS-Teen is a national, random-digit-dial telephone survey conducted by the Centers for Disease Control and Prevention to provide timely, detailed information regarding vaccination coverage among adolescents aged 13-17 years for vaccines recommended by the ACIP, including the HPV vaccine, and to evaluate factors associated with vaccination. Survey data are collected in 2 phases. In the first phase, a random-digit-dial telephone interview is conducted to identify households with age-eligible adolescents (aged 13-17 years at the time of the interview) and to collect demographic information from the parent or guardian on adolescent, maternal, and household characteristics. The interview also includes questions on the adolescent’s reported vaccination history. After completion of the interview, consent is requested to contact the vaccination provider. If consent is obtained, the adolescent’s vaccination provider is mailed a questionnaire to collect a provider-reported vaccination history for each recommended adolescent vaccine and selected childhood vaccines. The provider-reported histories are used to determine vaccination coverage estimates.8,11,12
In 2016, the NIS-Teen sampling plan included independent samples of households with a landline and households with a cell phone.9,11 There were a total of 18 948 adolescents with adequate provider data from landline and cell samples combined, excluding the US Virgin Islands and Guam.9–11 The Council of American Survey Research Organizations response rate in 2016 was 55.5% for landline (response rate for 2011-2015: 57.2%, 55.1%, 51.1%, 60.3%, and 56.4%, respectively) and 29.5% for cell phone (rate for 2011-2015: 22.4%, 23.6%, 23.3%, 31.2%, and 29.8%, respectively).9,11 In addition, provider recommendation status was assessed by asking parents/guardians whether they received a provider recommendation of the vaccine, and those who did not know or refused to answer this question were excluded from our analysis in 2016 (9.0%).
Covariates that were selected from survey questions to assess HPV vaccination (≥1 dose) included provider recommendation status as reported by the parent or guardian, age group, race/ethnicity, mother’s educational level, mother’s marital status, mother’s age, birth country, poverty level, health insurance status, number of physician contacts within the previous 12 months, provider-reported healthcare visit at age 1112 years, number of vaccination providers reported by parents, vaccination facility type (ie, public, private, hospital, sexually transmitted disease/school/teen clinics, mixed [including facilities in more than 1 category], and others [eg, military, Women, Infants, and Children clinics, pharmacies]), metropolitan statistical area (MSA, including MSA, central city; MSA, non-central city; and non-MSA), and US census region.
SUDAAN 11.0.1 (Research Triangle Institute, Research Triangle Park, North Carolina) was used to calculate point estimates and 95% CIs. All analyses account for the complex sampling design of the NIS-Teen and the survey sampling weights.9,11 The sampling weights for the combined 2011-2016 data equaled the original weights divided by6 (the number of years combined).11 The t test was used to examine associations with the significance level set at P < .05. Multivariable logistic regression and predictive marginal modeling were conducted to derive the adjusted prevalence difference. Multivariable logistic regression and predictive marginal models were also stratified by provider recommendation status. The models were checked for multicollinearity. The NIS-Teen has been approved by the Centers for Disease Control and Prevention, National Center for Health Statistics Research Ethics Review Board, and the NORC at the University of Chicago Institutional Review Board.
Results
The 2016 NIS-Teen included a total of 9712 male adolescents aged 13-17 years with adequate provider data. Of those, 65.5% received a provider recommendation for the HPV vaccine. Table I presents the demographic characteristics of the study population. Overall, a majority of adolescents were non-Hispanic white (54.6%), had a mother with more than a high school education (65.4%), had a mother who was currently married (66.2%), were born in the US (94.3%), were living in a household with an income >133% of the federal poverty level (69.2%), had private health insurance (52.7%), had 1 vaccination provider (58.9%), had at least 1 physician contact within the past year (83.8%), and had received all reported vaccinations from providers in a private facility (53.0%). Those with a provider recommendation for HPV vaccine differed significantly from those without a recommendation in all characteristics assessed except age, race/ethnicity, and mother’s marital status. In addition, the prevalence of provider recommendations was the lowest among adolescents without health insurance (47.9%) and those born outside the US (50.2%), and was highest among those living in a household with an income >503% of the federal poverty level (73.3%) (Table I).
Table I.
Sample characteristics of male adolescents aged 13-17 years in the US, by demographic and access-to-care variables, NIS-Teen 2016
| Overall |
Parental report of provider recommendation for HPV vaccine, weighted % |
Prevalence of provider recommendation for HPV vaccine, weighted % | |||
|---|---|---|---|---|---|
| Characteristics | n | Weighted % | Yes | No | |
| Total | 9712 | 100.0 | 100.0 | 100.0 | 65.5 |
| Age, y | |||||
| 13-15* | 5938 | 60.0 | 58.9 | 62.1 | 64.3 |
| 16-17 | 3774 | 40.0 | 41.1 | 37.9 | 67.3 |
| Race/ethnicity | |||||
| Non-Hispanic white* | 6267 | 54.6 | 55.5 | 52.9 | 66.6 |
| Non-Hispanic black | 923 | 13.0 | 12.9 | 13.0 | 65.3 |
| Hispanic | 1488 | 22.9 | 22.5 | 23.5 | 64.6 |
| American Indian/Alaskan Native | 144 | 0.9 | 0.9 | 0.9 | 63.8 |
| Asian | 319 | 3.5 | 3.1 | 4.2 | 58.6 |
| Other | 571 | 5.2 | 5.1 | 5.5 | 63.8 |
| Mother’s educational level | |||||
| Less than high school* | 931 | 13.2 | 11.9 | 15.8† | 58.8 |
| High school | 1478 | 21.3 | 18.7 | 26.2 | 57.6 |
| Some college or college graduate | 2554 | 24.6 | 25.2 | 23.6 | 67.0‡ |
| Beyond college graduate | 4749 | 40.8 | 44.2 | 34.4 | 70.9‡ |
| Mother’s marital status | |||||
| Married* | 6873 | 66.2 | 67.1 | 64.6 | 67.1 |
| Widowed/divorced/separated | 1634 | 24.3 | 23.4 | 26.1 | 63.8 |
| Never married | 591 | 9.4 | 9.5 | 9.2 | 66.9 |
| Mother’s age, y | |||||
| ≤34* | 801 | 9.4 | 7.7 | 12.5† | 54.1 |
| 35-44 | 3899 | 43.8 | 42.1 | 47.0 | 63.0‡ |
| ≥45 | 5012 | 46.9 | 50.2 | 40.6 | 70.2‡ |
| Country of birth | |||||
| Born in US* | 9235 | 94.3 | 95.6 | 91.8† | 66.4 |
| Born outside US | 401 | 5.7 | 4.4 | 8.2 | 50.2‡ |
| Income-to-poverty ratio, % | |||||
| <1.33* | 2308 | 30.8 | 28.3 | 35.7† | 60.1 |
| 1.33 to <3.22 | 2749 | 28.6 | 28.2 | 29.4 | 64.6 |
| 3.22 to <5.03 | 2033 | 18.4 | 18.7 | 17.8 | 66.6‡ |
| >5.03 | 2622 | 22.2 | 24.8 | 17.2 | 73.3‡ |
| Medical insurance§ | |||||
| Private only* | 5834 | 52.7 | 55.6 | 47.1† | 69.2 |
| Any Medicaid | 2842 | 36.4 | 33.8 | 41.3 | 60.8‡ |
| Other¶ | 727 | 7.6 | 8.1 | 6.5 | 70.3 |
| Uninsured | 309 | 3.4 | 2.5 | 5.1 | 47.9‡ |
| Physician contacts within past year | |||||
| None * | 1313 | 16.2 | 12.9 | 22.5† | 52.2 |
| 1 | 2933 | 31.4 | 32.2 | 29.9 | 67.3‡ |
| 2-3 | 3499 | 34.3 | 35.8 | 31.5 | 68.4‡ |
| ≥4 | 1901 | 18.1 | 19.1 | 16.0 | 69.5‡ |
| Well-child visit at age 11-12 y** | |||||
| Yes* | 4754 | 46.3 | 50.5 | 38.2† | 71.5 |
| No | 2267 | 22.1 | 18.8 | 28.4 | 55.7‡ |
| Don’t know | 2691 | 31.6 | 30.7 | 33.4 | 63.6‡ |
| Number of vaccination providers | |||||
| 1 | 5500 | 58.9 | 58.3 | 60.2† | 64.8 |
| 2 | 2733 | 26.5 | 26.9 | 25.9 | 66.4 |
| ≥3* | 1479 | 14.5 | 14.9 | 14.0 | 66.9 |
| MSA | |||||
| MSA, central city | 3831 | 40.9 | 42.1 | 38.6† | 67.5‡ |
| MSA, non-central city | 3876 | 46.4 | 46.6 | 45.9 | 65.9‡ |
| Non-MSA* | 2005 | 12.7 | 11.3 | 15.5 | 58.0 |
| Vaccination facility type | |||||
| All private facilities* | 4873 | 53.0 | 54.5 | 50.1† | 67.4 |
| All public facilities | 1339 | 14.5 | 12.2 | 18.9 | 55.0‡ |
| All hospital facilities | 1169 | 10.0 | 10.2 | 9.6 | 66.9 |
| All STD/school/teen clinics or other facilities | 157 | 2.3 | 2.1 | †† | 59.6 |
| Mixed‡‡ | 2044 | 18.8 | 19.2 | 18.1 | 66.9 |
| Other§§ | 130 | 1.4 | 1.8 | 0.6 | 85.4‡ |
Reference level.
P < .05 by the χ2 test.
P < .05 by the t test compared with the reference group.
Insurance categories are mutually exclusive.
Includes Indian Health Service (IHS), military, Children’s Health Insurance Program (CHIP), and some private.
Status of healthcare visit at age 11-12 years based on provider-reported data.
Data are not reliable due to sample size <30 or relative standard error (standard error/estimate) >0.3.
Mixed indicates that the facility is identified to be in more than 1 of the facility categories, such as private, public, hospital, and sexually transmitted disease (STD)/school/teen clinics.
Includes military, Women, Infants, and Children (WIC) clinics, and pharmacies.
Overall, HPV vaccination coverage among male adolescents increased significantly, from 8.3% in 2011 to 57.3% in 2016 (test for trend, P < .05) (Figure; available at www.jpeds.com). HPV vaccination coverage among male adolescents with a provider recommendation also increased significantly, from 36.0% in 2011 to 68.8% in 2016 (test for trend, P < .05), and vaccination coverage of male adolescents was significantly higher in those with a provider recommendation compared with those without a provider recommendation during this period. In addition, the prevalence of provider recommendations increased significantly, from 14.2% in 2011 to 65.5% in 2016 (test for trend, P < .05).
Figure.
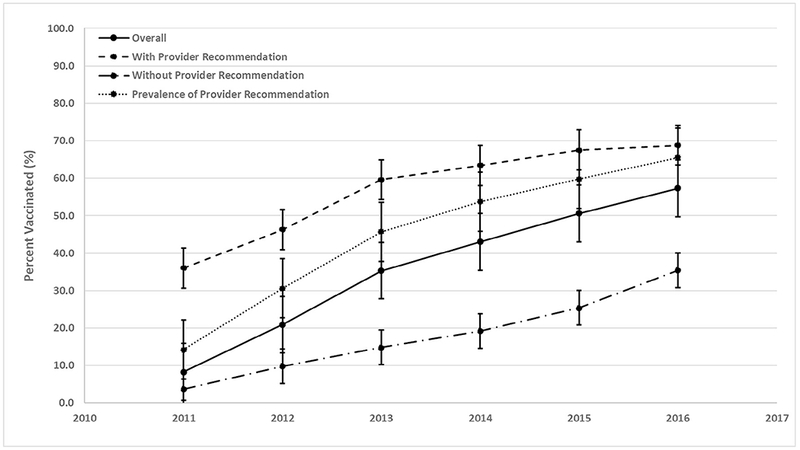
HPV vaccination coverage and prevalence of provider recommendation among male adolescents 13-17 years, United States, 2011-2016. Source: National Immunization Survey-Teen, 2011-2016.
By state, the prevalence of provider recommendations for HPV vaccine among all adolescents aged 13-17 years ranged from a low of 45.9% in Wyoming to a high of 82.4% in Maine, with a median of 67.0% (Table II). HPV vaccination coverage among all adolescent males aged 13-17 years ranged from 36.5% in Wyoming to 89.2% in Rhode Island, with a median of 58.4%. HPV coverage among those with a provider recommendation ranged from 48.5% in Indiana to 90.6% in Rhode Island (median, 69.6%), compared with a range of 20.1% in South Dakota to 82.6% in Rhode Island (median, 33.3%) among those without a provider recommendation (Table II). Point estimates of HPV vaccination coverage were significantly higher among adolescents with a provider recommendation compared with those without a provider recommendation in all but 4 states (California, Michigan, Rhode Island, and Washington, DC) (Table II). Coverage differences ranged from 8.0% in Rhode Island to 56.8% in Maine, with a median of 35.7%. State prevalence of receiving a provider recommendation also was positively correlated with overall state HPV vaccination coverage among male adolescents (r = 0.80; P < .01).
Table II.
HPV vaccination coverage of male adolescents aged 13-17 years in the US, by parental report of provider recommendation status, demographic, and access-to-care variables—NIS-Teen 2016
| State | Sample size, n | Prevalence of provider recommendation for HPV, % (95% CI) | HPV vaccination coverage, % (95% CI) |
|||
|---|---|---|---|---|---|---|
| Overall | With provider recommendation | Without provider recommendation | Percentage points difference* | |||
| National | 9712 | 65.5 (63.7-67.3) | 57.3 (55.5-59.1) | 68.8 (66.8-70.8) | 35.4 (32.1-38.7) | 33.5 (29.6-37.4)† |
| Wyoming | 186 | 45.9 (37.4-54.5) | 36.5 (28.5-45.4) | 51.5 (39.2-63.6) | 23.8 (14.5-36.7) | 27.7 (10.9-44.4)† |
| Mississippi | 180 | 48.4 (38.9-57.9) | 45.4 (36.1-54.9) | 65.7 (52.1-77.2) | 26.3 (16.5-39.2) | 39.4 (22.3-56.6)† |
| South Carolina | 150 | 52.0 (42.0-62.0) | 38.7 (29.7-48.6) | 54.4 (40.5-67.7) | 21.7 (11.7-36.6) | 32.7 (14.0-51.5)† |
| Kansas | 149 | 52.4 (42.6-62.2) | 43.1 (33.8-52.9) | 63.2 (50.7-74.2) | 20.9 (10.8-36.6) | 42.3 (24.7-60.0)† |
| Kentucky | 162 | 52.7 (43.3-62.0) | 42.3 (33.4-51.7) | 53.1 (40.7-65.1) | 30.2 (18.3-45.6) | 22.8 (4.2-41.5)† |
| Texas | 939 | 52.8 (47.4-58.2) | 46.0 (40.7-51.4) | 64.0 (56.6-70.9) | 25.7 (19.2-33.6) | 38.3 (28.1-48.5)† |
| Oklahoma | 137 | 53.4 (42.7-64.1) | 51.2 (40.6-61.6) | 66.0 (52.9-77.1) | 34.2 (20.3-51.4) | 31.8 (11.6-52.0)† |
| Missouri | 168 | 54.3 (44.5-64.2) | 49.2 (39.5-58.8) | 71.7 (59.7-81.2) | 22.3 (12.2-37.3) | 49.3 (32.7-65.9)† |
| Alabama | 155 | 54.5 (45.0-64.1) | 51.2 (41.7-60.6) | 76.1 (63.1-85.6) | 21.2 (11.5-35.8) | 54.9 (38.3-71.5)† |
| West Virginia | 149 | 56.2 (46.6-65.9) | 51.6 (42.0-61.1) | 69.3 (56.5-79.6) | 29.0 (16.9-45.0) | 40.3 (21.7-58.8)† |
| South Dakota | 167 | 56.2 (46.8-65.5) | 50.5 (41.3-59.7) | 74.2 (62.7-83.1) | 20.1 (10.6-34.7) | 54.1 (38.3-69.9)† |
| Montana | 173 | 57.0 (47.4-66.5) | 43.5 (34.6-52.9) | 56.5 (44.4-67.9) | 26.3 (15.3-41.4) | 30.2 (12.3-48.1)† |
| Arkansas | 190 | 58.0 (49.7-66.3) | 54.9 (46.5-63.0) | 67.8 (56.8-77.1) | 37.1 (25.5-50.4) | 30.7 (14.3-47.0)† |
| Utah | 151 | 60.2 (50.8-69.6) | 42.1 (33.1-51.6) | 54.4 (42.4-65.9) | 23.5 (13.3-38.1) | 30.9 (13.6-48.2)† |
| Florida | 183 | 61.1 (51.9-70.3) | 53.8 (44.5-62.9) | 62.1 (50.0-72.8) | 40.9 (26.8-56.6) | 21.2 (2.0-40.5)† |
| Indiana | 173 | 61.3 (52.2-70.4) | 39.0 (30.5-48.2) | 48.5 (37.3-60.0) | 23.8 (13.2-39.1) | 24.7 (7.3-42.2)† |
| Idaho | 178 | 61.3 (52.5-70.1) | 54.2 (45.2-63.0) | 63.6 (51.8-73.9) | 39.4 (25.9-54.7) | 24.2 (5.6-42.7)† |
| Tennessee | 144 | 61.7 (52.1-71.2) | 58.2 (48.5-67.3) | 68.0 (55.9-78.1) | 42.5 (27.7-58.7) | 25.5 (5.9-45.1)† |
| Nevada | 147 | 62.1 (52.4-71.9) | 66.1 (56.4-74.5) | 75.1 (63.3-84.1) | 51.2 (35.0-67.1) | 23.9 (4.3-43.6)† |
| Connecticut | 141 | 63.6 (53.5-73.6) | 58.8 (48.7-68.3) | 69.4 (57.4-79.2) | 40.5 (24.5-58.8) | 28.9 (7.9-49.8)† |
| Alaska | 187 | 64.4 (55.5-73.4) | 61.6 (52.6-69.9) | 77.2 (67.0-85.0) | 33.3 (19.2-51.3) | 43.9 (25.0-62.8)† |
| Virginia‡ | 219 | 64.8 (54.5-75.2) | 60.0 (49.3-69.8) | 68.9 (55.5-79.8) | 43.5 (26.5-62.1) | 25.5 (3.1-47.8)† |
| Nebraska | 161 | 66.4 (57.5-75.3) | 58.2 (48.7-67.2) | 70.3 (58.7-79.7) | 34.5 (20.8-51.4) | 35.8 (16.8-54.7)† |
| Hawaii | 143 | 66.5 (57.1-75.8) | 58.5 (48.6-67.8) | 71.0 (58.8-80.7) | 33.9 (19.8-51.6) | 37.1 (17.3-56.9)† |
| Arizona | 151 | 66.7 (57.1-76.3) | 61.5 (51.4-70.7) | 75.7 (63.6-84.7) | 33.0 (18.2-52.2) | 42.7 (22.2-63.2)† |
| Oregon | 156 | 67.0 (58.2-75.7) | 61.1 (51.8-69.6) | 77.1 (67.0-84.8) | 28.5 (16.3-45.0) | 48.6 (31.4-65.8)† |
| California | 166 | 67.2 (57.7-76.6) | 68.5 (59.0-76.6) | 74.6 (64.5-82.6) | 55.9 (37.6-72.7) | 18.6 (−1.8 to 39.1) |
| Minnesota | 177 | 67.2 (58.3-76.0) | 63.2 (54.1-71.4) | 69.9 (59.2-78.7) | 49.5 (33.4-65.7) | 20.4 (0.9-39.9)† |
| Louisiana | 155 | 67.5 (58.7-76.2) | 56.9 (47.7-65.7) | 65.4 (54.1-75.2) | 39.4 (24.7-56.4) | 25.9 (6.3-45.6)† |
| Illinois | 280 | 68.7 (62.0-75.5) | 60.6 (53.4-67.4) | 74.1 (65.6-81.2) | 31.0 (19.9-44.8) | 43.1 (28.2-58.1)† |
| Ohio | 137 | 69.0 (59.5-78.4) | 56.8 (46.5-66.6) | 73.0 (61.0-82.3) | 21.0 (10.7-36.9) | 52.0 (35.1-69.0)† |
| Wisconsin | 164 | 69.0 (60.1-77.8) | 56.5 (47.3-65.3) | 69.6 (59.6-78.1) | 27.4 (14.4-45.8) | 42.2 (23.6-60.7)† |
| Colorado | 151 | 70.3 (60.8-79.8) | 62.0 (51.9-71.2) | 74.0 (62.7-82.8) | 33.6 (18.0-53.8) | 40.4 (19.3-61.6)† |
| New York | 305 | 70.3 (63.7-76.8) | 68.1 (61.5-74.1) | 76.9 (69.4-83.0) | 47.4 (34.2-60.9) | 29.5 (14.2-44.8)† |
| Washington | 169 | 71.2 (62.6-79.8) | 60.7 (51.1-69.5) | 71.0 (60.0-80.0) | 35.1 (20.7-52.8) | 35.9 (16.4-55.4)† |
| New Mexico | 186 | 71.4 (63.6-79.3) | 58.4 (49.4-66.9) | 67.9 (57.1-77.1) | 34.7 (21.5-50.8) | 33.2 (15.0-51.3)† |
| North Carolina | 156 | 71.7 (63.0-80.5) | 60.0 (50.3-69.0) | 67.6 (56.3-77.2) | 40.7 (24.4-59.2) | 27.0 (6.0-47.9)† |
| New Jersey | 180 | 72.5 (63.8-81.3) | 53.6 (44.4-62.5) | 63.8 (53.2-73.1) | 26.6 (12.8-47.1) | 37.2 (16.9-57.5)† |
| Iowa | 183 | 72.6 (65.1-80.1) | 59.1 (50.5-67.2) | 68.9 (58.5-77.7) | 33.2 (19.6-50.4) | 35.7 (17.1-54.2)† |
| Pennsylvania | 436 | 73.1 (66.5-79.7) | 59.2 (51.7-66.3) | 70.0 (61.2-77.5) | 30.0 (18.8-44.2) | 40.0 (24.7-55.3)† |
| Maryland | 219 | 74.6 (66.2-83.1) | 60.4 (50.9-69.2) | 70.8 (60.4-79.4) | 29.7 (14.9-50.4) | 41.1 (20.4-61.8)† |
| Georgia | 158 | 74.8 (66.6-83.0) | 57.7 (47.7-67.2) | 66.8 (54.8-77.0) | 30.8 (17.0-49.2) | 36.0 (16.0-56.0)† |
| Massachusetts | 171 | 75.5 (67.3-83.7) | 65.3 (56.2-73.3) | 74.2 (64.5-81.9) | 37.9 (21.1-58.1) | 36.3 (15.0-57.6)† |
| New Hampshire | 133 | 76.1 (67.3-84.9) | 68.8 (58.9-77.3) | 81.4 (71.6-88.4) | 28.8 (13.8-50.5) | 52.6 (31.9-73.4)† |
| Delaware | 174 | 77.1 (69.4-84.8) | 61.4 (52.3-69.8) | 68.9 (58.7-77.6) | 36.2 (20.2-56.0) | 32.7 (11.7-53.7)† |
| Michigan | 129 | 78.7 (70.1-87.3) | 53.9 (43.5-63.9) | 57.0 (45.1-68.2) | 42.4 (21.9-65.9) | 14.6 (−11.8 to 41.0) |
| District of Columbia‡ | 182 | 81.4 (73.5-89.3) | 77.2 (68.5-84.1) | 76.3 (66.4-84.0) | 81.1 (60.0-92.4) | −4.7 (−23.2 to 13.7) |
| North Dakota | 146 | 82.0 (74.5-89.4) | 69.6 (60.2-77.6) | 75.8 (65.8-83.5) | 41.5 (21.8-64.5) | 34.2 (9.8-58.7)† |
| Rhode Island‡ | 160 | 82.0 (74.7-89.3) | 89.2 (82.8-93.4) | 90.6 (83.5-94.9) | 82.6 (63.9-92.7) | 8.0 (−7.2 to 23.2) |
| Vermont | 196 | 82.2 (76.2-88.3) | 69.0 (61.1-76.0) | 74.7 (66.1-81.7) | 42.9 (26.1-61.5) | 31.7 (11.6-51.8)† |
| Maine | 160 | 82.4 (75.4-89.4) | 69.0 (59.8-76.9) | 79.0 (69.6-86.1) | 22.2 (9.7-43.1) | 56.8 (38.0-75.6)† |
| Median | 67.0 | 58.4 | 69.6 | 33.3 | 35.7 | |
| Range | 45.9-82.4 | 36.5-89.2 | 48.5-90.0 | 20.1-82.6 | −4.7 to 56.8 | |
Coverage difference between with and without provider recommendation.
P< .05 comparing with and without provider recommendation.
Indicate that states with middle-school HPV vaccination requirement.
Overall HPV vaccination coverage was 57.3%, and coverage was significantly higher among non-Hispanic black (62.3%) and Hispanic (69.1%) males compared with non-Hispanic white (51.3%) males (P < .05) (Table III). Coverage was significantly higher among adolescents with a provider recommendation compared with those without a provider recommendation (68.8% vs 35.4%; P < .05) overall and in the majority of demographic and access-to-care characteristic categories (Table III).
Table III.
HPV vaccination coverage of male adolescents aged 13-17 years in the US, by parental report of provider recommendation status, demographic, and access-to-care variables—NIS-Teen 2016
| HPV vaccination coverage, % (95% CI) |
||||
|---|---|---|---|---|
| Parental report of provider recommendation for vaccine |
Percentage points difference* | |||
| Characteristics | Overall | Yes | No | |
| Total | 57.3 (55.5-59.1) | 68.8 (66.8-70.8) | 35.4 (32.1-38.7)§ | 33.5 (29.6-37.4)† |
| Age, y | ||||
| 13-15‡ | 55.8 (53.4-58.1) | 67.1 (64.4-69.6) | 35.4 (31.3-39.9) | 31.6 (26.6-36.7)† |
| 16-17 | 59.6 (56.7-62.4) | 71.4 (68.0-74.5)§ | 35.3 (30.3-40.6) | 36.1 (30.0-42.2)† |
| Race/ethnicity | ||||
| Non-Hispanic white‡ | 51.3 (49.2-53.3) | 64.9 (62.3-67.4) | 24.1 (20.9-27.7) | 40.8 (36.6-45.0)† |
| Non-Hispanic black | 62.3 (57.5-66.9)§ | 71.0 (65.2-76.2) | 45.9 (37.5-54.5)§ | 25.1 (15.0-35.3)† |
| Hispanic | 69.1 (64.6-73.2)§ | 77.5 (72.5-81.8)§ | 53.7 (45.3-61.9)§ | 23.8 (14.2-33.3)† |
| American Indian/Alaskan Native | 54.6 (39.7-68.6) | 57.3 (35.9-76.3) | 49.7 (31.8-67.7)§ | 7.5 (−20.9 to 36.0) |
| Asian | 55.6 (42.9-67.6) | 73.3 (62.2-82.0) | 30.7 (16.9-49.0) | 42.6 (23.3-61.9)† |
| Other | 58.2 (49.3-66.7) | 67.5 (57.1-76.5) | 41.8 (25.8-59.7)§ | 25.8 (5.6-46.0)† |
| Mother’s educational level | ||||
| Less than high school‡ | 70.1 (64.1-75.5) | 78.3 (71.5-83.8) | 58.4 (48.1-68.0) | 19.9 (8.0-31.7)† |
| High school | 58.6 (54.6-62.5)§ | 72.7 (67.8-77.0) | 39.5 (33.5-45.8)§ | 33.2 (25.5-40.9)† |
| Some college or college graduate | 53.9 (50.4-57.3)§ | 65.4 (61.2-69.4)§ | 30.5 (24.5-37.3)§ | 34.9 (27.3-42.5)† |
| Beyond college graduate | 54.5 (51.9-57.2)§ | 66.7 (63.7-69.5)§ | 25.0 (20.5-30.0)§ | 41.7 (36.1-47.3)† |
| Mother’s marital status | ||||
| Married‡ | 55.0 (52.9-57.1) | 66.2 (63.7-68.6) | 32.2 (28.3-36.3) | 34.0 (29.3-38.7)† |
| Widowed/divorced/separated | 57.0 (52.7-61.3) | 70.4 (65.3-75.1) | 33.4 (26.9-40.8) | 37.0 (28.5-45.6)† |
| Never married | 69.4 (63.0-75.2)§ | 74.4 (66.5-80.9) | 59.5 (46.9-70.9)§ | 14.8 (0.6-29.1)† |
| Mother’s age, y | ||||
| ≤34‡ | 63.6 (56.8-69.9) | 77.1 (70.8-82.4) | 47.7 (36.2-59.4) | 29.4 (16.3-42.6)† |
| 35-44 | 55.8 (53.0-58.6)§ | 65.3 (61.9-68.5)§ | 39.6 (34.8-44.6) | 25.7 (19.7-31.6)† |
| ≥45 | 57.5 (54.9-60.0)§ | 70.6 (67.7-73.2) | 26.7 (22.5-31.3)§ | 43.9 (38.7-49.1)† |
| Country of birth | ||||
| Born in US‡ | 57.0 (55.1-58.8) | 69.0 (66.9-71.0) | 33.2 (29.9-36.6) | 35.8 (31.9-39.7)† |
| Born outside US | 61.3 (50.2-71.3) | 64.9 (50.1-77.4) | 57.6 (41.3-72.4)§ | 7.3 (−14.0 to 28.6) |
| Income-to-poverty ratio, % | ||||
| <133‡ | 65.3 (61.8-68.6) | 74.7 (70.5-78.5) | 51.1 (45.1-57.1) | 23.6 (16.3-30.8)† |
| 133 to <322 | 54.7 (51.3-57.9)§ | 67.6 (63.7-71.3)§ | 31.1 (25.8-37.0)§ | 36.4 (29.7-43.2)† |
| 322 to <503 | 50.7 (47.0-54.4)§ | 64.1 (59.6-68.4)§ | 23.9 (17.8-31.3)§ | 40.2 (32.1-48.2)† |
| >503 | 55.1 (51.3-58.9)§ | 67.2 (63.1-71.0)§ | 21.8 (16.5-28.2)§ | 45.4 (38.3-52.5)† |
| Medical insurance¶ | ||||
| Private only‡ | 51.3 (49.0-53.7) | 63.5 (60.8-66.1) | 24.0 (20.2-28.4) | 39.5 (34.6-44.4)† |
| Any Medicaid | 66.3 (63.3-69.3)§ | 77.8 (74.5-80.7)§ | 48.6 (43.0-54.2)§ | 29.1 (22.7-35.6)† |
| Other** | 56.1 (49.7-62.3) | 66.8 (58.5-74.1) | 30.8 (21.7-41.7) | 35.9 (23.1-48.7)† |
| Uninsured | 55.7 (44.8-66.0) | 74.3 (58.5-85.6) | 38.6 (27.2-51.3)§ | 35.7 (17.3-54.1)† |
| Physician contacts within past year | ||||
| None‡ | 50.1 (44.7-55.5) | 62.1 (55.3-68.5) | 37.1 (29.2-45.7) | 25.1 (14.4-35.7)† |
| 1 | 55.1 (51.8-58.4)§ | 66.4 (62.5-70.0) | 31.9 (26.3-38.0) | 34.5 (27.6-41.4)† |
| 2-3 | 60.9 (58.0-63.8)§ | 71.4 (67.9-74.6)§ | 38.2 (32.7-44.0) | 33.2 (26.6-39.7)† |
| ≥4 | 60.5 (56.6-64.4)§ | 72.8 (68.6-76.6)§ | 32.6 (25.8-40.3) | 40.2 (31.8-48.5)† |
| Well-child visit at age 11-12 y†† | ||||
| Yes‡ | 63.0 (60.6-65.4) | 71.5 (68.9-74.0) | 41.6 (36.1-47.4) | 29.9 (23.7-36.1)† |
| No | 48.8 (45.0-52.6)§ | 64.1 (59.1-68.9)§ | 29.6 (24.8-34.8)§ | 34.5 (27.5-41.6)† |
| Don’t know | 54.9 (51.3-58.4)§ | 67.3 (63.1-71.3) | 33.1 (27.6-39.1)§ | 34.2 (27.1-41.3)† |
| Number of providers | ||||
| 1 | 58.9 (56.5-61.2) | 71.4 (68.8-73.8) | 35.9 (31.8-40.2) | 35.5 (30.5-40.4)† |
| 2 | 54.9 (51.6-58.1) | 65.5 (61.5-69.3) | 33.8 (27.8-40.4) | 31.7 (24.3-39.1)† |
| ≥3‡ | 55.3 (50.1-60.5) | 64.9 (58.5-70.8) | 35.9 (26.9-46.2) | 29.0 (17.4-40.5)† |
| MSA | ||||
| MSA central city | 62.7 (59.7-65.6)§ | 71.9 (68.6-75.1)§ | 43.5 (37.7-49.5)§ | 28.4 (21.6-35.2)† |
| MSA non-central city | 55.9 (53.3-58.6)§ | 67.6 (64.6-70.5) | 33.4 (28.9-38.2)§ | 34.2 (28.7-39.8)† |
| Non-MSA‡ | 45.0 (41.2-48.8) | 62.4 (57.3-67.2) | 21.0 (16.7-25.9) | 41.4 (34.6-48.2)† |
| Facility type | ||||
| All private facilities‡ | 56.8 (54.3-59.3) | 68.3 (65.5-71.0) | 33.1 (28.6-37.9) | 35.3 (29.8-40.7)† |
| All public facilities | 60.4 (55.7-64.9) | 71.0 (65.0-76.2) | 47.5 (40.0-55.2)§ | 23.5 (13.9-33.0)† |
| All hospital facilities | 61.6 (56.5-66.4) | 73.7 (68.4-78.4) | 37.1 (26.9-48.6) | 36.6 (24.5-48.7)† |
| All STD/school/teen clinics or other facilities | 53.3 (35.8-70.1) | 71.2 (54.7-83.6) | ‡‡ | ‡‡ |
| Mixed§§ | 56.0 (51.8-60.0) | 68.8 (63.8-73.3) | 30.1 (23.9-37.1) | 38.7 (30.5-46.8)† |
| Other¶¶ | 37.8 (24.5-53.2)§ | 41.5 (25.9-59.0)§ | ‡‡ | ‡‡ |
Difference between provider recommendation vs no provider recommendation.
P< .05 by the t test comparing provider recommendation vs no provider recommendation.
Reference level.
P< .05 by the t test compared with the reference level.
Insurance categories are mutually exclusive.
Includes IHS, military, CHIP, and some private.
Status of healthcare visit at age 11-12 years based on provider-reported data.
Data are not reliable due to sample size <30 or relative standard error (standard error/estimate) > 0.3.
Mixed indicates that the facility is identified to be in more than 1 of the facility categories, such as private, public, hospital, and STD/school/teen clinics.
Includes military, WIC clinics, and pharmacies.
In bivariable analyses, among all adolescents aged 13-17 years, other characteristics that were significantly associated with a higher level of HPV vaccination coverage compared with the referent group included having any Medicaid insurance, having ≥2 physician contacts in the previous 12 months, and living in an MSA central city area (P < .05). Adolescent males with a mother with at least a high school education and those with a mother aged 35-44 years had a lower likelihood of HPV vaccination (P < .05) (Table III). This was also the case for those with an income-to-poverty ratio >133% and those without a well-child visit at age 11-12 years. In all bivariable analyses, race/ethnicity, mother’s educational level, mother’s age, poverty level, medical insurance, well-child visit at age 11-12 years, MSA status, and facility type were significantly associated with HPV vaccination regardless of population group (overall, with a provider recommendation for HPV, and without a provider recommendation for HPV). Other factors associated with vaccination are listed in Table III.
In multivariable analyses, among all adolescents aged 13-17 years, characteristics independently associated with a higher likelihood of HPV vaccination included receipt of a provider recommendation, age 16-17 years, black or Hispanic race/ethnicity, any Medicaid insurance, ≥2 physician contacts in the previous 12 months, and urban or suburban residence (P < .05) (Table IV). Having a mother with some college or a college degree, having a mother aged 35-44 years, and not having a well-child visit at age 11-12 years were characteristics associated with a lower likelihood of HPV vaccination (P < .05) (Table IV). Overall, in all multivariable analyses, race/ethnicity, a well-child visit at age 11-12 years, and MSA, central city/MSA, non-central city residence were significantly associated with HPV vaccination regardless of population group (overall, with a provider recommendation for HPV, and without a provider recommendation for HPV). Other factors independently associated with vaccination are listed in Table IV. On statistical analysis, multicollinearity was not identified among the variables assessed in this multivariable logistic model.
Table IV.
Multivariable logistic regression and predictive marginal analysis of HPV vaccination of male adolescents aged 13-17 years in the US, by parental report of provider recommendation status, demographic, and access-to-care variables—NIS-Teen 2016
| Overall, adjusted prevalence difference (95% CI) | Parental report of provider recommendation for vaccine, adjusted prevalence difference, % (95% CI) |
||
|---|---|---|---|
| Characteristics | Yes | No | |
| Parental report of provider recommendation for vaccine | |||
| Yes* | Ref | ||
| No | −32.0 (−35.7 to −28.3)† | ||
| Age, y | |||
| 13-15* | Ref | Ref | Ref |
| 16-17 | 4.0 (0.5-7.6)† | 4.9 (0.9-9.0)† | 3.9 (−1.8 to 9.6) |
| Race/ethnicity | |||
| Non-Hispanic white* | Ref | Ref | Ref |
| Non-Hispanic black | 7.0 (1.2-12.8)† | 3.3 (−3.6 to 10.3) | 13.2 (4.0-22.4)† |
| Hispanic | 11.4 (6.0-16.9)† | 9.2 (2.8-15.5)† | 18.9 (10.1-27.8)† |
| American Indian/Alaskan Native | −4.2 (−24.2 to 15.7) | −20.3 (−43.0 to 2.4) | 20.1 (3.2-36.9)† |
| Asian | 4.4 (−5.3 to 14.1) | 8.9 (−1.5 to 19.3) | −1.2 (−15.7 to 13.3) |
| Other | 5.3 (−3.9 to 4.4) | 1.7 (−7.4 to 10.8) | 9.6 (−5.5 to 24.7) |
| Mother’s educational level | |||
| Less than high school* | Ref | Ref | Ref |
| High school | −3.1 (−10.4 to 4.3) | −0.7 (−10.0 to 8.6) | −5.7 (−16.2 to 4.7) |
| Some college or college graduate | −8.4 (−15.8 to −0.9)† | −4.8 (−14.3 to 4.6) | −11.5 (−21.7 to −1.4)† |
| Beyond college graduate | −6.2 (−13.8 to 1.3) | −2.8 (−12.3 to 6.7) | −10.7 (−21.3 to 0.0) |
| Mother’s marital status | |||
| Married* | Ref | Ref | Ref |
| Widowed/divorced/separated | −0.1 (−4.7 to 4.6) | 1.5 (−3.9 to 6.9) | 1.0 (−5.7 to 7.8) |
| Never married | 2.5 (−5.3 to 10.3) | 1.5 (−7.6 to 10.6) | 7.5 (−3.2 to 18.1) |
| Mother’s age, y | |||
| ≤34* | Ref | Ref | Ref |
| 35-44 | −8.0 (−14.2 to −1.8)† | −9.4 (−17.0 to −1.7)† | −3.3 (−12.5 to 6.0) |
| ≥45 | −5.2 (−11.6 to 1.1) | −3.3 (−11.0 to 4.4) | −6.1 (−15.8 to 3.6) |
| Country of birth | |||
| Born in US* | Ref | Ref | Ref |
| Born outside US | 3.2 (−7.6 to 14.1) | −6.6 (−19.9 to 6.7) | 17.6 (3.9-31.3)† |
| Income-to-poverty ratio, % | |||
| <133* | Ref | Ref | Ref |
| 133 to <322 | −0.9 (−6.4 to 4.6) | 2.5 (−4.5 to 9.5) | −7.2 (−15.3 to 0.9) |
| 322 to <503 | −1.2 (−7.8 to 5.5) | 4.8 (−3.4 to 12.9) | −11.7 (−21.5 to −2.0)† |
| >503 | 0.1 (−6.9 to 7.2) | 7.3 (−1.0 to 15.5) | −15.0 (−25.5 to −4.4)† |
| Medical insurance‡ | |||
| Private only* | Ref | Ref | Ref |
| Any Medicaid | 12.1 (6.9-17.4)† | 15.2 (9.0-21.5)† | 3.3 (−4.1 to 10.7) |
| Other§ | 4.9 (−1.9 to 11.8) | 9.2 (1.2-17.2)† | −1.3 (−12.8 to 10.1) |
| Uninsured | 5.7 (−4.8 to 16.2) | 13.8 (0.1-27.4)† | −6.6 (−17.6 to 4.5) |
| Physician contacts within past year | |||
| None* | Ref | Ref | Ref |
| 1 | 3.3 (−2.3 to 8.9) | 7.0 (−0.1 to 14.1) | −0.8 (−8.4 to 6.8) |
| 2-3 | 7.4 (1.9-13.0)† | 10.9 (3.9-17.9)† | 4.9 (−2.7 to 12.4) |
| ≥4 | 7.8 (1.8-13.8)† | 13.2 (5.8-20.6)† | 0.7 (−8.3 to −9.7) |
| Well child visit at age 11-12 y¶ | |||
| Yes* | Ref | Ref | Ref |
| No | −9.4 (−14.0 to −4.9)† | −6.4 (−12.0 to −0.8)† | −11.0 (−17.7 to −4.2)† |
| Don’t know | −7.9 (−12.0 to −3.9)† | −7.2 (−12.0 to −2.5)† | −7.7 (−14.4 to −0.9)† |
| Number of providers | |||
| 1 | 2.5 (−3.2 to 8.2) | 7.2 (0.6-13.9)† | −3.6 (−11.7 to 4.5) |
| 2 | −0.8 (−6.5 to 5.0) | 1.9 (−5.0 to 8.8) | −3.5 (−12.2 to 5.2) |
| ≥3* | Ref | Ref | Ref |
| MSA | |||
| MSA, central city | 11.3 (6.3-16.3)† | 7.9 (1.6-14.3)† | 11.7 (4.8-18.7)† |
| MSA, non-central city | 9.6 (4.6-14.6)† | 5.4 (−0.9 to 11.8) | 13.2 (6.5-20.0)† |
| Non-MSA* | Ref | Ref | Ref |
| Facility type | |||
| All private facilities* | Ref | Ref | Ref |
| All public facilities | 6.4 (0.8-12.0) | 4.5 (−2.8 to 11.8) | 8.8 (1.0-16.6)† |
| All hospital facilities | 4.7 (−0.4 to 9.9) | 5.6 (−0.5 to 11.6) | 1.7 (−7.1 to 10.5) |
| All STD/school/teen clinics or other facilities | 0.7 (−11.3 to 12.6) | 3.1 (−10.2 to 16.3) | 0.0 (−21.0 to 21.0) |
| Mixed** | 1.3 (−3.7 to 6.2) | 4.3 (−1.5 to 10.1) | −5.4 (−12.7 to 2.0) |
| Other†† | −17.4 (−32.6 to −2.2)† | −19.4 (−36.9 to −2.0)† | −11.9 (−33.3 to 9.4) |
Reference level.
P< .05 compared with the reference level.
Insurance categories are mutually exclusive.
Includes IHS, military, CHIP, and some private.
Status of healthcare visit at age 11-12 years based on provider-reported data.
Mixed indicates that the facility is identified to be in more than one of the facility categories such as private, public, hospital, and STD/school/teen clinics.
Includes military, WIC clinics, and pharmacies.
Discussion
Results from this national survey indicate that in 2016, HPV vaccination coverage among male adolescents was significantly higher among adolescents with a provider recommendation (68.8%) compared with those without a provider recommendation (35.4%). The prevalence of provider recommendations of the HPV vaccine to male adolescents increased over the study period from 2011 through 2016. Provider recommendation was associated with higher HPV vaccination coverage across the majority of states, and with many demographic and access-to-care factors. Based on the 2016 survey, 65.5% of parents reported ever having received a provider recommendation for HPV vaccination of their male adolescent aged 13-17 years. In a previous study, HPV vaccination coverage among female adolescents (≥1 dose) in 2008-2009 was 58.3% in those with a provider recommendation, compared with only 20.7% in those without a recommendation.13 Other studies have shown that recommendations from providers increase parental acceptance of vaccination, and that parents change their minds about delaying and refusing vaccines because of information or assurances from healthcare providers.14,15
Overall, HPV vaccination coverage among male adolescents aged 13-17 years in 2016 was 57.3%, a significant increase from the 8.3% in 2011, when the vaccine was first recommended by the ACIP. HPV vaccination coverage among male adolescents then increased from 20.9% in 2012 to 57.3% in 2016 (5 years after the vaccination was recommended). In comparison, among female adolescents, HPV vaccination coverage increased from 25.1% in 2007 (1 year after the recommendation) to 57.3% in 2013 (5-6 years later),16–19 and the rate of provider recommendations for HPV was 68.9% in 2013 (Centers for Disease Control and Prevention, unpublished data). As the vaccination program matures and the prevalence of provider recommendations increases, coverage should continue to increase.16–19 Providers should strongly recommend adolescent vaccines to parents and use every opportunity to assess vaccination status and vaccinate adolescents.
Wide differences in coverage among states were observed from our study for HPV vaccination among male adolescents. Substantial differences in the prevalence of provider recommendation across states were also observed. In addition, a state’s prevalence of receipt of provider recommendations was positively correlated with the state’s overall HPV vaccination coverage among male adolescents, further confirming the importance of provider recommendations on vaccination uptake. Variations in state coverage could be due to differences in medical care delivery infrastructure, socioeconomic factors, state laws, effectiveness of state and local immunization programs, population attitudes toward vaccination, immunization resources, reimbursement for vaccines, vaccine administration, prevalence of provider recommendations for HPV, and other factors.20–31 Some states achieved very high coverage and prevalence of provider recommendations. States with a low prevalence of provider recommendations and lower HPV coverage may especially benefit from provider-based interventions.
Studies have consistently identified provider recommendation as the strongest predictor of vaccination.13,32–36 A provider recommendations for vaccination is strongly associated with a patient’s decision to get vaccinated.13,32–36 One study found that an important pathway to achieving higher HPV vaccination coverage of female adolescents is by healthcare providers talking to parents about the HPV vaccine, giving parents time to discuss the vaccine, and making a strong recommendation for vaccination.36 However, our findings indicate that approximately 35% of parents of adolescents reported not receiving a provider recommendation for the vaccine. Various factors may have affected the prevalence of provider recommendations; for example, the parent may have forgotten about receiving a recommendation; the vaccine might have been recommended and offered, but the parent did not interpret the interaction as a recommendation; or the parent may have asked for the vaccine (eg, to comply with state immunization prematriculation requirements), and so a provider recommendation was not needed. Providers should strongly recommend the HPV vaccine to parents and adolescents. Parents usually trust physicians’ opinions above all others regarding vaccinations.14 Providers should use every opportunity to vaccinate adolescent patients, review medical records to assess vaccination status when they see adolescents for sick visits and sports physicals, use patient reminder and recall systems (eg, automated postcards, phone calls, text messages), educate adolescents and parents about the diseases that can be prevented by adolescent vaccines, and implement policies for standing orders so that patients can receive vaccines without a physician examination or individual physician order.37
Our present findings indicate that having more physician contacts in the previous 12 months and having a well-child visit at age 11-12 years were independently associated with higher vaccination coverage. Adolescents who have more physician contacts may have more opportunities to discuss their vaccination status and receive vaccination. The ACIP and partner organizations, including the American Academy of Pediatrics, American Medical Association, and Society for Adolescent Medicine, recommend a well-child visit for children aged 11-12 years to receive recommended vaccinations and other indicated preventive services.34,38,39 Even though well-child visits for children aged 11-12 years provide good opportunities to discuss vaccination status and receive vaccinations, in our study, only 46.3% of adolescents had a reported well-child visit at 11 or 12 years. Efforts are needed to increase preventive healthcare utilization, especially at age 11-12 years, so that preteens can receive recommended vaccinations and other preventive services. In addition, providers should be encouraged to review and, if necessary, administer recommended adolescent vaccinations at all healthcare visits, in addition to the preteen visit at age 11-12 years, to prevent missed opportunities for vaccination.
The findings in this study are subject to several limitations. First, the overall household response rate was 32.7% (55.5% for the landline samples and 29.5% for the cell phone samples), and only 53.9% of landline-completed and 47.4% of cell phone-completed interviews had adequate provider data.40 Second, bias in estimates might remain even after adjustment for household and provider nonresponse and phoneless households. Third, nonresponse bias might change, which could affect comparisons of estimates between survey years. Fourth, some provider-reported vaccination histories might not include all vaccines received (eg, vaccines administered in nontraditional settings, such as emergency departments) and might have underestimated vaccination coverage. Fifth, reporting of provider recommendation is subject to recall bias and to differing interpretations among respondents of a provider recommendation as discussed previously, and we did not have any information on the type or effectiveness of the provider recommendations.41 Finally, analysis of trends across 2011-2016 are subject to potential bias that may remain after weighting adjustments because of the expansions and reductions in the share of the total sample that came from the cell phone frame across these years and because of the change in the definition of adequate provider data in 2014.11
HPV vaccination coverage and prevalence of provider recommendation among male adolescents have increased since the vaccine was recommended. Receipt of a provider recommendation for HPV vaccine is significantly associated with vaccination, and this pattern remained when controlling for other demographic and access-to-care characteristics, indicating that provider recommendation plays a key role in vaccination uptake. To increase HPV vaccination coverage and improve recommendation quality, healthcare providers should endorse the HPV vaccine, recommend same-day vaccination, and emphasize HPV-related cancer prevention.42 Additional improvement is feasible, and opportunities for adolescent catch-up vaccination efforts should not be missed to ensure that more adolescents are protected from infection. Evidence-based strategies, such as standing orders and provider reminders alone or in combination with health system interventions should be taken to further improve HPV vaccination coverage. Providers, parents, and adolescents should view every healthcare visit as an opportunity to review adolescents’ vaccination histories and ensure that every adolescent receives HPV and other recommended vaccines.43,44
Acknowledgments
We thank Drs Stacie M. Greby and James A. Singleton of the Immunization Services Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention for their important review and contributions.
Glossary
- 4vHPV
Quadrivalent human papillomavirus vaccine
- ACIP
Advisory Committee on Immunization Practices
- HPV
Human papillomavirus
- MSA
Metropolitan statistical area
- NIS-Teen
National Immunization Survey-Teen
Footnotes
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. The authors declare no conflicts of interest.
References
- 1.Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56:No.RR-2. [PubMed] [Google Scholar]
- 2.Dunne EF, Markowitz LE, Saraiya M, Stokley S, Middleman A, Unger ER, et al. CDC grand rounds: reducing the burden of HPV-associated cancer and disease. MMWR Morb Mortal Wkly Rep 2014;63:69–72. [PMC free article] [PubMed] [Google Scholar]
- 3.Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 2013;40:187–93. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. HPV-associated cancer statistics. Available at: https://www.cdc.gov/cancer/hpv/statistics/index.htm Accessed March 26, 2018.
- 5.Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011;60:1705–8. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention.Cancers associated with human papillomavirus, United States—2011-2015. Available at: https://www.cdc.gov/cancer/hpv/pdf/USCS-DataBrief-No4-August2018-508.pdf Accessed September 17, 2018.
- 7.Centers for Disease Control and Prevention. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance fromthe Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010;59:630–2. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. The Advisory Committee on Immunization Practices (ACIP): summary report, October 27–28, 2010, Atlanta, Georgia: Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/min-archive/min-oct10.pdf Accessed May, 2018. [Google Scholar]
- 9.Centers for Disease Control and Prevention. National, regional, state, and local area vaccination coverage among adolescents aged 13-17 years—United States, 2016. MMWR Morb Mortal Wkly Rep 2017;66:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2017.MMWRMorbMortal Wkly Rep 2018;67:909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. National Immunization Survey-Teen. Available at: https://www.cdc.gov/vaccines/imz-managers/nis/downloads/NIS-TEEN-PUF16-DUG.pdf Accessed March 21, 2018.
- 12.Centers for Disease Control and Prevention. National Immunization Survey-Teen. Available at: https://www.cdc.gov/vaccines/imz-managers/nis/datasets-teen.html Accessed March 21, 2018.
- 13.Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008-2009. Pediatrics 2011;128:830–9. [DOI] [PubMed] [Google Scholar]
- 14.Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics 2008;122:718–25. [DOI] [PubMed] [Google Scholar]
- 15.Gerend MA, Weibley E, Bland H. Parental response to human papillomavirus vaccine availability: uptake and intentions. J Adolesc Health 2009;45:528–31. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention.National, state, and local area vaccination coverage among adolescents aged 13-17 years—United States, 2008. MMWR Morb Mortal Wkly Rep 2009;58:997–1001. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention.National, state, and local area vaccination coverage among adolescents aged 13-17 years—United States, 2009. MMWR Morb Mortal Wkly Rep 2010;59:1018–23. [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13-17 years—United States, 2011. MMWR Morb Mortal Wkly Rep 2012;61:671–7. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years–United States, 2013.MMWRMorbMortal Wkly Rep 2014;63:625–33. [PMC free article] [PubMed] [Google Scholar]
- 20.Lee GM, Santoli JM, Hannan C, Messonnier ML, Sabin JE, Rusinak D, et al. Gaps in vaccine financing for underinsured children in the United States. JAMA 2007;298:638–43. [DOI] [PubMed] [Google Scholar]
- 21.Freed GL, Cowan AE, Clark SJ. Primary care physician perspectives on reimbursement for childhood immunizations. Pediatrics 2008;122:1319–24. [DOI] [PubMed] [Google Scholar]
- 22.Rodewald LE Timing of the implementation of new vaccines in the VFC program. Presented at Centers for Disease Control and Prevention (US)/Agency for Toxic Substances and Disease Registry, Tribal Consultation Advisory Committee meeting February 10-12, 2009; Albuquerque, NM. [Google Scholar]
- 23.Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. Am J Prev Med 2010;38:525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freed GL, Cowan AE, Gregory S, Clark SJ. Variation in provider vaccine purchase prices and payer reimbursement. Pediatrics 2008;122:1325–31. [DOI] [PubMed] [Google Scholar]
- 25.Lindley MC, Smith PJ, Rodewald LE. Vaccination coverage among US adolescents aged 13-17 years eligible for the Vaccines for Children program, 2009. Public Health Rep 2011;126(Suppl 2):124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klaiman T, Ibrahim JK. State health department structure and pandemic planning. J Public Health Manag Pract 2010;16:E1–7. [DOI] [PubMed] [Google Scholar]
- 27.Nelson DE, Bland S, Powell-Griner E, Klein R, Wells HE, Hogelin G, et al. State trends in health risk factors and receipt of clinical preventive services among US adults during the 1990s. JAMA 2002;287:2659–67. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. State- and sex-specific prevalence of selected characteristics—Behavioral Risk Factor Surveillance System, 1996 and 1997. MMWR Surveill Summ 2000;49:1–39. [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Surveillance for certain health behaviors among states and selected local areas—Behavioral Risk Factor Surveillance System, United States, 2011.MMWRSurveill Summ 2014;63:1–149. [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Surveillance of influenza vaccination coverage—United States, 2007-08 through 2011-12 influenza seasons. MMWR Surveill Summ 2013;62(Suppl 4):1–29. [PubMed] [Google Scholar]
- 31.Immunization Action Coalition. Tdap booster requirements for secondary schools. Available at: http://www.immunize.org/laws/#dtap Accessed March 28, 2018.
- 32.Lu PJ, Yankey D, Jeyarajah J, O’Halloran A, Meyer SA, Elam-Evans LD, et al. Impact of provider recommendation on Tdap vaccination of adolescents aged 13-17 years. Am J Prev Med 2017;53:373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorell CG, Yankey D, Byrd KK, Murphy TV.Hepatitis A vaccination coverage among adolescents in the United States. Pediatrics 2012;129:213–21. [DOI] [PubMed] [Google Scholar]
- 34.Jain N, Hennessey K. Hepatitis B vaccination coverage among US adolescents, National Immunization Survey-Teen, 2006. J Adolesc Health 2009;44:561–7. [DOI] [PubMed] [Google Scholar]
- 35.Lu PJ, Jain N, Cohn AC.Meningococcal conjugate vaccination among adolescents aged 13-17 years, United States, 2007. Vaccine 2010;28:2350–5. [DOI] [PubMed] [Google Scholar]
- 36.Smith PJ, Stokley S, Bednarczyk RA, OrensteinWA, Omer SB. HPV vaccination coverage of teen girls: the influence of health care providers. Vaccine 2016;34:1604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention.Human papillomavirus (HPV). Available at: https://www.cdc.gov/hpv/hcp/index.html Accessed March 27, 2018.
- 38.Jain N, Stokley S, Cohn A. Receipt of tetanus-containing vaccinations among adolescents aged 13 to 17 years in the United States: National Immunization Survey-Teen 2007. Clin Ther 2010;32:1468–78. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. Immunization of adolescents: recommendations of the Advisory Committee on Immunization Practices, the American Academy of Pediatrics, the American Academy of Family Physicians, and the American Medical Association. MMWR Recomm Rep 1996;45(RR-13):1–16. [PubMed] [Google Scholar]
- 40.Dolson D Errors of non-observation: dwelling non-response and coverage error in traditional censuses. Available at: http://www.amstat.org/sections/srms/proceedings/y2012/files/303670_71713.pdf Accessed March 28, 2018.
- 41.Shay LA, Baldwin AS, Betts AC, Marks EG, Higashi RT, Street RL Jr, et al. Parent-provider communication of HPV vaccine hesitancy. Pediatrics 2018;141:e20172312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: the impact of recommendation quality. Vaccine 2016;34:1187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The Community Guide. Vaccination. Available at: http://www.thecommunityguide.org/index.html Accessed March 28, 2018.
- 44.National Center for Immunization and Respiratory Diseases. General recommendations on immunization–recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011;60:1–64. [PubMed] [Google Scholar]


