Abstract
Background and Purpose
Stress is known to reduce food intake. Many aspects of the stress response and feeding are regulated by the endocannabinoid system, but the roles of anandamide (AEA) and 2‐arachidonoyl glycerol (2‐AG) in stress‐induced anorexia are unclear.
Experimental Approach
Effects of acute restraint stress on endocannabinoids were investigated in male Sprague–Dawley rats. Systemic and central pharmacological inhibition of fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MAGL) was used to assess the effects of elevated AEA and 2‐AG on homeostatic feeding and on food consumption after stress. Animals were pretreated with the FAAH inhibitor, PF‐04457845, or the MAGL inhibitor, MJN110, before 2 h acute restraint stress or 2 h homecage period without food.
Key Results
Restraint stress decreased hypothalamic and circulating AEA, with no effect in the gastrointestinal tract, while 2‐AG content in the jejunum (but not duodenum) was reduced. PF‐04457845 (30 μg), given i.c.v., attenuated stress‐induced anorexia via CB1 receptors, but reduced homeostatic feeding in unstressed animals through an unknown mechanism. On the other hand, systemic administration of MJN110 (10 mg·kg−1) reduced feeding, regardless of stress or feeding status and inhibited basal intestinal transit in unstressed rats. The ability of MAGL inhibition to reduce feeding in combination with stress was independent of CB1 receptor signalling in the gut as the peripherally restricted CB1 receptor antagonist, AM6545 did not block this effect.
Conclusions and Implications
Our data reveal diverse roles for 2‐AG and AEA in homeostatic feeding and changes in energy intake following stress.
Linked Articles
This article is part of a themed section on 8th European Workshop on Cannabinoid Research. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.10/issuetoc
Abbreviations
- 2‐AG
2‐arachidonoyl glycerol
- AEA
anandamide
- CRH
corticotropin releasing hormone
- FAAH
fatty acid amide hydrolase
- GCs
glucocorticoids
- HPA
hypothalamic–pituitary–adrenal
- MAGL
monoacylglycerol lipase
- OEA
oleoylethanolamide
- PEA
palmitoylethanolamide
Introduction
Exposure to stress results in wide‐ranging effects on physiology and behaviour. In particular, energy homeostasis is profoundly affected by acute and chronic stress (Ulrich‐Lai and Ryan, 2014; Razzoli and Bartolomucci, 2016). These effects are complex and depend on a variety of factors such as stressor type, duration and frequency (Maniam and Morris, 2012; Razzoli et al., 2017). One of the best documented effects of stress is on homeostatic feeding, typically resulting in reduction of food intake – known as stress‐induced anorexia (Krahn et al., 1986).
Stress activates the hypothalamic–pituitary–adrenal (HPA) axis, which has a negative effect on appetite and feeding behaviour (Harris, 2015). The principal stress mediator, corticotropin releasing hormone (CRH) stimulates the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary and in turn triggers the synthesis of glucocorticoids (GCs). Activation of the HPA axis by CRH signalling results in anorexia, whereas GC release from the adrenal cortex serves as a negative feedback to reduce HPA activation and can stimulate food intake (Tempel et al., 1991; Santana et al., 1995). Early studies revealed that central administration of exogenous CRH in rats results in the short‐term reduction of feeding (Morley and Levine, 1982). Indeed, a single acute episode of restraint stress also produces anorexia (Krahn et al., 1986; Shibasaki et al., 1988), and the effects are attenuated by CRH antagonism (Krahn et al., 1986; Shibasaki et al., 1988; Smagin et al., 1999). The magnitude of anorexia depends on the frequency and exposure to stress (Krahn et al., 1990), as well as intensity of the stressor (Martí et al., 1994) with stress mirroring the effect of administering CRH. Taken together, activation of the HPA axis by stress (or exogenous CRH) reduces homeostatic feeding via activation of CRH receptors.
The endocannabinoid system also has a profound regulatory effect on energy balance (Silvestri and Di Marzo, 2013; Piazza et al., 2017). Two endocannabinoids, anandamide (AEA) and 2‐arachidonoyl glycerol (2‐AG), have been shown to play important homeostatic roles via the activation of cannabinoid CB1 receptors in distinct feeding circuits (Lau et al., 2017) and are increased in the brain (Kirkham et al., 2002) and gut (Dipatrizio et al., 2015) following food deprivation, likely due to their role in increasing appetite and motivation to feed. Indeed, manipulations that increase endocannabinoids also stimulate food intake: systemic (Williams and Kirkham, 1999; Hao et al., 2000) and central (Jamshidi and Taylor, 2001; Mahler et al., 2007) AEA administration increases feeding in rodents. Similarly, central administration of 2‐AG stimulates feeding of standard rat chow (Kirkham et al., 2002) and energy‐dense foods high in fat/sucrose (DiPatrizio and Simansky, 2008). These studies demonstrate the potent stimulatory effect of AEA and 2‐AG on feeding.
The role of the endocannabinoids in stress‐induced changes in feeding remains unclear. A number of studies have investigated how the endocannabinoid system influences the HPA axis (see Hill and Tasker, 2012; Morena et al., 2016), while also modulating homeostatic/hedonic feeding (see Lau et al., 2017). Moreover, stress has a robust effect on modulating both AEA and 2‐AG signalling (see Morena et al., 2016 for review), which involves the actions of both CRH and GCs (Evanson et al., 2010; Hill et al., 2011; Wang et al., 2012; Gray et al., 2015, 2016; Natividad et al., 2017).
The present study investigated the modulation, by the endocannabinoids, of food intake and body weight in both stressed and unstressed subjects. Specifically, we assessed how increases in 2‐AG and AEA affected homeostatic feeding and stress‐induced changes in energy intake via inhibition of their catabolic enzymes, monoacylglycerol lipase (MAGL) and fatty acid amide hydrolase (FAAH) respectively. Given that endocannabinoids modulate stress and feeding via distinct peripheral and CNS targets, we compared pharmacological manipulations following systemic and i.c.v. administration. Stress effects on tissue‐specific endocannabinoid levels were also investigated, as well as the effects of signalling by 2‐AG on intestinal transit.
Methods
Animals
All animal care and experimental procedures were approved by the Animal Care Committee of the University of Calgary and carried out in accordance with the recommendations of the Canadian Council on Animal Care. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Male Sprague–Dawley rats (400–450 g; Charles River Lab, St Constant, Quebec) were single‐housed in clear plastic cages (47 × 25 × 20 cm) and maintained on a reverse light/dark cycle (10:00 am lights off; 22:00 lights on) with free access to food (Prolab RMH 2500) except during behavioural testing.
Surgery for implantation of an i.c.v. cannula
The rats were allowed 1 week to acclimatise to the facility and then an i.c.v. cannula was implanted. Surgery was conducted as described previously (Gray et al., 2015; Sticht et al., 2015). Briefly, animals were anaesthetized under isoflurane and received an analgesic (meloxicam, 1 mg·kg−1) before the implantation of a 22 gauge cannula directed at the lateral ventricle (−0.9 mm, anteroposterior; +1.4 mm, mediolateral, −2.8 mm, dorso‐ventral from skull surface). The cannula was affixed to the skull using dental cement, and a blocker was inserted to prevent any occlusion.
Behavioural procedures
Experiment 1: Validation of stress model and stressor duration on feeding and body weight
Although many studies have assessed the effects of stress on feeding, there are often subtle differences in the stress and feeding manipulations that are employed, which can affect how stress modulates ingestive behaviour. Therefore, Experiment 1 investigated the extent to which stressor duration affected feeding and body weight gain in rats.
Experiment 1a: 1 h restraint stress
Rats were habituated to the handling/feeding procedures for 2 days before testing. On these habituation days, animals were weighed in the morning and remained in their homecage for the remainder of the day. Food intake was recorded by removing the chow from the wire cage lid and weighing at each interval. Feeding measurements began at the onset of the dark cycle and chow consumption was recorded at 1, 2, 4, 8 and 22 h following the light cycle change. On the test day, approximately half of the rats (1 h stress; n = 6) were removed from their homecage and placed into clear Plexiglas restraint tubes for 1 h. The remaining animals (HC; n = 7) remained in their homecage for the duration of the stress period without food. After 1 h, the stress group was returned to their homecage and all animals (stress group and control group) received approximately 10 g of chow on top of the wire cage lid, with additional food being replenished as necessary throughout the testing period. For 3 days after experimental manipulations, the animals were weighed daily in the morning to assess body weight gain. Following behavioural testing, the animals were killed with an overdose of pentobarbital (800 mg·kg−1).
Experiment 1b: 2 h restraint stress
Rats were habituated and tested as in Experiment 1a; however, animals received a 2 h restraint stress (2 h stress; n = 14) or homecage (HC; n = 13) food restriction period for 2 h.
Experiment 1c: Effect of 2 h restraint stress on tissue‐specific endocannabinoid levels
To determine the effects of the stress manipulation on the endocannabinoid system, levels of 2‐AG, AEA, palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) were analysed in different tissue compartments in the central and peripheral nervous system known to play a role in feeding and stress. Rats were balanced for body weight prior to experiments and assigned to two groups corresponding to a 2 h restraint stress (Stress; n = 10) or homecage period without access to food (HC; n = 10). Immediately following this 2 h period, the rats were killed by rapid decapitation and tissue was collected from distinct tissue compartments and immediately flash frozen on dry ice. The hypothalamus was selected given its role in mediating homeostatic feeding and response to stress. Trunk blood was collected to assay circulating endocannabinoid levels; blood was centrifuged at 1500× g for 20 min at 4°C, and serum was collected for endocannabinoid analysis. Gut tissue was collected from the jejunum (Dipatrizio et al., 2015) and duodenum (Sykaras et al., 2012) given that these subregions are thought to play an important regulatory role in feeding (DiPatrizio, 2016). Because 2‐AG levels have been found to be highest in the mucosal layer (Dipatrizio et al., 2015), endocannabinoids were quantified in this region. After laparotomy, the stomach was isolated outside the abdominal cavity and the small intestine was transected at the level of the pylorus. The first 8 cm were sampled as duodenum, 12 cm were discarded distally and another 8 cm segment was sampled as jejunum (20 cm from the pylorus). The intestinal segments were opened flat along the mesenteric border, and the adipose tissue was removed. The mucosa was separated from the muscularis externa and serosa by scraping with a glass slide. Samples were immediately flash frozen in liquid nitrogen and kept at −80°C until the time of processing.
Experiment 2: Effect of systemic endocannabinoid manipulations on feeding and body weight
The endocannabinoid system is known to exert regulatory effects on both feeding and appetite, as well as physiological response to stress. Therefore, to investigate how endocannabinoid manipulations potentially modulate stress‐induced changes in feeding, the effects of systemic inhibition of FAAH or MAGL were assessed separately on homeostatic (homecage) feeding and food intake following stress. Doses were selected based on previous studies in which systemic injection of MJN110 (10 mg·kg−1) was sufficient to increase cortical levels of 2‐AG (Parker et al., 2014). PF‐04457845 (10 mg·kg−1) has been shown to increase brain and plasma levels of anandamide to a similar degree in rats (Ahn et al., 2011).
Experiment 2a: Homecage feeding
On the test day, rats were injected systemically (i.p.) with vehicle (VEH; n = 13), PF‐04457845 (PF; 10 mg·kg−1; n = 10) or MJN110 (MJN; 10 mg·kg−1; n = 10) 2 h prior to undergoing a 2 h food restriction period. All chow was removed during this period to match the food restriction that animals experience during restraint stress. Following behavioural testing, the animals were killed with an overdose of pentobarbital (800 mg·kg−1).
Experiment 2b: Stress‐induced anorexia
Subjects were treated as described in Experiment 2a, with the exception that the rats were subjected to a 2 h restraint stress following pretreatment with vehicle (VEH; n = 13), PF‐04457845 (PF; 10 mg·kg−1; n = 10) or MJN110 (MJN; 10 mg·kg−1; n = 11). Following stress, the rats were immediately returned to their homecage and received approximately 10 g of chow on top of the wire cage lid. Feeding measurements were carried out as described above, and weight gain was monitored for 3 days following stress.
Experiment 3: Effect of antagonism of peripheral CB1 receptors on MJN110‐induced anorexia
This experiment assessed a potential peripheral mechanism underlying the differential changes in feeding and weight gain following stress and MAGL inhibition in Experiment 2. The extent to which peripheral CB1 receptor signalling mediates the anorectic effects of MAGL inhibition were investigated using the peripherally restricted CB1 receptor antagonist AM6545.
Experiment 3a: Homecage feeding
The animals were treated as described previously. However, in addition to receiving a systemic injection of vehicle (VEH; n = 15) or MJN110 (MJN; n = 11) 2 h prior to food restriction, all rats were additionally pretreated with AM6545 (5 mg·kg−1, i.p.) 30 min before the food was removed from their homecage. This dose of AM6545 was selected on the basis of previous studies indicating that 5 mg·kg−1 does not inhibit food intake (Cluny et al., 2010b). Following behavioural testing, the animals were killed with an overdose of pentobarbital (800 mg·kg−1).
Experiment 3b: Stress‐induced anorexia
Animals undergoing stress received an injection of vehicle (VEH; n = 17) or MJN110 (MJN; n = 10) 2 h prior to the stress manipulation. Administration of AM6545 (5 mg·kg−1, i.p.) occurred 30 min before the restraint period. Rats were returned to their homecage after stress, and intake was monitored as before. Body weight gain was monitored for 3 days following stress.
Experiment 4: Effect of systemic MAGL inhibition on small intestinal transit
To explore the basis by which MAGL inhibition differentially affects stress‐induced anorexia and body weight gain, Experiment 4 evaluated basal intestinal transit following MAGL inhibition as well as after a 2 h period of stress.
Experiment 4a: Unstressed transit
Rats received an injection of vehicle (VEH; n = 4) or 10 mg·kg−1 MJN110 (MJN; n = 5) 2 h prior to undergoing a 2 h food restriction period, as before. Immediately following the food restriction period, rats were administered a nonabsorbable marker containing 0.2 mL of 5% Evans blue (Sigma‐Aldrich, St. Louis, MO, USA) and 5% gum Arabic (Sigma‐Aldrich) in 0.9% saline, which was given via oral gavage. Rats were then placed back into their homecage with a pre‐weighed amount of food. After 45 min, rats were killed via rapid decapitation and small intestinal transit was determined following laparotomy. The small intestine was removed from the gastric pylorus to the caecum. Small intestinal transit was measured from the gastroduodenal junction to the most distal point where Evans blue dye was evident and expressed as a percentage of the total distance from the oral end of the small intestine to the ileocaecal junction.
Experiment 4b: Stressed transit
Two hours prior to undergoing restraint stress (2 h), rats were injected with vehicle (VEH; n = 12) or 10 mg·kg−1 MJN110 (MJN; n = 9). Immediately following termination of stress rats were administered the dye mixture via oral gavage and placed back into their homecage with access to chow. Animals were killed after 45 min, and small intestinal transit was measured as described above.
Experiment 5: Effect of i.c.v. FAAH/MAGL inhibition on feeding
The stress manipulation in the current study was found to negatively impact AEA and 2‐AG levels in distinct central and peripheral tissue compartments. Therefore, the aim of Experiment 5 was to differentiate between peripheral versus central manipulations targeting the endocannabinoid system, by limiting drug delivery to the brain via i.c.v. administration. Rats were allowed to recover for 1 week following surgery and were then habituated to the handling and feeding procedures, as before. For 2 days prior to testing, animals were weighed in the morning and remained in their homecage for the remainder of the day when food intake was recorded. To our knowledge, PF‐04457845 has not previously been administered centrally, although an analogous FAAH inhibitor, PF‐3845, has been shown to inhibit gut transit in mice, following a 30 μg i.c.v. infusion (Fichna et al., 2014). Therefore, a 10‐fold lower dose was selected for Experiment 5, whereas the higher dose of 30 μg was used for Experiment 6. To date, there are no reports assessing i.c.v. administration of MJN110, although several studies have demonstrated CB1 receptor‐dependent effects of localized injection (2 μg) into distinct brain regions (Limebeer et al., 2016; Sticht et al., 2016; Wills et al., 2016). As the current study assessed i.c.v. administration, we selected a 2.5× higher dose given the larger area for drug diffusion.
Experiment 5a: Homecage feeding
On the test day, rats were removed from their homecage and received an i.c.v. infusion of vehicle (VEH; n = 14), PF‐04457845 (PF; 3 μg; n = 9) or MJN110 (MJN; 5 μg; n = 7) at a rate of 0.25 μL·min−1 for 2 min. The infuser remained in place for an additional minute to allow the drug to diffuse into the ventricle. Animals were immediately placed back into their homecage for 1 h prior to undergoing the 2 h food restriction period. Following food restriction, all rats received approximately 10 g of chow on top of the wire cage lid, with additional food being replenished as necessary throughout the testing period. Body weight gain was monitored for 3 days. Following behavioural testing, the rats were killed via pentobarbital injection while also receiving an i.c.v. infusion of Evans blue dye to verify cannula placement. The brains were extracted and sectioned to reveal the extent of dye in the ventricle. If the infusion was outside the ventricle, the corresponding data were removed from the experiment. As such, the subject number for each treatment condition is representative of subjects for which there was confirmed targeting of the lateral ventricle with the implanted cannula.
Experiment 5b: Stressed‐induced anorexia
Animals received an i.c.v. infusion as described above with vehicle (VEH; n = 16), PF‐04457845 (PF; 3 μg; n = 9) or MJN110 (MJN; 5 μg; n = 8) and were placed into their homecage for 1 h prior restraint stress (2 h). Following termination of stress, the rats were immediately returned to their homecage for behavioural testing. All remaining procedures were identical to that of the homecage feeding animals in Experiment 5a.
Experiment 5c: Effect of dual i.c.v. FAAH and MAGL inhibition on homecage feeding
This experiment investigated whether dual inhibition of FAAH and MAGL would result in greater modulation of feeding as compared to selective enzyme inhibition. Vehicle‐pretreated rats from Experiment 6a were used as a comparison in the pretreatment analysis in this experiment. Rats were pretreated with a cocktail containing PF‐04457845 and MJN110 (3 μg PF; 5 μg MJN; n = 8) or vehicle (VEH; n = 14) 1 h prior to the 2 h food restriction period, as before.
Experiment 5d: Effect of dual i.c.v. FAAH and MAGL inhibition on stress‐induced anorexia
Rats were treated with PF‐04457845 and MJN110 (3 μg PF; 5 μg MJN; n = 8) or vehicle (VEH; n = 16) 1 h prior to restraint stress (2 h). All remaining procedures were identical to that of the previous experiment and vehicle‐pretreated rats from Experiment 6b were used as a comparison in the pretreatment analysis in this experiment.
Experiment 6: Effect of high dose PF‐04457845 on feeding
As we found that stress decreased AEA content within the hypothalamus, we reasoned that a higher dose of a FAAH inhibitor may be required to elevate AEA signalling back to normal levels. Accordingly, Experiment 7 assessed whether a higher dose of PF‐04457845 would modulate feeding under homecage and stress conditions, compared with the effects of the lower dose used in earlier experiments. The analogous FAAH inhibitor, PF3845, has been shown in mice to inhibit gut transit following a 30 μg i.c.v. infusion (Fichna et al., 2014); therefore, we assessed whether 30 μg of PF‐04457845 would be sufficient to induce a change in feeding under the current conditions. Given that antagonism of central CB1 receptors modulated feeding (Merroun et al., 2009), we also assessed whether the CB1 receptor antagonist, AM251, would modulate feeding alone or when combined with a high dose of PF‐04457845 in the current experiment.
Experiment 6a: Homecage feeding
Rats received an i.c.v. infusion as in the previous experiments. However, to accommodate a higher dose of PF‐04457845, drug was delivered in a total volume of 2 μL over 2 min (1 μL·min−1) with an additional minute to allow for diffusion in the ventricle. Rats were treated with vehicle (VEH; n = 12), PF‐04457845 (PF; 30 μg n = 13), AM251 (AM; 0.1 μg; n = 10) or a cocktail containing PF‐04457845 and AM251 (PF + AM; n = 8) and placed back into their homecage for 60 min. Animals were food restricted for 2 h, after which feeding measures began at the onset of the dark cycle.
Experiment 6b: Stress‐induced anorexia
Subjects received an i.c.v. infusion as described above with vehicle (VEH; n = 13), PF‐04457845 (PF; 30 μg n = 13), AM251 (AM; 0.1 μg; n = 8) or a cocktail containing PF‐04457845 and AM251 (PF + AM; n = 9) 60 min prior to restraint stress (2 h). Following termination of stress, the rats were immediately returned to their homecage for behavioural testing. All remaining procedures were identical to that of the homecage feeding animals in Experiment 5a.
Endocannabinoid extraction and analysis
Drug naïve rats were killed after stress or homecage food restriction by rapid decapitation, and tissues were collected as described above. Lipid extractions were carried out as previously described (Qi et al., 2015). Briefly, frozen brain and gut tissue was weighed and then manually homogenized (with a glass rod) in borosilicate glass culture tubes containing 2 mL of acetonitrile with 5 nmol of d8‐2‐AG, 5 pmol of d8‐AEA, 40 pmol d4‐PEA and 40 pmol d4‐OEA. For serum endocannabinoid levels, 500 μL of serum was added directly to the acetonitrile with the same preparation of internal standard as the tissue samples. All other steps of processing for serum were identical to those for tissue (described below). All samples were sonicated for 30 min in an ice bath and incubated overnight at −20°C to precipitate proteins. The following day samples were centrifuged at 1500× g to remove particulates. The supernatant from each sample was transferred to a new glass tube and evaporated under nitrogen, the tube was then washed once with 350 μL acetonitrile (to recapture any lipids adhering to the glass wall) and the acetonitrile was dried under nitrogen gas again. After complete drying, the samples were re‐suspended in 200 μL of acetonitrile and stored at −80°C until analysis by LC–MS. Analysis by MS was performed exactly as previously described (Qi et al., 2015).
Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018). Behavioural, biochemical and intestinal transit data are presented as means (±SEM) and were analysed in SPSS Statistics (IBM; version 22, Armonk, NY, USA). Results were considered significant at P < 0.05 for all statistical tests. The feeding data from early (1 h, 2 h) and later (4 h, 8 h, 22 h) time points were analysed using separate one‐way ANOVAs or independent t‐tests at each time point, as appropriate. Post hoc tests were conducted as required using Fisher's LSD. For the biochemical data, endocannabinoid levels were compared between stressed and unstressed animals using t‐tests. Differences in intestinal transit following drug pretreatment were compared using t‐tests.
Materials
All drugs were administered in a vehicle solution containing a 1:1:18 ratio of DMSO:Tween‐80:saline. For systemic administration, PF‐04457845 (10 mg·kg−1; provided by Pfizer, Cambridge, MA, USA), MJN110 (10 mg·kg−1; provided by B.F. Cravatt, The Scripps Research Institute, La Jolla, CA, USA) and AM6545 (5 mg·kg−1; provided by A. Makriyanis, Northeastern University, Boston, MA, USA) were injected at 1 mL·kg−1. For central administration, PF‐04457845 was prepared at a concentration of 6 μg·μL−1 and MJN110 was prepared at 10 μg·μL−1. Either drug was delivered in a final volume of 0.5 μL into the lateral ventricle. In Experiment 6, PF‐04457845 was prepared at a concentration of 15 μg·μL−1 alone or in combination with AM251 (0.05 μg·μL−1; Tocris, Bristol, UK) and delivered into the lateral ventricle in a total of 2 μL. In all cases, the drug was first dissolved in DMSO, and then warm Tween‐80 was added. After the DMSO and Tween‐80 were mixed, warm saline was added to make up the final concentration.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a, 2017b, 2017c, 2017d).
Results
Experiment 1: Validation of stress model and stressor duration on feeding and body weight
Stress effects on feeding depend on stressor intensity (and duration) and motivation to feed (appetite). Although 1 h restraint did not modify feeding, 2 h stress reduced cumulative intake and body weight, as well as reduced AEA and 2‐AG levels in different regions.
Experiment 1a: 1 h restraint stress
An acute 1 h restraint stress did not change cumulative feeding during early (1, 2 h; Figure 1A) or late (4, 8, 22 h; Figure 1B) post‐stress time points. However, analysis of the body weight revealed that 1 h stress significantly reduced body weight gain at 24 h, but not at 48 or 72 h.
Figure 1.
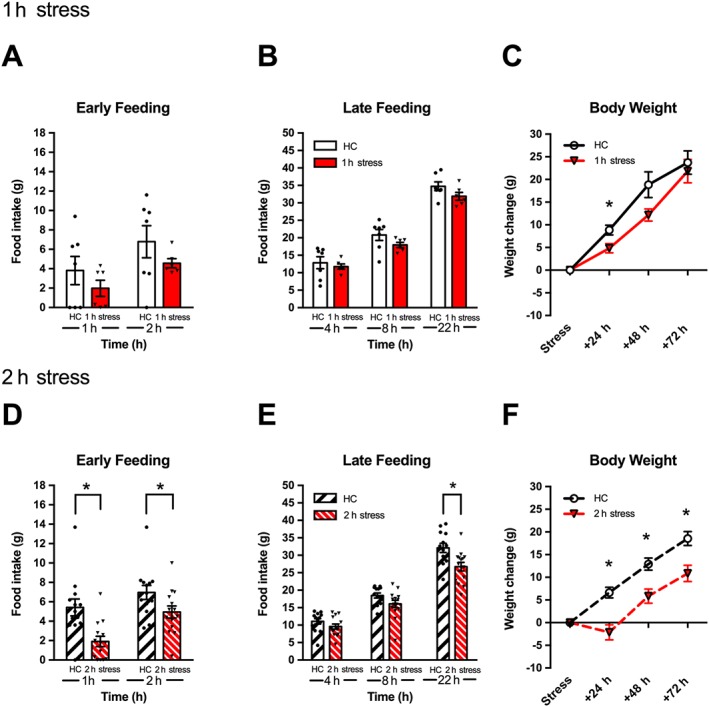
Acute stress reduces energy intake and body weight gain. Although a 1 h restraint stress (n = 6) did not reduce feeding at early (A) or late (B) time points compared to 1 h homecage without food (HC; n = 7), a 2 h restraint (n = 14) resulted in decreased food intake at 1 and 2 h (D; early feeding), and 22 h post‐stress (E; late feeding) compared to 2 h homecage without food (HC; n = 13). Body weight gain was reduced at all time points after a 2 h stress episode (F) and at 24 h after 1 h of restraint (C). Individual results are shown with mean ± SEM indicated by the bars. *P < 0.05, significantly different from HC unstressed group.
Experiment 1b: 2 h restraint stress
Analysis of cumulative feeding following 2 h stress revealed that restraint significantly decreased intake during early post‐stress time points (1 h) (Figure 2A). Although feeding was not different at 4 or 8 h, overall intake at the 22 h time point was significantly reduced (Figure 2B). There was also a significant effect of stress on body weight at all points following stress.
Figure 2.
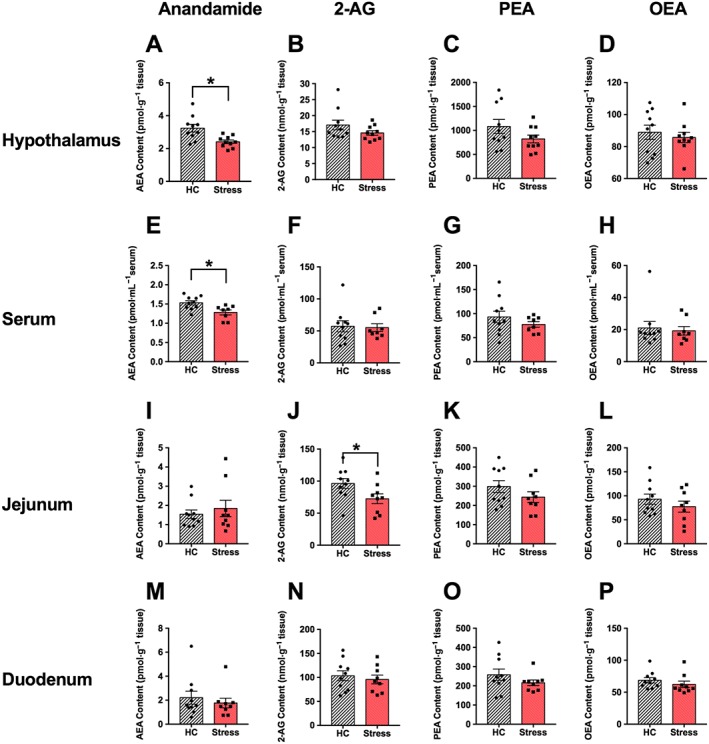
The effects of 2 h restraint stress on AEA, 2‐AG, PEA and OEA content in the hypothalamus (A–D), serum (E–H), jejunum (I–L) and duodenum (M–P). Acute restraint (n = 10) resulted in decreased AEA content in the hypothalamus (A) and in serum (E) compared to unstressed homecage animals (HC; n = 10). However, no other changes in endocannabinoids or levels of PEA or OEA were observed in these regions. In the small intestine, 2‐AG content was selectively decreased in the jejunum following stress, but not in duodenum. Intestinal content of AEA, PEA and OEA in these regions were unaffected by stress. Individual results are shown with mean ± SEM indicated by the bars. * P < 0.05, significantly different from homecage unstressed group.
Experiment 1c: Effect of 2 h restraint stress on tissue‐specific endocannabinoid levels
Given that 2 h restraint stress reduced feeding and weight gain, this stressor duration was used for the remaining experiments. We examined the effects of 2 h restraint stress on endocannabinoids in distinct tissue compartments involved in response to stress and feeding. Figure 2 presents the mean (±SEM) endocannabinoid (and PEA/OEA) content in the hypothalamus (A–D), blood (E–H), jejunum (I–L) and duodenum (M–P). AEA content was significantly reduced in the hypothalamus (Figure 2A) and serum (Figure 2E) following stress. However, no other changes in endocannabinoids or levels of PEA or OEA were observed in these regions. In the small intestine, 2‐AG content was selectively decreased in the jejunum following stress (Figure 2J), but not in duodenum. Intestinal content of AEA/PEA/OEA in these regions were unaffected by stress.
Experiment 2: Effect of systemic endocannabinoid manipulations on feeding and body weight
Because endocannabinoids regulate feeding and appetite, as well as response to stress, the effects of pharmacological manipulations in the current study were assessed independently on homecage feeding and food intake following stress.
Experiment 2a: Homecage feeding
Analysis of cumulative feeding during early time points (Figure 3A) revealed a main effect of drug pretreatment at 2 h, and post hoc analysis revealed that MJN110 reduced intake relative to vehicle‐treated rats and those given PF‐04457845. Analysis of later time points (Figure 3B) revealed a significant pretreatment effect at 8 h and 22 h with MJN110‐treated rats consuming significantly less chow than vehicle controls at both time points (8 h and 22 h), as well as at 22 h feeding among those administered PF‐04457845. However, analysis of body weight (Figure 3C) did not reveal an effect of pretreatment at any time point.
Figure 3.
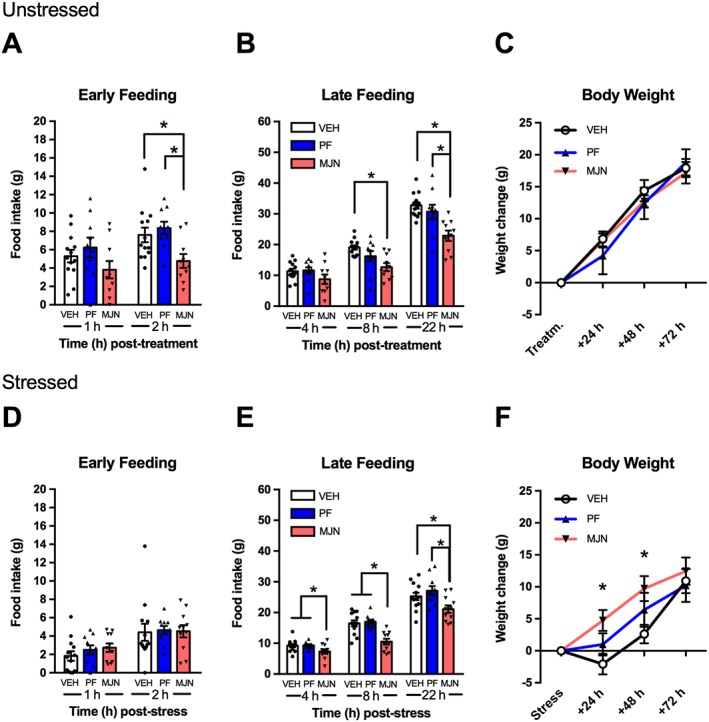
Effect of systemic endocannabinoid manipulations on feeding and body weight in unstressed (A–C) and stressed (D–F) rats. The MAGL inhibitor, MJN110 (MJN; 10 mg·kg−1, n = 10), reduced early (2 h; A) and late (8 h, 22 h; B) feeding in unstressed animals compared to vehicle (VEH; n = 13) without affecting weight gain (C). In stressed rats, MJN110 (10 mg·kg−1; n = 11) pretreatment reduced late feeding at all time points compared to vehicle (n = 13) but increased body weight at 24 and 48 h following stress. The FAAH inhibitor, PF‐0447845 (PF; 10 mg·kg−1), did not have any effect on feeding and body weight in stressed (n = 10) or unstressed (n = 10) conditions. Individual results are shown with mean ± SEM indicated by the bars. *P < 0.05, significantly different as indicated .
Experiment 2b: Stress‐induced anorexia
Although there was no drug effect during early feeding (Figure 3C), analysis of later time points (Figure 3D) revealed a main effect of pretreatment at 4 h, 8 h and 22 h. Animals pretreated with MJN110 ate significantly less than either the vehicle or PF‐04457845 group at 4 h, 8 h and 22 h. Analysis of body weight (Figure 3E) revealed pretreatment effects at 24 h and 48 h. Post hoc comparisons revealed that MJN110‐treated rats gained significantly more weight than vehicle controls at 24 h and 48 h after stress.
Experiment 3: Effect of antagonism of peripheral CB1 receptors on MJN110‐induced anorexia
The finding that MJN110 produced anorexia yet attenuated stress‐induced decreases in body weight gain suggested that MAGL inhibition exerts distinct effects on gut function. To explore whether signalling by peripheral CB1 receptors underlies MJN110‐induced anorexia, the peripherally restricted, CB1 receptor antagonist AM6545 was administered to all rats. Although the anorectic effects of MAGL inhibition were no longer evident in homecage feeding, MJN110 still decreased feeding in animals exposed to stress.
Experiment 3a: Homecage feeding
Analysis of cumulative feeding did not reveal an effect of MJN110 during early time points (1, 2 h; Figure 4A) or later time points (4, 8, 22 h; Figure 4B) throughout the testing period. Similarly, there was no effect of MJN110 on body weight (24, 48, 72 h; Figure 4C).
Figure 4.
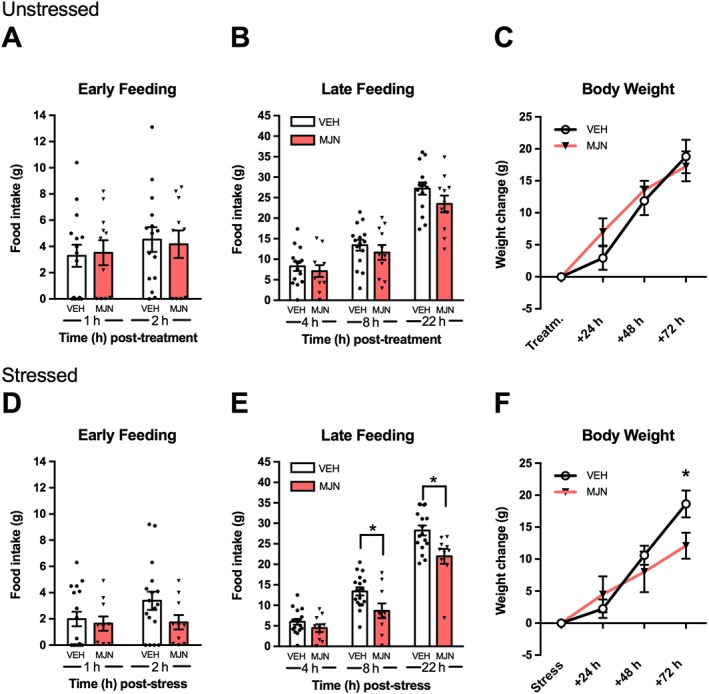
Inhibition of MAGL by MJN110 decreases feeding independent of peripheral CB1 receptors following stress. Although MJN110 pretreatment (MJN; 10 mg·kg−1; n = 11) did not affect intake (A,B) and body weight gain (C) in unstressed rats receiving the peripherally restricted CB1 antagonist, AM6545 (5 mg·kg−1), the MAGL inhibitor (n = 10) reduced feeding at 8 h (D) and 22 h (E) post‐stress compared to vehicle pretreated animals (n = 17). Post‐stress body weight (F) was reduced 72 h following MJN110 pretreatment. All animals received AM6545 (5 mg·kg−1). Individual results are shown with mean ± SEM indicated by the bars. *P < 0.05, significantly different from VEH.
Experiment 3b: Stress‐induced anorexia
There was no effect of pretreatment during early time points of testing (1, 2 h; Figure 4D). Although feeding remained unchanged at 4 h (Figure 4E), MJN110 reduced food intake at 8 h and 22 h post‐stress. Analysis of body weight (Figure 4F) revealed a significant drug effect with MJN110‐treated rats gaining significantly less weight at 72 h following stress, although body weight change at earlier periods was not significantly different (24, 48 h).
Experiment 4: Effect of systemic MAGL inhibition on small intestinal transit
To further explore how MAGL inhibition differentially affected stress‐induced anorexia and body weight gain, the effects of MJN110 on small intestinal transit were explored. Despite reducing gut transit in unstressed conditions, MAGL inhibition did not modulate motility after stress.
Experiment 4a: Unstressed transit
Analysis of pretreatment feeding (Figure 5A) and food intake following gavage (Figure 5B) did not reveal any effect of MJN110 administration. However, MAGL inhibition significantly reduced small intestinal transit in rats (Figure 5C).
Figure 5.
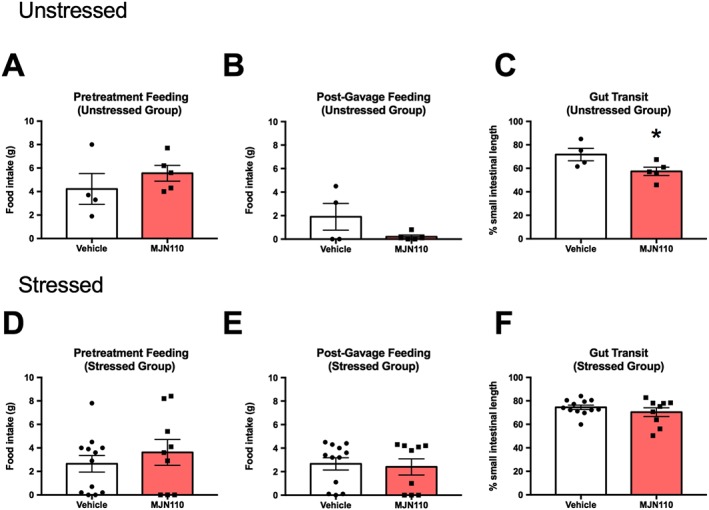
Inhibition of MAGL by MJN110 reduces basal small intestinal transit. Pretreatment feeding and food intake following gavage were not affected by MJN110 (MJN; 10 mg·kg−1) administration in unstressed (A,B; n = 5) and stressed rats (D,E; n = 9), compared to vehicle pretreated animals that underwent 2 h restraint (n = 12) or remained in their homecage (n = 4). However, MAGL inhibition significantly reduced small intestinal transit in unstressed rats (C), while transit remained unchanged by MJN110 pretreatment following stress (F). Individual results are shown with mean ± SEM indicated by the bars. *P = 0.05, significantly different from VEH.
Experiment 4b: Stressed transit
Analysis of pretreatment feeding (Figure 5D) and food intake following gavage (Figure 5E) did not reveal any effect of MJN110 administration, nor was there an effect on small intestinal transit in rats.
Experiment 5: Effect of i.c.v. FAAH and MAGL inhibition on feeding
Two hour restraint stress reduced the levels of endocannabinoids in distinct central and peripheral compartments. Thus, in order to assess if central inhibition of FAAH and MAGL could modulate feeding, PF‐04457845 and MJN110 were delivered via icv administration. Although neither manipulation affected feeding under stressed conditions, combined dual inhibition of FAAH and MAGL stimulated early homeostatic feeding.
Experiment 5a: Homecage feeding
Analysis of cumulative feeding did not reveal an effect for the treatment drug during early feeding, (1, 2 h; Figure 6A). Likewise, there was no drug effect at later time points (4, 8, 22 h; Figure 6B) or an effect on body weight at any time point (24, 48, 72 h; Figure 6C).
Figure 6.
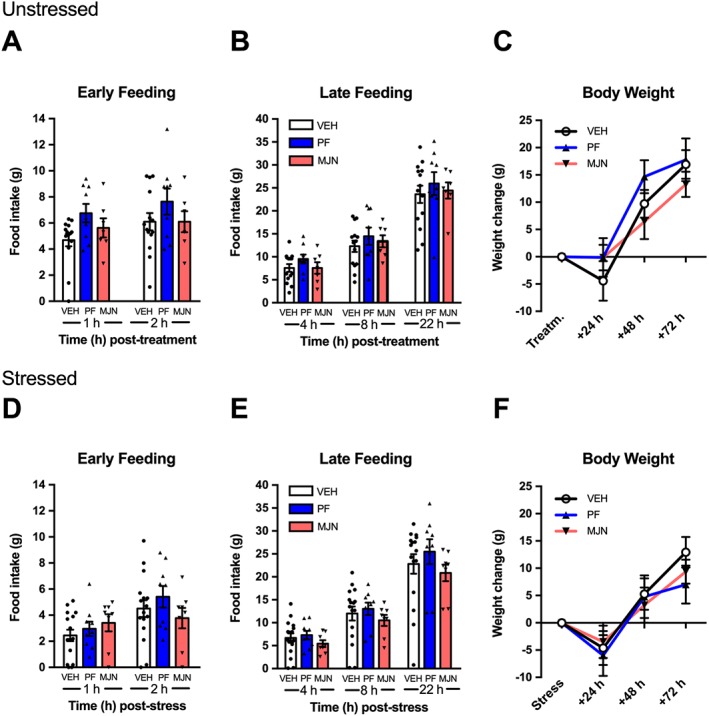
Effect of i.c.v. FAAH and MAGL inhibition on feeding and body weight in unstressed (A–C) and stressed (D–R) rats. Administration of PF‐04457845 (PF; 3 μg) or MJN110 (MJN; 5 μg) did not modify food intake or body weight in rats that were exposed to a 2 h restraint stress (VEH, n = 16; PF, n = 9; MJN, n = 8) or 2 h confinement to homecage without food (VEH, n = 14; PF, n = 9; MJN, n = 7). Individual results are shown with mean ± SEM indicated by the bars.
Experiment 5b: Stressed‐induced anorexia
Analysis of cumulative feeding following stress did not reveal an effect of pretreatment during early time points (1, 2 h; Figure 6D) or later time points (4, 8, 22 h; Figure 6E). Similarly, there was no effect of drug administration on body weight (24, 48, 22 h; Figure 6F).
Experiment 5c: Effect of dual i.c.v. FAAH and MAGL inhibition on homecage feeding
Analysis of cumulative feeding during early time points revealed that combined PF‐04457845 + MJN110 pretreatment stimulated feeding during 1 h (Figure 7A); but not at 2 h. There were no significant effects at later time points for feeding (4, 22 h; Figure 7B) or body weight gain (24, 48, 72 h; Figure 7C).
Figure 7.
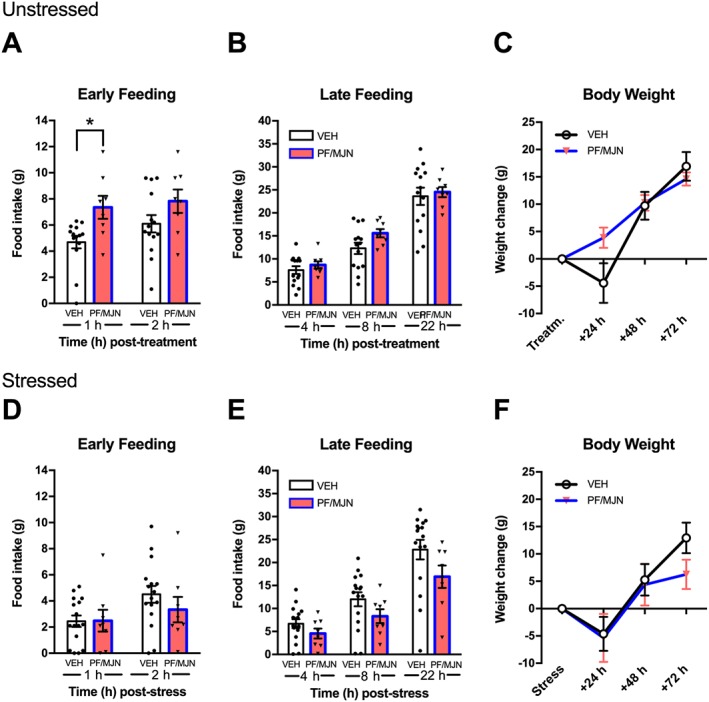
Dual FAAH/MAGL inhibition stimulates food intake. In unstressed rats, combined i.c.v. administration of PF‐04457845 (PF; 3 μg) and MJN110 (MJN; 5 μg) (n = 8) increased early feeding at 1 h (A) compared to animals that received vehicle (n = 14), however, body weight was not significantly increased with combined treatment (C). In stressed animals, combined PF‐04457845 and MJN110 pretreatment (n = 8) had no effect on feeding (D,E) or body weight (E) compared to vehicle pretreated rats (n = 16). Individual results are shown with mean ± SEM indicated by the bars. *P < 0.05, significantly different from VEH.
Experiment 5d: Effect of dual i.c.v. FAAH and MAGL inhibition on stress‐induced anorexia
Analysis of cumulative feeding following stress did not reveal an effect of pretreatment during the early time points (1, 2 h; Figure 7D) or later time points (4, 8, 22 h; Figure 7E) throughout the testing period. Similarly, there was no drug effect on body weight (24, 48, 72 h; Figure 7F).
Experiment 6: Effect of high dose PF‐04457845 on feeding
Experiment 6 assessed whether a higher dose of PF‐04457845 would modulate feeding under homecage and stress conditions given that a 10‐fold higher dose of the analogous FAAH inhibitor, PF3845, has been reported to elicit central effects in mice (Fichna et al., 2014). The i.c.v. administration of PF‐04457845 differentially affected homecage feeding compared to stress‐induced changes in food intake. Specifically, central FAAH inhibition reduced homeostatic feeding independent of CB1 receptors but attenuated stress‐induced anorexia through a CB1 receptor‐dependent mechanism.
Experiment 6a: Homecage feeding
There was a significant pretreatment effect at 1 h (Figure 8A). Animals injected with PF‐04457845 consumed significantly less chow than vehicle treated control rats during the first hour of feeding. Similarly, rats given the combined PF‐04457845/AM251 also consumed less chow than vehicle controls. Analysis of later time points did not reveal any differences in food intake (2 h; 4, 8, 22 h; Figure 8B) or body weight (24, 48, 72 h; Figure 8C).
Figure 8.
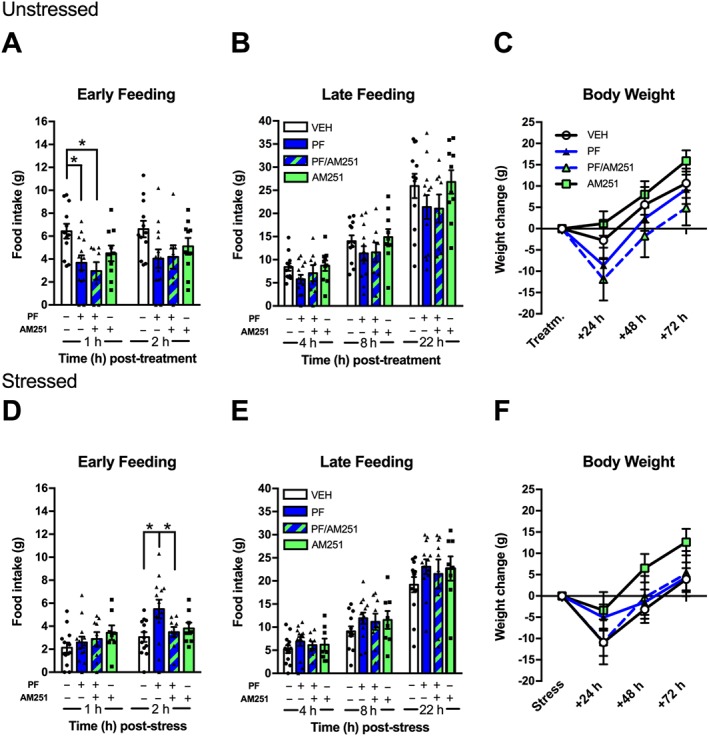
Central FAAH inhibition attenuates stress‐induced anorexia via CB1 receptors and reduces homeostatic feeding independent of CB1 signalling. In unstressed rats, i.c.v. administration of PF‐04457845 (PF; 30 μg; n = 13) or PF‐04457845 combined with AM251 (0.1 μg; n = 8) reduced early feeding (A) compared to animals receiving vehicle (n = 12). Among stressed animals, rats administered i.c.v. PF‐04457845 (PF; n = 13) consumed more food at 2 h post‐stress (D) compared to rats receiving vehicle (n = 13) or PF‐04457845 combined with AM251 (n = 9). Individual results are shown with mean ± SEM indicated by the bars. *P < 0.05, significantly different as indicated.
Experiment 6b: Stress‐induced anorexia
Although there was no drug effect on feeding at 1 h (Figure 8D), analysis revealed a significant effect at 2 h with PF‐04457845‐injected rats consuming significantly more chow than vehicle controls. The attenuation of stress‐induced anorexia by PF‐04457845 was CB1‐dependent, as rats co‐administered PF + AM251 consumed significantly less chow at 2 h post‐stress than animals receiving PF‐04457845 alone, but did not differ from vehicle controls. Analysis of later time points did not reveal any differences in food intake (4, 8, 22 h; Figure 8E), nor was there any effect on body weight (24, 8, 22 h; Figure 8F).
Discussion
The current study investigated how inhibition of endocannabinoid hydrolysis, and thus increased AEA and 2‐AG signalling, modulated homeostatic feeding and consumption in response to stress – a known modulator of energy intake. Acute stress leads to inhibition of food intake (Harris, 2015), and the data from the current experiments are consistent with these findings. One hour stress modestly reduced feeding but significantly reduced body weight gain. It is possible that due to the relatively small sample size (n = 6–7 per group), this experiment was under‐powered; although this shorter stressor did not produce a robust anorectic effect. However, a single 2 h acute restraint stress had immediate anorectic effects within the 2 h post‐stress period, consistent with previous studies (e.g. Krahn et al., 1990). Stressed animals did not differ from homecage controls at 4 h, most likely because they overconsumed chow, thus making up for lost intake. However, overall consumption remained lower at 22 h. As stressed animals reduced their intake, body weight was consequently affected by stress, resulting in a sustained shift in weight gain for the duration of the study.
Activation of the endocannabinoid system and the HPA axis modulates energy balance in opposite ways. Manipulations that increase endocannabinoids or activation of CB1 receptors stimulate feeding (Lau et al., 2017). In particular, feeding status appears to be regulated by endocannabinoid levels in the brain (Kirkham et al., 2002) and gut (Dipatrizio et al., 2015), suggesting that increased AEA and 2‐AG levels drive feeding through separate circuits. Conversely, stress exerts a negative effect on food intake (Harris, 2015), which corresponds to reduced AEA levels (Hill and Tasker, 2012; Morena et al., 2016). Indeed, in the current study, restraint stress decreased AEA in the hypothalamus (and circulating blood) while 2‐AG content was reduced in the jejunum. Given that fasting has been shown to increase jejunal 2‐AG (Dipatrizio et al., 2015), the stress‐induced decrease observed in the current study and ensuing anorexia is consistent with a role for 2‐AG in regulating feeding in this region. Fasting has also been shown to increase brain endocannabinoid levels (Kirkham et al., 2002); therefore, a decrease in hypothalamic AEA following stress may reflect a reduction in feeding drive after exposure to a stressor.
Given that restraint decreased AEA and 2‐AG levels in the brain and gut, we assessed whether inhibition of FAAH and MAGL modulates stress‐induced anorexia. While systemic administration of the FAAH inhibitor, PF‐04457845, did not affect intake, surprisingly the MAGL inhibitor, MJN110, reduced consumption. The endocannabinoid system has previously been shown to increase/decrease feeding via CB1 receptors expressed on glutamatergic and GABAergic neurons respectively (Bellocchio et al., 2010). Some studies have suggested that 2‐AG could preferentially signal at CB1 receptors on GABAergic terminals, while AEA could preferentially signal at CB1 receptors on glutamatergic terminals (Llorente‐Berzal et al., 2015; Di et al., 2016; Natividad et al., 2017). In light of these findings, it is plausible that the ability of AEA signalling to reverse stress‐induced anorexia is due to activation of CB1 receptors on glutamatergic terminals (which promote feeding; Bellocchio et al., 2010), while the anorectic effects of MJN110 could be via 2‐AG acting at CB1 receptors on GABA neurons (which have been found to suppress feeding; Bellocchio et al., 2010).
Despite reducing food intake in both unstressed and stress conditions, MJN110 actually reversed stress‐induced weight loss. This paradoxical effect on feeding and weight gain could be attributable to changes in gastrointestinal function following systemic MAGL inhibition. Interestingly, in unstressed animals, MJN110 reduced GI transit suggesting that the dissociation between feeding and weight gain in unstressed animals is due to reduced clearance of faecal matter, which prevents weight loss in light of reduced food intake. As treatment with CB1 receptor agonists inhibit, whereas antagonists increase, basal gut transit in rats (Mathison et al., 2004), the finding that MAGL inhibition reduces basal motility is consistent with previous reports on the endocannabinoid system in regulating small intestine function (Storr et al., 2010; Cluny et al., 2010b, 2010a; Keenan et al., 2015). Inhibition of MAGL has also been shown in mice to reduce whole gut transit (Duncan et al., 2008). It is unclear why MAGL inhibition did not affect transit with stress. However, the biochemical data indicate that jejunal 2‐AG is reduced following stress, which could be attributable to changes in the hydrolytic activity of MAGL or reduced 2‐AG biosynthesis. In the latter case, the MAGL inhibitor could be less effective in increasing 2‐AG levels, which may underlie the difference of MJN110 on gut transit in the current study.
To explore the contribution of signalling by peripheral CB1 receptors in mediating the effects of MJN110 on feeding and weight changes, we assessed concomitant pretreatment with the peripherally restricted neutral CB1 receptor antagonist, AM6545. Antagonism of CB1 receptors is well known to exert anorectic effects with inverse agonist/antagonists (Chambers et al., 2004, 2006; McLaughlin et al., 2005; Merroun et al., 2009) and neutral antagonists (Randall et al., 2010; Cluny et al., 2010b, 2011) all reducing feeding, including peripherally restricted compounds such as AM6545 (Randall et al., 2010; Cluny et al., 2010b). In this case, MJN110 did not further reduce unstressed food intake among animals pretreated with AM6545 but still decreased feeding in rats exposed to restraint stress. These findings suggest that 2‐AG may exert CB1 receptor‐dependent and ‐independent effects in the periphery under stressed/unstressed conditions respectively. Interestingly, AM6545 prevented the ability of MJN110 to reverse stress‐induced weight loss, suggesting that 2‐AG signalling on body weight gain is mediated by peripheral CB1 receptors.
Systemic PF‐04457845 was ineffective in attenuating stress‐induced anorexia, which could be attributable to FAAH inhibition elevating two distinct – and opposing – regulators of feeding. The FAAH enzyme catalyses the hydrolysis not only of AEA but also other fatty acid amides (Cravatt et al., 1996), including the anorexic lipid, OEA (Rodríguez de Fonseca et al., 2001). The satiety‐inducing effects of OEA are mediated by activation of the nuclear transcription factor, PPAR‐α (Fu et al., 2003), which occurs, in part, through peripheral regulation of the gut (Rodríguez de Fonseca et al., 2001; Fu et al., 2003). It is likely that the effect of increased AEA on feeding is opposed by concomitant elevation of OEA following global FAAH inhibition. Increased levels of acyl ethanolamides could also activate other receptor targets previously shown to play a role in feeding/satiety (Hansen and Diep, 2009; Hansen, 2014), such as TRPV1 (Motter and Ahern, 2008) or GPR119 (Overton et al., 2006), and could thus modulate feeding independent of CB1 receptor (and PPAR‐α) signalling under unstressed feeding conditions, as well.
Because OEA is known to reduce feeding via a peripheral mechanism, we sought to differentiate between peripheral and central FAAH inhibition to unmask a potential role of AEA in modulating feeding. Systemic FAAH inhibition was without effect, and the current biochemical data suggested that a drop in central AEA signalling may underlie anorexia following stress. Therefore, we explored whether manipulations that increase central AEA could overcome stress‐induced decreases in hypothalamic levels and reduce anorexia. Indeed, i.c.v. administration of PF‐04457845 (30 μg) attenuated stress‐induced anorexia, and this was blocked by co‐administration of the CB1 receptor antagonist, AM251. Given that stress‐induced reductions in AEA are mediated by increases in CRH signalling (Gray et al., 2015; Natividad et al., 2017) and that inhibition of FAAH can counteract the behavioural effects of CRH (Gray et al., 2015; Natividad et al., 2017), it is interesting to view these findings in light of the fact that CRH mediates the effects of acute stress on feeding behaviour (Krahn et al., 1986; Shibasaki et al., 1988; Smagin et al., 1999). As such, these data suggest that stress promotes CRH release, thereby triggering FAAH‐mediated AEA hydrolysis. As reduced AEA signalling contributes to the generation of an anxiety state (Gray et al., 2015; Natividad et al., 2017), it is likely also to suppress food intake. Accordingly, these data support the potential importance of CRH and FAAH/AEA dynamics in contributing to several aspects of the stress response.
It is interesting to note that central administration of PF‐04457845 resulted in opposing effects on food intake depending on the dose and feeding condition. Specifically, 30 μg of PF‐04457845 was sufficient to attenuate stress‐induced anorexia, although the same dose produced hypophagia among animals in the unstressed homecage feeding condition. Although the effect of central FAAH inhibition on stress‐feeding was via a CB1 receptor‐dependent mechanism, the anorectic effects were not blocked by co‐administration of a CB1 receptor antagonist. These opposing effects indicate that central FAAH inhibition may stimulate or inhibit food intake depending on the relative increase in AEA and related acyl ethanolamides, which activate distinct receptor targets within the CNS to control energy intake.
Although a lower dose of PF‐04457845 (3 μg) was ineffective alone, combined pretreatment with MJN110 (5 μg) increased food intake during the early feeding period. This is noteworthy given that MAGL inhibition alone did not have an effect on feeding in either condition after i.c.v. administration. In this case, the lack of effect(s) of central MJN110 alone could be attributable to differences in administering an exogenous agonist/ligand compared to elevating endogenous 2‐AG via inhibition of its catabolic enzyme, MAGL. This latter approach requires endocannabinoid biosynthesis, gradually increasing levels of 2‐AG. Moreover, Kirkham et al. (2002) reported that, although 2‐AG infusions into the nucleus accumbens stimulated feeding in rats, administration into the lateral ventricle had no effect. Therefore, it is possible that i.c.v. MJN110 was not sufficient to increase 2‐AG levels in critical feeding centres to stimulate intake.
In summary, the current study revealed important differences in AEA and 2‐AG signalling in homeostatic feeding and changes in energy intake following stress. Restraint stress resulted in a decrease in hypothalamic and circulating AEA, while 2‐AG in the jejunum was reduced. Increasing central AEA via FAAH inhibition attenuated stress effects on food intake via CB1 receptors yet reduced homeostatic feeding in unstressed animals independent of CB1 receptors. On the other hand, increased 2‐AG following MAGL inhibition decreased energy intake regardless of stress or feeding condition and inhibited basal intestinal transit in unstressed rats. The effect of MAGL inhibition to reduce feeding appears to be independent of signalling by CB1 receptors following stress. It remains to be determined how FAAH and MAGL inhibition exert effects on feeding independent of CB1 receptors.
Author contributions
M.A.S., K.A.S. and M.N.H. participated in research design. M.A.S., D.J.L., C.M.K., J.‐B.C., M.M. and M.N.H. conducted the experiments. V.K.V., A.M. and B.F.C. contributed reagents or analytical tools. M.A.S., K.A.S. and M.N.H. performed data analyses. M.A.S., K.A.S. and M.N.H. wrote the manuscript. All authors contributed to the final manuscript and approved its submission.
Conflict of interest
M.N.H. is a consultant for Pfizer and GW Pharmaceuticals. All other authors declare no competing interests.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
Supported by grants from the Canadian Institutes of Health Research (CIHR; M.N.H., K.A.S.) and National Institutes of Health (NIH; A.M, B.F.C.), and a CIHR postdoctoral fellowship (M.A.S.). M.N.H. receives salary support from CIHR in the form of a tier II Canada Research Chair. K.A.S. is the Crohn's and Colitis Foundation of Canada Chair in Inflammatory Bowel Disease Research. The PF‐04457845 used in this study was kindly donated by Pfizer International. The authors would like to acknowledge the Southern Alberta Mass Spectrometry Centre, located in and supported by the Cumming School of Medicine, University of Calgary, for their services in targeted LC tandem MS.
Sticht, M. A. , Lau, D. J. , Keenan, C. M. , Cavin, J.‐B. , Morena, M. , Vemuri, V. K. , Makriyannis, A. , Cravatt, B. F. , Sharkey, K. A. , and Hill, M. N. (2019) Endocannabinoid regulation of homeostatic feeding and stress‐induced alterations in food intake in male rats. British Journal of Pharmacology, 176: 1524–1540. 10.1111/bph.14453.
Contributor Information
Keith A Sharkey, Email: ksharkey@ucalgary.ca.
Matthew N Hill, Email: mnhill@ucalgary.ca.
References
- Ahn K, Smith SE, Liimatta MB, Beidler D, Sadagopan N, Dudley DT et al (2011). Mechanistic and pharmacological characterization of PF‐04457845: A highly potent and selective fatty acid amide hydrolase inhibitor that reduces inflammatory and noninflammatory pain. J Pharmacol Exp Ther 338: 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. Br J Pharmacol 174: S208–S224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017d). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. Br J Pharmacol 174: S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio L, Lafentre P, Cannich A, Cota D, Puente N, Grandes P et al (2010). Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci 13: 281–283. [DOI] [PubMed] [Google Scholar]
- Chambers AP, Koopmans HS, Pittman QJ, Sharkey KA (2006). AM 251 produces sustained reductions in food intake and body weight that are resistant to tolerance and conditioned taste aversion. Br J Pharmacol 147: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AP, Sharkey KA, Koopmans HS (2004). Cannabinoid (CB)1receptor antagonist, AM 251, causes a sustained reduction of daily food intake in the rat. Physiol Behav 82: 863–869. [DOI] [PubMed] [Google Scholar]
- Cluny NL, Chambers AP, Vemuri VK, Wood JT, Eller LK, Freni C et al (2011). The neutral cannabinoid CB1 receptor antagonist AM4113 regulates body weight through changes in energy intake in the rat. Pharmacol Biochem Behav 97: 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluny NL, Keenan CM, Duncan M, Fox A, Lutz B, Sharkey KA (2010a). Naphthalen‐1‐yl‐(4‐pentyloxynaphthalen‐1‐yl)methanone (SAB378), a peripherally restricted cannabinoid CB1/CB2 receptor agonist, inhibits gastrointestinal motility but has no effect on experimental colitis in mice. J Pharmacol Exp Ther 334: 973–980. [DOI] [PubMed] [Google Scholar]
- Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT et al (2010b). A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol 161: 629–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB (1996). Molecular characterization of an enzyme that degrades neuromodulatory fatty‐acid amides. Nature 384: 83–87. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA et al (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. Brit J Pharmacol 175: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Itoga CA, Fisher MO, Solomonow J, Roltsch EA, Gilpin NW et al (2016). Acute stress suppresses synaptic inhibition and increases anxiety via endocannabinoid release in the basolateral amygdala. J Neurosci 36: 8461–8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPatrizio NV (2016). Endocannabinoids in the gut. Cannabis Cannabinoid Res 1: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipatrizio NV, Igarashi M, Narayanaswami V, Murray C, Gancayco J, Russell A et al (2015). Fasting stimulates 2‐AG biosynthesis in the small intestine: Role of cholinergic pathways. Am J Physiol Regul Integr Comp Physiol 309: R805–R813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPatrizio NV, Simansky KJ (2008). Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J Neurosci 28: 9702–9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M, Thomas AD, Cluny NL, Patel A, Patel KD, Lutz B et al (2008). Distribution and function of monoacylglycerol lipase in the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 295: G1255–G1265. [DOI] [PubMed] [Google Scholar]
- Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP (2010). Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology 151: 4811–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichna J, Sałaga M, Stuart J, Saur D, Sobczak M, Zatorski H et al (2014). Selective inhibition of FAAH produces antidiarrheal and antinociceptive effect mediated by endocannabinoids and cannabinoid‐like fatty acid amides. Neurogastroenterol Motil 26: 470–481. [DOI] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Verme JL, Serrano A, Rodríguez de Fonseca F et al (2003). Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR‐α. Nature 425: 90–93. [DOI] [PubMed] [Google Scholar]
- Gray JM, Vecchiarelli HA, Morena M, Lee TT‐Y, Hermanson DJ, Kim AB et al (2015). Corticotropin‐releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci 35: 3879–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Wilson CD, Lee TTY, Pittman QJ, Deussing JM, Hillard CJ et al (2016). Sustained glucocorticoid exposure recruits cortico‐limbic CRH signaling to modulate endocannabinoid function. Psychoneuroendocrinology 66: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HS (2014). Role of anorectic N‐acylethanolamines in intestinal physiology and satiety control with respect to dietary fat. Pharmacol Res 86: 18–25. [DOI] [PubMed] [Google Scholar]
- Hansen HS, Diep TA (2009). N‐acylethanolamines, anandamide and food intake. Biochem Pharmacol 78: 553–560. [DOI] [PubMed] [Google Scholar]
- Hao S, Avraham Y, Mechoulam R, Berry EM (2000). Low dose anandamide affects food intake, cognitive function, neurotransmitter and corticosterone levels in diet‐restricted mice. Eur J Pharmacol 392: 147–156. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RBS (2015). Chronic and acute effects of stress on energy balance: Are there appropriate animal models? Am J Physiol ‐ Regul Integr Comp Physiol 308: R250–R265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Mclaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT‐Y et al (2011). Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci 31: 10506–10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Tasker JG (2012). Endocannabinoid signaling, glucocorticoid‐mediated negative feedback, and regulation of the hypothalamic‐pituitary‐adrenal axis. Neuroscience 204: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi N, Taylor DA (2001). Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol 134: 1151–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan CM, Storr MA, Thakur GA, Wood JT, Wager‐Miller J, Straiker A et al (2015). AM841, a covalent cannabinoid ligand, powerfully slows gastrointestinal motility in normal and stressed mice in a peripherally restricted manner. Br J Pharmacol 172: 2406–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V (2002). Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: Stimulation of eating by 2‐arachidonoyl glycerol. Br J Pharmacol 136: 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA, Grace M, Levine AS (1986). CRF antagonist partially reverses CRF‐ and stress‐induced effects on feeding. Brain Res Bull 17: 285–289. [DOI] [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA, Majchrzak MJ (1990). The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biol Psychiatry 27: 1094–1102. [DOI] [PubMed] [Google Scholar]
- Lau BK, Cota D, Cristino L, Borgland SL (2017). Endocannabinoid modulation of homeostatic and non‐homeostatic feeding circuits. Neuropharmacology 124: 38–51. [DOI] [PubMed] [Google Scholar]
- Limebeer CL, Rock EM, Puvanenthirarajah N, Niphakis MJ, Cravatt BF, Parker LA (2016). Elevation of 2‐AG by monoacylglycerol lipase inhibition in the visceral insular cortex interferes with anticipatory nausea in a rat model. Behav Neurosci 130: 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente‐Berzal A, Terzian ALB, di Marzo V, Micale V, Viveros MP, Wotjak CT (2015). 2‐AG promotes the expression of conditioned fear via cannabinoid receptor type 1 on GABAergic neurons. Psychopharmacology (Berl) 232: 2811–2825. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC (2007). Endocannabinoid hedonic hotspot for sensory pleasure: Anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology 32: 2267–2278. [DOI] [PubMed] [Google Scholar]
- Maniam J, Morris MJ (2012). The link between stress and feeding behaviour. Neuropharmacology 63: 97–110. [DOI] [PubMed] [Google Scholar]
- Martí O, Martí J, Armario A (1994). Effects of chronic stress on food intake in rats: Influence of stressor intensity and duration of daily exposure. Physiol Behav 55: 747–753. [DOI] [PubMed] [Google Scholar]
- Mathison R, Ho W, Pittman QJ, Davison JS, Sharkey KA, Pittman QJ et al (2004). Effects of cannabinoid receptor‐2 activation on accelerated gastrointestinal transit in lipopolysaccharide‐treated rats. Br J Pharmacol 142: 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD (2005). The cannabinoid CB1 antagonist AM 251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology (Berl) 180: 286–293. [DOI] [PubMed] [Google Scholar]
- Merroun I, Errami M, Hoddah H, Urbano G, Porres JM, Aranda P et al (2009). Influence of intracerebroventricular or intraperitoneal administration of cannabinoid receptor agonist (WIN 55,212‐2) and inverse agonist (AM 251) on the regulation of food intake and hypothalamic serotonin levels. Br J Nutr 101: 1569–1578. [DOI] [PubMed] [Google Scholar]
- Morena M, Patel S, Bains JS, Hill MN (2016). Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology 41: 80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Levine AS (1982). Corticotrophin releasing factor, grooming and ingestive behavior. Life Sci 31: 1459–1464. [DOI] [PubMed] [Google Scholar]
- Motter AL, Ahern GP (2008). TRPV1‐null mice are protected from diet‐induced obesity. FEBS Lett 582: 2257–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Buczynski MW, Herman MA, Kirson D, Oleata CS, Irimia C et al (2017). Constitutive increases in amygdalar corticotropin‐releasing factor and fatty acid amide hydrolase drive an anxious phenotype. Biol Psychiatry 82: 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton HA, Babbs AJ, Doel SM, Fyfe MCT, Gardner LS, Griffin G et al (2006). Deorphanization of a G protein‐coupled receptor for oleoylethanolamide and its use in the discovery of small‐molecule hypophagic agents. Cell Metab 3: 167–175. [DOI] [PubMed] [Google Scholar]
- Parker LA, Niphakis MJ, Downey R, Limebeer CL, Rock EM, Sticht MA et al (2014). Effect of selective inhibition of monoacylglycerol lipase (MAGL) on acute nausea, anticipatory nausea, and vomiting in rats and Suncus murinus. Psychopharmacology (Berl) 232: 583–593. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Cota D, Marsicano G (2017). The CB1 receptor as the cornerstone of exostasis. Neuron 93: 1252–1274. [DOI] [PubMed] [Google Scholar]
- Qi M, Morena M, Vecchiarelli HA, Hill MN, Schriemer DC (2015). A robust capillary liquid chromatography/tandem mass spectrometry method for quantitation of neuromodulatory endocannabinoids. Rapid Commun Mass Spectrom 29: 1889–1897. [DOI] [PubMed] [Google Scholar]
- Randall PA, Vemuri VK, Segovia KN, Torres EF, Hosmer S, Nunes EJ et al (2010). The novel cannabinoid CB1 antagonist AM6545 suppresses food intake and food‐reinforced behavior. Pharmacol Biochem Behav 97: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzoli M, Bartolomucci A (2016). The dichotomous effect of chronic stress on obesity. Trends Endocrinol Metab 27: 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzoli M, Pearson C, Crow S, Bartolomucci A (2017). Stress, overeating, and obesity: Insights from human studies and preclinical models. Neurosci Biobehav Rev 76: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Navarro M, Gómez R, Escuredo L, Nava F, Fu J et al (2001). An anorexic lipid mediator regulated by feeding. Nature 414: 209–212. [DOI] [PubMed] [Google Scholar]
- Santana P, Akana SF, Hanson ES, Strack AM, Sebastian RJ, Dallman MF (1995). Aldosterone and dexamethasone both stimulate energy acquisition whereas only the glucocorticoid alters energy storage. Endocrinology 136: 2214–2222. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Yamauchi N, Kato Y, Masuda A, Imaki T, Hotta M et al (1988). Involvement of corticotropin‐releasing factor in restraint stress‐induced anorexia and reversion of the anorexia by somatostatin in the rat. Life Sci 43: 1103–1110. [DOI] [PubMed] [Google Scholar]
- Silvestri C, Di Marzo V (2013). The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab 17: 475–490. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Howell LA, Redmann S, Ryan DH, Harris RB (1999). Prevention of stress‐induced weight loss by third ventricle CRF receptor antagonist. Am J Physiol 276: R1461–R1468. [DOI] [PubMed] [Google Scholar]
- Sticht MA, Limebeer CL, Rafla B, Parker LA (2015). Intra‐visceral insular cortex 2‐arachidonoylglycerol, but not N‐arachidonoylethanolamide, suppresses acute nausea‐induced conditioned gaping in rats. Neuroscience 286: 338–344. [DOI] [PubMed] [Google Scholar]
- Sticht MA, Limebeer CL, Rafla BR, Abdullah RA, Poklis JL, Ho W et al (2016). Endocannabinoid regulation of nausea is mediated by 2‐arachidonoylglycerol (2‐AG) in the rat visceral insular cortex. Neuropharmacology 102: 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storr MA, Bashashati M, Hirota C, Vemuri VK, Keenan CM, Duncan M et al (2010). Differential effects of CB1 neutral antagonists and inverse agonists on gastrointestinal motility in mice. Neurogastroenterol Motil 22: 787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykaras AG, Demenis C, Case RM, Mclaughlin JT, Smith CP (2012). Duodenal enteroendocrine I‐cells contain mRNA transcripts encoding key endocannabinoid and fatty acid receptors. PLoS One 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempel DL, Yamamoto M, Kim T, Leibowitz SF (1991). Effects of adrenalectomy on macronutrient selection patterns in the rat. Pharmacol Biochem Behav 40: 861–866. [DOI] [PubMed] [Google Scholar]
- Ulrich‐Lai YM, Ryan KK (2014). Neuroendocrine circuits governing energy balance and stress regulation: Functional overlap and therapeutic implications. Cell Metab 19: 910–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Hill MN, Zhang L, Gorzalka BB, Hillard CJ, Alger BE (2012). Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J Psychopharmacol 26: 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC (1999). Anandamide induces overeating: Mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 143: 315–317. [DOI] [PubMed] [Google Scholar]
- Wills KL, Petrie GN, Millett G, Limebeer CL, Rock EM, Niphakis MJ et al (2016). Double dissociation of monoacylglycerol lipase inhibition and CB 1 antagonism in the central amygdala, basolateral amygdala, and the interoceptive insular cortex on the affective properties of acute naloxone‐precipitated morphine withdrawal in rats. Neuropsychopharmacology 41: 1865–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]


