Abstract
Purpose
Current approaches to prostate cancer screening and diagnosis are plagued with limitations in diagnostic accuracy. There is a compelling need for biomolecular imaging that will not only detect prostate cancer early but also distinguish prostate cancer from benign lesions accurately. In this topic paper, we review evidence that supports further investigation of VPAC1-targeted PET/CT imaging in the primary diagnosis of prostate cancer.
Methods
A non-systematic review of Medline/PubMed was performed. English language guidelines on prostate cancer diagnosis and management, original articles, and review articles were selected based on their clinical relevance.
Results
VPAC1 receptors were overexpressed 1000 times more in prostate cancer than benign prostatic stromal tissue. In vitro and in vivo studies showed that Copper-64 labeled analogs of VPAC1 ligands can be synthesized with high radiochemical efficiency and purity. The radioactive probes had excellent VPAC1 receptor binding specificity and affinity. They had good biochemical stability in vitro and in mouse and human serum. They had minimal urinary excretion, which made them favorable for prostate cancer imaging. Initial feasibility study in men with prostate cancer showed that the probes were safe with no reported adverse reaction. 64Cu-TP3805 PET/CT detected 98% of prostate cancer lesions and nodal metastasis as confirmed with whole mount histopathological evaluation.
Conclusions
VPAC1 receptors are promising targets for biomolecular imaging of primary prostate cancer that can distinguish malignant from benign lesions non-invasively. Further investigations are warranted to validate initial findings and define the clinical utilities of VPAC1-targeted PET imaging for prostate cancer diagnosis and management.
Keywords: Prostate cancer, Positron-emission tomography, VPAC1 receptors, Copper-64, Biomolecular imaging
Introduction
Prostate cancer is a heterogeneous spectrum of disease ranging from indolent to lethal malignancy. Early diagnosis of prostate cancer or early detection of prostate cancer recurrence remains challenging. Current screening strategies with serum prostate specific antigen (PSA) and digital rectal exam (DRE) are plagued with limitations. The specificity of PSA is limited with false positives from benign conditions such as prostatic enlargement and prostatic inflammation. The reported positive predictive value (PPV) of PSA in asymptomatic men is low, ranging from 28 to 35% [1, 2]. In the European Randomized Study of Screening for Prostate Cancer (ERSPC) involving 162,243 men from 1991 to 2003, the authors found that to prevent one prostate cancer death at a median follow up of 9 years, 1410 men would need to be screened and 48 additional cases of prostate cancer would need to be treated [3]. Due to its lack of specificity, a PSA-based approach to prostate cancer screening prompts many men to undergo unnecessary prostate biopsies. Transrectal ultrasound (TRUS) guided biopsy remains the gold standard for histologic diagnosis of prostate cancer. TRUS-guided biopsy is an invasive and expensive procedure with inherent morbidities of urinary tract infection, sepsis, and psychologic stress for patients. Over two-thirds of the 1.5 million prostate biopsies performed annually in the United States may not identify any malignant lesions [4, 5]. Overall, there is considerable controversy surrounding serum PSA for early detection of prostate cancer, with no consistent recommendations from major medical organizations for best approach to PSA screening.
Molecular profiling of prostate cancer has emerged as a novel multidisciplinary approach to characterize prostate cancer, elucidate mechanisms of disease at the molecular level, and personalized selection of therapy. The prostate cancer antigen 3 (PCA3) multiplex gene test is based on voided urine sample after prostate massage on DRE. The test targets prostate cancer molecular signature. The predictive accuracy of PCA3 test have been questioned with sensitivity ranging from 62 to 94% and specificity ranging from 37 to 99% [6]. Another screening test is 4Kscore test, which measures serum total PSA, free PSA, intact PSA, and human kallikrein levels. Early results of the 4Kscore test in detecting high-grade prostate cancer appear promising [7, 8]. Despite great strides have been made formulating molecular assays for the detection of prostate cancer, these tests are either cost prohibitive, controversial or have not yet become widely accepted for routine clinical practice.
While numerous imaging modalities have been evaluated for staging prostate cancer, the role of current imaging in primary prostate cancer diagnosis has been limited. Traditional TRUS has major limitations in evaluating prostate cancer outside its of role of providing guidance for prostate biopsy and prostate volume measurement. Color Doppler ultrasonography and elastography have been used to enhance the diagnostic performance of TRUS biopsy but not as stand-alone method in diagnosing prostate cancer. Likewise, cross-sectional imaging with computed tomography (CT) scan has no role in detecting prostate cancer, its primary role is in diagnosis of regional and distant metastasis in patient with unfavorable intermediate or high risk disease [9–11]. Multiparametric magnetic resonance imaging (MRI) has garnered significant interest in its ability to provide high-resolution images to delineates prostate anatomy as well as Prostate Imaging Reporting and Data System (PIRADS) correlation with histopathologic grading. However, the diagnostic performance of multiparametric MRI is highly variable and subject to significant interobserver variation, even among specialists with high level of expertise in reading MRI [12, 13]. The reported accuracy of MRI ranges from 44 to 87% with sensitivity of 58–97% and specificity of 23–87% [14].
Over the past decade, positron-emission tomography (PET) has emerged as a powerful modality for imaging oncologic lesions. PET scan can non-invasively visualize cancer and measure selective metabolism and gene product overexpressed on malignant cells. Current metabolic and biochemical agents such as 18F-FDG, 11C-acetate, 18F-acetate, 11C-choline, 18F-choline, and PSMA have serious limitations. 18F-FDG has limited role in prostate cancer imaging because prostate cancer has few Glut-1 binding sites and slow metabolic rate, which cause notoriously poor and unreliable uptake of 18F-FDG. 11C-choline and 18F-choline have well known high uptake in benign prostatic hyperplasia and poor ability to discriminate between clinically significant and insignificant prostate cancer [15]. Prostate specific membrane antigen (PSMA) targeted PET has yielded promising results in imaging patients with biochemical recurrence post-radical prostatectomy. PSMA is a transmembrane protein with more abundant expression in malignant prostatic cells than in normal prostatic tissue. Hybrid 68Ga-PSMA ligand PET/CT has been shown to detect PCa recurrence and metastasis at low PSA values in comparison with conventional imaging or PET imaging with other tracers [16, 17]. However, the role of PSMA ligand PET/CT in primary prostate cancer diagnosis is yet to be determined.
There exists a dire need for a biomolecule that will not only detect prostate cancer early but also distinguish prostate cancer from benign lesions accurately. Such biomolecular imaging approach to prostate cancer diagnosis could minimize the need for invasive biopsies and significantly reduce the health care cost and patient’s stress and anxiety induced by prostate cancer biopsy. Based on a decade of studies on VPAC1 receptors, a G protein that intimately involved in cell proliferation, differentiation, and survival, we hypothesized that VPAC1 oncogene product, which is overexpressed on all prostate cancer cells but exists in negligible amount in benign stromal cells, will serve as an excellent biomarker for early and accurate detection of prostate cancer. In this topic paper, we will review the pre-clinical and clinical data that support the utility of VPAC1 targeted PET/CT imaging in the primary diagnosis of prostate cancer.
Cancer diagnosis via endogenous VPAC gene expression
The human VPAC receptors are regulatory G protein coupled receptors that bind both vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating peptide (PACAP) related peptides with high affinity. VPAC receptors form a regulatory network in cell proliferation and transformation. Reubi and colleagues studied VPAC’s roles in oncogenesis in more than 600 tumors and their metastases. They found that VPAC1 and VPAC2 receptors are overexpressed in common human malignancies including prostate, breast, lung, and colon cancer [18–20]. Importantly, Reubi et al. found VPAC1 receptors were predominantly overexpressed on 100% of prostate cancer tissues whereas VPAC2 receptors were expressed in prostatic stoma [20]. VPAC receptors are expressed in high density on the order of 104–105/cell at the onset of oncogenesis and alterations in cell morphology. On stroma, normal, benign, and inflammatory cells, VPAC receptors are minimally expressed at a density of 10/cell [21, 22]. In other words, VPAC1 receptors are overexpressed at least 1000 times more on prostate cancer than on benign prostatic cells.
Our initial experience with VPAC1 receptor imaging was from studies involving breast cancer. Early in vitro studies of four VPAC1 ligand analogs TP3982, TP3939, TP 3805, and TP4200, named according to their molecular weights, revealed that these analogs can be made with high radiolabeling efficiency at greater than 92% and radiochemical purity of at least 94.6% that do not require further purification prior to injection for PET imaging [23]. Using muscle relaxant assay on the resting tension of internal anal sphincter smooth muscle in rats, functional studies of the VIP/PACAP analogs showed these radiolabeled probes are functionally stable compared to unaltered VIP28. In vitro receptor autoradiography studies showed 64Cu-labeled peptides preferentially bound to breast cancer tissues compared to normal breast tissue at a ratio of 2.17–10.93 [23]. VPAC1 targeted probes were first investigated in breast cancer in women in 2013. In 19 female patients with breast cancer, VPAC1 targeted probes were found to have excellent safety profile; no toxicity or reaction was observed in any of the patients [24]. 64Cu-TP3805 PET/CT detected 100% of all primary lesions and involved sentinel lymph nodes [24]. Interestingly, 64Cu-TP3805 was able to delineate primary breast cancer lesions irrespective of their hormonal status [24].
Pre-clinical studies of 64Cu-TP3939 PET imaging of prostate cancer
We first investigated the biomolecular activity of radioactive probes for VPAC1 receptors in prostate cancer xenografts in athymic nude mice and spontaneous prostate cancer in transgenic adenocarcinoma of the mouse prostate (TRAMP) mice [25]. We radiolabeled TP3939 probe, an analog of the VIP peptide with molecular weight of 3939.4 Da, with Copper-64, which is commercially available and a long half-life of 12.8 h.
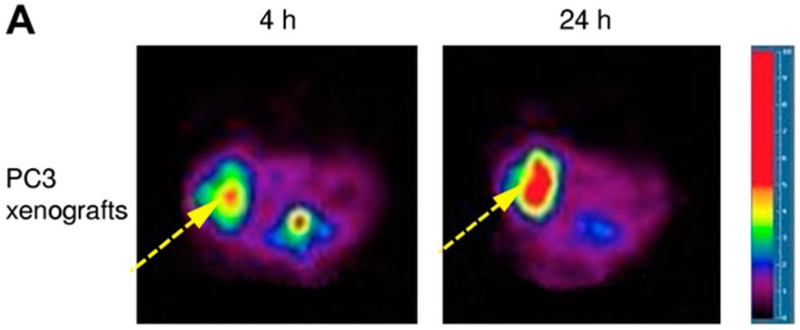
In the prostate cancer xenograft model, we implanted approximately 4 × 106 viable androgen receptor-positive PC3 prostate cancer cells in 200 μl subcutaneously in the right thigh of male athymic nude mice. PET images revealed high uptake of PC3 tumors. The ratios of tumor to contralateral muscle as determined by region of interest analysis was 3.357 and 4.205 at 4 and 24 h of implantation, respectively (Fig. 1) [25].
Fig. 1.
Tranaxial PET images demonstrate high uptake of 64Cu-TP3939 in xenografted PC3 tumor in athymic nude mice. Images were taken at 4 and 24 h after injection of 64Cu-TP3939. The ratios of tumor to background ratio as determined by region of interest analysis was 3.357 and 4.205 at 4 and 24 h, respectively (This research was originally published in Zhang et al. [25]. Copyrighted by the Society of Nuclear Medicine and Molecular Imaging, Inc.)
To study tumor to normal prostate distribution level, we used male TRAMP mice, which uniformly and spontaneously develop orthotopic prostate tumors following the onset of puberty. We obtained the TRAMP mice at the age of 9 weeks. We serially imaged them once a month from the age of 11 weeks-5 months. We used 18F-FDG as control. Prior to 64Cu-TP3939 imagin, TRAMP mice received 18F-FDG intravenously and were imaged 1 h later. We waited 24 h before imaging TRAMP mice with 64Cu-TP3939 to allow for complete decay of Fluoride-18.
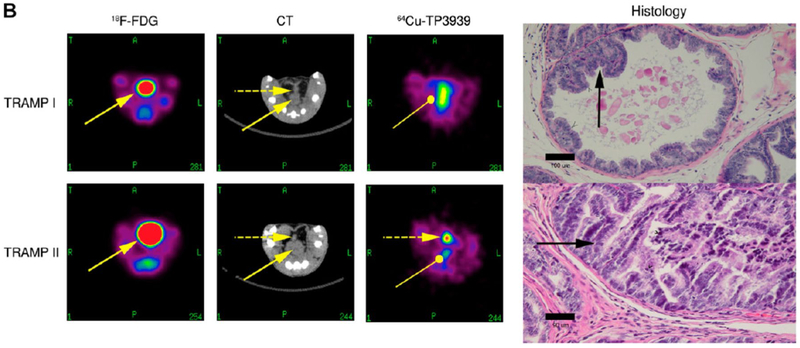
We found that in TRAMP mice, 64Cu-TP3939 uptake correlates with histopathologic grading of TRAMP tumors [25]. In mouse with grade IV well-differentiated prostate adenocarcinoma, the image was clearly imaged with 64Cu-TP3939 PET but not CT or 18F-FDG (Fig. 2, TRAMP II). In mouse with grade II early intraepithelial neoplasia, the lesion was not imaged on 64Cu-TP3939 PET, 18F-FDG, or CT scan (Fig. 2, TRAM I).
Fig. 2.
18F-FDG, CT, and 64Cu-TP3939 PET of TRAMP mouse. TRAMP I mouse harbored grade II early intraepithelial neoplasia. The benign lesion was not visible on 18F-FDG, CT, and 64Cu-TP3939 PET. TRAMP II mouse harbored grade IV well-differentiated prostate adenocarcinoma. The malignant prostate lesion was detected on 64Cu-TP3939 but missed on 18F-FDG and CT. The solid arrow indicates high bladder uptake of 18F-FDG. In contrast, bladder uptake is absent with 64Cu-TP3939. As the primary route of excretion is fecal, there are some background activity of 64Cu-TP3939 in the colon (oval-head arrow) (This research was originally published in Zhang et al. [25]. Copyrighted by the Society of Nuclear Medicine and Molecular Imaging, Inc.)
Our pre-clinical study demonstrated 64Cu-TP3939 probe has a high target to background ratio at 24 h after injection and is promising agent for imaging prostate cancer not only for primary diagnosis but also for detecting tumor recurrence and metastases.
VPAC1-targeted PET/CT imaging for primary prostate cancer diagnosis in men
The promising data from our pre-clinical investigation of 64Cu-VIP analogs formed the basis of an exploratory Investigational New Drug (eIND 101550), Institutional Review Board, Clinical Cancer Research Committee, and Radioactive Drug Research Committee approvals to initiate a feasibility study of VPAC1 targeted PET/CT imaging for prostate cancer in men.
We used 64Cu-TP3805, a 28-amino acid PACAP analog that was labeled with positron emitting Copper-64 (t½ = 12.8 h). Our pre-clinical evaluation of 64Cu-TP3805 showed that this agent has a strong binding affinity to VPAC1, Kd = 3.3 × 10−9 M. It also has good receptor specificity and functional response, IC50 = 5.3 × 10−8 M versus 9.0 × 10−8 M for 64Cu-TP3805 and unaltered VIP28, respectively [23]. 64Cu-TP3805 has excellent (97%) stability in mouse and human serum [23]. Like 64Cu-TP3939, 64Cu-TP3805 is excreted in feces and has less than 2% urinary excretion, making it a favorable probe for prostate cancer imaging [25].
In this first exploratory study of VPAC1 targeted PET/CT imaging for prostate cancer in men, we recruited 25 patients (6 African Americans, 19 Caucasians, mean age 63.4 ± 7.6 years) with localized prostate cancer who elected to undergo radical prostatectomy for definitive treatment. All patients had standard sextant TRUS-guided prostate biopsy for cancer diagnosis. They underwent 64Cu-TP3805 PET/CT 1–3 weeks prior to surgery. They did not have to fast for the study. 64Cu-TP3805 was given intravenously at a dose of 148 ± 10% MBq, 4 ± 10% mCi, which was previously determined based on dose escalation study and approved by the Food and Drug Administration [24]. Patients underwent whole body scans at 30-min and 2-h post-injection. To further investigate the histologic status of 64Cu-TP3805 PET positive lesions, we performed digital autoradiography (DAR) on whole mount pathology slides incubated in 64Cu-TP3805 solution of 6 patients with prostate cancer, 3 with benign prostate hyperplasia, 1 malignant and 1 benign lymph node. We compared the DAR analysis with histologic hematoxylin and eosin (H&E) stained slides read by experienced urologic pathologists.
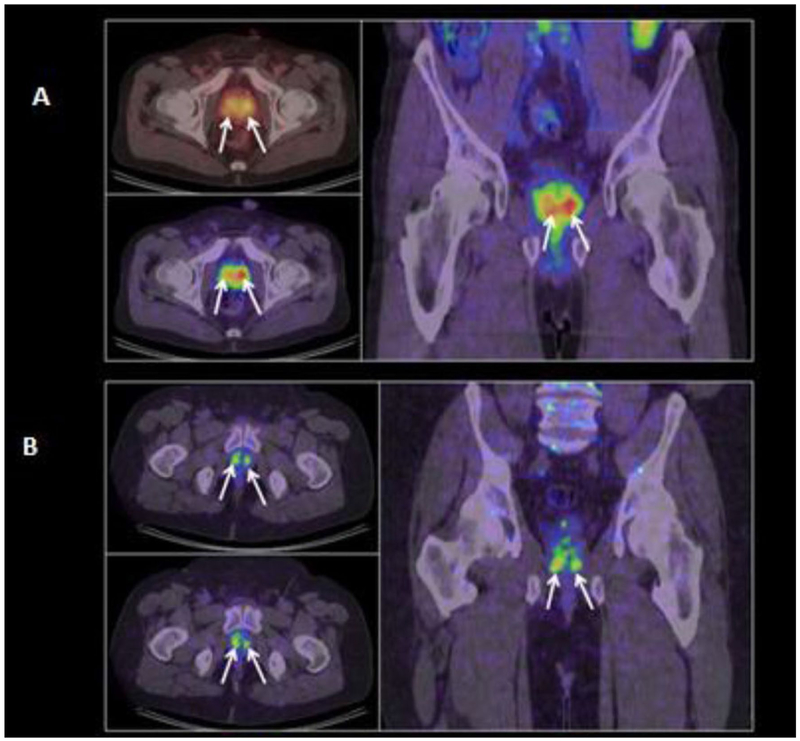
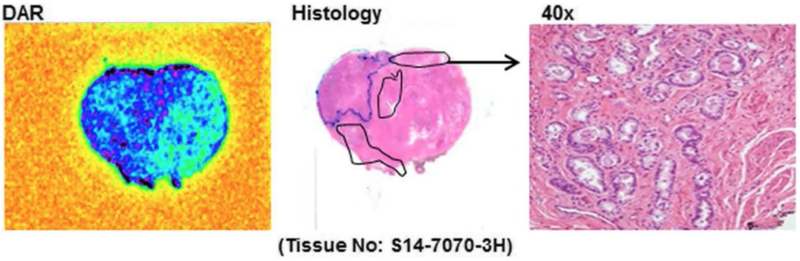
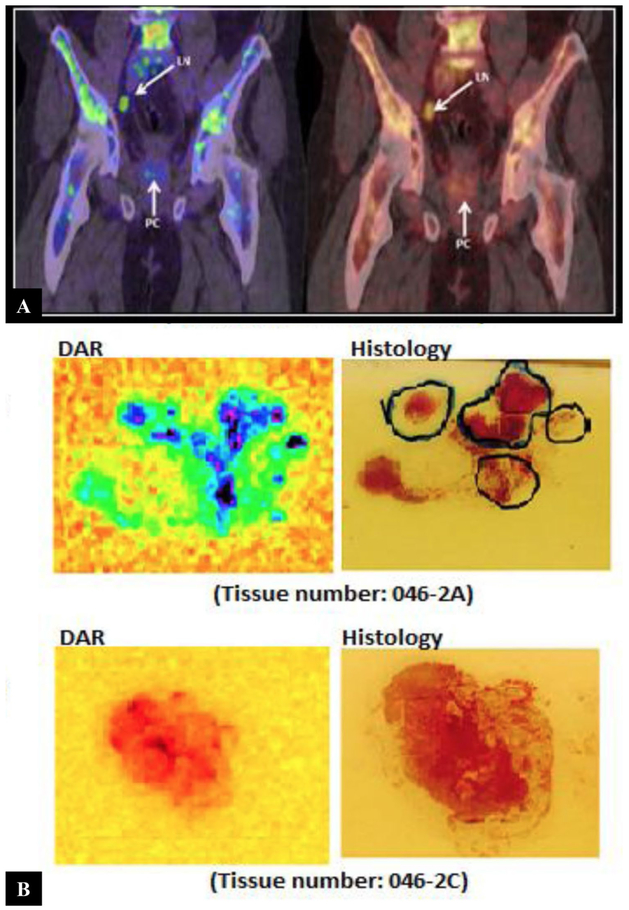
In 25 patients, 64Cu-TP3805 identified more (n = 212) lesions with SUVmax > 1.0 than histologically confirmed malignant lesions (n = 127) [26]. The status of the additional 85 64Cu-TP3805 PET identified prostate lesions has not been fully determined. There was no significant difference in SUV in prostate cancer lesions between PET images obtained at 30 min compared to 2 h after 64Cu-TP3805 injection. Two representative cross-sectional images of men with prostate cancer on 64Cu-TP3805 PET/CT were shown in Fig. 3. Of note, since less than 2% of 64Cu-TP3805 was renally excreted, no bladder uptake of 64Cu-TP3805 was noted in any image in any of the patients. In a sub-analysis with DAR, 105 (98%) out of 107 prostate cancer accurately showed 64Cu-TP3805 uptake (Fig. 4). DAR also showed uptakes in prostatic carcinoma involving the ejaculatory ducts and verumontanum and appropriately no signal in benign cystic lesions [26]. DAR missed two prostate lesions due to technical artifacts. Traditional histopathologic evaluation initially missed nine small prostate cancer foci that were identified positively on DAR; these foci were confirmed malignant on subsequent close histologic evaluation. DAR correctly identified malignant from benign lymph node (Fig. 5). For 3 patients with BPH and no evidence of prostate cancer, DAR analysis of their whole mounts was negative.
Fig. 3.
Coronal PET/CT images of two patients a 68 years old man with Gleason 3 + 4 = 7 prostate cancer and b 51 years old man with Gleason 3 + 3 = 6 prostate cancer, obtained at 30-min post-injection of 64Cu-TP3805 Multiple prostate cancer lesions are indicated with arrows. Unlike 18F-FDG, there was negligible radioactivity in the bladder (This research was originally published in Tripathi et al. [28])
Fig. 4.
Whole mount digital audioradiography (DAR) and hematoxylin and eosin (H&E) stained slides of a patient with prostate cancer who underwent radical prostatectomy. Malignant foci with 64Cu-TP3805 updates on DAR were confirmed on histology (This research was originally published in Tripathi et al. [28])
Fig. 5.
a Coronal images of positive lymph node on 64Cu-TP3805 which was proven malignant by histology. b Comparison of 64Cu-TP3805 DAR analysis of malignant and benign lymph nodes versus histologic H&E analysis. Top—uptake of 64Cu-TP3805 in prostate cancer foci in a malignant lymph node. Bottom—no uptake of 64Cu-TP3805 in benign node (This research was originally published in Tripathi et al. [28])
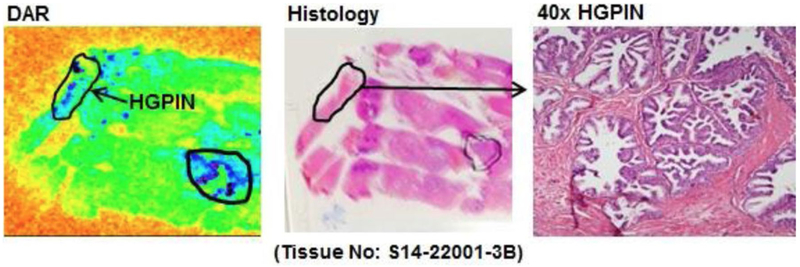
One potential significant limitation of 64Cu-TP3805 PET/CT in management of prostate cancer patient was its false positive uptakes in high-grade prostatic intraepithelial neoplasia (HGPIN) lesions [26]. DAR identified 19 lesions corresponding to HGPIN on histology (Fig. 6). At the biomolecular level, VPAC1 receptors were expressed early in HGPIN prior to the modulation of cell morphology. While HGPIN may be a precursor to invasive prostate carcinoma, HGPIN lesions themselves are not malignant and do not warrant treatment.
Fig. 6.
Digital audioradiography (DAR) of whole mount tissues from a patient demonstrates 64Cu-TP3805 update on HGPIN lesions (This research was originally published in Tripathi et al. [28])
In our first feasibility, clinical study of VPAC1 receptor targeted PET/CT of men with prostate cancer, 64Cu-TP3805 PET/CT accurately diagnosed 98% of prostate cancer lesions when confirmed with whole mount histopathology [26]. There was no uptake of 64Cu-TP3805 in patient with benign prostatic hypertrophy. 64Cu-TP3805 PET/CT was also able to detect nodal metastasis accurately.
Conclusion
Given the high prevalence of prostate cancer and benign prostatic hyperplasia in aging men, there is a critical need for a biomolecule that accurately differentiates prostate cancer lesions from benign nodules. The investigation of VPAC1 receptor analogs as biomolecular imaging agents arose from decades of data that support the consistent and reliable expression of VPAC1 in prostate cancer cells but not in benign stromal cells. Pre-clinical data and the first feasibility study in men with prostate cancer provide compelling evidence that 64Cu-TP3805 reliably detected prostate cancer and precancerous prostatic lesions. Since 64Cu-TP3805 and other VPAC1 analogs tagged with Copper-64 were not renally excreted, they bypassed the urinary bladder and presented a favorable target for prostate cancer imaging.
The success from pre-clinical study and the first preliminary study in men warrants further investigation of VPAC1 targeted PET/CT imaging in men with prostate cancer on a larger scale to determine its sensitivity, specificity, and accuracy in detecting primary prostate cancer. In addition, it may represent an attractive target to diagnosis early prostate cancer recurrence post-definitive therapy and nodal metastasis. Another exciting application of VPAC1 targeted approach to prostate cancer detection is circulating tumor cell assay in urinary specimens. In a pilot study of voided urine assay for prostate cancer detection using a radioactive ligand that bound to VPAC1 receptor on cell surface, we found the assay detected VPAC positive cancer cells in 98.6% of men with prostate cancer with 99.3% sensitivity and 100% specificity [27]. This represents a potential major leap in non-invasive approach to prostate cancer diagnosis.
We currently have an ongoing clinical study to investigate the clinical utility of VPAC1 targeted PET/CT imaging. This study aims to determine the role of 64Cu-TP3805 PET/CT in the diagnosis of prostate cancer in men with persistently elevated PSA, comparing 64Cu-TP3805 PET/CT findings with multiparametric MRI, MR/ultrasound fusion biopsy, and histology, as well as men with biochemical recurrence after treatment who are undergoing standard of care biopsy of suspected metastatic lesions (NCT02989623). With improved understanding of the biochemical pathways that govern the pathogenesis of urinary malignancy at the molecular level, biomolecular based imaging has the potential to improve cancer diagnosis and management, reducing the financial, health, and emotional toll of prostate cancer.
Acknowledgments
Funding The studies were supported by a grant from the National Institutes of Health (NIH/NCI-R01-157372, PI—MLT).
Abbreviation
- CT
Computed tomography
- Da
Dalton, atomic mass unit
- DAR
Digital autoradiography
- DRE
Digital rectal exam
- eIND
Exploratory Investigational New Drug
- H&E
Hematoxylin and eosin
- MRI
Magnetic resonance imaging
- PET
Positron-emission tomography
- PIRADS
Prostate Imaging Reporting and Data System
- PPV
Positive predictive value
- PSA
Prostate specific antigen
- SUV
Standard uptake value
- TRAMP
Transgenic adenocarcinoma of the mouse prostate
- TRUS
Transrectal ultrasound
- VIP
Vasoactive intestinal peptide
- PACAP
Pituitary adenylate cyclase activating peptide
Footnotes
Conflict of interest MLT is a consultant to NuView Life Sciences. HT, LGG, and EJT declare no relevant financial interests.
Research involving human participants All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJJ et al. (1991) Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med 324(17):1156–1161 [DOI] [PubMed] [Google Scholar]
- 2.Catalona WJ (1993) Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA J Am Med Assoc 270(8):948–954 [PubMed] [Google Scholar]
- 3.Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V et al. (2009) Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 360(13):1320–1328 [DOI] [PubMed] [Google Scholar]
- 4.Bjurlin MA, Carter HB, Schellhammer P, Cookson MS, Gomella LG, Troyer D et al. (2013) Optimization of initial prostate biopsy in clinical practice: sampling, labeling and specimen processing. J Urol. 189(6):2039–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loeb S, Roehl KA, Antenor JAV, Catalona WJ, Suarez BK, Nadler RB (2006) Baseline prostate-specific antigen compared with median prostate-specific antigen for age group as predictor of prostate cancer risk in men younger than 60 years old. Urology 67(2):316–320 [DOI] [PubMed] [Google Scholar]
- 6.Dijkstra S, Mulders PFA, Schalken JA (2014) Clinical use of novel urine and blood based prostate cancer biomarkers: a review. Clin Biochem 47(10–11):889–896 [DOI] [PubMed] [Google Scholar]
- 7.Bryant RJ, Sjoberg DD, Vickers AJ, Robinson MC, Kumar R, Marsden L et al. (2015) Predicting high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured in blood in the ProtecT study. J Natl Cancer Inst 107(7):djv095. 10.1093/jnci/djv095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parekh DJ, Punnen S, Sjoberg DD, Asroff SW, Bailen JL, Cochran JS et al. (2015) A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. Eur Urol 68(3):464–470 [DOI] [PubMed] [Google Scholar]
- 9.Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V et al. (2011) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol 59:61–71 [DOI] [PubMed] [Google Scholar]
- 10.Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, Freedland SJ, Greene K, Klotz LH, Makarov DV, Nelson JB, Rodrigues G, Sandler HM, Taplin ME, Treadwell JR (2017) Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J Urol. 10.1016/j.juro.2017.11.095 [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines®). Prostate cancer. (Version 2.2017). Prostate cancer. Version 02.2017 [Google Scholar]
- 12.Muller BG, Shih JH, Sankineni S, Marko J, Rais-Bahrami S, George AK et al. (2015) Prostate cancer: interobserver agreement and accuracy with the revised prostate imaging reporting and data system at multiparametric mr imaging. Radiol Radiol Soc N Am 277(3):741–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenkrantz AB, Ginocchio LA, Cornfeld D, Froemming AT, Gupta RT, Turkbey B et al. (2016) Interobserver reproducibility of the PI-RADS version 2 Lexicon: a multicenter study of six experienced prostate radiologists. Radiology 280(3):793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fütterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A et al. (2015) Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A Systematic Review Of The Literature. Eur Urol 68(6):1045–1053 [DOI] [PubMed] [Google Scholar]
- 15.Krause BJ, Souvatzoglou M, Treiber U (2013) Imaging of prostate cancer with PET/CT and radioactively labeled choline derivates. Urol Oncol 31(4):427–435 [DOI] [PubMed] [Google Scholar]
- 16.Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B et al. (2015) Evaluation of HYBRID 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med 56(5):668–674 [DOI] [PubMed] [Google Scholar]
- 17.Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG et al. (2014) Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 41(1):11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reubi JC (1995) In vitro identification of vasoactive intestinal peptide receptors in human tumors: implications for tumor imaging. J Nucl Med 36(10):1846–1853 [PubMed] [Google Scholar]
- 19.Reubi JC, Läderach U, Waser B, Gebbers JO, Robberecht P, Laissue JA (2000) Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor subtypes in human tumors and their tissues of origin. Cancer Res 60(11):3105–3112 [PubMed] [Google Scholar]
- 20.Reubi JC (2000) In vitro evaluation of VIP/PACAP receptors in healthy and diseased human tissues. Clinical implications. Ann N Y Acad Sci 921:1–25 [DOI] [PubMed] [Google Scholar]
- 21.Zia H, Hida T, Jakowlew S, Birrer M, Gozes Y, Reubi JC et al. (1996) Breast cancer growth is inhibited by vasoactive intestinal peptide (VIP) hybrid, a synthetic VIP receptor antagonist. Cancer Res 56(15):3486–3489 [PubMed] [Google Scholar]
- 22.Leyton J, Gozes Y, Pisegna J, Coy D, Purdom S, Casibang M et al. (1999) PACAP(6-38) is a PACAP receptor antagonist for breast cancer cells. Breast Cancer Res Treat 56(2):177–186 [DOI] [PubMed] [Google Scholar]
- 23.Zhang K, Aruva MR, Shanthly N, Cardi CA, Patel CA, Rattan S et al. (2007) Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating peptide (PACAP) receptor specific peptide analogues for PET imaging of breast cancer: in vitro/in vivo evaluation. Regul Pept 144(1–3):91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thakur ML, Zhang K, Berger A, Cavanaugh B, Kim S, Channappa C et al. (2013) VPAC1 receptors for imaging breast cancer: a feasibility study. J Nucl Med 54(7):1019–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang K, Aruva MR, Shanthly N, Cardi CA, Rattan S, Patel C et al. (2008) PET imaging of VPAC1 expression in experimental and spontaneous prostate cancer. J Nucl Med Soc Nucl Med 49(1):112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakr WA, Partin AW (2001) Histological markers of risk and the role of high-grade prostatic intraepithelial neoplasia. Urology 57(4 Suppl 1):115–120 [DOI] [PubMed] [Google Scholar]
- 27.Trabulsi EJ, Tripathi SK, Gomella L, Solomides C, Wickstrom E, Thakur ML (2017) Development of a voided urine assay for detecting prostate cancer non-invasively: a pilot study. BJU Int 119(6):885–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tripathi S, Trabulsi EJ, Gomella L, Kim S, McCue P, Intenzo C et al. (2016) VPAC1 targeted (64)Cu-TP3805 positron emission tomography imaging of prostate cancer: preliminary evaluation in man. Urology 88:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]