Abstract
Introduction
There is a continuing worldwide shortage of organs from deceased human donors for transplantation into patients with end-stage organ failure. Genetically engineered pigs could resolve this problem, and could also provide tissues and cells for the treatment of conditions such as diabetes, Parkinson’s disease and corneal blindness.
Sources of data
The current literature has been reviewed.
Areas of agreement
The pathobiologic barriers are now largely defined. Research progress has advanced through the increasing availability of genetically engineered pigs and novel immunosuppressive agents. Life-supporting pig kidneys and islets have functioned for months or years in nonhuman primates.
Areas of controversy
The potential risk of transfer of a pig infectious microorganism to the recipient continues to be debated.
Growing points
Increased attention is being paid to selection of patients for initial clinical trials.
Areas timely for developing research
Most of the advances required to justify a clinical trial have now been met.
Keywords: cells, genetic-engineering, organs, pig, xenotransplantation
Introduction
The current shortage of donor organs for transplantation in patients with terminal organ failure is exemplified by the situation with kidney transplantation.1 Transplantation of a kidney from a deceased or living donor is optimal treatment for patients with irreversible kidney failure (end-stage renal disease, ESRD). Regardless of age, kidney recipients on average live twice as long as comparable patients on dialysis, with less morbidity and superior quality of life. Compared to financial costs associated with dialysis, a transplant is also estimated to save payers and society over $1 million per patient during an individual recipient’s lifetime. For at least a quarter of a century, the limiting factor precluding successful transplantation for thousands of wait-listed ESRD patients, and thousands more never listed, has been the relative shortage of available kidneys.
The transplant community has responded with a full-court-press of approaches to remedy the imbalance between supply and demand. To enhance availability of kidneys from deceased donors, most Western countries have developed elaborate organ recovery systems, some voluntary and others mandatory, with intricate allocation algorithms to promote both equity and optimal outcomes in recipients. Efforts are made to allocate organs to patients likely to survive the longest and also to those difficult to tissue match. Donated kidneys are also allocated on the basis of organ quality, tacitly acknowledging that growth in supply is likely to come from non-traditional sources such as donors after cardiac death or older donors with more comorbidity. There is also growing interest in improving organ quality via pump preservation and normothermic perfusion.
Nowadays, the greatest potential for growth in numbers may reside in living kidney donation. Donor nephrectomy is now widely performed as a minimally invasive procedure, with rapid postoperative recovery. Criteria for donor/recipient relationships have liberalized, with many living kidney donors now genetically unrelated to their recipient. An increasing percentage (15% in the USA) of transplants are carried out in patients in whom it has been necessary to ‘desensitize’ to their donor because of ABO blood group or major histocompatibility complex (MHC) (human leukocyte antigen (HLA)) incompatibility, or this incompatibility has been avoided by kidney paired donation.
Nonetheless, prospects for ESRD patients seeking transplantation remain bleak, and continue to worsen. In the USA alone, in 2016, 98 000 patients started the year on the waiting list, with 19 800 (20%) transplanted after a median waiting time essentially too long to calculate. Since 2005, over 9000 wait-listed patients died or became too sick to transplant. Thus, the current system supporting recovery of human kidneys for transplantation must be viewed as woefully inadequate.
It is against this backdrop that recent efforts utilizing genetically modified pig kidneys (and other organs) are moving towards clinical trials in humans.2
History of xenotransplantation
Clinical cross-species transplantation (xenotransplantation) has a long history going back to blood transfusions across species in the 17th century.3 Following the pioneering surgical work of Carrel, who developed the technique of blood vessel anastomosis, numerous attempts at nonhuman primate (NHP) organ transplantation in patients were carried out in the 20th century. In 1963–64, one patient returned to work for almost 9 months supported by a pair of chimpanzee kidneys. In 1964, the first (unsuccessful) heart transplant utilized a chimpanzee as the ‘donor’. A patient with a baboon liver transplant survived for 70 days in 1992.
However, there are several disadvantages with the use of NHPs as sources of organs and, with the advent of genetic engineering and cloning technologies, pigs are currently considered the animals most likely to resolve the problem of donor organ shortage (Table 1).
Table 1.
The advantages and disadvantages of the pig as a potential source of organs and cells for humans, in contrast with those of the baboon in this role
| Pig | Baboon | |
|---|---|---|
| Availability | Unlimited | Limited |
| Breeding potential | Good | Poor |
| Period to reproductive maturity | 4–8 months | 3–5 years |
| Length of pregnancy | 114 ± 2 days | 173–193 days |
| Number of offspring | 5–12 | 1–2 |
| Growth | Rapid (adult human size within 6 months)** | Slow (9 years to reach maximum size) |
| Size of adult organs | Adequate | Inadequate* |
| Cost of maintenance | Significantly lower | High |
| Anatomical similarity to humans | Moderately close | Close |
| Physiological similarity to humans | Moderately close | Close |
| Relationship of immune system to humans | Distant | Close |
| Knowledge of tissue typing | Considerable (in selected herds) | Limited |
| Necessity for blood type compatibility with humans | Probably unimportant | Important |
| Experience with genetic engineering | Considerable | None |
| Risk of transfer of infection (xenozoonosis) | Low | High |
| Availability of specific pathogen-free animals | Yes | No |
| Public opinion | More in favor | Mixed |
*The size of certain organs, e.g. the heart, would be inadequate for transplantation into adult humans. **Breeds of miniature swine are ~50% of the weight of domestic pigs at birth and sexual maturity, and reach a maximum weight of ~30% of standard breeds.
Pathobiology of xenotransplantation
The pathobiological barriers to successful pig organ transplantation in primates include activation of the innate and adaptive immune systems, coagulation dysregulation, and inflammation.4 A rapid innate immune response (natural anti-pig antibody, complement activation, and an innate cellular response, e.g. neutrophils, monocytes, macrophages, natural killer [NK] cells) is followed by an adaptive immune response, although T cell infiltration of the graft has rarely been reported even though T cell activation of a B cell elicited antibody response is well-documented. Factors such as coagulation dysregulation and inflammation appear to play a significantly greater role than in allotransplantation. The immune responses to a pig xenograft cannot, therefore, be controlled simply by suppression of T cell activity.
The immunologic barriers to successful xenotransplantation are primarily related to the presence of natural anti-pig antibodies in humans and NHPs that bind to antigens expressed on the transplanted pig organ (the most important of which is galactose-α1,3-galactose [Gal]),5 and activate the complement cascade, which results in rapid destruction of the graft, a process known as hyperacute rejection. Even if this initial antibody-mediated rejection is avoided through genetic modification of the pig, if the adaptive immune response is not prevented by adequate immunosuppressive therapy, high levels of elicited anti-pig IgG may develop.
The transplantation of organs and cells from pigs that do not express Gal,6 but express one or more human complement-regulatory proteins (see below), when combined with an effective immunosuppressive regimen, prevents early antibody-mediated and cellular rejection. However, low levels of anti-nonGal antibody and innate immune cells and/or platelets may initiate the development of a thrombotic microangiopathy in the graft that may be associated with a consumptive coagulopathy in the recipient. This pathogenic process is accentuated by the dysregulation of the coagulation–anticoagulation systems between pigs and primates. In pigs in which the Gal antigen has been deleted, the expression of a human coagulation-regulatory protein, e.g. thrombomodulin, is increasingly being associated with prolonged pig graft survival in NHPs.
Recently, a sustained inflammatory response has been documented in NHP recipients of pig grafts,7 and it is likely this will also need to be prevented.
Two other (nonGal) antigens expressed on pig endothelial cells have been definitively identified, namely N-glycolylneuraminic acid (Neu5Gc, a product of cytidine monophosphate-N-acetylneuraminic acid hydroxylase [CMAH])8 and Sda (a product of β1,4-N-acetylgalactosaminyl transferase 2 [β4GalNT2]).9 It is anticipated that when transplants are carried out using triple knockout pig organs, then in many patients an absence of antibody binding to the vascular endothelium of the organ will negate all of the sequelae, and features such as thrombotic microangiopathy will not develop. However, this has not yet been confirmed in pig-to-NHP preclinical models.
Genetically engineered pigs
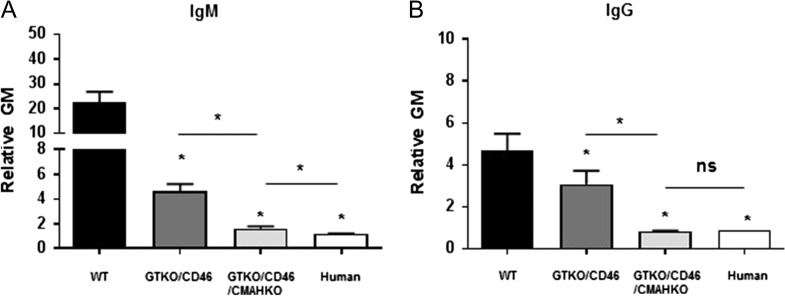
Importantly, for the first time, xenotransplantation allows modification of the donor and not only treatment of the recipient. Genetic engineering of the organ-source pig has largely involved (i) deletion of expression of one or more of the three key pig antigens, and/or (ii) insertion of a human transgene that provides protection from human complement and/or coagulation activity.10,11 Deletion of expression of two or all three key pig antigens has been achieved by simultaneous knockout of their respective genes.12,13 This is associated with markedly reduced human antibody binding to these cells (Fig. 1).
Fig. 1.
Human IgM and IgG antibody binding to wild-type (WT), GTKO/hCD46, and GTKO/hCD46/NeuGc-KO pig and human AECs by flow cytometry. Binding to GTKO/hCD46/NeuGc-KO pig cells is almost as low as to human AECs.
Genetic engineering may also contribute to overcome any of the physiological barriers that might be identified as well as in reducing the risks of transfer of a potentially infectious microorganism with the organ (see below). There are now an estimated 25 or more genetic manipulations that have been carried out in pigs, with some pigs expressing five or six manipulations. With the new technology now available (e.g. CRISPR-Cas9), it is becoming quicker and cheaper to achieve multiple genetic manipulations in pigs, thus accelerating progress towards clinical implementation of the technology.11
Immunosuppressive therapy
The T cell and elicited antibody responses to a pig xenograft can be prevented by the biologic and/or pharmacologic agents currently available, in particular, by the relatively new costimulation blockade-based regimens. Most regimens have been similar to those used in clinical allotransplantation by including a potent agent that depletes T cells, e.g. anti-thymocyte globulin (ATG), but have also included a B cell-depleting agent, e.g. anti-CD20mAb (which is frequently administered to patients HLA-sensitized to a potential allograft). However, maintenance immunosuppressive therapy with conventional pharmacologic agents, e.g. tacrolimus, rapamycin, corticosteroids, was until recently less successful than in allotransplantation, and treatment with one of the major costimulation blockade agents, e.g. anti-CD154mAb, anti-CD40mAb, was required. However, with a reduction in the expression of the three key pig antigens, conventional therapy is once again being explored. In addition, adjunctive therapy with an anti-inflammatory agent (e.g. anti-tumor necrosis factor-α and/or anti-interleukin-6 [IL-6] receptor) has been administered.
Judicious targeted genetic manipulation of the pig may allow the exogenous immunosuppressive regimen to be significantly reduced.14 For example, deletion of one or more pig antigens, e.g. Gal, in addition to reducing the humoral response, also reduces the cellular response to the graft, as does expression of a human complement-regulatory protein. In addition, there are specific modifications that can be made to reduce the cellular response, e.g. (i) insertion of a mutant (human) MHC class II transactivator gene, resulting in downregulation of swine leukocyte antigen (SLA) class II expression,15 or (ii) insertion of an immunosuppressive gene, e.g. CTLA4-Ig.16
Experience of organ transplantation in NHPs
The immune systems of Old World NHPs, e.g. baboons, rhesus monkeys, have many similarities to humans and, although not identical, are adequate surrogates in experimental models of xenotransplantation. There is now extensive experience in these models, from which some results will be highlighted
Kidney
Failure of wild-type (i.e. genetically unmodified) pig kidneys in NHPs generally occurs within minutes. Life-supporting kidneys from pigs expressing a single human complement-regulatory protein have functioned for up to 90 days.17 Deletion of expression of Gal together with the expression of one or more complement-regulatory proteins and/or coagulation-regulatory proteins has extended graft and recipient survival to >6 months, with some recipients remaining alive and well for >12 months.18–20 Renal graft function has remained normal with minimal proteinuria and no accompanying hypoalbuminemia.
Heart
Most experiments involving pig hearts have involved their transplantation as a non-life-supporting organ in the abdomen (heterotopic heart xenotransplantation) where the heart can be monitored, biopsied, and, if required, can be excised after rejection to continue to monitor the host’s immune response. Survival now generally extends for months and has been documented for >2 years).21,22
There have been relatively few reports of successful life-supporting (orthotopic) heart xenotransplantation,21,22 with the longest survival being only 54 days. However, this is likely associated with surgical technical problems and idiosyncracies of pig hearts that are likely to be resolved in the near future.
Liver
As there is no system comparable to dialysis or ventricular assist device support to maintain a patient with severe liver failure until an allograft is identified, pig liver xenotransplantation may initially be employed as a ‘bridge’ to allotransplantation. For example, in the event of fulminant hepatic failure, heterotopic liver transplantation could be carried out to support the patient until either (i) spontaneous recovery of the native liver occurred or (ii) orthotopic liver allotransplantation could be performed.
However, pig liver transplantation presents additional problems to kidney or heart transplantation, the most important of which is the rapid loss of platelets, resulting in spontaneous hemorrhage.23 Several mechanisms for this thrombocytopenia have been proposed and explored, the most likely being that recipient platelets are phagocytosed by pig liver sinusoidal endothelial cells and/or macrophages (Kupffer cells), or lost in platelet-leukocyte aggregates in the graft. To date, maximum survival has been restricted to less than a month.24 Further genetic manipulation of the pig will almost certainly resolve this problem.
Additionally, pigs could be a source of hepatocytes for the treatment of patients with inborn errors of metabolism, with genetic modifications being carried out to express high levels of the desired enzymes. Pig hepatocytes could also be employed in ex vivo perfusion liver assist devices which would enable repeated periods of temporary support using fresh hepatocytes on each occasion.
Lung
Transplantation of pig lungs in NHPs is proving even more problematic than pig livers. Dysfunctional coagulation is a major barrier, and graft failure may also be enhanced by the very fragile structure of the lung.25 Graft survival is currently measured in days rather than weeks or months.
Pancreatic islets
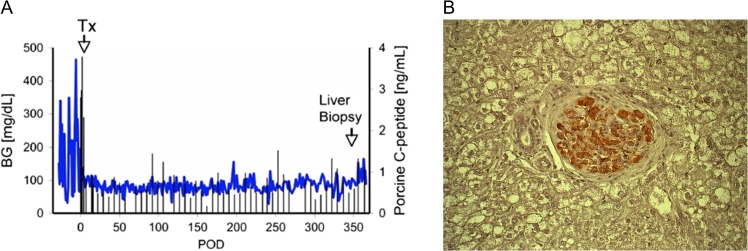
Diabetes is increasing in frequency not only in the Western world, but worldwide, affecting the health of millions or people. Expenditure on patients with diabetes now absorbs 10% of the health care budget in the USA. Advances in medical therapy, including newer glucose-lowering agents and insulin pumps, have improved the management of diabetes, but tight glucose control remains difficult. Islet allotransplantation is proving increasingly successful, but is severely limited by a lack of suitable deceased human donors. Genetically engineered pigs could resolve the problem, and several groups have reported pig islet transplantation in diabetic NHPs followed by maintenance of normoglycemia for periods of >1 year26 (Fig. 2).
Fig. 2.
(A) Blood glucose and pig C-peptide levels in a streptozotocin-induced diabetic cynomolgus monkey before and after intraportal transplantation of islets from a pig expressing the human complement-regulatory protein, CD46. No exogenous insulin was administered after the transplant. The normoglycemic monkey was electively euthanized after 12 months. Tx = day of islet transplantation. (B) Insulin immunostaining (in red) of a liver section in a monkey recipient of islets from a pig transgenic for human CD46, showing a healthy pig islet 12 months after transplantation (magnification ×200).
Parkinson’s disease
Encouraging results have been obtained in the treatment of a Parkinson-like condition induced in monkeys by the transplantation of pig dopamine-producing cells into the substantia nigra.27 Improvement in the clinical features of the condition were obtained in a significant percentage of immunosuppressed monkeys, and persisted for several months.
Skin
In the treatment of patients with severe burns, if sufficient autologous skin is not available, skin allografts from deceased donors, various artificial dermal substitutes, or skin xenografts may be transplanted to provide temporary coverage. Despite the fact that, in many patients with burns, the immune system is already suppressed, rejection of a skin xenograft is almost immediate, preventing revascularization (a so-called ‘white graft’). The transplantation of genetically engineered pig skin in a NHP (in the absence of exogenous immunosuppressive therapy), however, is followed by comparable graft survival to a skin allograft (~11–14 days).28 Furthermore, failure of a pig skin graft does not impact the success of a subsequent allograft, or vice versa, thus doubling the period of time during which adequate skin cover could be provided.29
As the administration of immunosuppressive agents to a patient with burns would almost certainly be detrimental to outcome (by increasing the risk of infection), truly prolonged pig skin graft survival will probably have to wait until immunological tolerance to the graft can be achieved, a state in which the recipient’s immune system no longer takes steps to reject the graft.
Cornea
In many countries in the world, a shortage of corneas from deceased donors greatly limits corneal transplantation in the millions of people with corneal blindness.30,31 The transplantation of decellularized pig corneal anterior lamellar grafts (which are recellularized by the patient’s own keratocytes) is already being undertaken clinically in China, with encouraging results. Full-thickness or endothelial cell corneal grafts await the same solutions as organ grafts.
Red blood cell transfusion
Even in the Western world, there are not infrequent shortages of erythrocytes for clinical transfusion, and access to HIV-negative blood is increasingly difficult in several countries, e.g. South Africa. This problem, as well as ensuring the transfused cells are not harboring an infectious microorganism, could be negated by the use of pigs as donors of erythrocytes.32 The potential risk of the transfer of a porcine endogenous retrovirus (see below) is negated as erythrocytes do not have a nucleus.
Cross-sensitization
One important question that remains to be fully answered relates to whether a pig graft will be successful in a patient highly-sensitized to human leukocyte antigens (HLA) (which prevents the patient from receiving a graft from a human donor) or will cross-sensitization result in early failure of the pig graft. Conversely, if a pig graft is transplanted and is rejected (associated with the development of antibodies to SLA), will this preclude a subsequent allograft?
The limited data are conflicting, with several studies indicating that cross-sensitization will not be problematic (as summarized above29), but others strongly suggesting that some anti-HLA antibodies cross-react with SLA.33 However, pigs in which SLA class I antigens have been deleted remain healthy,34 and there is every possibility that in due course genetic engineering techniques can be used to knockout any SLA class II gene against which a patient may have antibodies, in effect tailoring the pig specifically for the needs of the patient. This will open the possibility of a suitable organ for every highly-HLA-sensitized patient, a group of patients who currently may never receive a compatible allograft.
It should also be noted that all pigs to be used for clinical xenotransplantation will be of blood type O, thus negating the effects of ABO-incompatibility.
Physiological aspects of xenotransplantation
In early studies of kidney xenotransplantation, a high incidence of proteinuria was observed, raising the question that this resulted from physiological differences between pig and primate. In more recent studies, proteinuria has not been seen,19 suggesting that in the earlier studies it was associated with the immune response, which has now been minimized. It is likely, however, that not all of the 2000 proteins produced in the pig liver will function adequately in humans.35 If this proves to be the case, then the pig can be genetically engineered to produce the specific human protein required.
Potential for infection with a porcine agent
Zoonosis is a concern because of the potential transmission of infectious agents, including porcine endogenous retroviruses, with the pig organ to the human recipient and possibly to those who come into contact with the patient.36–38 It is now recognized that this risk is small, particularly as the source-pigs will be housed under specific pathogen-free, biosecure conditions and monitored regularly. From an infection perspective, these pigs should be greatly superior to the average deceased human donor who almost ubiquitously carries agents such as cytomegalovirus and Epstein-Barr virus that can jeopardize the recipient’s health.
There remains some concern with regard to the transmission of porcine endogenous retroviruses (PERV) as these are incorporated in the genome of every pig cell, and thus will inevitably be transplanted with the organ or cells.37–39 Although they are likely to be able to be eliminated by genetic engineering techniques, numerous studies (both experimental and clinical) have suggested that a pathologic effect is unlikely. Furthermore, several drugs are now available that would be effective in treating any adverse clinical syndrome that resulted from their transfer.
Selection of the first patients
Patient selection will be key to the initial clinical trials of xenotransplantation40 (Table 2). Potential candidates include organ (particularly kidney) transplant candidates who are allosensitized. Older patients, i.e. >60 years-old, awaiting kidney transplantation often die before a suitable allograft becomes available and so might well benefit from xenotransplantation, which would allow them at least a prolonged period without dependency on dialysis. Infants with complex congenital heart disease in whom the need for a donor heart is urgent would be strong candidates for xenotransplantation, where a suitably-sized heart will always be available. Patients dying of fulminant hepatic failure, for whom no alternative therapy is available, may be candidates for a pig liver, even if only as a bridge until an allograft becomes available.
Table 2.
Potential conditions for which initial clinical trials of pig organ xenotransplantation may be justified
| Kidney |
|
| Heart |
|
| Liver |
|
| Lung |
|
The patients most likely to benefit from an islet transplant are ‘brittle’ diabetics with hypoglycemic unawareness, particularly those experiencing life-threatening hypoglycemic episodes. In addition, diabetic patients with a previous kidney allotransplant (or who are about to receive a kidney allograft) who are already receiving immunosuppressive therapy would be candidates, as that would allay concern about submitting a patient to life-long immunosuppressive therapy simply for the treatment of a condition such as diabetes.
Comment
The timing of the first clinical trials of pig organ or cell transplantation will depend on a number of factors.
Whether the current experimental trials of conventional immunosuppressive therapy in NHPs with genetically engineered pig grafts successfully prevent a T cell-mediated elicited antibody response.
Whether the transplantation of organs from pigs in which the key antigens have been deleted is fully successful in experimental NHP models, or whether the expression of human complement- and/or coagulation-regulatory proteins is also required.
What requirements relating to porcine endogenous retroviruses will the regulatory agencies, e.g. the FDA in the USA, place on those initiating the trial.
With the recent relatively rapid advances in the (i) genetic engineering of pigs, (ii) introduction and testing of novel immunosuppressive agents in experimental models and (iii) results of pig organ transplantation in NHPs, it seems likely that clinical trials of pig organ or cell transplantation will be initiated within the next couple of years. These will initiate a new era in the treatment of patients with terminal organ failure or with conditions such as diabetes and Parkinson’s disease.
‘History tells us that procedures
that were inconceivable yesterday,
and are barely achievable today,
often become routine tomorrow.’
Thomas E. Starzl, 1982
(US transplant pioneer).
Conflict of interest statement
No author declares a conflict of interest.
Funding
Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 grant AI090959.
Authors' biographical
All authors are members of the team at the University of Alabama at Birmingham attempting to overcome the remaining barriers to xenotransplantation.
Abbreviations
- CRISPR
clustered regularly-interspaced short palindromic repeates
- Gal
galactose-α1,3-galactose
- HLA
human leukocyte antigen
- MHC
major histocompatibility complex
- NHP
nonhuman primate
- PERV
porcine endogenous retroviruses
- SLA
swine leukocyte antigen
References
- 1.United States Renal Data System. USRDS 2016 Annual Data Report. https://www.usrds.org/2016/view/Default.aspx. (9 June 2017, date last accessed).
- 2. Ekser B, Cooper DKC, Tector AJ. The need for xenotransplantation as a source of organs and cells for clinical transplantation. Int J Surg 2015;23:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cooper DKC. A brief history of cross-species organ transplantation. Proc Bayl Univ Med Cent 2012;25:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooper DKC, Ezzelarab MB, Hara H, et al. The pathobiology of pig-to-primate xenotransplantation: a historical review. Xenotransplantation 2016;23:83–105. [DOI] [PubMed] [Google Scholar]
- 5. Good AH, Cooper DKC, Malcolm AJ, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in man. Transplant Proc 1992;24:559–62. [PubMed] [Google Scholar]
- 6. Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3 galactosyltransferase-deficient pigs. Science 2003;299:411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ezzelarab MB, Ekser B, Azimzadeh A, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation 2015;22:32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bouhours D, Pourcel C, Bouhours JE. Simultaneous expression by porcine aorta endothelial cells of glycosphingolipids bearing the major epitope for human xenoreactive antibodies (Gal alpha 1-3Gal), blood group H determinant and N-glycolylneuraminic acid. Glycoconj J 1996;13:947–53. [DOI] [PubMed] [Google Scholar]
- 9. Byrne GW, Du Z, Stalboerger P, et al. Cloning and expression of porcine beta1,4 n-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation 2014;21:543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cooper DKC, Ekser B, Ramsoondar J, et al. The role of genetically-engineered pigs in xenotransplantation research. J Pathol 2015; 10.1002/path.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Butler JR, Tector AJ. CRISPR genome-editing: a medical revolution. J Thorac Cardiovasc Surg 2017;153:488–91. [DOI] [PubMed] [Google Scholar]
- 12. Lutz AJ, Li P, Estrada JL, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3- galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation 2013;20:27–35. [DOI] [PubMed] [Google Scholar]
- 13. Estrada JL, Martens G, Li P, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/Beta4GalNT2 genes. Xenotransplantation 2015;22:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Satyananda V, Hara H, Ezzelarab MB, et al. New concepts of immune modulation in xenotransplantation. Transplantation 2013;96:937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hara H, Witt W, Crossley T, et al. Human dominant-negative class II transactivator transgenic pigs – effect on the human anti-pig T cell immune response and immune status. Immunology 2013;140:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Phelps CJ, Ball SF, Vaught TD, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation 2009;16:477–85. [DOI] [PubMed] [Google Scholar]
- 17. Baldan N, Rigotti P, Calabrese F, et al. Ureteral stenosis in HDAF pig-to-primate renal xenotransplantation: a phenomenon related to immunological events? Am J Transpl 2004;4:475–81. [DOI] [PubMed] [Google Scholar]
- 18. Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation 2015;22:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iwase H, Hara H, Ezzelarab M, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation 2017;24: 10.1111/xen.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wijkstrom M, Iwase H, Paris W, et al. Renal xenotransplantation: experimental progress and clinical prospects. Kidney int 2017;91:790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mohiuddin MM, Reichart B, Byrne GW, et al. Current status of pig heart xenotransplantation. Int J Surg 2015;23:234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mohiuddin MM, Singh AK, Corcoran PC, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun 2016;7:11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ekser B, Markmann JF, Tector AJ. Current status of pig liver xenotransplantation. Int J Surg 2015;23:240–6. [DOI] [PubMed] [Google Scholar]
- 24. Shah JA, Navarro-Alvarez N, DeFazio M, et al. A bridge to somewhere: 25-day survival after pig to baboon liver xenotransplantation. Ann Surg 2016;263:1069–71. [DOI] [PubMed] [Google Scholar]
- 25. Laird C, Burdorf L, Pierson RN III. Lung xenotransplantation: a review. Curr Opin Organ Transplant 2016;21:272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park C-G, Bottino R, Hawthorne WJ. Current status of islet xenotransplantation. Int J Surg 2015;23:261–6. [DOI] [PubMed] [Google Scholar]
- 27. Vadori M, Denaro L, D’Avella D, et al. Indications and prospects of neural transplantation for chronic neurological diseases. Curr Opin Organ Transplant 2016;21:490–6. [DOI] [PubMed] [Google Scholar]
- 28. Leto Barone AA, Mastroianni M, Farkash EA, et al. Genetically modified porcine split-thickness skin grafts as an alternative to allograft for provision of temporary wound coverage: preliminary characterization. Burns 2015;41:565–74. [DOI] [PubMed] [Google Scholar]
- 29. Albritton A, Leonard DA, Leto Barone A, et al. Lack of cross-sensitization between alpha-1,3-galactosyltransferase knockout porcine and allogeneic skin grafts permits serial grafting. Transplantation 2014;97:1209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hara H, Cooper DKC. Xenotransplantation – the future of corneal transplantation? Cornea 2011;30:371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim MK, Hara H. Current status of corneal xenotransplantation. Int J Surg 2015;23:255–60. [DOI] [PubMed] [Google Scholar]
- 32. Cooper DKC, Hara H, Yazer M. Genetically engineered pigs as a source for clinical red blood cell transfusion. Clin Lab Med 2010;30:365–80. [DOI] [PubMed] [Google Scholar]
- 33. Martens GR, Reyes LM, Butler JR, et al. Humoral reactivity of renal transplant-waitlisted patients to cells from GGTA1/CMAH/B4GalNT2, and SLA Class I knockout pigs. Transplantation 2017;101:e86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reyes LM, Estrada JL, Wang ZY, et al. Creating class I MHC-null pigs using guide RNA and the Cas9 endonuclease. J Immunol 2014;193:5751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ibrahim Z, Busch J, Awwad M, et al. Selected physiologic compatibilities and incompatibilities between human and porcine organ systems. Xenotransplantation 2006;13:488–99. [DOI] [PubMed] [Google Scholar]
- 36. Onions D, Cooper DKC, Alexander TJL, et al. An approach to the control of disease transmission in pig-to-human xenotransplantation. Xenotransplantation 2000;7:143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Denner J, Mueller NJ. Preventing transfer of infectious agents. Int J Surg 2015;23:306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Denner J. Recent progress in xenotransplantation, with emphasis on virological safety. Ann Transplant 2016;21:717–27. [DOI] [PubMed] [Google Scholar]
- 39. Denner J. How active are porcine endogenous retroviruses (PERVs)? Viruses 2016;8:pii: E215, 10.3390/v8080215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cooper DKC, Wijkstrom M, Hariharan S, et al. Selection of patients for initial clinical trials of solid organ xenotransplantation. Transplantation 2017;101:1551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]