Abstract
Although use of oral contraceptives (OCs) is common, their influence on carcinogenesis is not fully understood. We used Cox proportional hazards models to examine OC use (never/<1 year (referent), 1–4, 5–9, ≥10 years) and development of incident cancers across body sites within the same base population: women in the prospective National Institutes of Health-AARP Diet and Health Study (enrolled 1995–1996 and followed until 2011). Adjustment for confounding varied by outcome; all models accounted for age, race, body mass index, and smoking status, and included at least 100,000 women. Any OC use conferred a 3% reduction in the risk for any cancer (hazard ratio = 0.97, 95% confidence interval: 0.95, 0.99). Expected risk reductions that strengthened with duration of use were identified for ovarian and endometrial cancers and were suggested for kidney cancer (all P for trend < 0.05). Non-Hodgkin lymphoma risk (hazard ratio = 0.79, 95% confidence interval: 0.64, 0.97) was reduced with 10 or more years of OC use. There was a 37% reduced risk for bladder cancer and 46% increased risk for pancreatic cancer among long-term OC users who were 60 years of age or younger at baseline. OC use did not influence risks for most other cancers evaluated. Given the high prevalence of use and changing formulations, additional studies are warranted to fully understand the chemopreventive effects of these medications.
Keywords: cancer, chemoprevention, oral contraceptives, prospective studies
Oral contraceptive (OC) use is ubiquitous in the United States and the benefits and risks associated with this use are fairly well documented (1, 2). However, the influence of OCs on processes such as carcinogenesis is not fully understood—a problem complicated by the long pathogenesis of cancer and the lack of detailed information on OC use in cancer cohorts. OCs could potentially influence cancer development by altering estradiol-to-progesterone ratios, immune responses, and even 1-carbon metabolism (3, 4).
Current OC use is associated with increased risk of breast cancer and longer duration of OC use reduces risks for ovarian and endometrial cancers (5, 6). Less is understood about risks for other cancers, and even among cancers for which enough literature exists to merit reviews and meta-analyses, there is considerable heterogeneity across studies. There is increasing evidence that suggests a hormonal etiology for many cancers in women, yet much remains to be learned about the influence of exogenous hormones. Furthermore, the formulation of OCs has changed considerably since their debut, which may mean that associations between OC use and cancer development will change over time (2, 7). As such, it behooves us to continue investigating OC use and cancer.
Relative to meta-analyses on OC use and individual cancers, using data from the same base population to explore cancer risks minimizes residual confounding and systematic bias through standardized recruitment and data collection. Therefore, we examined the associations between OC use and risks for cancer across organ sites in the large prospective National Institutes of Health (NIH)-AARP Diet and Health Study. To our knowledge, this is the largest US cohort used to explore OC use and cancer, and women also provided information on the duration of OC use.
METHODS
Study population
The NIH-AARP Diet and Health Study (www.clinicaltrials.gov; NCT00340015) is a prospective cohort study that began in 1995–1996; methods for the study were approved by The National Cancer Institute Special Studies Institutional Review Board (8). Approximately 3.5 million AARP members between the ages of 50 and 71 years and residing in 6 states (California, Florida, Louisiana, New Jersey, North Carolina, or Pennsylvania) or 2 metropolitan areas (Atlanta, Georgia, or Detroit, Michigan) were mailed a baseline questionnaire. Of these, 566,398 members returned satisfactorily completed questionnaires, provided informed consent, and did not die or move before study entry. We excluded participants who completed questionnaires by proxy respondents (n = 15,760), were men (n = 325,171), had a previous history of cancer (n = 23,998), were identified as having cancer through death reports only (n = 1,430), showed disagreement between reported sex (n = 136), indicated that their menses had stopped due to chemotherapy or radiation (n = 157), and who did not provide information on OC use (n = 3,210). The final analytic population was 196,536 women. For analyses of ovarian cancer, we additionally removed women who had a bilateral oophorectomy or were missing this information (n = 45,675); for endometrial cancer, we removed from analysis women who had a hysterectomy or were missing this information (n = 81,935). Information on the duration of OC use and other demographic, health, and lifestyle characteristics was queried at baseline. Information on timing of OC use and drug formulation was unavailable. An additional questionnaire that collected information on body mass index (BMI) as a teenager was sent approximately 6 months after the baseline questionnaire; this information was available for 122,986 women and used only in sensitivity analyses.
Cohort follow-up and case ascertainment
Cancer cases were ascertained through linkage with state registries for the original 8 states plus Arizona, Texas, and Nevada, which are states to which cohort members commonly migrated. Vital status was determined by linkage to the Social Security Administration Death Master File, the National Death Index Plus, and the state registries. Participants were followed from enrollment until the first date of diagnosis of any cancer, the date of death, the end of study follow-up (December 31, 2011), or the date of loss to follow-up, whichever occurred first.
We examined risks for registry-confirmed, incident, invasive cancers with approximately 200 or more cases: any cancer (n = 33,618), acute myeloid leukemia (n = 204), bladder (n = 547), brain (n = 357), breast (n = 10,599), chronic lymphocytic leukemia (n = 327), colorectal (n = 3,246), endometrial (n = 2,189), head and neck (n = 599), kidney and renal pelvis (n = 815), lung and bronchus (n = 5,423), multiple myeloma (n = 437), non-Hodgkin lymphoma (n = 1,494), ovarian (n = 1,223), pancreatic (n = 1,000), stomach (n = 304), and thyroid (n = 500). We did not analyze cancers for which crucial risk factor information was unavailable (e.g., sun exposure and melanoma or human papillomavirus and cervical cancer). Head and neck cancers included those in the lip, tongue, salivary gland, gum/mouth, pharynx, tonsil, and larynx. Ovarian cancers included those identified as ovarian (n = 1,066), fallopian tube (n = 40), or peritoneal (n = 117). We used International Classification of Diseases for Oncology, Third Edition, histology codes to identify cases of acute myeloid leukemia (9840, 9861, 9865–9867, 9869, 9871–9874, 9895–9897, 9898, 9910–9911, 9920) and chronic lymphocytic leukemia (9823). We did not make any exclusions based on histology (e.g., endometrial cancer cases include those with epithelial tumors and sarcomas).
Statistical analyses
We defined OC use as any use or the duration of use (1–4, 5–9, or ≥10 years) relative to never using OCs or using them for less than 1 year. We used Cox proportional hazards models to estimate hazard ratios and 95% confidence intervals for the associations between OC use and time to diagnosis of cancer. Age was our time metric and we adjusted for baseline age to minimize the impact of left truncation (9). The exact method was used to handle ties. We report P values for trend across categories of duration of OC use. Tests of significance were 2-sided and an α of 0.05 indicated statistical significance. To account for multiple comparisons across the 17 cancer outcomes in our overall models, we used a Bonferroni corrected P value of 0.003 to assess whether associations with 10 or more years of OC use remained statistically significant. All other analyses were exploratory and not corrected for multiple comparisons.
We used knowledge of the literature and directed acyclic graphs to select potential confounders. To address our primary goal of comparing OC associations across cancer sites, we chose to use a consistent adjustment strategy in our models, adjusting for confounders but not mediators. All models were adjusted for age, race, BMI, and smoking status at the time of the baseline questionnaire. We additionally adjusted for age at menarche, alcohol use, number of cigarettes smoked per day, and/or physical activity at baseline, depending on the cancer outcome. All adjustment factors were categorized as presented in Table 1.
Table 1.
Characteristics of the Study Population Across Categories of Oral Contraceptive Use Duration, National Institutes of Health-AARP Diet and Health Study, United States, 1995–2011a
| Characteristic | Duration of Oral Contraceptive Use, years | |||||||
|---|---|---|---|---|---|---|---|---|
| No Use or <1 (n = 118,144) | 1–4 (n = 34,866) | 5–9 (n = 24,564) | ≥10 (n = 18,962) | |||||
| No. | % | No. | % | No. | % | No. | % | |
| Age at baseline, yearsb | 64 (60–67) | 59 (55–64) | 59 (55–64) | 60 (56–64) | ||||
| Race | ||||||||
| White | 105,285 | 90.4 | 31,156 | 90.6 | 22,121 | 91.2 | 17,152 | 91.5 |
| Black | 6,608 | 5.7 | 2,067 | 6.0 | 1,438 | 5.9 | 1,117 | 6.0 |
| Other | 4,530 | 3.9 | 1,184 | 3.4 | 689 | 2.8 | 469 | 2.5 |
| Educational level | ||||||||
| Did not complete high school | 8,972 | 7.9 | 1,593 | 4.7 | 959 | 4.0 | 669 | 3.6 |
| High school | 33,332 | 29.2 | 7,525 | 22.2 | 5,232 | 21.9 | 3,912 | 21.2 |
| Some college/post–high school | 40,286 | 35.3 | 12,842 | 37.9 | 9,273 | 38.8 | 7,218 | 39.0 |
| College | 15,919 | 14.0 | 5,816 | 17.2 | 4,070 | 17.0 | 3,120 | 16.9 |
| Postgraduate | 15,584 | 13.7 | 6,071 | 17.9 | 4,343 | 18.2 | 3,577 | 19.3 |
| Smoking status | ||||||||
| Nonsmoker | 54,778 | 47.8 | 14,605 | 43.2 | 9,914 | 41.5 | 7,794 | 42.3 |
| Former smoker | 43,728 | 38.2 | 13,814 | 40.8 | 10,201 | 42.7 | 7,927 | 43.0 |
| Current smoker | 16,065 | 14.0 | 5,424 | 16.0 | 3,753 | 15.7 | 2,722 | 14.8 |
| No. of cigarettes smoked per dayc | ||||||||
| Never smoked | 54,778 | 47.8 | 14,605 | 43.2 | 9,914 | 41.5 | 7,794 | 42.3 |
| 1–10 | 21,780 | 19.0 | 6,978 | 20.6 | 4,930 | 20.7 | 3,622 | 19.6 |
| 11–20 | 20,534 | 17.9 | 6,548 | 19.3 | 4,647 | 19.5 | 3,443 | 18.7 |
| 21–30 | 9,705 | 8.5 | 3,198 | 9.4 | 2,374 | 9.9 | 1,865 | 10.1 |
| 31–40 | 4,963 | 4.3 | 1,610 | 4.8 | 1,258 | 5.3 | 993 | 5.4 |
| 41–60 | 2,272 | 2.0 | 758 | 2.2 | 630 | 2.6 | 579 | 3.1 |
| ≥61 | 539 | 0.5 | 146 | 0.4 | 115 | 0.5 | 147 | 0.8 |
| Current alcohol use | ||||||||
| Nondrinker | 38,421 | 32.7 | 9,167 | 26.4 | 5,799 | 23.7 | 4,080 | 21.6 |
| ≤2 servings per day | 74,038 | 63.0 | 23,797 | 68.5 | 17,259 | 70.5 | 13,558 | 71.7 |
| >2 servings per day | 5,100 | 4.3 | 1,789 | 5.1 | 1,429 | 5.8 | 1,279 | 6.8 |
| Current body mass indexd | ||||||||
| <25 | 48,467 | 42.5 | 15,214 | 44.9 | 11,301 | 47.2 | 9,147 | 49.3 |
| 25–29 | 37,654 | 33.0 | 10,788 | 31.8 | 7,609 | 31.8 | 5,830 | 31.4 |
| ≥30 | 27,867 | 24.4 | 7,915 | 23.3 | 5,018 | 21.0 | 3,562 | 19.2 |
| Current physical activity | ||||||||
| ≥3 times per week | 48,368 | 41.5 | 14,245 | 41.2 | 10,105 | 41.5 | 8,093 | 43.0 |
| 1–2 times per week | 24,067 | 20.6 | 7,587 | 22.0 | 5,417 | 22.3 | 4,115 | 21.9 |
| ≤3 times per month | 44,149 | 37.9 | 12,709 | 36.8 | 8,823 | 36.2 | 6,598 | 35.1 |
| Age at menarche, years | ||||||||
| ≤12 | 57,460 | 48.8 | 17,343 | 49.9 | 12,002 | 49.0 | 9,259 | 48.9 |
| 13–14 | 49,035 | 41.6 | 14,297 | 41.1 | 10,341 | 42.2 | 7,791 | 41.2 |
| ≥15 years | 11,273 | 9.6 | 3,135 | 9.0 | 2,166 | 8.8 | 1,873 | 9.9 |
| Menopausal status | ||||||||
| Premenopausal | 2,224 | 1.9 | 2,256 | 6.5 | 1,606 | 6.6 | 1,130 | 6.0 |
| Postmenopausal | 115,568 | 98.1 | 32,492 | 93.5 | 22,881 | 93.4 | 17,758 | 94.0 |
| Has first degree relative with breast cancer | ||||||||
| No | 98,138 | 87.0 | 29,082 | 87.2 | 20,499 | 87.1 | 15,930 | 87.6 |
| Yes | 14,606 | 13.0 | 4,258 | 12.8 | 3,025 | 12.9 | 2,265 | 12.5 |
| Has first degree relative with colon cancer | ||||||||
| No | 101,351 | 89.9 | 30,072 | 90.2 | 21,312 | 90.6 | 16,482 | 90.6 |
| Yes | 11,393 | 10.1 | 3,268 | 9.8 | 2,212 | 9.4 | 1,713 | 9.4 |
| Has a history of diabetes | ||||||||
| No | 108,219 | 91.6 | 32,605 | 93.5 | 23,118 | 94.1 | 18,035 | 95.1 |
| Yes | 9,925 | 8.4 | 2,261 | 6.5 | 1,446 | 5.9 | 927 | 4.9 |
a Characteristics reflect the analytic population before exclusions for specific cancer analyses (e.g., women with bilateral oophorectomy were excluded from Cox models for ovarian cancer). Percentages in the table refer to proportions of the population who were not missing information on a given covariate. Missingness was less than 5% for all covariates and is not tabulated.
b Values expressed as median (interquartile range). The overall age range for the analytic population at baseline was 50–72 years.
c Number of cigarettes smoked per day was treated as a continuous variable in our models.
d Weight (kg)/height (m)2.
We performed the following sensitivity analyses: 1) Information on BMI and physical activity as a teenager was available for some participants. We adjusted for these factors in a series of models rather than BMI and activity at baseline, because the latter would not directly affect prior OC use. 2) We excluded the first 2 years of follow-up in the event that clinically undetected malignancies influenced reporting of information at baseline. 3) We excluded women with end-stage renal disease in our kidney cancer models and 4) those with emphysema in our lung and bronchial cancer models. 5) We adjusted for a history of diabetes in the pancreatic cancer model. 6) We evaluated risk for all cancers stratified by age at baseline (≤60 years, >60 years) to assess the potential influence of OC formulation or recency of use. Analyses were performed with SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina) and forest plots were generated with R Studio (10).
RESULTS
Sixty percent of the participants never used OCs or used them for less than 1 year (Table 1). Only 10% of the population were long-term users of OCs (≥10 years). Long-term users were more likely to be younger and premenopausal, have completed more years of education, drink alcohol, and have lower BMI at baseline than women who used OCs for 1 year or less.
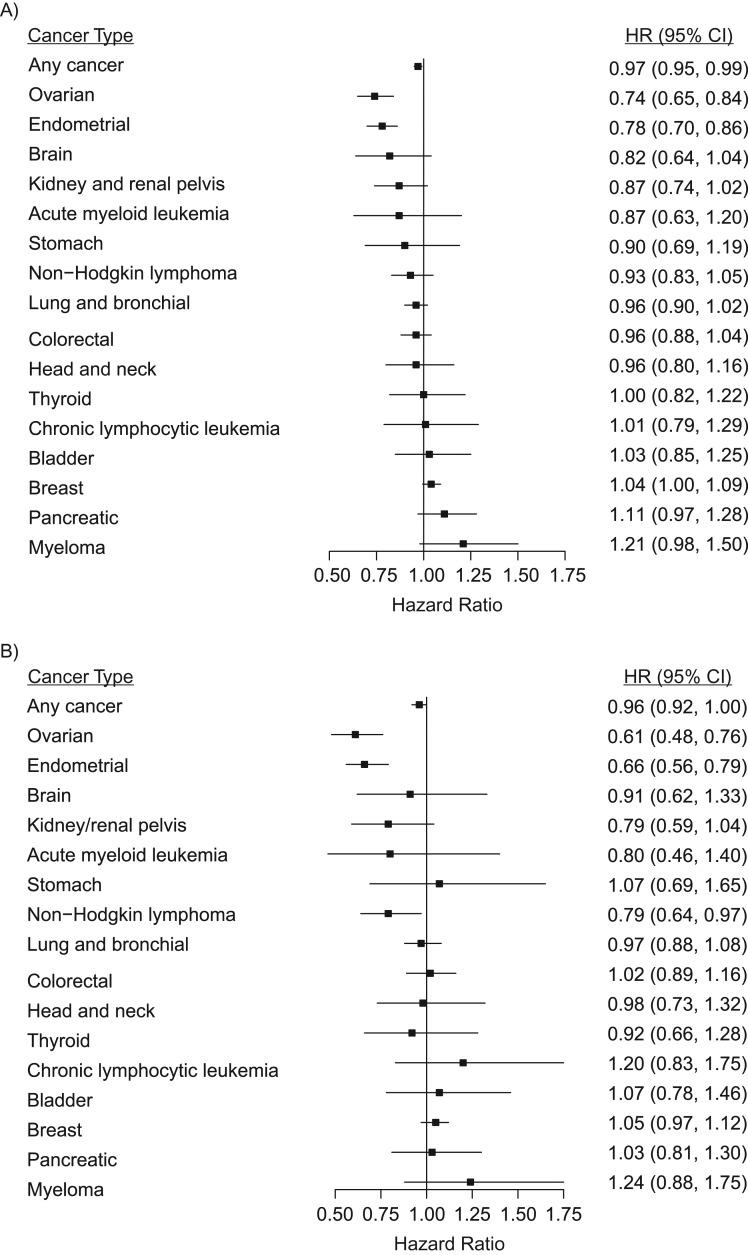
Any OC use conferred a 3% reduction in the risk for any cancer (95% confidence interval (CI): 0.95, 0.99) (Table 2, Figure 1A). Overall, we identified null associations, in terms of magnitude, between any OC use and most cancers. Risk reductions were observed for ovarian (hazard ratio (HR) = 0.74, 95% CI: 0.65, 0.84) and endometrial cancer (HR = 0.78, 95% CI: 0.70, 0.86) and were suggested, but not statistically significant, for brain (HR = 0.82, 95% CI: 0.64, 1.04), colorectal (HR = 0.96, 95% CI: 0.88, 1.04), kidney and renal pelvis (HR = 0.87, 95% CI: 0.74, 1.02), and lung and bronchial cancers (HR = 0.96, 95% CI: 0.90, 1.02). Elevated risks for breast (HR = 1.04, 95% CI: 1.00, 1.09) and pancreatic (HR = 1.11, 95% CI: 0.97, 1.28) cancers as well as multiple myeloma (HR = 1.21, 95% CI: 0.98, 1.50) were suggested.
Table 2.
Oral Contraceptive Use and the Time to Diagnosis of Incident Invasive Cancers, Overall Results and Those Stratified by Age at Baseline, National Institutes of Health-AARP Diet and Health Study, United States, 1995–2011
| Cancer Outcome/OC Use | Overall (n = 196,536) | ≤60 Years Old (n = 74,608) | >60 Years Old (n = 121,928) | |||||
|---|---|---|---|---|---|---|---|---|
| No. of Noncases | No. of Cases | Adjusted HRa | 95% CI | Adjusted HRa | 95% CI | Adjusted HRa | 95% CI | |
| Any cancer | ||||||||
| Any OC use (vs. none/<1 year) | 162,918 | 33,618 | 0.97 | 0.95, 0.99 | 0.94 | 0.91, 0.98 | 0.99 | 0.96, 1.02 |
| Duration of use, years | ||||||||
| None/<1 | 96,913 | 21,231 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 29,309 | 5,557 | 0.99 | 0.95, 1.02 | 0.95 | 0.90, 1.00b | 1.01 | 0.97, 1.05 |
| 5–9 | 20,723 | 3,841 | 0.96 | 0.93, 1.00b | 0.95 | 0.90, 1.01 | 0.96 | 0.91, 1.01 |
| ≥10 | 15,973 | 2,989 | 0.96 | 0.92, 1.00b,c | 0.92 | 0.86, 0.98c | 0.98 | 0.93, 1.04 |
| Acute myeloid leukemia | ||||||||
| Any use | 196,332 | 204 | 0.87 | 0.63, 1.20 | 0.60 | 0.35, 1.05 | 1.05 | 0.72, 1.52 |
| Duration of use, years | ||||||||
| None/<1 | 118,008 | 136 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 34,839 | 27 | 0.80 | 0.52, 1.24 | 0.46 | 0.21, 1.00 | 1.07 | 0.65, 1.78 |
| 5–9 | 24,538 | 26 | 1.03 | 0.65, 1.62 | 0.92 | 0.46, 1.85 | 1.03 | 0.56, 1.88 |
| ≥10 | 18,947 | 15 | 0.80 | 0.46, 1.40 | 0.47 | 0.16, 1.34 | 1.02 | 0.53, 1.96 |
| Bladder | ||||||||
| Any use | 195,989 | 547 | 1.04 | 0.86, 1.26 | 0.75 | 0.58, 0.99 | 0.92 | 0.76, 1.12 |
| Duration of use, years | ||||||||
| None/<1 | 117,790 | 354 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 34,779 | 87 | 1.06 | 0.83, 1.36 | 0.97 | 0.71, 1.32 | 0.89 | 0.68, 1.17 |
| 5–9 | 24,508 | 56 | 0.97 | 0.72, 1.31 | 0.54 | 0.35, 0.84 | 1.00 | 0.74, 1.36 |
| ≥10 | 18,912 | 50 | 1.09 | 0.80, 1.48 | 0.63 | 0.39, 1.01c | 0.87 | 0.61, 1.23 |
| Brain | ||||||||
| Any use | 196,179 | 357 | 0.82 | 0.64, 1.04 | 0.83 | 0.58, 1.20 | 0.81 | 0.59, 1.12 |
| Duration of use, years | ||||||||
| None/<1 | 117,909 | 235 | 1.00 | Referent | 1.000 | Referent | 1.00 | Referent |
| 1–4 | 34,812 | 54 | 0.83 | 0.60, 1.14 | 0.67 | 0.41, 1.10 | 1.03 | 0.69, 1.54 |
| 5–9 | 24,528 | 36 | 0.73 | 0.50, 1.08 | 1.00 | 0.61, 1.62 | 0.42 | 0.20, 0.85 |
| ≥10 | 18,930 | 32 | 0.91 | 0.62, 1.33 | 0.91 | 0.52, 1.60 | 0.91 | 0.53, 1.54 |
| Breast | ||||||||
| Any use | 185,937 | 10,599 | 1.04 | 1.00, 1.09 | 1.03 | 0.96, 1.10 | 1.05 | 0.99, 1.11 |
| Duration of use, years | ||||||||
| None/<1 | 111,797 | 6,347 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 32,970 | 1,896 | 1.04 | 0.98, 1.10 | 1.03 | 0.95, 1.12 | 1.05 | 0.97, 1.13 |
| 5–9 | 23,235 | 1,329 | 1.03 | 0.97, 1.10 | 1.04 | 0.95, 1.15 | 1.02 | 0.93, 1.11 |
| ≥10 | 17,935 | 1,027 | 1.05 | 0.97, 1.12 | 1.01 | 0.91, 1.12 | 1.08 | 0.98, 1.18 |
| Chronic lymphocytic leukemia | ||||||||
| Any use | 196,209 | 327 | 1.01 | 0.79, 1.29 | 0.91 | 0.59, 1.40 | 1.07 | 0.79, 1.44 |
| Duration of use, years | ||||||||
| None/<1 | 117,934 | 210 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 34,820 | 46 | 0.91 | 0.65, 1.28 | 0.66 | 0.36, 1.20 | 1.10 | 0.74, 1.65 |
| 5–9 | 24,527 | 37 | 1.00 | 0.69, 1.45 | 1.19 | 0.68, 2.07 | 0.81 | 0.47, 1.37 |
| ≥10 | 18,928 | 34 | 1.20 | 0.83, 1.75 | 1.01 | 0.53, 1.94 | 1.31 | 0.83, 2.08 |
| Colorectal | ||||||||
| Any use | 193,290 | 3,246 | 0.96 | 0.88, 1.04 | 0.98 | 0.84, 1.15 | 0.95 | 0.86, 1.05 |
| Duration of use, years | ||||||||
| None/<1 | 115,988 | 2,156 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 34,400 | 466 | 0.93 | 0.83, 1.04 | 0.94 | 0.77, 1.14 | 0.93 | 0.81, 1.07 |
| 5–9 | 24,226 | 338 | 0.96 | 0.84, 1.08 | 1.03 | 0.84, 1.27 | 0.91 | 0.78, 1.07 |
| ≥10 | 18,676 | 286 | 1.02 | 0.89, 1.16 | 1.00 | 0.79, 1.27 | 1.03 | 0.88, 1.21 |
| Endometrial | ||||||||
| Any use | 112,412 | 2,189 | 0.78 | 0.70, 0.86 | 0.71 | 0.62, 0.82 | 0.83 | 0.73, 0.94 |
| Duration of use, years | ||||||||
| None/<1 | 66,595 | 1,455 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 19,884 | 333 | 0.78 | 0.69, 0.89 | 0.75 | 0.63, 0.90 | 0.78 | 0.65, 0.95 |
| 5–9 | 14,219 | 245 | 0.86 | 0.75, 0.99 | 0.76 | 0.63, 0.93 | 0.96 | 0.78, 1.17 |
| ≥10 | 11,714 | 156 | 0.66 | 0.56, 0.79c | 0.57 | 0.45, 0.74c | 0.75 | 0.59, 0.95c |
| Head and neck | ||||||||
| Any use | 195,937 | 599 | 0.96 | 0.80, 1.16 | 1.01 | 0.75, 1.37 | 0.94 | 0.75, 1.18 |
| Duration of use, years | ||||||||
| None/<1 | 117,775 | 369 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 34,764 | 102 | 0.97 | 0.77, 1.23 | 0.93 | 0.63, 1.36 | 1.03 | 0.76, 1.40 |
| 5–9 | 24,492 | 72 | 0.94 | 0.71, 1.24 | 1.05 | 0.70, 1.57 | 0.86 | 0.58, 1.26 |
| ≥10 | 18,906 | 56 | 0.98 | 0.73, 1.32 | 1.12 | 0.72, 1.75 | 0.87 | 0.58, 1.32 |
| Kidney and renal pelvis | ||||||||
| Any use | 195,721 | 815 | 0.87 | 0.74, 1.02 | 0.77 | 0.58, 1.00 | 0.93 | 0.77, 1.13 |
| Duration of use, years | ||||||||
| None/<1 | 117,606 | 538 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 34,731 | 135 | 0.95 | 0.78, 1.17 | 0.97 | 0.71, 1.33 | 0.90 | 0.69, 1.18 |
| 5–9 | 24,482 | 82 | 0.80 | 0.62, 1.03 | 0.55 | 0.35, 0.85 | 1.01 | 0.75, 1.37 |
| ≥10 | 18,902 | 60 | 0.79 | 0.59, 1.04c | 0.65 | 0.40, 1.03c | 0.88 | 0.62, 1.24 |
| Lung and bronchial | ||||||||
| Any use | 191,113 | 5,423 | 0.96 | 0.90, 1.02 | 0.95 | 0.85, 1.06 | 0.96 | 0.90, 1.04 |
| Duration of use, years | ||||||||
| None/<1 | 114,681 | 3,463 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 33,980 | 886 | 0.97 | 0.90, 1.05 | 0.92 | 0.80, 1.05 | 1.01 | 0.92, 1.12 |
| 5–9 | 23,986 | 578 | 0.92 | 0.84, 1.01 | 0.94 | 0.80, 1.09 | 0.90 | 0.80, 1.02 |
| ≥10 | 18,466 | 496 | 0.97 | 0.88, 1.08 | 1.01 | 0.86, 1.20 | 0.95 | 0.84, 1.08 |
| Multiple myeloma | ||||||||
| Any use | 196,099 | 437 | 1.21 | 0.98, 1.50 | 1.05 | 0.72, 1.55 | 1.29 | 1.001, 1.67 |
| Duration of use, years | ||||||||
| None/<1 | 117,878 | 266 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 34,793 | 73 | 1.17 | 0.89, 1.55 | 1.06 | 0.66, 1.69 | 1.21 | 0.85, 1.72 |
| 5–9 | 24,510 | 54 | 1.25 | 0.91, 1.71 | 1.04 | 0.61, 1.76 | 1.38 | 0.93, 2.03 |
| ≥10 | 18,918 | 44 | 1.24 | 0.88, 1.75 | 1.07 | 0.60, 1.93 | 1.33 | 0.88, 2.03 |
| Non-Hodgkin lymphoma | ||||||||
| Any use | 195,042 | 1,494 | 0.93 | 0.83, 1.05 | 0.97 | 0.79, 1.17 | 0.91 | 0.79, 1.06 |
| Duration of use, years | ||||||||
| None/<1 | 117,176 | 968 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 34,622 | 244 | 1.00 | 0.86, 1.16 | 1.07 | 0.85, 1.35 | 0.94 | 0.76, 1.14 |
| 5–9 | 24,392 | 172 | 0.95 | 0.80, 1.13 | 0.96 | 0.73, 1.26 | 0.96 | 0.76, 1.21 |
| ≥10 | 18,852 | 110 | 0.79 | 0.64, 0.97 | 0.76 | 0.54, 1.05 | 0.83 | 0.63, 1.08 |
| Ovarian | ||||||||
| Any use | 149,638 | 1,223 | 0.74 | 0.65, 0.84 | 0.77 | 0.63, 0.94 | 0.72 | 0.60, 0.85 |
| Duration of use of use, years | ||||||||
| None/<1 | 88,612 | 827 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 26,980 | 200 | 0.84 | 0.71, 0.996 | 0.84 | 0.66, 1.08 | 0.85 | 0.67, 1.07 |
| 5–9 | 19,192 | 116 | 0.70 | 0.57, 0.86 | 0.69 | 0.51, 0.93 | 0.72 | 0.54, 0.96 |
| ≥10 | 14,854 | 80 | 0.61 | 0.48, 0.76c | 0.73 | 0.52, 1.01c | 0.50 | 0.34, 0.72c |
| Pancreatic | ||||||||
| Any use | 195,536 | 1,000 | 1.11 | 0.97, 1.28 | 1.37 | 1.05, 1.78 | 1.02 | 0.86, 1.20 |
| Duration of use, years | ||||||||
| None/<1 | 117,518 | 626 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 34,693 | 173 | 1.16 | 0.97, 1.38 | 1.33 | 0.97, 1.83 | 1.11 | 0.89, 1.39 |
| 5–9 | 24,446 | 118 | 1.11 | 0.90, 1.37 | 1.35 | 0.95, 1.92 | 1.03 | 0.78, 1.35 |
| ≥10 | 18,879 | 83 | 1.03 | 0.81, 1.30 | 1.46 | 1.00, 2.14c | 0.84 | 0.61, 1.15 |
| Stomach | ||||||||
| Any use | 196,232 | 304 | 0.90 | 0.69, 1.19 | 0.84 | 0.50, 1.40 | 0.93 | 0.67, 1.28 |
| Duration of use, years | ||||||||
| None/<1 | 117,935 | 209 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 34,826 | 40 | 0.86 | 0.59, 1.25 | 0.83 | 0.44, 1.58 | 0.85 | 0.53, 1.36 |
| 5–9 | 24,534 | 30 | 0.83 | 0.53, 1.30 | 0.77 | 0.36, 1.63 | 0.85 | 0.49, 1.48 |
| ≥10 | 18,937 | 25 | 1.07 | 0.69, 1.65 | 0.95 | 0.43, 2.08 | 1.14 | 0.68, 1.92 |
| Thyroid | ||||||||
| Any use | 196,036 | 500 | 1.00 | 0.82, 1.22 | 0.91 | 0.68, 1.21 | 1.09 | 0.84, 1.42 |
| Duration of use, years | ||||||||
| None/<1 | 117,849 | 295 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| 1–4 | 34,757 | 109 | 1.23 | 0.97, 1.56 | 1.08 | 0.77, 1.52 | 1.39 | 1.00, 1.94 |
| 5–9 | 24,512 | 52 | 0.75 | 0.54, 1.04 | 0.69 | 0.44, 1.08 | 0.79 | 0.49, 1.29 |
| ≥10 | 18,918 | 44 | 0.92 | 0.66, 1.28 | 0.87 | 0.55, 1.38 | 0.93 | 0.57, 1.52 |
Abbreviations: CI, confidence interval; HR, hazard ratio; OC, oral contraceptive.
a All models were adjusted for age, race, body mass index, and smoking status (from the baseline questionnaire). Additional adjustment was made for specific cancer outcomes, as follows: any cancer (age at menarche, alcohol use, number of cigarettes smoked per day, physical activity); acute myeloid leukemia (alcohol use, number of cigarettes smoked per day, physical activity); bladder (age at menarche, cigarettes smoked per day); brain (none); breast (age at menarche, alcohol use, physical activity, family history of breast cancer); chronic lymphocytic leukemia (alcohol use, number of cigarettes smoked per day, physical activity); colorectal (age at menarche, alcohol use, family history of colon cancer); endometrial (age at menarche); head and neck (alcohol use, number of cigarettes smoked per day); kidney and renal pelvis (age at menarche, alcohol use); lung and bronchial (age at menarche, number of cigarettes smoked per day); multiple myeloma (alcohol use, physical activity); non-Hodgkin lymphoma (alcohol use, number of cigarettes smoked per day, physical activity); ovarian (age at menarche); pancreatic (alcohol use, number of cigarettes smoked per day); stomach (alcohol use, number of cigarettes smoked per day, physical activity); thyroid (alcohol use).
b Statistically significant (P < 0.05).
c Trend across categories of duration is statistically significant (P < 0.05).
Figure 1.
Oral contraceptive use and time to diagnosis of incident invasive cancers in the National Institutes of Health-AARP Diet and Health Study, United States, 1995–2011. A) Estimates for any oral contraceptive use. B) Estimates for 10 or more years of oral contraceptive use. No use/less than 1 year of use is the comparison group in both panels. All hazard ratios (HRs) are adjusted; see Table 2 for details. CI, confidence interval.
The risk reduction for any cancer strengthened with duration of OC use (long-term OC use: HR = 0.96, 95% CI: 0.92, 1.00; P for trend < 0.01) (Table 2, Figure 1B). Longer duration of use conferred stronger risk reductions for ovarian and endometrial cancer (P-trends < 0.01). The long-term OC use associations for endometrial and ovarian cancers were statistically significant at a Bonferroni corrected P value of <0.003. The reductions in risk suggested for any OC use and brain, colorectal, and lung and bronchial cancers were not statistically significant across durations of use and were close to the null in magnitude. The reductions in risk for kidney and renal pelvis cancer strengthened monotonically across strata of OC use duration but were imprecise (reductions ranged from 5% to 21%; P for trend = 0.03). Long-term OC use was associated with a reduced risk of non-Hodgkin lymphoma (HR = 0.79, 95% CI: 0.64, 0.97; P for trend = 0.05). The magnitudes of association indicated increased risks for breast cancer and multiple myeloma across durations of OC use, but null associations could not be ruled out.
Reductions in risk for any cancer, as well as endometrial and kidney and renal pelvis cancers, were strongest among women who were 60 years or younger at baseline (Table 2). Similarly, reductions in risk of bladder cancer were noted among this group of women and strengthened with increasing duration of OC use (long-term OC use: HR = 0.63, 95% CI: 0.39, 1.01; P for trend < 0.01). The reduced risk for non-Hodgkin lymphoma associated with long-term OC use was visible regardless of age at baseline but became imprecise. Among women 60 years or younger at baseline, we identified increased risk for pancreatic cancer associated with any OC use and the association strengthened with increasing duration of use (long-term OC use: HR = 1.46, 95% CI: 1.00, 2.14; P for trend = 0.03). The increased risks for multiple myeloma suggested in the overall models were attributable to women who were older than 60 years at baseline, but confidence intervals were imprecise and no trend was observed. Our overall findings were consistent after performing the other sensitivity analyses described in the Methods.
DISCUSSION
The chemopreventive effects of OCs may stem from the decreased production of and exposure to endogenous estradiol across the menstrual cycle, which is a result of biofeedback changes to the hypothalamus-pituitary-adrenal axis and the continuous progestin dose provided by these medications (11). OCs may exert long-term influences on hormone metabolism (12; B.T., unpublished data, 2017). Though the impact of these medications is not fully understood, it is possible that changes in sex-steroid hormone metabolism influence later health by altering epigenetic regulation/transcription, cellular proliferation, lipid metabolism, cytokine signaling, and even the microbiome (13–17).
We estimated OC-associated cancer risks for a range of organ sites among participants from the same base population. Our findings were robust across sensitivity analyses. We identified cancer risk reductions and increases associated with OC use, which contributed to our overall effect estimate for risk of any cancer being relatively small (a 3% decrease). However, at the population level, OC use is very common and has the potential to affect the health of many women; 40% of our participants used OCs for at least a year. To our knowledge, the NIH-AARP study is the largest single US data source that has been used to explore OC use and cancer risk. One group has reported on OC associations with cancer across organ sites within the same study population in the United Kingdom (among approximately 46,000 women) (18). That study, by Iversen et al. (18), had longer follow-up time than ours but lacked information on important confounders like BMI, and the authors adjusted for potential mediators like parity and did not present results by duration of OC use. OC use and cancer risk have been looked at in other published analyses using data from the NIH-AARP study; however, we included the most recent information on cases and used a systematic adjustment strategy across cancer sites (e.g., selecting confounders based on the literature and directed acyclic graphing, not adjusting for mediators). Compared with consortia data and meta-analyses, using the same base population across analyses allowed for consistent measurement of confounders in all models.
Similar to what has been reported in other studies (see below), we identified risk reductions that strengthened with longer durations of OC use for ovarian, endometrial, and kidney cancers. Our results expand on other work in which more than 5 years of OC use reduced risk for non-Hodgkin lymphoma, but was not statistically significant; in our study, this association reached significance among women who used OCs for at least 10 years. Effect magnitudes for multiple myeloma were in the direction of increased risk, but estimates were imprecise and we did not observe a trend with increasing duration of OC use. Additional research into this novel association and potential mechanisms is needed. Interestingly, we noted stronger OC-associated risk reductions for any cancer and for endometrial, kidney and renal pelvis, and bladder cancers, as well as greater risk for pancreatic cancer among younger women, which may suggest that recency of use or drug formulation is important.
The OC-associated risk reductions for ovarian and endometrial cancers that we observed are comparable to those reported in an Agency for Healthcare Research and Quality Evidence Report on OC use and cancer (6). We did not observe a risk reduction for colorectal cancer that was as strong in magnitude (odds ratio for any OC use = 0.86, 95% CI: 0.79, 0.95) and the slightly elevated risks for breast cancer that we identified were attenuated and imprecise relative to the Agency for Healthcare Research and Quality findings (odds ratio for any OC use = 1.08, 95% CI: 1.00, 1.17) (6). This may be explained by the high proportion of postmenopausal women in our population, many of whom likely stopped using OCs years before our study began. According to research from a consortium (5), increased risks for breast cancer may be limited to current OC users and those who used these drugs within the past 10 years.
Our observations regarding kidney cancer risk reductions agree with a the findings of a meta-analysis (19), in which a 20% reduction in risk for long-term OC use was found, though the definition of “long-term” varied across studies (risk ratio = 0.80, 95% CI: 0.68, 0.94). Karami et al. (20) used NIH-AARP study data and observed similar kidney cancer risk reductions, though they excluded premenopausal women and renal pelvis cancers and did not adjust for age at menarche. According to animal studies, estrogens, particularly catechol metabolites, may contribute to renal cancers through the production of oxidative stress and DNA damage (21, 22). Our group found that estrone and catechol estrogen levels were significantly lower among postmenopausal women who were prior OC users compared with never users (B.T., unpublished data, 2017).
Results of animal studies also indicate that sex steroid hormones influence lymphomagenesis; higher lymphocyte levels among women who had recently used OCs were observed in some studies, but the impact of OC use on lymphoma risk is not understood (15, 23–25). In the study from the United Kingdom (18) in which cancers were evaluated across organ sites, a strong reduction in risk was found for lymphatic and hematopoietic cancers (combined) associated with any OC use. A risk reduction for non-Hodgkin lymphoma that was of borderline statistical significance among women with more than 5 years of OC use (OR = 0.86, 95% CI: 0.73, 1.02) was reported in a meta-analysis (26).
Sex steroid hormones and their receptors are hypothesized to play a role in bladder carcinogenesis because there are sex disparities in its incidence and fatality (i.e., more common in men, but diagnosed at advanced stages in women) (27). Increasing parity is reported to reduce risk for bladder cancer in many studies (28). In contrast with our finding of an OC-associated risk reduction for bladder cancer among younger women, authors of 2 meta-analyses concluded that OC use is not associated with bladder cancer risk (28, 29). However, the influence of recency of OC use, drug formulation, or participant age group was not evaluated in most studies we identified (28, 30–35).
We estimated elevated risks for pancreatic cancer that strengthened with duration of OC use among women who were 60 years or younger at baseline. Our results were similar after adjusting for a history of diabetes. Sex steroid hormones, estrogen, in particular, may play important roles in endocrine and exocrine pancreatic functioning; changes in parameters like insulin sensitivity associated with hormonal contraceptives were observed in several studies, though the contraceptive pills and methods used were likely newer than were those used by our population (36–41). In the California Teacher’s Study (42), an increased risk for pancreatic cancer was noted with long-term OC use (HR = 1.72 95% CI: 1.19, 2.49), but increased risks with longer duration of use were not found in a study using data from the European Prospective Investigation Into Cancer and Nutrition (EPIC) (43). Results that were stratified by age were presented in neither study, and both studies enrolled women younger than those in the NIH-AARP study.
We did not find associations between OC use or duration of use and thyroid cancer. In another NIH-AARP study analysis and in the EPIC cohort, significant risk reductions were found with long-term use (52% and 44% reductions, respectively) (44, 45). The earlier NIH-AARP analysis, which included 312 cancer cases, had 5 fewer years of follow-up and was limited to postmenopausal women, and the authors arguably overadjusted for potential mediators and factors having an uncertain relationship with OC use (i.e., parity, menopause status, menopausal hormone therapy use, and age at last menstrual period) (44). The updated estimates in our analysis for thyroid cancer were nearly identical to the earlier analysis for women who used OCs for 1–4 years; however, the reduction in risk with long-term use emphasized in the prior publication was attenuated, likely as a result of increased follow-up and case accrual. Age and study site were adjusted for in the EPIC study, but other key confounders were missed and OC use was measured as years the drugs were taken continuously, rather than cumulatively (45). Authors of 2 meta-analyses reached different conclusions, despite overlap in the studies included; in the first, it was determined that any OC use was not related to thyroid cancer and in the other, that long-term use conferred a 16% reduction in risk (46, 47). In many cancer studies, including NIH-AARP, older populations are enrolled. As such, our data may not be best suited to fully estimate the associations between OCs and thyroid cancer given that the incidence for papillary thyroid carcinoma first peaks among women of reproductive age (48). Interestingly, in a study in which reproductive-age women were enrolled, researchers did not find an increased incidence of thyroid cancer (18).
Our goal was to estimate the total effect of OC use on cancer risk, avoiding overadjustment by not including factors that were potential mediators in our models (i.e., on a causal pathway between OC use and cancer risk) (49, 50). Adjustment for confounding differs considerably across publications, which makes comparisons with our results difficult. We were able to adjust for age at menarche, number of cigarettes smoked per day, alcohol use, and physical activity when estimating risks, unlike the study from the United Kingdom in which OC use and cancer associations across organ sites were reported (18). In contrast with some studies, we did not adjust for parity, because OC use may influence it. Adjusting for lifetime parity, which was measured in our study at baseline, would not allow for the estimation of the total effect of OC use. An individual’s pregnancy history before her first OC use might influence the decision to use these medications and cause confounding, but we did not have information on age at first birth and first OC use; this is a limitation of all study reports we examined.
Our study population was predominately composed of non-Hispanic white, postmenopausal women and as such, generalizability to similar populations is limited. We also did not have information on recency of OC use and drug formulation—something that is not evaluated in most studies. Our participants were likely using first- and second-generation OCs, which were marketed before 1989 (2, 7). Relative to first- and second-generation OCs, third-generation (1990–2000) and fourth-generation (after 2000) drugs tend to contain lower doses of estradiol and progestins with less androgenic activity (2, 7). As such, we might expect that cancer associations with first- and second-generation drugs are the strongest. Interestingly, we found that risk reductions for endometrial, kidney, and bladder cancers and risk increases for pancreatic cancer were strongest among younger women in our population, which may mean that recency of use is a greater influence on cancer development. Studies in which women using newer drugs are enrolled do not have sufficient follow-up time to evaluate many cancer outcomes associated with these drugs. Additionally, the prevalence of OC use—only 40% of our population used these medications—and patterns of use will differ in future cohorts (e.g., more frequent use before first pregnancies). To improve our understanding of the hormonal etiology of many cancers and the chemopreventive effects of these medications, we should continue to study OC use and carcinogenesis.
ACKNOWLEDGMENTS
Author affiliations: Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, Maryland (Kara A. Michels, Louise A. Brinton, Ruth M. Pfeiffer, Britton Trabert).
This research was supported by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health.
Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia. Cancer incidence data from California were collected by the California Cancer Registry, California Department of Public Health's Cancer Surveillance and Research Branch, Sacramento, California. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, Lansing, Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (Miami, Florida) under contract with the Florida Department of Health, Tallahassee, Florida. Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Health Sciences Center School of Public Health, New Orleans, Louisiana. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, The Rutgers Cancer Institute of New Jersey, New Brunswick, New Jersey. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry, Raleigh, North Carolina. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, Arizona Department of Health Services, Phoenix, Arizona. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, Austin, Texas. Cancer incidence data from Nevada were collected by the Nevada Central Cancer Registry, Division of Public and Behavioral Health, State of Nevada Department of Health and Human Services, Carson City, Nevada.
We thank Sigurd Hermansen and Kerry Grace Morrissey from Westat for study outcomes ascertainment and management and Leslie Carroll at Information Management Services for data support and analysis.
Portions of this research were presented at the Society for Epidemiologic Research annual meeting in Seattle, Washington, June 20–23, 2017.
The views expressed herein are solely those of the authors and do not necessarily reflect those of the Florida Cancer Data System or Florida Department of Health. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Conflict of interest: none declared.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- EPIC
European Prospective Investigation Into Cancer and Nutrition
- HR
hazard ratio
- NIH
National Institutes of Health
- OC
oral contraceptive
REFERENCES
- 1. De Leo V, Musacchio MC, Cappelli V, et al. Hormonal contraceptives: pharmacology tailored to women’s health. Hum Reprod Update. 2016;22(5):634–646. [DOI] [PubMed] [Google Scholar]
- 2. Petitti DB. Clinical practice. Combination estrogen-progestin oral contraceptives. N Engl J Med. 2003;349(15):1443–1450. [DOI] [PubMed] [Google Scholar]
- 3. Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11(4):411–423. [DOI] [PubMed] [Google Scholar]
- 4. Shere M, Bapat P, Nickel C, et al. Association between use of oral contraceptives and folate status: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2015;37(5):430–438. [DOI] [PubMed] [Google Scholar]
- 5. Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713–1727. [DOI] [PubMed] [Google Scholar]
- 6. Havrilesky LJ, Gierisch JM, Moorman PG, et al. Oral Contraceptive Use for the Primary Prevention of Ovarian Cancer Rockville, MD: Agency for Healthcare Research and Quality; 2013. (Evidence Report/Technology Assessment No. 212). [PMC free article] [PubMed]
- 7. Golobof A, Kiley J. The current status of oral contraceptives: progress and recent innovations. Semin Reprod Med. 2016;34(3):145–151. [DOI] [PubMed] [Google Scholar]
- 8. Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. [DOI] [PubMed] [Google Scholar]
- 9. Kleinbaum DG, Klein M. Computer appendix: survival analysis on the computer In: Survival Analysis: A Self-Learning Text. 3rd ed New York: Springer; 2012:585–588. [Google Scholar]
- 10. R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. http://www.R-project.org/. [Google Scholar]
- 11. Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1531–1543. [PubMed] [Google Scholar]
- 12. Chan MF, Dowsett M, Folkerd E, et al. Past oral contraceptive and hormone therapy use and endogenous hormone concentrations in postmenopausal women. Menopause. 2008;15(2):332–339. [DOI] [PubMed] [Google Scholar]
- 13. Igunnu A, Seok YM, Olatunji LA, et al. Combined oral contraceptive synergistically activates mineralocorticoid receptor through histone code modifications. Eur J Pharmacol. 2015;769:48–54. [DOI] [PubMed] [Google Scholar]
- 14. Berenson AB, Rahman M, Wilkinson G. Effect of injectable and oral contraceptives on serum lipids. Obstet Gynecol. 2009;114(4):786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Campesi I, Sanna M, Zinellu A, et al. Oral contraceptives modify DNA methylation and monocyte-derived macrophage function. Biol Sex Differ. 2012;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fleming DC, King AE, Williams AR, et al. Hormonal contraception can suppress natural antimicrobial gene transcription in human endometrium. Fertil Steril. 2003;79(4):856–863. [DOI] [PubMed] [Google Scholar]
- 17. Brooks JP, Edwards DJ, Blithe DL, et al. Effects of combined oral contraceptives, depot medroxyprogesterone acetate and the levonorgestrel-releasing intrauterine system on the vaginal microbiome. Contraception. 2017;95(4):405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iversen L, Sivasubramaniam S, Lee AJ, et al. Lifetime cancer risk and combined oral contraceptives: the Royal College of General Practitioners’ Oral Contraception Study. Am J Obstet Gynecol. 2017;216(6):580.e1–580.e9. [DOI] [PubMed] [Google Scholar]
- 19. Liu H, Wang XC, Hu GH, et al. Oral contraceptive use and kidney cancer risk among women: evidence from a meta-analysis. Int J Clin Exp Med. 2014;7(11):3954–3963. [PMC free article] [PubMed] [Google Scholar]
- 20. Karami S, Daugherty SE, Schonfeld SJ, et al. Reproductive factors and kidney cancer risk in 2 US cohort studies, 1993–2010. Am J Epidemiol. 2013;177(12):1368–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Butterworth M, Lau SS, Monks TJ. 2-Hydroxy-4-glutathion-S-yl-17beta-estradiol and 2-hydroxy-1-glutathion-S-yl-17beta-estradiol produce oxidative stress and renal toxicity in an animal model of 17beta-estradiol-mediated nephrocarcinogenicity. Carcinogenesis. 1998;19(1):133–139. [DOI] [PubMed] [Google Scholar]
- 22. Weisz J, Fritz-Wolz G, Clawson GA, et al. Induction of nuclear catechol-O-methyltransferase by estrogens in hamster kidney: implications for estrogen-induced renal cancer. Carcinogenesis. 1998;19(7):1307–1312. [DOI] [PubMed] [Google Scholar]
- 23. Auerbach L, Hafner T, Huber JC, et al. Influence of low-dose oral contraception on peripheral blood lymphocyte subsets at particular phases of the hormonal cycle. Fertil Steril. 2002;78(1):83–89. [DOI] [PubMed] [Google Scholar]
- 24. Kincade PW, Medina KL, Payne KJ, et al. Early B-lymphocyte precursors and their regulation by sex steroids. Immunol Rev. 2000;175:128–137. [PubMed] [Google Scholar]
- 25. Lu Y, Wang SS, Sullivan-Halley J, et al. Oral contraceptives, menopausal hormone therapy use and risk of B-cell non-Hodgkin lymphoma in the California Teachers Study. Int J Cancer. 2011;129(4):974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kane EV, Roman E, Becker N, et al. Menstrual and reproductive factors, and hormonal contraception use: associations with non-Hodgkin lymphoma in a pooled analysis of InterLymph case-control studies. Ann Oncol. 2012;23(9):2362–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Godoy G, Gakis G, Smith CL, et al. Effects of androgen and estrogen receptor signaling pathways on bladder cancer initiation and progression. Bladder Cancer. 2016;2(2):127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dietrich K, Demidenko E, Schned A, et al. Parity, early menopause and the incidence of bladder cancer in women: a case-control study and meta-analysis. Eur J Cancer. 2011;47(4):592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davis-Dao CA, Henderson KD, Sullivan-Halley J, et al. Lower risk in parous women suggests that hormonal factors are important in bladder cancer etiology. Cancer Epidemiol Biomarkers Prev. 2011;20(6):1156–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cantwell MM, Lacey JV Jr, Schairer C, et al. Reproductive factors, exogenous hormone use and bladder cancer risk in a prospective study. Int J Cancer. 2006;119(10):2398–2401. [DOI] [PubMed] [Google Scholar]
- 31. McGrath M, Michaud DS, De Vivo I. Hormonal and reproductive factors and the risk of bladder cancer in women. Am J Epidemiol. 2006;163(3):236–244. [DOI] [PubMed] [Google Scholar]
- 32. Kabat GC, Kim MY, Luo J, et al. Menstrual and reproductive factors and exogenous hormone use and risk of transitional cell bladder cancer in postmenopausal women. Eur J Cancer Prev. 2013;22(5):409–416. [DOI] [PubMed] [Google Scholar]
- 33. Prizment AE, Anderson KE, Harlow BL, et al. Reproductive risk factors for incident bladder cancer: Iowa Women’s Health Study. Int J Cancer. 2007;120(5):1093–1098. [DOI] [PubMed] [Google Scholar]
- 34. Daugherty SE, Lacey JV Jr, Pfeiffer RM, et al. Reproductive factors and menopausal hormone therapy and bladder cancer risk in the NIH-AARP Diet and Health Study. Int J Cancer. 2013;133(2):462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wolpert BJ, Amr S, Ezzat S, et al. Estrogen exposure and bladder cancer risk in Egyptian women. Maturitas. 2010;67(4):353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andrén-Sandberg A. Estrogens and pancreatic cancer: some recent aspects. Scand J Gastroenterol. 1986;21(2):129–133. [DOI] [PubMed] [Google Scholar]
- 37. Morimoto S, Morales A, Zambrano E, et al. Sex steroids effects on the endocrine pancreas. J Steroid Biochem Mol Biol. 2010;122(4):107–113. [DOI] [PubMed] [Google Scholar]
- 38. Morin-Papunen L, Martikainen H, McCarthy MI, et al. Comparison of metabolic and inflammatory outcomes in women who used oral contraceptives and the levonorgestrel-releasing intrauterine device in a general population. Am J Obstet Gynecol. 2008;199(5):529.e521–529.e510. [DOI] [PubMed] [Google Scholar]
- 39. Bender NM, Segall-Gutierrez P, Najera SO, et al. Effects of progestin-only long-acting contraception on metabolic markers in obese women. Contraception. 2013;88(3):418–425. [DOI] [PubMed] [Google Scholar]
- 40. Straznicky NE, Barrington VE, Branley P, et al. A study of the interactive effects of oral contraceptive use and dietary fat intake on blood pressure, cardiovascular reactivity and glucose tolerance in normotensive women. J Hypertens. 1998;16(3):357–368. [DOI] [PubMed] [Google Scholar]
- 41. Piltonen T, Puurunen J, Hedberg P, et al. Oral, transdermal and vaginal combined contraceptives induce an increase in markers of chronic inflammation and impair insulin sensitivity in young healthy normal-weight women: a randomized study. Hum Reprod. 2012;27(10):3046–3056. [DOI] [PubMed] [Google Scholar]
- 42. Lee E, Horn-Ross PL, Rull RP, et al. Reproductive factors, exogenous hormones, and pancreatic cancer risk in the CTS. Am J Epidemiol. 2013;178(9):1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duell EJ, Travier N, Lujan-Barroso L, et al. Menstrual and reproductive factors in women, genetic variation in CYP17A1, and pancreatic cancer risk in the European Prospective Investigation Into Cancer and Nutrition (EPIC) cohort. Int J Cancer. 2013;132(9):2164–2175. [DOI] [PubMed] [Google Scholar]
- 44. Schonfeld SJ, Ron E, Kitahara CM, et al. Hormonal and reproductive factors and risk of postmenopausal thyroid cancer in the NIH-AARP Diet and Health Study. Cancer Epidemiol. 2011;35(6):e85–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zamora-Ros R, Rinaldi S, Biessy C, et al. Reproductive and menstrual factors and risk of differentiated thyroid carcinoma: the EPIC study. Int J Cancer. 2015;136(5):1218–1227. [DOI] [PubMed] [Google Scholar]
- 46. Wu L, Zhu J. Linear reduction in thyroid cancer risk by oral contraceptive use: a dose-response meta-analysis of prospective cohort studies. Hum Reprod. 2015;30(9):2234–2240. [DOI] [PubMed] [Google Scholar]
- 47. Cao Y, Wang Z, Gu J, et al. Reproductive factors but not hormonal factors associated with thyroid cancer risk: a systematic review and meta-analysis. Biomed Res Int. 2015;2015:103515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aschebrook-Kilfoy B, Ward MH, Sabra MM, et al. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid. 2011;21(2):125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- 50. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]