Abstract
Background:
Since special efforts are necessary to vaccinate people living far from fixed vaccination posts, decision makers are interested in knowing the economic value of such efforts.
Methods:
Using our immunization geospatial information system platform and a measles compartment model, we quantified the health and economic value of a 2-dose measles immunization outreach strategy for children <24 months of age in Kenya who are geographically hard-to-reach (i.e., those living outside a specified catchment radius from fixed vaccination posts, which served as a proxy for access to services).
Findings:
When geographically hard-to-reach children were not vaccinated, there were 1427 total measles cases from 2016 to 2020, resulting in $9.5 million ($3.1–$18.1 million) in direct medical costs and productivity losses and 7504 (3338–12,903) disability-adjusted life years (DALYs). The outreach strategy cost $76 ($23–$142)/DALY averted (compared to no outreach) when 25% of geographically hard-to-reach children received MCV1, $122 ($40–$226)/DALY averted when 50% received MCV1, and $274 ($123–$478)/DALY averted when 100% received MCV1.
Conclusion:
Outreach vaccination among geographically hard-to-reach populations was highly cost-effective in a wide variety of scenarios, offering support for investment in an effective outreach vaccination strategy.
Keywords: Vaccination, Economic, Hard-to-reach populations
1. Introduction
The 2020 Measles and Rubella Strategic Plan aims to achieve at least 95% coverage with the first and second dose of the measles-containing vaccine (MCV) in all countries and districts globally by 2020 [1]. As special efforts are necessary to vaccinate people who live far from fixed vaccination posts, decision makers are interested in knowing the economic value of efforts to place vaccination sessions closer to these hard-to-reach populations. WHO and UNICEF use coverage for the first dose of diphtheria-tetanus-pertussis vaccine (DTP1) as an indicator of population access to routine immunization services [2]. Children who fail to receive DTP1 are generally considered to be hard-to-reach [3]. In 2017, 13.7 million children (10% of the yearly target for routine childhood immunization services) failed to receive DTP1 globally and can be considered hard-to-reach for the purpose of this analysis [4]. From 2003 to 2016, the annual incidence of confirmed measles cases in Kenya has ranged from 2 to 65 cases/million persons, with a consistently higher incidence among those in urban compared to rural residences [5].
Vaccinating such geographically hard-to-reach populations requires allocating personnel time and other resources to make trips to sparsely populated locations, resulting in a higher cost per person vaccinated but may be necessary to achieve measles elimination goals. Long distances to vaccination posts can be a barrier to vaccine access, especially in regions with transportation barriers (e.g., poor road conditions or lack of public transit). Not vaccinating geographically hard-to-reach target populations means deaths and suffering that could have been avoided, as well as increased risk for measles outbreaks that may spread to other locations as people travel, even affecting those who are vaccinated but may not be immune [6].
Therefore, determining the appropriate level of vaccination service resources for geographically hard-to-reach populations involves balancing the corresponding benefits and costs. To reach populations geographically distant from fixed vaccination posts (i.e., health facilities) generally requires an outreach approach, whereby a health worker travels to these distant communities and conducts an outreach vaccination session [7]. Although outreach is a key component of the Reaching Every District strategy [8,9], there is limited economic evidence of conducting outreach sessions. We developed and utilized the Strategic Integrated Geotemporal Mapping Application (SIGMA) to quantify the number of children eligible for measles vaccination located beyond the fixed vaccination post catchment areas needing to be reached through outreach sessions, the costs entailed in reaching these children, and the economic value of vaccinating these children in a sample country setting.
2. Methods
2.1. Study setting
Vaccinating hard-to-reach populations is an important consideration in Kenya (test case for the modeling application based on SIGMA), a low-income African country with 43 million people [10] and 2728 health centers (Fig. 1a-b) that administer Expanded Program on Immunization (EPI) vaccines. While 74% [11] of the population reside in rural areas, health centers are clustered in more densely populated areas [Fig. 1a-b shows by overlaying the current fixed vaccination posts (i.e., health centers) onto the population density]. Kenya’s EPI schedule includes a MCV first dose (MCV1) for infants 9 months old, followed by a second dose (MCV2) at 18 months [12]. The MCV2 was introduced into the EPI schedule in 2013. Although Kenya reached 93% coverage for MCV1 in 2012, coverage has decreased in subsequent years; in 2015, 75% of children 12–23 months old had received MCV1, and 28% of children 24–35 months had received MCV2 [13]. We defined the target populations of interest as eligible children <12 months of age (i.e., surviving infants) for MCV1 and eligible children 12–23 months for MCV2.
Fig. 1.
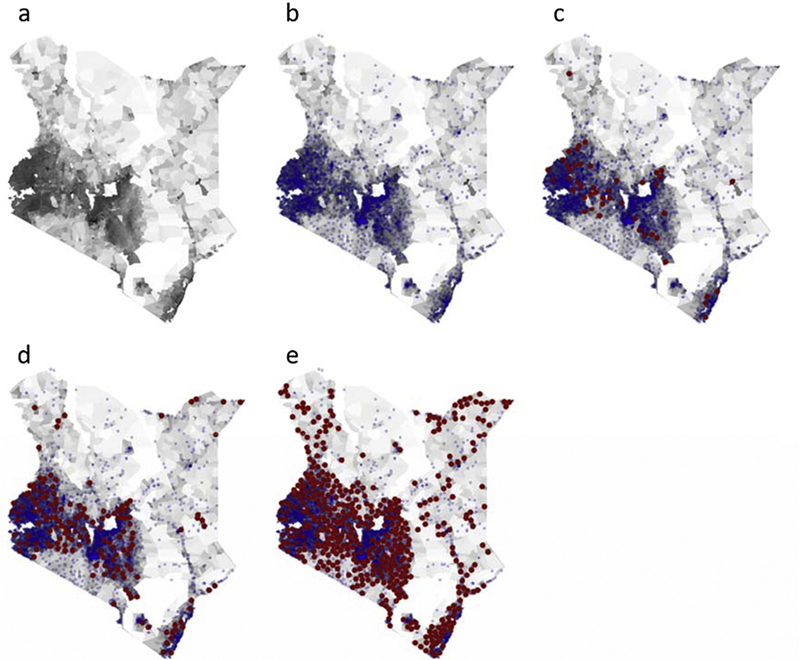
Maps of Kenyan population and fixed and proposed outreach vaccination locations used to model cost-effectiveness of vaccinating geographically hard-to-reach target populations with measles 1st and 2nd doses, 2016–2020. (a) Displays the population density where darker shaded regions are more densely populated; (b) shows in blue the assumed 5 km (km) in radius catchment areas of each existing fixed vaccination post; (c), (d), and (e) overlay in red the 10 km in radius catchment areas for proposed outreach locations necessary to include 25%, 50% and 75% of the geographically hard-to-reach children <12months, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. Identifying geographically hard-to-reach populations
To identify geographically hard-to-reach target populations, we used our SIGMA for Immunization geospatial information system (GIS) platform. SIGMA assigns fixed-immunization post vaccination locations to a designated geographic area and then generates catchment radii of specified distances around each location. These catchment areas serve to quantify how many individuals reside within a feasible distance to immunization locations versus how many individuals do not (i.e. hard-to-reach populations) when overlaid onto geospatially explicit population data [14]. Kenyan population geospatial distribution data came from the 2000 Global Rural-Urban Mapping Project (GRUMP) Population Count Grid, v1 dataset [15]. The 2010 Cold Chain Equipment Manager [16] assessment provided the fixed vaccination posts. We assigned each fixed vaccination post a circular catchment area (radius varied: baseline 5 km, sensitivity analyses ranged from 2.5–10 km) [17]. Geographically hard-to-reach children were those who did not fall within any fixed vaccination post catchment area (21% of children <24 months with baseline fixed post catchment radius). These hard-to-reach target populations were assumed to be unreachable by existing fixed posts, thus unvaccinated against measles at baseline where no outreach vaccination occurred [18]. In each scenario, 100% of eligible children <12 months who were located within fixed or outreach vaccination location catchment areas received the MCV1 at these locations (sensitivity analyses varied MCV1 coverage 80–100%). Among eligible children <24 months who received MCV1, we assumed 50% of those within fixed or outreach vaccination location catchment areas received MCV2 (varied 25–100%).
2.3. Outreach vaccination
We modeled outreach vaccination as a strategy to vaccinate geographically hard-to-reach target populations. Each session, a health worker would transport MCV in ≤2 vaccine carriers via motorbike to an outreach site, including hard-to-reach target populations located within a specified radius (assumed people would travel short distances to the outreach session and the health worker would roam the vicinity to vaccinate others). We assumed each session took one day and, on average, one child was vaccinated every 15 min [19] (sensitivity analyses ranged 2–30 min), and that each site would host as many sessions as needed to achieve the specified coverage level. In the event the number of outreach sessions exceeded one day, modeled outreach continues to the next day with the costs accruing daily in order to achieve the specified coverage for that location. Appendix Table 1 enumerates the logistics costs of vaccination outreach (e.g. per diems, vaccine carrier, motorbike, etc.).
To place potential outreach locations, we first defined the catchment area for an outreach location, with a baseline radius of 10 km [sensitivity analyses varied 7.5–20 km (independent from the radius for fixed posts)]. A mathematical algorithm placed outreach locations to include a percentage (10–100%) of the hard-to-reach target populations while minimizing overlap with other fixed and outreach catchment areas. The algorithm utilized an iterative scoring approach where locations were randomly placed on the map and a score was computed based on the objective criteria of maximizing the target populations included and minimizing the overlap with other locations. As inclusion of the target populations approached 100%, this algorithm became unstable and a modified procedure was used once adding a new outreach location resulted in including a fraction of a child. Then, the remaining land area was divided hexagonally and aggregated until an outreach site could be placed so that at least one child was included.
2.4. Measles transmission model
For this study, we developed a susceptible-exposed-infectious-recovered (SEIR) compartment model to determine the number of measles cases across all ages for each scenario from 2016 to 2020, capturing health impacts beyond those children vaccinated. The Appendix describes SEIR model, while Appendix Table 1 shows its inputs. The number of measles-related deaths were calculated by multiplying the number of cases by a fixed case fatality rate.
2.5. Costs and health effects
The Appendix also provides details on the linked economic model we developed for this study that translates cases into costs and health effects. Briefly, the cost of vaccination included the costs of vaccines, transport, portable vaccine storage, and personnel time. We assumed vaccination costs at fixed vaccination posts were the same for each strategy and were not included. The cost per measles case included the costs of care (either outpatient or hospitalization, based on disease severity), transportation, and caretakers’ lost productivity. We used the human capital approach to quantify productivity losses due to disability and death, by discounting the life expectancy at age 15 (i.e., age at labor force entry) to the year of vaccination and using the gross domestic product (GDP) per capita as a proxy for wages as not to undervalue the individuals deemed hard-to-reach. Additionally, we used daily minimum wage to calculate the caretaker wages lost. Cost and treatment parameters came from the published literature and other public data sources such as WHO-CHOICE, which serves to provide a conservative estimate for the impact of this strategy (Appendix Table 1). Sensitivity analyses varied disability-adjusted life years (DALYs) incurred per measles case, medical costs per case, time taken per dose administered, and proportion of geographically hard-to-reach target populations included.
For each scenario, the following formula calculated the incremental cost-effectiveness ratio (ICER) of vaccinating geographically hard-to-reach target populations through outreach:
Outreach vaccination was considered cost-effective if the ICER was <3 times Kenya’s GDP per capita ($5595), highly cost-effective if <GDP per capita ($1865) [36], and economically dominant when it saved both costs and health effects. All costs are presented in 2018 $US, with future costs discounted using a 3% rate.
3. Results
3.1. No outreach to hard-to-reach populations
Assuming a 5 km catchment radius around each fixed vaccination post, 21% of eligible children <12 months were located outside the catchment areas and not vaccinated with MCV1 (Fig. 1b). If 50% of eligible children 12–23 months within the fixed vaccination catchment areas who received MCV1 received MCV2, failure to reach the remaining target population (i.e., those within both fixed and outreach catchment areas) resulted in 1427 measles cases and 257 deaths. These cases accrued $39,000 [$18,000–$69,000 (range represents minimum and maximum across sensitivity analyses of key parameters)] in direct medical costs, $9.5 million ($3.1–$180 million) in productivity losses, and 7504 (3338–14,366) DALYs. A 2.5 km fixed post catchment radius yielded 52% not vaccinated with MCV1, resulting in 46,584 cases, 8385 deaths, $1.3 million ($590,000–$2.3 million) in direct medical costs, $290.8million ($94.3–$550.8 million) in productivity losses and 245,033 (109,007–469,103) DALYs. While a 10 km radius yielded 7% not receiving MCV1, resulting in 449 cases, 81 deaths, $12,200 ($5600–$22,000) in direct medical costs, $3.0 million ($962,000–$5.7 million) in productivity losses, and 2360 (1050–4518) DALYs. The resulting number of cases for the various catchment radii were not linear as the number of people who could reach fixed-post immunization locations differed substantially. Thus, the subsequent number of children left uncovered who remained susceptible differed by catchment radius (e.g., three-times as many are susceptible in a 2.5 km radius than the 5 km radius).
3.2. Outreach included 25% of Hard-to-Reach MCV1 target population
Table 1 shows the resulting cases and costs averted. Compared to not vaccinating the geographically hard-to-reach target populations, vaccinating 25% of the hard-to-reach eligible children <12 months with MCV1 and half of the hard-to-reach eligible children 12–23 months who had received MCV1 with MCV2 (Fig. 1c) cost $76/DALY averted (range: $23–$142/DALY averted) with a 5 km fixed vaccination post catchment radius. ICERs were $0.56/DALY averted (dominant–$5/DALY averted) for a 2.5 km radius, and $1312/DALY averted ($252–$3277/DALY averted) for a 10 km radius.
Table 1.
Measles cases, costs ($US), and DALYs averted through outreach vaccination when 25% of geographically hard-to reach children are included in catchment areas of proposed outreach vaccination sessions, Kenya 2016–2020.
| Fixed vaccination posts catchment radius | Proportion of target population vaccinated within fixed post catchments* | Proportion of hard-to-reach target population vaccinated* | Proportion of total target population vaccinated* | Measles cases averted† | Deaths averted† | DALYs averted† | Direct medical costs averted† | Productivity losses averted† | Total societal costs averted† | Outreach vaccination strategy costs† |
|---|---|---|---|---|---|---|---|---|---|---|
| 25% of Population that Received MCV1 Receives MCV2 | ||||||||||
| 2.5 | MCV1: 100% | MCV1: 25% | MCV1: 61% | 22,699 | 4086 | 119,399 | 617,653 | 150,497,104 | 151,114,757 | 583,866 |
| MCV2: 25% | MCV2: 6% | MCV2: 15% | (21,161–22,734) | (1693–9321) | (49,518–228,934) | (266,422–1,098,063) | (45,093,474–285,685,096) | (45,359,896–286,783,159) | (219,641–984,953) | |
| 5 | MCV1: 100% | MCV1: 25% | MCV1: 84% | 2247 | 404 | 11,818 | 61,135 | 14,896,019 | 14,957,154 | 264,027 |
| MCV2: 25% | MCV2: 6% | MCV2: 21% | (2234–2306) | (179–9450) | (5227–23,217) | (28,124–111,357) | (4,760,108–27,371,447) | (4,788,231–27,482,804) | (94,140–457,439) | |
| 10 | MCV1: 100% | MCV1: 25% | MCV1: 95% | 22 | <5 | 116 | 601 | 146,389 | 146,989 | 130,790 |
| MCV2: 25% | MCV2: 6% | MCV2: 24% | (28–18) | (65–184) | (352–882) | (229,422–229,422) | (230,304–230,304) | (37,849–242,704) | ||
| 50% of Population that Received MCV1 Receives MCV2 | ||||||||||
| 2.5 | MCV1: 100% | MCV1: 25% | MCV1: 61% | 23,234 | 4182 | 122,213 | 632,210 | 154,044,054 | 154,676,264 | 700,639 |
| MCV2: 50% | MCV2: 13% | MCV2: 31% | (21,806–24,194) | (1,744–9920) | (51,027–243,631) | (274,543–1,168,558) | (46,467,920–304,025,953) | (46,742,463–305,194,511) | (263,569–1,181,943) | |
| 5 | MCV1: 100% | MCV1: 25% | MCV1: 84% | 747 | 134 | 3927 | 20,313 | 4,949,355 | 4,969,667 | 316,832 |
| MCV2: 50% | MCV2: 13% | MCV2: 42% | (752–758) | (60–311) | (1759–7633) | (9466–36,612) | (1,602,250–8,999,175) | (1,611,717–9,035,787) | (112,968–548,927) | |
| 10 | MCV1: 100% | MCV1: 25% | MCV1: 95% | 23 | <5 | 119 | 616 | 150,211 | 150,827 | 156,948 |
| MCV2: 50% | MCV2: 13% | MCV2: 47% | (30–29) | (69–293) | (372–1405) | (365,669–365,669) | (367,074–367,074) | (45,419–291,245) | ||
| 75% of Population that Received MCV1 Receives MCV2 | ||||||||||
| 2.5 | MCV1: 100% | MCV1: 25% | MCV1: 61% | 26,505 | 4771 | 139,416 | 721,197 | 175,726,729 | 176,447,927 | 817,413 |
| MCV2: 75% | MCV2: 19% | MCV2: 46% | (25,662–26,135) | (2038–10,867) | (60,048–263,177) | (323,080–1,262,309) | (56,575,100–328,417,316) | (56,898,180–329,679,626) | (307,497–1,378,934) | |
| 5 | MCV1: 100% | MCV1: 25% | MCV1: 84% | 217 | 39 | 1143 | 5913 | 1,440,702 | 1,446,615 | 369,638 |
| MCV2: 75% | MCV2: 19% | MCV2: 63% | (242–226) | (17–99) | (566–2281) | (3045–10,939) | (515,453–2,688,855) | (518,498–2,699,794) | (131,797–640,415) | |
| 10 | MCV1: 100% | MCV1: 25% | MCV1: 95% | 22 | <5 | 115 | 596 | 145,279 | 145,875 | 183,106 |
| MCV2: 75% | MCV2: 19% | MCV2: 71% | (24–27) | (56–275) | (304–1318) | (342,906–342,906) | (344,224–344,224) | (52,989–339,786) | ||
| 100% of Population that Received MCV1 Receives MCV2 | ||||||||||
| 2.5 | MCV1: 100% | MCV1: 25% | MCV1: 61% | 25,806 | 4645 | 135,739 | 702,180 | 171,093,010 | 171,795,190 | 934,186 |
| MCV2: 100% | MCV2: 25% | MCV2: 61% | (25,424–25,923) | (2034–10,780) | (59,493–261,047) | (320,093–1,252,093) | (54,177,661–325,759,373) | (54,497,754–327,011,466) | (351,426–1,575,924) | |
| 5 | MCV1: 100% | MCV1: 25% | MCV1: 84% | 92 | 17 | 482 | 2494 | 607,660 | 610,154 | 422,443 |
| MCV2: 100% | MCV2: 25% | MCV2: 84% | (83–97) | (7–40) | (195–981) | (1050–4707) | (177,700–1,156,867) | (178,750–1,161,573) | (150,625–731,903) | |
| 10 | MCV1: 100% | MCV1: 25% | MCV1: 95% | 26 | <5 | 138 | 713 | 173,712 | 174,425 | 209,264 |
| MCV2: 100% | MCV2: 25% | MCV2: 95% | (24–22) | (55–226) | (297–1086) | (282,621–282,621) | (283,707–283,707) | (60,559–388,327) | ||
MCV1 = first dose of measles-containing vaccine; MCV2 = second dose of measles-containing vaccine; MCV1 target population = eligible children <12 months (i.e., surviving infants); MCV2 target population = eligible children 12–23 months;
Values are rounded to nearest whole number and cannot be used to reconstruct incremental cost-effectiveness ratios (ICERs) reported in the text, which used unrounded values. Note: Base value correspond to an outreach vaccination location catchment area radius of 10 km. Ranges represent the minimum and maximum values when varying parameters across their ranges, for an outreach vaccination location catchment area radius of 7.5–20 km. Logistics cost assumes a child is vaccinated every 15 min(2–30 min).
3.3. Outreach included 50% of hard-to-reach MCV1 target population
Fig. 1d shows outreach locations needed to include 50% of the hard-to-reach children <12 months. Compared to not vaccinating the geographically hard-to-reach target populations, vaccinating 50% of hard-to-reach children <12 months with MCV1 and 50% of hard-to-reach children 12–23 months who received MCV1 with MCV2 cost $122/DALY averted ($40–$226/DALY averted) with a 5 km fixed vaccination post catchment radius, $1.06/DALY averted (dominant–$10/DALY averted) with a 2.5 km radius, and $1803/DALY averted ($303–$5650/DALY averted) with a 10 km radius (Table 2).
Table 2.
Measles cases, costs ($US), and DALYs averted through outreach vaccination when 50% of geographically hard-to reach children are included in catchment areas of proposed outreach vaccination sessions, Kenya 2016–2020.
| Fixed vaccination posts catchment radius | Proportion of target population vaccinated within fixed post catchments* | Proportion of hard-to-reach target population vaccinated* | Proportion of total target population vaccinated* | Measles cases averted† | Deaths averted† | DALYs averted† | Direct medical costs averted† | Productivity losses averted† | Total societal costs averted† | Outreach vaccination strategy costs† |
|---|---|---|---|---|---|---|---|---|---|---|
| 25% of Population that Received MCV! Receives MCV2 | ||||||||||
| 2.5 | MCV1: 100% | MCV1: 50% | MCV1: 74% | 43,858 | 7894 | 230,694 | 1,193,382 | 290,778,977 | 291,972,358 | 1,153,637 |
| MCV2: 25% | MCV2: 13% | MCV2: 19% | (43,742–43,929) | (3,463–18,011) | (102,355–442,365) | (550,706–2,121,772) | (93,210,173–552,025,216) | (93,760,879–554,146,987) | (439,591–1,955,085) | |
| 5 | MCV1: 100% | MCV1: 50% | MCV1: 90% | 2877 | 518 | 15,133 | 78,285 | 19,074,967 | 19,153,252 | 521,940 |
| MCV2: 25% | MCV2: 13% | MCV2: 22% | (2852–2863) | (228–1189) | (6673–28,834) | (35,901–138,301) | (6,076,371–33,994,263) | (6,112,272–34,132,564) | (187,397–891,469) | |
| 10 | MCV1: 100% | MCV1: 50% | MCV1: 97% | 55 | 10 | 290 | 1499 | 365,291 | 366,791 | 266,949 |
| MCV2: 25% | MCV2: 13% | MCV2: 24% | (49–40) | (3–23) | (115–405) | (617–1941) | (504,891–504,891) | (506,831–506,831) | (79,432–497,655) | |
| 50% of Population that Received MCVI Receives MCV2 | ||||||||||
| 2.5 | MCV1: 100% | MCV1: 50% | MCV1: 74% | 42,246 | 7604 | 222,212 | 1,149,501 | 280,087,153 | 281,236,654 | 1,384,364 |
| MCV2: 50% | MCV2: 25% | MCV2: 37% | (42,366–42,404) | (3380–17,431) | (99,136–427,009) | (533,383–2,048,117) | (90,278,170–532,862,383) | (90,811,553–534,910,500) | (527,509–2,346,102) | |
| 5 | MCV1: 100% | MCV1: 50% | MCV1: 90% | 938 | 169 | 4934 | 25,523 | 6,218,811 | 6,244,333 | 626,328 |
| MCV2: 50% | MCV2: 25% | MCV2: 45% | (938–934) | (75–388) | (2196–9405) | (11,815–45,109) | (1,999,720–11,087,687) | (2,011,535–11,132,796) | (224,876–1,069,763) | |
| 10 | MCV1: 100% | MCV1: 50% | MCV1: 97% | 34 | 6 | 177 | 917 | 223,347 | 224,264 | 320,339 |
| MCV2: 50% | MCV2: 25% | MCV2: 48% | (29–38) | (2–16) | (68–380) | (368–1821) | (473,834–473,834) | (475,656–475,656) | (95,319–597,187) | |
| 75% of Population that Received MCVI Receives MCV2 | ||||||||||
| 2.5 | MCV1: 100% | MCV1: 50% | MCV1: 74% | 41,620 | 7492 | 218,923 | 1,132,490 | 275,942,065 | 277,074,554 | 1,615,092 |
| MCV2: 75% | MCV2: 38% | MCV2: 56% | (41,587–41,538) | (3323–17,064) | (97,313–418,286) | (523,578–2,006,276) | (88,618,544–521,976,488) | (89,142,122–523,982,763) | (615,427–2,737,119) | |
| 5 | MCV1: 100% | MCV1: 50% | MCV1: 90% | 313 | 56 | 1647 | 8519 | 2,075,659 | 2,084,178 | 730,716 |
| MCV2: 75% | MCV2: 38% | MCV2: 67% | (319–313) | (25–131) | (746–3153) | (4013–15,121) | (679,144–3,716,806) | (683,157–3,731,927) | (262,356–1,328,667) | |
| 10 | MCV1: 100% | MCV1: 50% | MCV1: 97% | 33 | 6 | 171 | 886 | 215,862 | 216,748 | 373,729 |
| MCV2: 75% | MCV2: 38% | MCV2: 72% | (37–36) | (2–15) | (87–367) | (468–1762) | (458,382–458,382) | (460,144–460,144) | (111,205–696,718) | |
| 100% of Population that Received MCVl Receives MCV2 | ||||||||||
| 2.5 | MCV1: 100% | MCV1: 50% | MCV1: 74% | 37,489 | 6748 | 197,192 | 1,020,077 | 248,551,653 | 249,571,730 | 1,845,819 |
| MCV2: 100% | MCV2: 50% | MCV2: 74% | (37,513–37,466) | (2997–15,380) | (87,781–377,282) | (472,294–1,809,603) | (79,938,493–470,807,872) | (80,410,787–472,617,476) | (703,346–3,128,136) | |
| 5 | MCV1: 100% | MCV1: 50% | MCV1: 90% | 163 | 29 | 859 | 4446 | 1,083,204 | 1,087,650 | 835,104 |
| MCV2: 100% | MCV2: 50% | MCV2: 90% | (163–160) | (13–67) | (382–1612) | (2057–7731) | (348,156–1,900,251) | (350,213–1,907,981) | (299,835–1,426,351) | |
| 10 | MCV1: 100% | MCV1: 50% | MCV1: 97% | 33 | 6 | 171 | 886 | 215,888 | 216,774 | 427,119 |
| MCV2: 100% | MCV2: 50% | MCV2: 97% | (28–27) | (2–14) | (64–270) | (347–1293) | (336,361–336,361) | (337,654–337,654) | (127,092–796,249) | |
MCV1 = first dose of measles-containing vaccine; MCV2 = second dose of measles-containing vaccine; MCV1 target population = eligible children < 12 months (i.e., surviving infants); MCV2 target population = eligible children 12–23 months;
Values are rounded to nearest whole number and cannot be used to reconstruct incremental cost-effectiveness ratios (ICERs) reported in the text, which used unrounded values. Note: Base value correspond to an outreach vaccination location catchment area radius of 10 km. Ranges represent the minimum and maximum values when varying parameters across their ranges, for an outreach vaccination location catchment area radius of 7.5–20 km. Logistics cost assumes a child is vaccinated every 15 min (2–30 min).
3.4. Outreach included 100% of hard-to-reach MCV1 target population
Outreach locations reached 100% of the geographically hard-to-reach children <12 months (Fig. 1e) and all of these children were vaccinated with MCV1. Compared to not vaccinating these children, vaccinating 100% of hard-to-reach children <12 months with MCV1 and 50% of hard-to-reach children 12–23 months who received MCV1 with MCV2 cost $274/DALY averted ($123–$478/DALY averted), $8/DALY averted (dominant–$31/DALY averted), and $1832/DALY averted ($1087–$4461/DALY averted) for a 5 km, 2.5 km, and 10 km fixed vaccination post catchment radius, respectively (Table 3).
Table 3.
Measles cases, costs ($US), and DALYs averted through outreach vaccination when 100% of geographically hard-to reach children are included in catchment areas of proposed outreach vaccination sessions, Kenya 2016–2020.
| Fixed vaccination posts catchment radius | Proportion of target population vaccinated within fixed post catchments* | Proportion of hard-to-reach target population vaccinated* | Proportion of total target population vaccinated* | Measles cases averted† | Deaths averted† | DALYs averted† | Direct medical costs averted† | Productivity losses averted† | Total societal costs averted† | Outreach vaccination strategy costs† |
|---|---|---|---|---|---|---|---|---|---|---|
| 25% of Population that Received MCVl Receives MCV2 | ||||||||||
| 2.5 | MCV1: 100% | MCV1: 100% | MCV1: 100% | 51,336 | 9240 | 270,028 | 1,396,854 | 340,357,107 | 341,753,961 | 2,657,448 |
| MCV2: 25% | MCV2: 25% | MCV2: 25% | (51,332–51,335) | (4107–21,048) | (120,117–516,947) | (646,269–2,479,496) | (109,384,724–645,095,021) | (110,030,993–647,574,517) | (1,293,480–4,543,823) | |
| 5 | MCV1: 100% | MCV1: 100% | MCV1: 100% | 3036 | 546 | 15,972 | 82,622 | 20,131,546 | 20,214,167 | 1,293,179 |
| MCV2: 25% | MCV2: 25% | MCV2: 25% | (3038–3041) | (243–1247) | (7109–30,624) | (38,249–146,886) | (6,473,920–38,215,576) | (6,512,169–38,362,462) | (783,431–2,139,250) | |
| 10 | MCV1: 100% | MCV1: 100% | MCV1: 100% | 88 | 16 | 462 | 2390 | 582,254 | 584,643 | 671,015 |
| MCV2: 25% | MCV2: 25% | MCV2: 25% | (90–102) | (7–43) | (211–1030) | (1137–4940) | (1,285,198–1,285,198) | (1,290,138–1,290,138) | (518,396–1,032,957) | |
| 50% of Population that Received MCVl Receives MCV2 | ||||||||||
| 2.5 | MCV1: 100% | MCV1: 100% | MCV1: 100% | 46,203 | 8317 | 243,026 | 1,257,176 | 306,323,003 | 307,580,179 | 3,188,938 |
| MCV2: 50% | MCV2: 50% | MCV2: 50% | (46,219–46,221) | (3696–18,951) | (108,151–465,447) | (581,891–2,232,484) | (98,488,447–580,829,430) | (99,070,339–583,061,914) | (1,552,176–5,452,588) | |
| 5 | MCV1: 100% | MCV1: 100% | MCV1: 100% | 1058 | 190 | 5563 | 28,778 | 7,012,001 | 7,040,779 | 1,551,815 |
| MCV2: 50% | MCV2: 50% | MCV2: 50% | (1048–1055) | (84–437) | (2453–10,625) | (13,200–50,964) | (2,234,220–13,259,432) | (2,247,420–13,310,397) | (940,117–2,567,099) | |
| 10 | MCV1: 100% | MCV1: 100% | MCV1: 100% | 83 | 15 | 438 | 2268 | 552,539 | 554,806 | 805,218 |
| MCV2: 50% | MCV2: 50% | MCV2: 50% | (86–83) | (6–36) | (202- | (1087- | (1,047,268- | (1,051,294- | (622,076- | |
| 839) | 4025) | 1,047,268) | 1,051,294) | 1,363,756) | ||||||
| 75% of Population that Received MCVl Receives MCV2 | ||||||||||
| 2.5 | MCV1: 100% | MCV1: 100% | MCV1: 100% | 43,194 | 7775 | 227,198 | 1,175,297 | 286,372,490 | 287,547,787 | 3,720,428 |
| MCV2: 75% | MCV2: 75% | MCV2: 75% | (43,192–43,196) | (3455–17,710) | (101,069–434,988) | (543,787–2,086,386) | (92,039,150–542,819,005) | (92,582,938–544,905,391) | (1,810,872–6,361,352) | |
| 5 | MCV1: 100% | MCV1: 100% | MCV1: 100% | 426 | 77 | 2242 | 11,598 | 2,825,929 | 2,837,527 | 1,810,450 |
| MCV2: 75% | MCV2: 75% | MCV2: 75% | (427–428) | (34–176) | (998–4315) | (5372–20,695) | (909,173–5,384,127) | (914,544–5,404,822) | (1,096,803–2,994,949) | |
| 10 | MCV1: 100% | MCV1: 100% | MCV1: 100% | 74 | 13 | 387 | 2004 | 488,328 | 490,332 | 939,421 |
| MCV2: 75% | MCV2: 75% | MCV2: 75% | (73–76) | (6–32) | (171–761) | (921–3648) | (949,110–949,110) | (952,758–952,758) | (725,755–1,446,139) | |
| 100% of Population that Received MCVl Receives MCV2 | ||||||||||
| 2.5 | MCV1: 100% | MCV1: 100% | MCV1: 100% | 38,130 | 6863 | 200,566 | 1,037,531 | 252,804,407 | 253,841,938 | 4,251,917 |
| MCV2: 100% | MCV2: 100% | MCV2: 100% | (38,134–38,132) | (3050–15,635) | (89,233–383,993) | (480,103–1,841,793) | (81,260,201–479,182,696) | (81,740,304–481,024,489) | (2,069,568–7,270,117) | |
| 5 | MCV1: 100% | MCV1: 100% | MCV1: 100% | 265 | 48 | 1395 | 7217 | 1,758,534 | 1,765,751 | 2,069,086 |
| MCV2: 100% | MCV2: 100% | MCV2: 100% | (266–267) | (21–111) | (622–2694) | (3348–12,919) | (566,744–3,361,247) | (570,093–3,374,166) | (1,253,489–3,422,799) | |
| 10 | MCV1: 100% | MCV1: 100% | MCV1: 100% | 70 | 13 | 367 | 1898 | 462,349 | 464,247 | 1,073,624 |
| MCV2: 100% | MCV2: 100% | MCV2: 100% | (69–70) | (6–30) | (162–708) | (874–3398) | (883,976–883,976) | (887,374–887,374) | (829,434–1,652,731) | |
MCV1 = first dose of measles containing vaccine; MCV2 = second dose of measles containing vaccine; MCV1 target population = eligible children <12 months (i.e., surviving infants); MCV2 target population = eligible children 12–23 months;
Values are rounded to nearest whole number and cannot be used to reconstruct ICERs reported in the text, which used unrounded values. Note: Base value correspond to an outreach vaccination location catchment area radius of 10 km. Ranges represent the minimum and maximum values when varying parameters across their ranges, for an outreach vaccination location catchment area radius of 7.5–20 km. Logistics cost assumes a child is vaccinated every 15 min (2–30 min).
3.5. Sensitivity analyses
Fig. 2 shows the impact of key parameters (varied from their minimum to maximum) on the cost-effectiveness of outreach when 25% of the geographically hard-to-reach children <12 months were included in outreach catchment areas. Fixed catchment area radius and the proportion vaccinated with MCV2 had the greatest impact on cost-effectiveness. Increasing either of these parameters increased the ICER for any given scenario, while also causing the ICER to rise at a steeper rate as coverage of hard-to-reach populations increased. The trends (i.e., the parameters that have the greatest impact on the ICER) hold when increasing the proportion of the hard-to-reach target populations. Fig. 3 shows how the ICER varies when ranging the proportion of hard-to-reach children <12 months included in the outreach catchment areas from 10% to 100%.
Fig. 2.
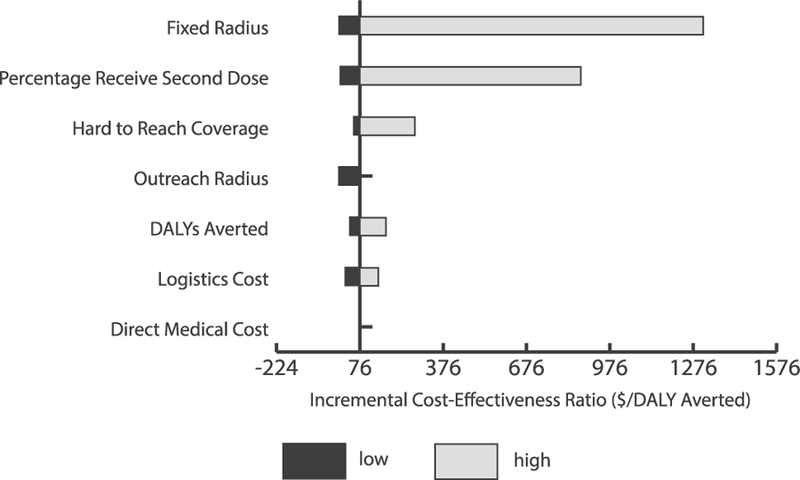
Univariate sensitivity analysis for incremental cost-effectiveness ratios (ICERs) of vaccinating 25% of the hard-to-reach eligible children < 12 months with MCV1 and 50% of hard-to-reach eligible children 12–23 months who had received MCV1 with MCV2 through proposed outreach vaccination sessions in Kenya. Bars show how ICERs change from the baseline of $76 with varying inputs for each parameter. The most impactful parameters are displayed at the top of the diagram. Black bars represent parameter estimates lower than base case, while grey bars represent parameter estimates higher than base case. Note: Base case estimate ranges were calculated by varying the following variables by the given values listed: Fixed vaccination post radius of 5 km (2–10km); Outreach vaccination location catchment area radius of 10 km (0–20 km); Measles second dose coverage among individuals who receive a first measles vaccination dose at 50% (0–100%); Disability-adjusted life years (DALYs) averted mean (minimum-maximum); Logistics cost assuming a child is vaccinated every 15 min (2–30 min); Hard-to-reach target population for MCV1 included in outreach vaccination catchment areas at 25% (10–100%); Direct medical cost mean (minimum-maximum).
Fig. 3.
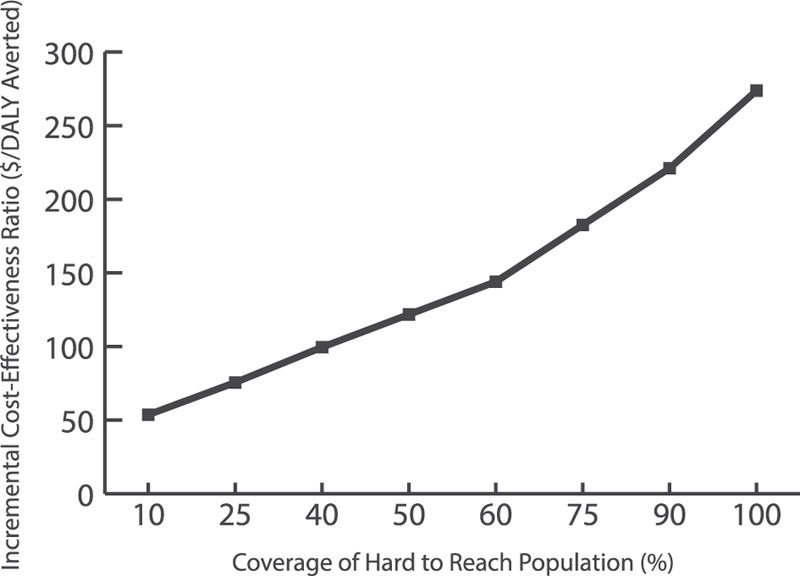
Incremental cost-effectiveness ratio (ICER) for vaccinating the geographically hard-to-reach target populations with measles 1st and 2nd dose vaccines through proposed outreach vaccination sessions in Kenya. Note: Results assume fixed vaccination post catchment radius of 5 km (km), outreach vaccination post catchment radius of 10 km, measles second dose vaccination coverage among individuals who receive a first measles vaccine dose of 50%, and logistics costs assuming a child is immunized every 15 min. The target population refers to children under 24 months of age.
Reduced MCV1 coverage among eligible children <12 months located within fixed catchment areas increased the number of measles cases in each scenario. MCV1 coverage of 95% of children <12 months located within fixed post catchment areas resulted in 3180 measles cases, yielding $87,000 ($40,000–$154,000) in direct medical costs, $16.9 million ($6.8–$40.1 million) in productivity losses, and 16,729 (7442–32,026) DALYs (assuming no outreach vaccination, a 5 km catchment radius for fixed posts, and 50% MCV2 coverage among children 12–23 months who received MCV1). Compared to not vaccinating the geographically hard-to-reach target populations, vaccinating 25% of hard-to-reach eligible children <12 months with MCV1 was cost-effective [ICER: $25 (dominant–$507)/DALY averted] with a 5 km fixed vaccination post catchment. Outreach cost $40/DALY averted ($1–$754/DALY averted) when vaccinating 50% of hard-to-reach children <12 months and $101/DALY averted ($19–1376/DALY averted) when vaccinating 100% compared to no vaccination.
An MCV1 coverage of 80% of the target population within fixed catchment areas led to 18,756 cases, resulting in $510,000 ($236,000–$906,000) in direct medical costs, $124.4 million ($40.2–$236.6 million) in productivity losses, and 98,657 (43,889–188,873) DALYs. This reduction resulted in an ICER of $2/DALY averted (dominant–$21/DALY averted) when vaccinating 25% of hard-to-reach eligible children <12 months with MCV1, $3/DALY averted (dominant–$30/DALY averted) when vaccinating 50% of children and $11/DALY averted ($1–$74/DALY averted) when achieving 100% coverage.
4. Discussion
Our results quantified the potential cost-effectiveness and health benefits of immunizing geographically hard-to-reach target populations in Kenya against measles and showed that doing so in most scenarios would be highly cost-effective. This stems from the substantial costs and early mortality resulting from measles cases, which ends up overshadowing the costs associated with outreach vaccination sessions. Outreach vaccination sessions require personnel time, transport, and storage devices to carry and administer the vaccines, so they can appear costly since special arrangements need to be made to vaccinate relatively few people. When conducting outreach sessions to vaccinate hard-to-reach populations, our study found each additional increase in the proportion of these children vaccinated yielded a greater increase in ICER, as compared to lower levels of the proportion vaccinated. For example, a 15% increase in the proportion of geographically hard-to-reach eligible children <12 months vaccinated from 10% to 25% would raise the ICER from $54 to $76/DALY averted; in contrast, a 15% increase from 75% to 90% would raise the ICER from $183 to $221/DALY averted. To put this into context, a universal childhood rotavirus vaccination program in Kenya cost $142–288/DALY averted (in 2011 $US) [21] and routine infant vaccination with a typhoid conjugate vaccine $2390/DALY averted in urban and $6931/DALY averted in rural Kenya (2015 international dollars) [22].
Based on GDP per capita thresholds, our results demonstrated that immunizing geographically hard-to-reach populations in Kenya would be highly cost-effective. However, it should be noted that appropriate thresholds may depend on the context and resources [23,24]. Other thresholds proposed are opportunity-cost based (i.e., the cost of not prioritizing outreach over other health interventions), which can inform resource allocation decisions and suggest that the routinely used GDP thresholds may be too high [24]. Even with a lower threshold, our results suggest that immunizing hard-to-reach populations could be an efficient investment.
The two key drivers of outreach vaccination cost-effectiveness were the fixed vaccination post catchment area size and the proportion of the hard-to-reach target population that received MCV2. Even though hard-to-reach populations are a small proportion of most countries’ populations and located in remote areas, if not immunized they can still produce measles outbreaks because of mobility and mixing. Outbreaks costs can surpass the additional costs of conducting outreach. Computational modeling to quantify the costs and early mortality averted by vaccination can demonstrate how these benefits may outweigh the costs associated with outreach programs. Such evidence can support decision makers in planning and investing resources to have the greatest potential impact and ultimately reduce inequities in vaccination access.
Although global investment for immunization programs over the past 15 years have focused extensively on vaccine introduction, a key objective of both the 2011–2020 Global Vaccine Action Plan [25] as well as Gavi, the Vaccine Alliance, 2016–2020 Strategy [26] is to ensure equitable vaccination coverage within countries. While many studies have demonstrated the cost-effectiveness of vaccination, few studies capture the costs of interventions to increase immunization coverage [27] such as additional efforts to reach subpopulations who may be isolated by geographic or cultural regions.
The total outreach vaccination strategy costs in our application ranged from $131,000 (when 25% of hard-to-reach children were included and only 25% received MCV2 outside of a fixed post catchment radius of 10 km) to $4.3 million (when 100% of hard-to-reach children were included and reached with MCV2 outside of a 2.5 km fixed post radius). By comparison, the total budget across all counties allocated to health in FY2016/17 was approximately $917.7 million (2018 USD) while government budget allocations to the national Ministry of Health were $601.5 million (2018 USD) [28]; assuming our hypothetical intervention costs were entirely incremental to the existing system (i.e., not accounting for any current investments in outreach vaccination), this would represent approximately 0.01% to 0.28% of total national and county government health budgets. An important consideration for the affordability of any such intervention to strengthen immunization is ensuring that budgeted amounts are actually disbursed as planned to provide sufficient resources for primary health care service delivery, especially at county level in Kenya in the context of devolution.
Our findings support the utility of ensuring national immunization programs incorporate an effective outreach vaccination strategy. As national coverage rates increase from augmenting coverage in areas that have adequate access to established vaccination locations, countries should also ensure they address hard-to-reach populations, including those geographically distant from fixed vaccination posts. Better quantifying the value of outreach can help national immunization programs and supporting organizations justify investment in an effective outreach vaccination strategy to ensure sufficient routine vaccination of hard-to-reach target populations.
While this study focused on geographically hard-to-reach populations, future studies could examine the value of reaching other hard-to-reach populations, such as those based on socioeconomic, educational, or religious/cultural barriers (which may overlap with one another and with geographic barriers). Additionally, some populations may be difficult to reach given their itinerant status. This may be relevant especially in the context of Kenya where lower socio-economic status and lower levels of parents’ education have been found to be associated with lower measles immunization [29].
4.1. Limitations
The measles transmission model assumed homogenous mixing and did not incorporate the effects of death from infection, although these features are not expected to substantially alter results. This analysis projected currently available population data assuming a uniform population growth rate, which should provide reasonable estimates but may miss heterogeneity in population growth. We mapped fixed post-catchment areas based on straight-line distances from the posts, which does not capture variations in travel times that may have a greater impact on accessibility than distance alone; additionally, the 5 km radius was not necessarily an exact 5 km radius for every location. When Kenya-specific measles cost and treatment parameters were not available, our inputs drew from proxy countries. Our model did not represent all of the possible factors that may affect the costs and impacts of outreach programs. For example, traveling across particular terrain can increase costs by substantially decreasing travel speed, and different information, education, and communication (IEC) or social mobilization activities may have varying costs and effectiveness in raising awareness and facilitating vaccination acceptance among the target population. Vaccine costs did not account for the cost of syringes, safety boxes, or other administration supplies. Furthermore, our study did not consider the feasibility of conducting outreach sessions, such as trying to reach places that are inaccessible by the model transport means and overcoming cultural or linguistic barriers that can lead to vaccine refusal and ultimately diminish the health and economic benefits of the outreach sessions; rather, we assume a best-case scenario (100% of the target population in the defined outreach catchment area would be reached). Thus, scenarios with smaller radii for outreach catchment areas may therefore be more realistic estimates of the hard-to-reach target population that would be vaccinated (which would make the ICER more favorable but decrease the number of children vaccinated via outreach compared to larger radii). Additionally, our baseline scenario assumed no outreach sessions occurred; in reality, Kenya currently includes outreach strategies as part of its interventions package to ensure high vaccination coverage, so our baseline scenario would underestimate the current reality of immunization program performance. Conversely, our study may underestimate the value of reaching hard-to-reach target populations, as it assumed no cross-border measles transmission (potentially underestimating measles cases) and included only the benefits of increasing coverage for MCV and no other vaccines. Finally, while many of the conditions in Kenya are not unique to Kenya, future studies may explore similar questions in other countries to determine how results may vary from country to country.
5. Conclusions
Immunizing geographically hard-to-reach target populations in Kenya with the measles vaccine can be cost-effective and even highly cost-effective under a wide range of modeling scenarios. This provides support for investment into effective strategies to vaccinate these populations, even though they do not represent the majority of Kenya’s population and outreach sessions are more expensive per person vaccinated compared to fixed sessions.
Acknowledgments
Research reported in this publication was supported by the Centers for Disease Control and Prevention (CDC) via contract 200-2015-M-63169, the International Society for Infectious Diseases (ISID) and Pfizer via the SIGMA grant and the Agency for Healthcare Research and Quality (AHRQ) via grant R01HS023317, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Office of Behavioral and Social Sciences Research (OBSSR) and the Global Obesity Prevention Center (GOPC) via grant U54HD070725, NICHD via grant U01HD086861, and the National Institute for General Medical Science (NIGMS) via the MIDAS 5U24GM110707 grant. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Appendix
Measles transmission model
The SEIR model divided the country into 1 square kilometer (km) cells with homogeneous mixing occurring among the population of each cell. The following set of equations governed the change in the number of people in each compartment (Susceptible (S), Exposed (E), Infectious (I), and Recovered (R)) in each cell over time:
µ is the birth rate for the population and ε the mortality rate, which were assumed to be equal to maintain a constant population. γ and σ are the reciprocal of the mean latent (8 days) and infectious periods (7 days), respectively. β is the transmission coefficient and relates to the basic reproduction number (Ro) through the formula β = Ro (γ + ε) and varied seasonally according to kernel similar to the one proposed by Ferrari et al. in their metapopulation model of Niger [30] with the form (t) = (1 + 0.6cos(2πt)). We assumed an Ro of 15 for measles. The following formula determined, ν, the immunization rate:
We assumed an initial population immunity to measles of 95%, due to either prior illness or vaccination, among people outside of the MCV target populations of children <24 months. Immunity within the target populations was based on vaccination coverage rates as estimated by SIGMA: 100% of eligible children <12 months located within fixed or outreach location catchment areas were assumed to be vaccinated with MCV1 in every scenario with sensitivity analyses varying the proportion of children 12–23 months who had already received MCV1 who were vaccinated with MCV2 from 25 to 100%), while no individuals in the target populations located outside of fixed or outreach catchment areas received either dose of MCV. Our model assumed no cross-border disease transmission between Kenya and neighboring countries. P is the probability of ξ infectious people migrating from one cell to an adjacent cell, which would allow measles to spread between cells. Transmission between cells in the model was accomplished by a coupling term that allowed infectious people and susceptible people to migrate between cells. To keep the population constant per cell, an equal number of infectious and susceptible people were transferred between each cell. Each simulation run entailed annual seeds of 10 infectious persons per year in random grid cells with a preference towards grid cells with the largest populations. We determined the number of seeds to use through calibration, which entailed running the model to match the number ofcases to historical surveillance data.
Table 1.
Model inputs, ranges, and sources.
| Parameter | Value (Range) | Point estimate Source |
|---|---|---|
| Acute Cost of Illness* | ||
| Bed days per hospitalization | 1.33 (0.67–2.00) | Thompson et al. [31] (Global data) |
| Outpatient visits per non-hospitalized case | 0.50 (0.25–0.75) | Thompson et al. [31] (Global data) |
| Outpatient home care days per non-hospitalized case | 3.50 (1.75–5.25) | Thompson et al. [31] (Global data) |
| Post-hospital home care days per hospitalized case | 1.00 (0.50–1.50) | Thompson et al. [31] (Global data) |
| Inpatient secondary hospital cost per bed day | $7.11 (6.75–7.47) | WHO-CHOICE [32] (Country specific data) |
| Outpatient health center without any inpatient beds cost per visit, rural | $1.77 (1.66–1.87) | WHO-CHOICE [32] (Country specific data) |
| Outpatient primary hospital cost per visit, urban | $2.48 (2.33–2.64) | WHO-CHOICE [32] (Country specific data) |
| Percentage living in urban areas | 0.25% | World Bank [11] (Country specific data) |
| Daily minimum wage | $1.99 (1.81–3.65) | US State Department [33] (Country specific data) |
| Medication costs (acute measles) | $3.32 (2.09–5.46) | Thompson et al. [31] (Low and middle-income country data) |
| Transportation costs per patient | $3.54 (1.56–6.24) | Kim et al. [34] (Low and middle income country data) |
| Probability of survival from age 1–15 | 0.95 (0.93–1.00) | WHO Global Health Observatory [35] (Country specific data) |
| GDP per capita in 2018 (USD) | $1865 (12882378) | World Bank [36] (Country specific data) |
| Life expectancy at age 15 (productive age) | 54.19 (52.5755.82) | UN Population Division [37] (Country specific data) |
| Duration acute infection (days) | 14 (7–21) | WHO Measles Model [38] CDC (Global data) |
| Disability weight, acute infection (untreated) | 0.13 (0.09–0.19) | Salomon et al. [39] (Global data) |
| Disability weight, acute infection (treated) | 0.05 (0.03–0.08) | Salomon et al. [39] (Global data) |
| Discount rate for costs and DALYs◊ | 3% | Gold et al. [40] (Global data) |
| Age at vaccination (years) | 2.88 (0.75–5.00) | WHO Measles Model [38] (Global data) |
| Percentage of care-seeking patients hospitalized | 0.25% (0.13–0.38) | Thompson et al. [31] (Low and middle income country data) |
| Percentage of patients with fever seeking care | 0.63% (0.14–0.72) | World Bank [11] (Country specific data) |
| Age of infection (years) | 2.14 (2.00–2.31) | WHO Measles Model [38] (Jordan data) |
| Case fatality rate (CFR) | 0.18 (0.08–0.41) | Wolfson et al. [41] (Country specific data) |
| Country Parameters | ||
| Total population in 2015 | 46,100,000 | World Bank [36] (Country specific data) |
| Birth rates (per 1000 persons) from 2000 to 2015 | 34.1–38.4 | World Bank [36] (Country specific data) |
| Birth rates (per 1000 persons) from 2016 to 2020 | 30.7–32.9 | Projected from World Bank data [36] (Country specific data) |
| Neonatal mortality rate (per 1000 live births) from 2000 to 2015 | 31.7–53.1 | World Bank [36] (Country specific data) |
| Infant mortality rate (per 1000 live births) from 2000 to 2015 | 35.5–66.5 | World Bank [36] (Country specific data) |
| Logistics | ||
| Cost of vial of 10 dose measles vaccine | $1.28 | UNICEF [42] (Low and middle income country data) |
| Cost of health care worker per day of outreach | $7.0 | cMYP§ [43] (Country specific data) |
| Amortization of motorbike per km | $0.13 | cMYP§ [43] (Country specific data) |
| Amortization of vaccine carrier per year | $2.6 | WHO PQS [44]^ (Global data) |
| Time (minutes) required per vaccine dose administered in outreach | 15 (2–30) | WHO [19] (Global data) |
| Catchment area radius of each fixed vaccination post (km) | 5 (2.5–10) | WHO [45] (Global data) |
| Catchment area radius of each outreach vaccination site (km) | 10 (7.5–20) | Webber [46] (Global data) |
| Transmission Model Parameters | ||
| Mean latent period of measles (days) | 8.0 | Anderson and May [47] (Global data) |
| Mean infectious period of measles (days) | 7.0 | Anderson and May [47] (Global data) |
| Basic reproduction number (RO) of measles | 15 | Anderson and May [47] (Global data) |
| Efficacy of first dose of measles vaccine | 77% | Uzicanin and Zimmerman [48] (Global data) |
| Efficacy of second dose of measles vaccine | 94% | Uzicanin and Zimmerman [48] (Global data) |
| Initial population immunity to measles | 95% | Ferrari et al. [30] (Niger data) |
| P, probability of infectious measles cases being migrated | 0.07 | Model calibration |
| ξ, magnitude of infectious measles case migration | 0.012 | Model calibration |
| MCV1 coverage across Kenya population from 2011 to 2015 | 75–87% | WHO and UNICEF [13] (coverage for target population assumed for as a whole) population |
| MCV2 coverage across Kenya population in 2015 | 28% | WHO and UNICEF [13] (coverage for target population assumed for as a whole) population |
| Percentage of vaccinated population that receives second dose of MCV | 50% (25–100%) | Sensitivity analyses |
Parameters shown are for acute infection. Sequelae (blindness, diarrhea, acute encephalitis, pneumonia, and subacute sclerosing panencephalitis) are omitted from the table as they contributed a relatively small proportion of disease burden and costs but were included in the model. Acute infection was used as a proxy for measles infection, as the Global Burden of Disease Study 2013 does not include a specific measles weight.
DALY = Disability-adjusted life-year, defined as the number of years lost due to ill-health, disability or early death.
cMYP = Comprehensive multi-year plan.
PQS = World Health Organization Performance, Quality and Safety process.
As validation, the model and its parameterization was able to reproduce reported measles surveillance data from 2011 to 2015 for Kenya using World Health Organization (WHO) and United Nations Children’s Fund (UNICEF) vaccine coverage estimates.
Costs and health effects
Appendix Table 1 shows input parameters related to cost and health effects using Kenya-specific values when available. Costs of vaccination included the costs of vaccines and transport (based on the roundtrip distance between the closest fixed vaccination post and the outreach location; included driver per diems and vehicle maintenance, amortization, and fuel), portable vaccine storage (equipment maintenance and amortization), and personnel time.
To estimate the costs per measles case, we summed costs of care, transportation, and caretakers’ lost productivity. We applied the hospital admittance rate based on disease severity to outpatients seeking care from hospitals to determine the facility level case receiving care. We estimated treatment costs for these cases by location and facility level, based on facility level estimated costs of care. The cost per trip to a healthcare facility for each outpatient visit and hospital stay was used to estimate transportation costs. To calculate caretaker productivity losses, we multiplied a caretaker’s estimated daily productivity by the duration of hospitalization.
We used the human capital approach to quantify productivity losses due to disability and death. Measles cost and treatment parameters came from the published literature and other public data sources (Appendix). Estimating the number of productive life years lost due to disability entailed multiplying total cases of disability by life expectancy from age 15 (i.e., age at labor force entry), discounted to the vaccination year. We multiplied this discounted life expectancy by the Gross Domestic Product (GDP) per capita to quantify productivity losses due to disability. The same approach estimated productivity losses due to death, with total deaths multiplied by the probability of survival to age 15 and by the measles-specific life expectancy at death (discounted to year of vaccination) and GDP per capita. Sensitivity analyses varied disability-adjusted life years (DALYs) incurred per measles case, medical costs per measles case, time taken per dose administered in outreach, and proportion of geographically hard-to-reach target populations included.
For each scenario, we calculated the incremental cost-effectiveness ratio (ICER) of vaccinating geographically hard-to-reach target populations through the outreach strategy compared to not vaccinating, by taking the ratio between additional costs and DALYs averted due to outreach vaccination. We assumed vaccination costs at fixed vaccination posts were the same for each strategy and were not included. Outreach vaccination was considered cost-effective if the ICER was < 3*GDP per capita for Kenya ($5595) and highly cost-effective if < GDP per capita ($1865) [36]. Outreach vaccination was economically dominant when it saved both costs and health effects.
Footnotes
Declaration of interests
We have nothing to disclose.
References
- [1].World Health Organization. Global measles and rubella strategic plan: 2012; 2012.
- [2].Organization, W.H. and UNICEF. Increasing immunization coverage at the health facility level Geneva: World Health Organization; 2002. [Google Scholar]
- [3].Feldstein LR et al. Global routine vaccination coverage, 2016, in Morbidity and Mortality Weekly Report (MMWR) Atlanta: Centers for Disease Control and Prevention; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].VanderEnde K et al. Global routine vaccination coverage — 2017, in Morbidity and Mortality Weekly Report (MMWR) Atlanta: Centers for Disease Control and Prevention; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kisangau N et al. Progress towards elimination of measles in Kenya, 2003—2016. Pan Afr Med J 2018;31(65). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vijayaraghavan M et al. Measles supplemental immunization activities improve measles vaccine coverage and equity: Evidence from Kenya, 2002. Health Policy 2007;83(1):27–36. [DOI] [PubMed] [Google Scholar]
- [7].World Health Organization. The RED Strategy Available from: http://www.who.int/immunization/programmes_systems/service_delivery/red/en/; 2016.
- [8].Vandelaer J, Bilous J, Nshimirimana D. Reaching Every District (RED) approach: a way to improve immunization performance. Bull World Health Organ 2008:86(3). p. A–b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ryman T et al. Reaching every district (RED) approach to strengthen routine immunization services: evaluation in the African region, 2005. J Public Health (Oxf) 2010;32(1):18–25. [DOI] [PubMed] [Google Scholar]
- [10].Instituto Nacional de Estatística Moҫambique. 2015 [cited 2015 April]; Available from: http://www.ine.gov.mz/.
- [11].World Bank. The 2013 World Bank Development Indicators Washington, DC: World Bank; 2014. [Google Scholar]
- [12].WHO. Immunization schedules by antigens Available from: http://apps.who.int/immunization_monitoring/globalsummary/schedules; 2016.
- [13].WHO and UNICEF. WHO/UNICEF estimates of national immunization coverage (WUENIC) Available from: ; 2015.
- [14].Haidari LA et al. The economic value of increasing geospatial access to tetanus toxoid immunization in Mozambique. Vaccine 2016;34(35):4161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].CIESIN, FAO, and CIAT. Global Rural-Urban Mapping Project, Version 1 (GRUMPv1): Population count grid Palisades, NY: NASA Socioeconomic Data and Applications Center (SEDAC); 2000. [Google Scholar]
- [16].World Bank. GNI per capita, Atlas method; 2015.
- [17].Toikilik S et al. Are hard-to-reach populations being reached with immunization services? Findings from the 2005 Papua New Guinea national immunization coverage survey. Vaccine 2010;28(29):4673–9. [DOI] [PubMed] [Google Scholar]
- [18].Rainey JJ et al. Reasons related to non-vaccination and under-vaccination of children in low and middle income countries: findings from a systematic review of the published literature, 1999–2009. Vaccine 2011;29(46):8215–21. [DOI] [PubMed] [Google Scholar]
- [19].WHO. Immunization in practice: a practical guide for health staff, 2015 update Available from: http://apps.who.int/iris/bitstream/10665/193412/1/9789241549097_eng.pdf.
- [20].van Hoek AJ et al. A cost effectiveness and capacity analysis for the introduction of universal rotavirus vaccination in Kenya: comparison between Rotarix and RotaTeq vaccines. PLoS ONE 2012;7(10):e47511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].van Hoek AJ et al. A cost effectiveness and capacity analysis for the introduction of universal rotavirus vaccination in Kenya: comparison between Rotarix and RotaTeq vaccines. PLoS ONE 2012;7(10):e47511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Antillón M et al. Cost-effectiveness analysis of typhoid conjugate vaccines in five endemic low-and middle-income settings. Vaccine 2017;35(27):3506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Woods B et al. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value in Health 2016;19(8):929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bertram MY et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ 2016;94(12):925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Global Vaccine Action Plan 2011–2020 Available from: http://who.int/iris/bitstream/10665/78141/1/9789241504980_eng.pdf; 2013.
- [26].Gavi, the Vaccine Alliance, 2016–2020 Strategy Geneva: Gavi, the Vaccine Alliance; 2014. [Google Scholar]
- [27].Ozawa S, Yemeke TT, Thompson KM. Systematic review of the incremental costs of interventions that increase immunization coverage. Vaccine 2018;36 (25):3641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].National and County Health Budget Analysis FY 2016/17 Nairobi: Kenya Ministry of Health; 2017. [Google Scholar]
- [29].Van Malderen C et al. Decomposing Kenyan socio-economic inequalities in skilled birth attendance and measles immunization. Int J Equity Health 2013;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ferrari MJ et al. The dynamics of measles in sub-Saharan Africa. Nature 2008;451(7179):679–84. [DOI] [PubMed] [Google Scholar]
- [31].Thompson KM, Odahowski CL. The costs and valuation of health impacts of measles and rubella risk management policies. Risk Anal 2016;36(7):1357–82. [DOI] [PubMed] [Google Scholar]
- [32].WHO-CHOICE Country specific unit costs [cited 2016 25 Feb]; Available from: http://www.who.int/choice/costs/en/; 2008.
- [33].US State Department. Country Reports on Human Rights Practices for 2012 Available from: http://www.state.gov/j/drl/rls/hrrpt/humanrightsreport/index.htm; 2014.
- [34].Kim SY et al. Health and economic impact of rotavirus vaccination in GAVI-eligible countries. BMC Public Health 2010;10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].WHO. Global Health Observatory Life tables Available from: http://www.who.int/gho/mortality_burden_disease/life_tables/life_tables/en/; 2012.
- [36].World Bank. World Development Indicators, Kenya Available from: http://data.worldbank.org/country/kenya.
- [37].United Nations. Department of Economic and Social Affairs, Population Division, in World Population Prospects: The 2015 Revision, DVD Edition; 2015. [Google Scholar]
- [38].Centers for Disease Control and Prevention. Manual for the surveillance of vaccine-preventable diseases Centers for Disease Control and Prevention, Atlanta, GA, 2008. [Google Scholar]
- [39].Salomon JA et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health 2015;3(11):e712–23. [DOI] [PubMed] [Google Scholar]
- [40].Cost-effectiveness in health and medicine In: Gold M, et al. , editor. New York, NY: Oxford University Press; 1996. [Google Scholar]
- [41].Wolfson LJ et al. Estimates of measles case fatality ratios: a comprehensive review of community-based studies. Int J Epidemiol 2009;38(1):192–205. [DOI] [PubMed] [Google Scholar]
- [42].UNICEF. Vaccine price data Available from: http://www.unicef.org/supply/index_57476.html; 2016.
- [43].Republic of Kenya Ministry of Health Division of Vaccines and Immunization. Kenya comprehensive multi-year plan 2011–2015; 2011.
- [44].World Health Organization. WHO prequalified vaccines; April 2016.
- [45].WHO. Global reference list of 100 core health indicators Available from: http://www.who.int/healthinfo/indicators/2015/en/; 2015. [DOI] [PMC free article] [PubMed]
- [46].Webber R Communicable diseases: a global perspective 4th ed. Cambridge MA: CABI; 2012. [Google Scholar]
- [47].Anderson R, May R. Infectious disease of humans Oxford: Oxford University Press; 1991. [Google Scholar]
- [48].Uzicanin A, Zimmerman L. Field effectiveness of live attenuated measles-containing vaccines: a review of published literature. J Infect Dis 2011;204 (Suppl 1):S133–48. [DOI] [PubMed] [Google Scholar]


