Abstract
This study uses Medicaid drug utilization data to describe reimbursement and market share of long-acting insulins before and after approval of new insulin products and to estimate savings associated with a “biosimilar” for insulin glargine approved in 2015.
Insulin prices increased by 320% from 2001 to 2014,1,2 partly because of limited competition and barriers to generic entry.2,3 There were only 2 long-acting insulins available before 2015, insulin glargine (Lantus [Sanofi]), and insulin detemir (Levemir [Novo Nordisk]). In December 2015, a “biosimilar” for insulin glargine (Basaglar [Lilly]) gained US Food and Drug Administration approval. Although approved as a new drug rather than through the biosimilar pathway,4 it is the first substitute for Lantus. Basaglar’s approval coincided with approvals of highly concentrated insulin glargine (Toujeo [Sanofi]; February 2015), ultra long-acting insulin degludec (Tresiba [Novo Nordisk]; September 2015), and a combination of insulin glargine and lixisenatide (Soliqua [Sanofi]; November 2016).
We examined changes in reimbursement and market share of long-acting insulins in Medicaid following the approval of these new products. Additionally, we estimated savings associated with the use of Basaglar instead of Lantus.
Methods
Using Medicaid state drug utilization data, we extracted fee-for-service and managed care reimbursement records for long-acting insulins1 from quarter 1 (Q1) 2005 to Q2 2018, including insulin glargine, 100 international units (IU)/mL (Basaglar and Lantus); insulin detemir; insulin glargine–lixisenatide; insulin glargine, 300 IU/mL; and insulin degludec. For every product and quarter, we calculated the mean amount reimbursed per 100 IU and the market share, defined as the proportion of all long-acting IU reimbursed for the particular product. All outcomes were reported at the product level.
To estimate Medicaid savings associated with Basaglar, we multiplied the number of milliliters (100 IU) reimbursed for Basaglar each quarter by the lower reimbursement rate of Basaglar compared with Lantus (ie, the difference between postrebate reimbursement rates for Lantus and Basaglar). Because Basaglar was approved as a new drug,4 it receives the base rebate of 23.1% of average manufacturer price.5 Our estimates do not account for inflationary or supplemental rebates, which remain proprietary.
Results
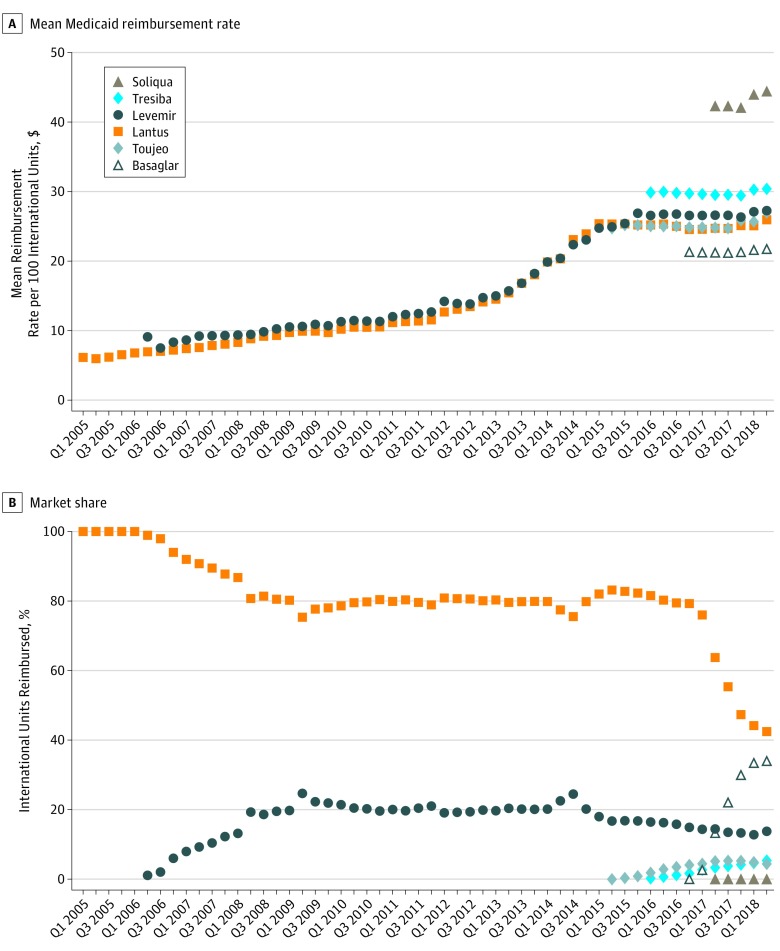
In 2005-2007, Lantus was the predominant long-acting insulin (Figure). From 2008-2014, Lantus accounted for approximately 80% of IU reimbursed for long-acting insulins, and insulin detemir accounted for the remaining 20%. Following the approval of new products, the market shares of Lantus and insulin detemir decreased to 42% and 14%, respectively. By Q1 2018, the market share of Basaglar reached 34% of all IU for long-acting insulins, or 44% of all IU for insulin glargine, 100 IU/mL (Table). In Q1 2018, insulin glargine, 300 IU/mL, and insulin degludec each accounted for 5% of IU for long-acting insulins and insulin glargine–lixisenatide for 0.1%.
Figure. Mean Medicaid Reimbursement Rate and Market Share of Long-Acting Insulin Products, 2005-2018.
The upper panel shows the mean reimbursement per 100 international units for each long-acting insulin product and quarter, before minimum statutory rebates, and expressed in nominal dollars. The lower panel shows the market share of each long-acting insulin product, which was defined as the proportion of international units for long-acting insulins reimbursed by Medicaid in each quarter for each insulin product. Lantus is insulin glargine; Basaglar, “biosimilar” for insulin glargine; Toujeo, highly concentrated insulin glargine; Levemir, insulin detemir; Tresiba, insulin degludec; and Soliqua, combination insulin glargine and lixisenatide.
Table. Changes in Spending Associated With Use of Basaglar Instead of Lantus in Medicaida.
| Quarter | No. of Milliliters (100 IU) Reimbursed | % of Milliliters (100 IU) Basaglar | Mean Amount Reimbursed per Milliliter (100 IU) After Minimum Statutory Rebates, $b | Spending if 100% Brand-Name Use, $ | Savings From Biosimilar Usec | |||
|---|---|---|---|---|---|---|---|---|
| Lantus | Basaglar | Lantus | Basaglar | $ | % | |||
| Q4 2016 | 15 388 234 | 1062 | 0 | 18.90 | 16.37 | 290 854 469 | 2688 | 0.0 |
| Q1 2017 | 14 854 227 | 528 227 | 3 | 18.89 | 16.31 | 290 533 522 | 1 359 898 | 0.5 |
| Q2 2017 | 13 160 463 | 2 708 533 | 17 | 19.01 | 16.29 | 301 641 334 | 7 349 793 | 2.4 |
| Q3 2017 | 10 782 516 | 4 297 872 | 28 | 19.01 | 16.29 | 286 682 620 | 11 711 823 | 4.1 |
| Q4 2017 | 8 924 676 | 5 656 298 | 39 | 19.31 | 16.33 | 281 555 989 | 16 873 763 | 6.0 |
| Q1 2018 | 8 635 776 | 6 526 285 | 43 | 19.28 | 16.60 | 292 340 602 | 17 489 712 | 6.0 |
| Q2 2018 | 6 993 888 | 5 593 548 | 44 | 19.97 | 16.72 | 251 333 434 | 18 140 025 | 7.2 |
| Total, Q4 2016 to Q2 2018 | 1 704 087 501 | 72 925 015 | 4.3 | |||||
Lantus is insulin glargine and Basaglar is the “biosimilar” for insulin glargine.
Under the Medicaid Drug Rebate Program,5 innovator products are subject to a minimum statutory rebate of 23.1% of average manufacturer price. In analyses, the 23.1% rebate base was applied to the mean reimbursement amount per milliliter (100 international units [IU]) before rebates, which was the estimate available in the data. Because Basaglar was approved through a new drug application, it is treated as an innovator drug by Medicaid and therefore subject to the 23.1% rebate rate. These estimates do not account for additional rebates due to price increases above inflation or other supplemental rebates negotiated between manufacturers and Medicaid.
Number of milliliters (100 IU) reimbursed for Basaglar × (postrebate mean amount reimbursed for Lantus minus postrebate mean amount reimbursed for Basaglar).
Reimbursement rates for Lantus and insulin determir increased in parallel in 2006-2014 by an average of 13% annually but stabilized following new product entry. Without accounting for inflationary or supplemental rebates, Basaglar reimbursement rates have been 15% to 16% lower than those of Lantus since its entry. From Q4 2016 to Q2 2018, Basaglar entry resulted in savings of more than $70 million to Medicaid, or around 4.4% of expenditures on insulin glargine, 100 IU/mL.
Discussion
In the long-acting insulin market, new product entry was associated with a halt in increases in reimbursement levels for incumbent products. The first biosimilar insulin had a large uptake and was associated with modest reductions in insulin expenditures for Medicaid.
The present analysis has important limitations, including the unavailability of inflationary and supplemental rebate data and the potential effect on prices of external forces such as public pressure. Savings associated with Basaglar would be lower if supplemental rebates were larger for Lantus than for Basaglar. Additionally, it is not possible to determine whether the slowing of price increases for incumbent products was a result of Basaglar entry or due to the approval of branded competitors, which has not been shown to lower inflation in other therapeutic classes.6
Nevertheless, these findings suggest that increased competition in the long-acting insulin market was associated with lower per-milliliter reimbursements in Medicaid, lending support to policies that expedite biosimilar approval and market entry. More savings should be expected once biosimilars are labeled as “interchangeable” and can be automatically substituted at the pharmacy, which is not currently the case for Basaglar.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Luo J, Avorn J, Kesselheim AS. Trends in Medicaid reimbursements for insulin from 1991 through 2014. JAMA Intern Med. 2015;175(10):1681-1686. doi: 10.1001/jamainternmed.2015.4338 [DOI] [PubMed] [Google Scholar]
- 2.Hua X, Carvalho N, Tew M, Huang ES, Herman WH, Clarke P. Expenditures and prices of antihyperglycemic medications in the United States: 2002-2013. JAMA. 2016;315(13):1400-1402. doi: 10.1001/jama.2016.0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greene JA, Riggs KR. Why is there no generic insulin? historical origins of a modern problem. N Engl J Med. 2015;372(12):1171-1175. doi: 10.1056/NEJMms1411398 [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration Center for Drug Evaluation and Research Approval package for Basaglar. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/205692Orig1s000Approv.pdf. Accessed January 7, 2019.
- 5.Centers for Medicare & Medicaid Services Medicaid drug rebate program. https://www.medicaid.gov/medicaid/prescription-drugs/medicaid-drug-rebate-program/index.html. Accessed September 25, 2018.
- 6.San-Juan-Rodriguez A, Prokopovich MV, Shrank WH, Good CB, Hernandez I. Assessment of price changes of existing tumor necrosis factor inhibitors after the market entry of competitors. JAMA Intern Med. 2019. doi: 10.1001/jamainternmed.2018.7656 [DOI] [PMC free article] [PubMed] [Google Scholar]