Abstract
Background and Purpose
It has been suggested that the non‐euphorogenic phytocannabinoid cannabidiol (CBD) can ameliorate adverse effects of Δ9‐tetrahydrocannabinol (THC). We determined whether CBD ameliorates cognitive deficits and withdrawal signs induced by cannabinoid CB1/CB2 receptor agonists or produces these pharmacological effects on its own.
Experimental Approach
The effects of THC or the CB1/CB2 receptor full agonist WIN55212 alone, CBD alone or their combination were tested across a range of doses. Cognitive effects were assessed in C57BL/6 mice in a conditional discrimination task and in the Barnes maze. Cannabinoid withdrawal signs were assessed following precipitated withdrawal by acute administration of the CB1 receptor antagonist SR141716, the 5‐HT1A receptor antagonist WAY100635, the TRPV1 receptor antagonist capsazepine or the adenosine A2A receptor antagonist SCH58261.
Key Results
THC produced significant motor and cognitive impairment in the Barnes maze task, none of which were attenuated by the addition of CBD. CBD alone did not affect cognitive performance. Precipitation of withdrawal signs by SR141716 occurred in mice chronically treated with THC or WIN55,212. These withdrawal signs were not attenuated by addition of chronic CBD. Chronic treatment with CBD alone did not induce withdrawal signs precipitated by SR141716 or WAY100635. Chronic CBD treatment also produced anxiolysis, which was not altered by attempting to precipitate withdrawal‐induced anxiety with a range of antagonists.
Conclusions and Implications
CBD as a monotherapy may prove to be a safer pharmacological agent, than CB1 receptor agonists alone or in combination with CBD, for the treatment of several disorders.
Linked Articles
This article is part of a themed section on 8th European Workshop on Cannabinoid Research. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.10/issuetoc
Abbreviations
- CBD
cannabidiol
- EPM
elevated plus maze
- THC
Δ9‐tetrahydrocannabinol
Introduction
The non‐euphorogenic cannabinoid cannabidiol (CBD) is emerging as a promising therapeutic agent for the treatment of a range of psychiatric and inflammatory disorders (see Campos et al., 2016). This is driven not only by its anti‐inflammatory and neuroprotective pharmacological profile but by that fact that it is devoid of many of the psychoactive effects produced by the primary phytocannabinoid Δ9‐tetrahydrocannabinol (THC). In spite of other shared pharmacological actions, CBD exhibits much lower affinity than THC for cannabinoid CB1 receptors (Showalter et al., 1996; Bisogno et al., 2001; Thomas et al., 2004; Pertwee et al., 2005). Currently, over 90 clinical trials are planned, ongoing or completed in which CBD is a primary intervention (https%3A%2F%2Fwww.clinicaltrials.gov%2Fct2%2Fresults%3Fcond%3D%26term%3Dcannabidiol%26cntry1%3D%26state1%3D%26recrs%3D).
Sativex is an oromucosal spray extract containing THC, CBD and specific minor cannabinoids and other non‐cannabinoid components developed by GW Pharma, and a search for ‘Sativex’ on the clinicaltrials.gov website yields over 500 results for a vast array of safety and efficacy trials for this cannabinoid‐based therapeutic. Part of the rationale for Sativex was the idea that the incorporation of additional compounds in the Cannabis plant, especially CBD, would mitigate some of the adverse side effects associated with THC. In addition, in a growing number of states where medical marijuana is approved, more products are being made available which contain moderate to high doses of CBD, and more marijuana strains are being produced which yield high levels of CBD in combination with low to high combinations of THC. Therefore, an overarching strategy has been to harness the therapeutic potential of CBD while lessening the adverse effects associated with THC. This strategy is exemplified by the term ‘entourage effect’, used to describe how phytocannabinoids (and other chemicals classes such as terpenes and flavonoids) may produce more than additive actions. For example, CBD may either synergize with or antagonize THC effects to enhance wanted actions and/or antagonize adverse actions (Russo, 2011). We have recently demonstrated, for example, that a 1:1 ratio of CBD to THC synergistically prevented development of mechanical sensitivity in a mouse model of chemotherapy‐induced neuropathic pain, greatly decreasing the dose of each drug required to produce efficacy (King et al., 2017). A continuation of quantitative research is required to determine the scope of these interactions, including how they affect the adverse effects of THC.
Alterations in learning and memory are of the most common adverse pharmacological effects linked with cannabis use. In humans, acute exposure to cannabis or THC has been shown to impair working and episodic memory (Curran et al., 2002; Bossong et al., 2012; Crane et al., 2013). Long‐term impairments in memory have been reported mainly in frequent, heavy users, but confounding factors make it difficult to establish cause–effect relationships between cannabis use and changes in neurocognitive function (Curran et al., 2016). Animal studies have revealed a high density of cannabinoid receptors in memory‐associated brain regions such as the hippocampus, amygdala, striatum and prefrontal cortex (Herkenham et al., 1991; Matsuda et al., 1993), and THC disrupts hippocampal LTP in animal models as well (Hoffman et al., 2007; Misner and Sullivan, 1999). THC administration is associated with cognitive impairment in a variety of experimental rodent assays, including operant and spatial maze models of memory (Brodkin and Moerschbaecher, 1997; Ferrari et al., 1999; Heyser et al., 1993; Lichtman et al., 1995; Mallet and Beninger, 1998; Nakamura et al., 1991; Varvel et al., 2001). Therefore, one objective of the present study was to determine the effects of CBD alone and in combination with THC or the synthetic CB1/CB2 receptor agonist WIN55,212 to define effects of single and combined cannabinoid regimens on instrumental conditioning and spatial learning. Previous reports have described impairing effects of CB1 receptor agonists on both types of learning (see Lichtman et al., 1995; Sokolic et al., 2011). Cannabinoid agonists and CBD were co‐administered in a 1:1 ratio based on dose to model the content of the major phytocannabinoids in Sativex.
Withdrawal upon abstinence is another pharmacological effect associated with chronic marijuana use in humans (Cooper and Haney, 2008; Jones, 1983). A specific cannabis withdrawal syndrome is well recognized and affects approximately 50% of daily users upon cessation of use (Budney et al., 2004), and common symptoms include craving, poor sleep quality, irritability and dysphoria (Allsop et al., 2011). Withdrawal symptoms associated with chronic exposure to CB1 receptor agonists have also been observed in rodent models (Cook et al., 1998; Rubino et al., 1998; Castane et al., 2004; Huang et al., 2009; Marusich et al., 2014). In the majority of these experiments, administration of the CB1 receptor selective antagonist SR141716 is used to precipitate withdrawal in animals chronically treated with cannabinoid agonists such as THC or WIN55212. Characteristic observations of cannabis‐associated withdrawal in rodents include the presence or absence of head shakes, paw flutter or scratching, and hypolocomotion. Therefore, another objective of the present study was to determine whether chronic CBD treatment leads to physical dependence and subsequent withdrawal symptoms mediated by actions on the CB1 receptor, and whether chronic co‐administration of CBD in combination with the CB receptor agonists THC or WIN55212 altered the observed signs of CB1 receptor‐associated withdrawal. Cannabinoid agonists and CBD were co‐administered in an approximately 1:1 ratio, based on the phytocannabinoid ratio in Sativex, while also testing relevant doses of cannabinoid agonists known to induce physical dependence.
While evidence supporting the pharmacological effects of CBD as being mediated through CB1 receptors is limited (but see Bisogno et al., 2001; Casarotto et al., 2010; Sartim et al., 2016), more compelling evidence attributes many of CBD's actions to interactions with other receptors. Three commonly reported receptor mechanisms for the pharmacological effects of CBD are (i) direct agonist action at 5‐HT1A receptors (Russo et al., 2005; Gomes et al., 2011; Ward et al., 2014), (ii) direct agonist action at TRPV1 receptors (Costa et al., 2004; Pertwee et al., 2005) and (iii) inhibition of the equilibrative nucleoside transporter leading to indirect agonist activity at adenosine A2A receptors (Carrier et al., 2006; El‐Remessy et al., 2008; Liou et al., 2008; Pandolfo et al., 2011). Therefore, a third objective of the present study was to determine whether chronic CBD administration could lead to traditional signs of physical dependence through a mechanism other than through CB1 receptors. In these studies, we have focused on a potential 5‐HT1A receptor mechanism as this is the receptor most indicated in the demonstrated CNS effects of CBD, such as anxiolysis and analgesia. As cannabinoid withdrawal has also been associated with anxiety in humans (Ramesh et al. 2011) and mouse models (Huang et al., 2010), we also tested the potential for 5‐HT1A, TRPV1 or A2A receptors antagonism to precipitate anxiogenesis in chronically treated CBD mice.
Methods
Animals
All animal care and experimental procedures were approved by the Temple University Institutional Animal Care and Use Committee. The animal facilities at Temple University are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and sentinel animals are tested quarterly to maintain a pathogen‐free environment. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
A total of 335 male C57Bl6 mice (Taconic, 6–8 weeks upon delivery) were used the experiments described here. Animals were group‐housed, four to a cage except where noted, in a temperature‐controlled animal facility. Cages are polycarbonate with cobb bedding on ventilated racks, and each cage contains enrichment items such as bedding materials and chew sticks. Mice were kept in the animal facility for at least 1 week prior to experiments with a light–dark cycle of 12:12 h and had access to food and water ad libitum except where noted. The number of animals per treatment used in the experiments was based on several years of experience with the experimental protocols by the principal investigators.
Conditional discrimination
Instrumental discrimination learning was assessed in mice using an appetitive‐motivated operant conditional discrimination procedure (Bisen‐Hersh et al., 2013) that used a tone/light compound stimulus and temporal duration. Following the habituation period, mice were separated into individual cages, weighed and food‐restricted for 24 h prior to the start of experimental testing. Water remained available ad libitum. On day 1 of training, each mouse was weighed and placed inside an experimental chamber for 15 min before the session started. During each session, the house light illuminated to indicate the availability of a vanilla‐flavoured liquid nutritional drink, Ensure Plus/water (50:50) solution, which would be presented in a 0.01‐cc dipper. These food deliveries were contingent upon correct responding, as defined separately for each phase described below. Each session lasted for 1 h, following which mice were fed 3 g of food and returned to their cages.
The conditional discrimination procedure involved a series of 8 phases, and specific criteria were completed for each prior to progressing to the next phase. ‘Extensive training’ was defined as completion of Phases 1–5, followed by a test in which a discrimination ratio was measured (Phase 6). ‘Rapid training’ was defined as Phase 7, followed by a test in which a discrimination ratio was measured (Phase 8). Briefly, during Phases 1 and 2, a nose‐poke response into the left or right hole, respectively, was reinforced on a fixed ratio schedule of reinforcement. During Phases 3 and 4, an audible tone was presented along with an illuminated stimulus light above the left or right poke hole for 2 or 8 s, respectively, and a nose‐poke response into the correct hole was reinforced. During Phase 5, the duration of the tone/light cue was randomly alternated between short (2 s) and long (8 s), and a nose‐poke response into the correct hole was reinforced. Mice were trained on this set of contingencies to 70% correct responding over two consecutive sessions before the discrimination ratio was assessed during the Phase 6 test session. During Phase 7, the contingencies previously described were reversed and this new set of contingencies was trained for one session before the discrimination ratio was assessed during the Phase 8 test session.
After separate groups of mice were trained to criteria (n = 8 per group), they were pretreated with THC (0–10 mg·kg−1 i.p.), CBD (0–10 mg·kg−1) or CBD + THC (0–10 mg·kg−1 each drug), or WIN55212 (0–3.0 mg·kg−1), CBD (0–3 mg·kg−1) or CBD + WIN55212 (0–3 mg·kg−1 each drug), 15 min prior to days 6 and 8 to test the acute effect of single and combined cannabinoids on discrimination and reversal learning (Veh and THC only). These doses and pretreatment time were selected based on published data (Lichtman et al., 1995, Lichtman and Martin, 1996, Varvel et al., 2001 and Wise et al., 2009).
Barnes maze
Spatial learning was assessed in mice using an aversively‐motivated standard, automated Barnes maze. A commercially available maze was used (Stoelting Co.). Dark curtains were hung to form a 177 × 177 cm square around the maze to exclude extraneous cues. Four spatial cues consisting of simple geometric figures (plus, square, circle and triangle) were hung on the four curtains. Mice were transported to the testing room and allowed 1–2 h to acclimatize. The first habituation trial lasted for 1 min, before the experimenter guided the mouse to the target hole. If the mouse did not enter on its own volition, a gentle tail‐pull was used to encourage entry into the escape tunnel. The escape tunnel was then covered, and the mouse rested for 1 min before moving the mouse to a holding cage for a 3 min inter‐trial interval during which time the maze was cleaned with 70% isopropanol. Acquisition training began 1 h later, with mice being placed on the maze and permitted to explore for 5 min. If the mouse entered the escape tunnel, the trial was terminated, the target hole covered and the mouse permitted 1 min of rest in the target hole. If the mouse did not exit the maze within the trial, the experimenter guided the mouse to the target hole as before and the mouse was kept there for 1 min. Four acquisition trials were performed per day for 4 days, for a total of 16 acquisition trials. The memory retention trial occurred 24 h later on the fifth day. For this, the escape tunnel was removed from the target hole and replaced with a shallow tray. The ability of the mice in finding the target hole was tested in a 5 min trial. Animals were pretreated with THC (5.0–20 mg·kg−1), CBD (5.0–20 mg·kg−1) or a combination of THC + CBD (5.0–20 mg·kg−1) for 30 min prior to the retention trial. Trials were recorded using a CCD monochrome camera and analysed using ANY‐Maze tracking software. Using nose‐point tracking, latency to entrance of the animal's head into the target hole, total time spent in the target zone, total number of entries into the target zone, average speed around the maze and distance travelled were recorded per trial. These doses and pretreatment times were selected based on published data (Lichtman et al., 1995, Lichtman and Martin, 1996, Varvel et al., 2001 and Wise et al., 2009).
Chronic cannabinoid administration and precipitated withdrawal
Separate groups of mice (n = 8 per group) were injected i.p. with vehicle, CBD (5.0 or 20 mg·kg−1), THC (20 mg·kg−1) or WIN55212 (3.0 mg·kg−1) alone or in combination (20 mg·kg−1 CBD + 20 mg·kg−1 THC, or 5.0 mg·kg−1 CBD + 3.0 mg·kg−1 WIN55212) every 12 h for 4.5 days. Selective antagonists for the CB1 receptor(10 mg·kg−1 SR141716), 5‐HT1A receptor (0.5 mg·kg−1 WAY 100635), TRPV1 receptor (10 mg·kg−1 capsazepine) or adenosine A2A receptor(0.05 mg·kg−1 SCH 58261) were administered 2 h following the last cannabinoid injection. Doses of THC, WIN55212 and SR141716 were based on extensive pilot work in our laboratory as well as Cook et al. (1998), Huang et al. (2009, 2010) and Lichtman et al. (2001). The dose of WAY 100635 was determined by previous published work by the principal investigators as well as Fogaca et al. (2014) and Rock et al. (2017). The dose of capsazepine was based on Costa et al. (2004). The dose of SCH 58261 was based on Cunha et al. (2008).
Immediately following injection of the antagonist, mice were placed in open field apparatus with clear Plexiglas walls (Med Associates ENV 510, 10.75″ L × 10.75″ W × 8″ H). They were video‐taped for 30 min, and a blinded observer subsequently rated their behaviours in the following four categories: head shakes (turning or twisting of the head side to side), rearing, head scratches (repetitive movements around the neck with the hind paw) and paw flutters (episodic rapid lateral movements of the paws). These withdrawal behaviours were selected based on reports of the most robust withdrawal behaviours seen following chronic cannabinoid treatment precipitated by SR141716, as cited above.
Elevated plus maze
Thirty minutes following injection of the antagonist, mice were placed on the mouse elevated plus maze [EPM; 50 cm high, consisting of two open and two closed arms (28 × 7 cm) in dim light conditions (7 lux)]. To begin the session, mice were placed in the centre of the maze facing an open arm. Exploration of the mouse was videotaped for 5 min. The measure of anxiety is the per cent time spent on the open arms, whereas the total number of open and closed arm entries is considered a measure of locomotor/exploratory activity.
Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). All statistical analyses were run using GraphPad Prism version 6 and as described below for the different sets of data. Significance for all statistical tests was set at P < 0.05.
Conditional discrimination. The discrimination ratio defined as [correct responding / (correct + incorrect responding)]*100 was calculated for each mouse at Phases 6 and 8 to measure response accuracy following extensive training and reversal learning respectively. Only mice that reached the 70% criteria at Phase 5 were included in the following analyses. Separate repeated measures one‐way ANOVAs were used to assess the effect of a range of THC, WIN, CBD or combination doses on correct discrimination. In addition, two‐way ANOVAs with Tukey's multiple comparisons tests were run to determine whether single or combined treatments were significantly different from one another.
Barnes maze. Separate one‐way ANOVAs and Dunnett's multiple comparisons tests were run to assess the effect of a range of THC, CBD or combination doses on each variable measured. In addition, two‐way ANOVAs with Tukey's multiple comparisons tests were run to determine whether single or combined treatments were significantly different from one another.
Withdrawal behaviours. One‐way ANOVA was used to determine the effect of acute administration of SR141716 or WAY 100635 and chronic dosing of CBD, THC, WIN‐55212 or combinations on head shakes, paw flutters and scratching. Post hoc analysis was performed using Dunnett's multiple comparisons test to determine the following: (i) which treatment groups were significantly different from vehicle+vehicle treatment; (ii) which chronic treatment groups were significantly different from vehicle+SR141716 or vehicle+WAY 100635 treatment; and (iii) whether combination CBD groups were significantly different from chronic THC or WIN55212 alone groups. EPM: Un‐paired Student's t‐tests were used to determine the effects of acute antagonist treatments (WAY 100635, capsazepine or SCH 58261) in chronic CBD treated mice as compared with their vehicle‐treated controls.
Materials
Δ9‐THC, CBD, WIN55212 and SR141716 were provided by the NIDA drug supply programme (Rockville, MD, USA). All cannabinoid compounds were dissolved in a vehicle of 1:1:18 ethyl alcohol: cremophor:saline (v/v). The 5‐HT1A receptor antagonist WAY 100635 (dissolved in saline), the TRPV1 receptor antagonist capsazepine (dissolved in the cremophor vehicle described above) and the adenosine A2A receptor antagonist SCH 58621 (dissolved in saline) were purchased from Tocris Bioscience (Ellisville, MO, USA). All agents were injected i.p. based on body weight.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c).
Results
Conditional discrimination
No effects of THC, CBD or THC + CBD treatment were observed on the test for extensive training (Figure 1A) or reversal learning (data not shown). For the WIN/CBD experiment, WIN55212 alone showed a dose‐dependent decrease in performance (Figure 1B). Two‐way ANOVA showed a significant effect of treatment [F (2, 63) = 3.66] but no post hoc differences between treatments. In addition, separate one‐way ANOVAs showed no significant effect of THC, WIN55212, CBD or combinations, compared to their vehicle control.
Figure 1.
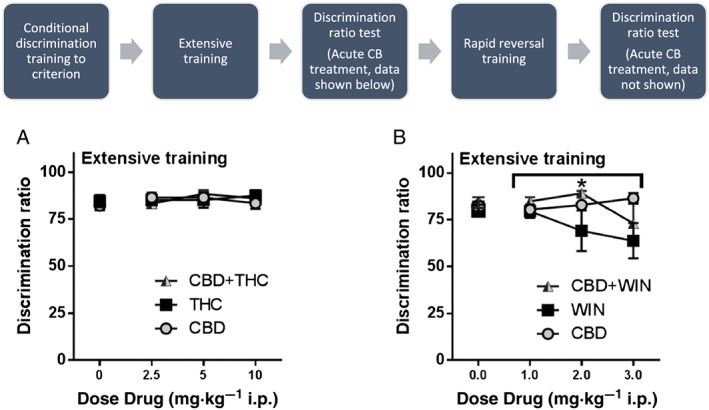
Effects of THC or WIN55212 alone or in combination on retention of a tone discrimination task. (A) Treatment with THC, CBD or their combination did not affect performance in the discrimination task following training. (B) Although a decrease was observed for WIN55212 on discrimination performance and a less potent decrease for co‐administration with CBD to reverse this affect, one‐way ANOVAs showed no significant effects of each treatment. Two‐way ANOVA showed a significant effect of treatment across groups, but post hoc analysis determined no significant differences between two particular treatment groups. N = 8 per group. Underneath overarching bracket, * P < 0.0.5, main effect of dose.
Barnes maze
Latency to first target zone entry
THC alone and in combination showed a dose‐dependent increase in the time taken to enter the target zone for the first time. One‐way ANOVA for each compound showed no significant effect of CBD or THC + CBD treatment, but a significant effect of THC on latency to first target zone entry [F(3,24) = 2.735]. Dunnett's multiple comparisons post test showed a significant effect of 20 mg·kg−1 THC compared with vehicle control. Two‐way ANOVA showed a significant effect of cannabinoid treatment on latency [F(2,54) = 3.965], but no post test significance between specific treatment groups. There were no significant main effects of dose or significant interaction (Figure 2A).
Figure 2.
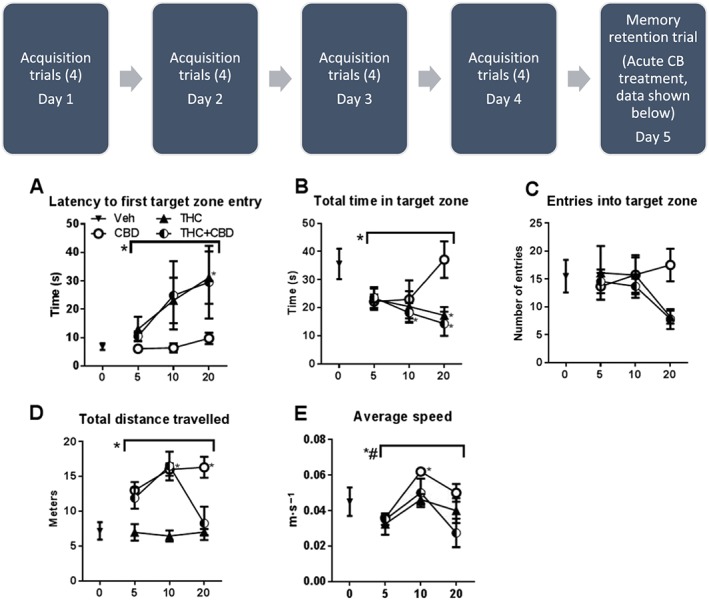
Effects of THC or CBD + THC administered in 1:1 ratio based on dose performance during retention of spatial memory. Top panel: THC and CBD + THC dose‐dependently decrease latency to first target zone entry, total time in target zone and # of entries into target zone. CBD did not alter retention of spatial memory. Bottom panel: THC did not affect total distance travelled or average speed, while CBD increased both locomotor measurements. N = 7 per group. To the left of overarching bracket, * P < 0.0.5, main effect of dose; # P < 0.0.5, main effect of treatment. Next to individual data points, * P < 0.0.5, significantly different from vehicle‐treated group.
Total time in target zone
THC alone and in combination showed a dose‐dependent decrease in the total time spent in the target zone across the test session. One‐way ANOVA for each compound showed no significant effect of CBD treatment, but a significant effect of THC [F(3,24) = 3.132] and THC + CBD [F(3,24) = 4.683] treatment. Dunnett's multiple comparisons post test showed a significant effect of 20 mg·kg−1 THC compared with vehicle control and significant effects of 10 and 20 mg·kg−1 THC + CBD. Two‐way ANOVA showed a significant effect of cannabinoid treatment on latency [F(2,54) = 3.045], but no post test significance between specific treatment groups. There were no significant main effects of dose or significant interaction (Figure 2B).
Number of entries into target zone
THC alone and in combination showed a dose‐dependent decrease in the total number of times the mice entered the target zone during the test period. One‐way ANOVAs for each compound showed no significant effect of THC, CBD or THC + CBD treatment. Two‐way ANOVA showed no significant main effects or significant interaction (Figure 2C).
Total distance travelled
CBD produced a dose‐dependent increase in total distance travelled, while the combination produced an inverted U‐shaped function. THC did not affect total distance travelled on the maze. One‐way ANOVA for each compound showed a significant effect of CBD treatment on increasing total distance travelled [F(3,24) = 3.999] and no significant effect of THC treatment or THC + CBD. Dunnett's multiple comparisons post test showed a significant effect of 10 and 20 mg·kg−1 CBD compared with vehicle control. Two‐way ANOVA showed a significant effect of cannabinoid treatment on total distance [F(2,54) = 27.27] and a significant interaction [F(4,54) = 2.905] (Figure 2D).
Average speed
CBD, THC and their combination all produced an inverted U‐shaped dose effect on average speed on the maze. One‐way ANOVA for each compound showed a significant effect of CBD treatment on increasing average speed [F(3,24) = 9.089] and no significant effect of THC treatment or THC + CBD. Dunnett's multiple comparisons post test showed a significant effect of 10 and 20 mg·kg−1 CBD compared with vehicle control. Two‐way ANOVA showed a significant effect of cannabinoid treatment on total distance [F(2,54) = 24.07], a significant main effect of dose [F(2,54) = 6.034] and no significant interaction (Figure 2E).
Withdrawal behaviours
CB1 receptor antagonist precipitated withdrawal – THC and CBD
The ability of an acute injection of the CB1 receptor antagonist SR141716 (10 mg·kg−1 i.p.) to elicit withdrawal signs following chronic administration of 20 mg·kg−1 THC, 20 mg·kg−1 CBD or the combination was tested. Scratching: One‐way ANOVA and Tukey's multiple comparison test showed a significant overall effect of drug treatment and several post hoc significant differences [F(4,35) = 13.9]. The following treatment groups were significantly different from vehicle‐treated mice: vehicle+SR, CBD + SR showed significant increases in scratching behaviour. The following treatment groups were significantly different from SR‐treated mice, suggesting an effect of precipitated withdrawal: THC + SR, CBD + THC + SR showed an attenuation of scratching behaviour elicited by SR alone. The THC + CBD + SR group showed significantly less scratching than the CBD + SR group but no difference as compared with the THC + SR group. Paw tremor: One‐way ANOVA and Tukey's multiple comparison test showed a significant overall effect of drug treatment and several post hoc significant differences [F(4,35) = 6.91]. The following treatment groups were significantly different from vehicle‐treated mice: THC + SR led to a significant increase in paw tremor behaviour. The following treatment groups were significantly different from SR‐treated mice, suggesting an effect of precipitated withdrawal: THC + SR led to a significant increase in paw tremor behaviour compared with SR alone. The THC + CBD + SR group showed a trend toward less paw tremor behaviour as compared with THC + SR, but there was no post hoc significant effect between these two groups. Head shakes: One‐way ANOVA and Tukey's multiple comparison test showed a significant overall effect of drug treatment and several post hoc significant differences [F(4,35) = 3.59]. The following treatment groups were significantly different from vehicle‐treated mice: vehicle+SR, CBD + SR and THC + CBD + SR showed significant increases in head shake behaviour. No treatment groups were significantly different from SR‐treated mice. Lastly, the THC + CBD + SR group showed no difference in rearing compared with the CBD + SR and THC + SR groups. Rearing: One‐way ANOVA and Tukey's multiple comparison test showed a significant overall effect of drug treatment [F(4,35) = 3.69]. The following treatment groups were significantly different from vehicle‐treated mice: THC+ CBD + SR showed significant decreases in rearing behaviour. No treatment groups were significantly different from SR‐treated mice. Lastly, the THC + CBD group showed no difference in rearing compared with the CBD + SR and THC + SR groups (Figure 3).
Figure 3.
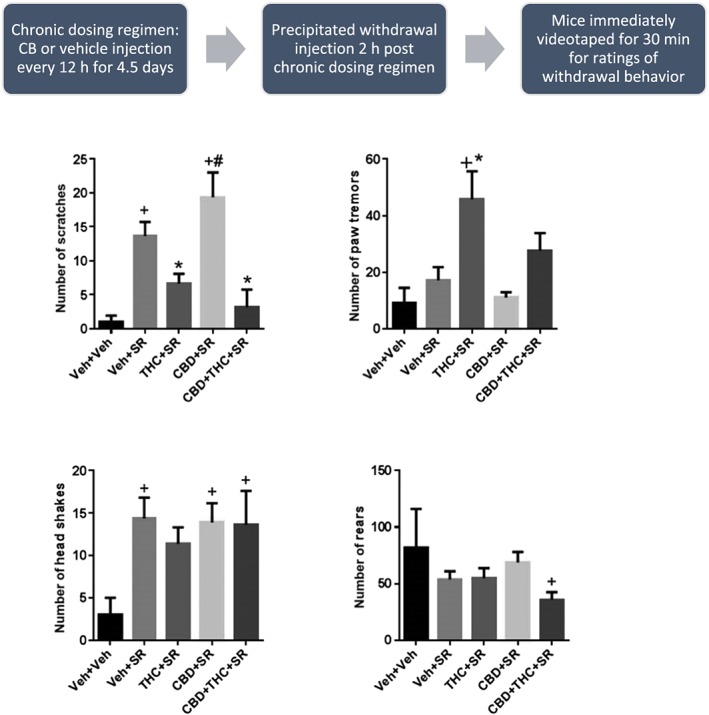
Effects of SR‐141716‐precipitated withdrawal from chronic administration of THC, CBD or their combination. Acute administration of SR141716 significantly increased scratching and head shakes compared to acute vehicle administration. Scratching is attenuated in mice chronically treated with THC or THC + CBD. Chronic THC administration also led to significant paw tremor, compared with vehicle or acute SR administration. N = 8 per group. + P < 0.05, significantly different from Veh + Veh; * P < 0.05, significantly different from Veh + SR; # P < 0.05, significantly different from CBD + THC; one‐way ANOVA.
CB1 receptor antagonist precipitated withdrawal – WIN 55212 and CBD
The ability of an acute injection of the CB1 receptor antagonist SR141716 (10 mg·kg−1 i.p.) to elicit withdrawal signs following chronic administration of 3.0 mg·kg−1 WIN 55212, 5.0 mg·kg−1 CBD or the combination was tested. Scratching: One‐way ANOVA and Tukey's multiple comparison test showed a significant overall effect of drug treatment and several post hoc significant differences [F(4,38) = 10.2]. The following treatment groups were significantly different from vehicle‐treated mice: vehicle+SR, CBD + SR showed significant increases in scratching behaviour. The following treatment groups were significantly different from SR‐treated mice, suggesting an effect of precipitated withdrawal: WIN+SR, WIN+CBD + SR showed an attenuation of scratching behaviour elicited by SR alone. The WIN+CBD + SR group showed no difference as compared with the WIN+SR group. Paw tremor: One‐way ANOVA and Tukey's multiple comparison test showed a significant overall effect of drug treatment [F(4,38) = 3.20]. The following treatment groups were significantly different from vehicle‐treated mice: veh + SR led to a significant increase in paw tremor behaviour. No treatment groups were significantly different from SR‐treated mice beside vehicle. Head shakes: One‐way ANOVA and Tukey's multiple comparison test showed a significant overall effect of drug treatment and several post hoc significant differences [F(4,38) = 7.39]. The following treatment groups were significantly different from vehicle‐treated mice: vehicle+SR, CBD + SR showed significant increases in head shake behaviour. The following treatment groups were significantly different from SR‐treated mice, suggesting an effect of precipitated withdrawal: WIN+SR, WIN+CBD + SR showed an attenuation of head shake behaviour elicited by SR alone. Lastly, the WIN+CBD + SR group showed no difference in rearing compared with the CBD + SR and WIN+SR groups. Rearing: One‐way ANOVA and Tukey's multiple comparison test showed a significant overall effect of drug treatment [F(4,38) = 7.53]. The following treatment groups were significantly different from vehicle‐treated mice: WIN+SR and WIN+CBD + SR showed significant decreases in rearing behaviour. No treatment groups were significantly different from SR‐treated mice. Lastly, the WIN+CBD + SR group showed no difference in rearing compared with the CBD + SR and WIN+SR groups (Figure 4).
Figure 4.
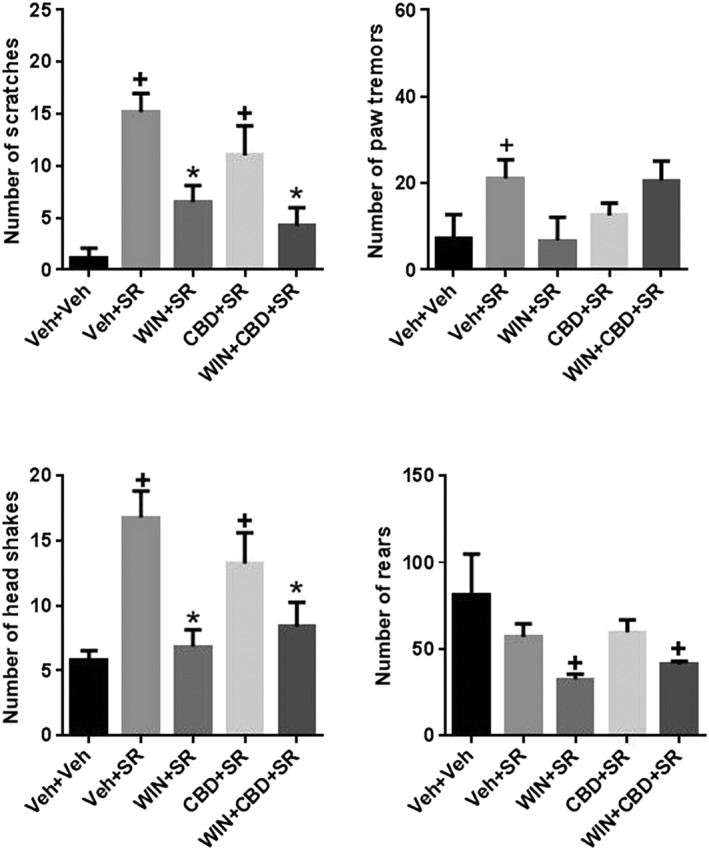
Effects of SR141716‐precipitated withdrawal from chronic administration of WIN‐55212, CBD or their combination. Acute administration of SR141716 significantly increase scratching, paw tremor and head shakes compared to acute vehicle administration. Scratching and head shakes are attenuated in mice chronically treated with WIN or WIN+CBD. Chronic WIN and WIN+CBD administration also led to significant decreases in rearing. N = 8 per group. + P < 0.05, significantly different from Veh + Veh; * P < 0.05, significantly different from Veh + SR; # P < 0.05, significantly different from WIN+CBD; one‐way ANOVA.
5‐HT1A receptor antagonist precipitated withdrawal – THC and CBD
One‐way ANOVA showed no significant effects of chronic phytocannabinoid treatment and acute injection of the 5‐HT1A receptor antagonist WAY 100635 on scratching, head shakes or paw tremors. There was a significant overall effect of treatment on rearing behaviour [F(4,35) = 3.88], but no post hoc significant differences (Figure 5).
Figure 5.
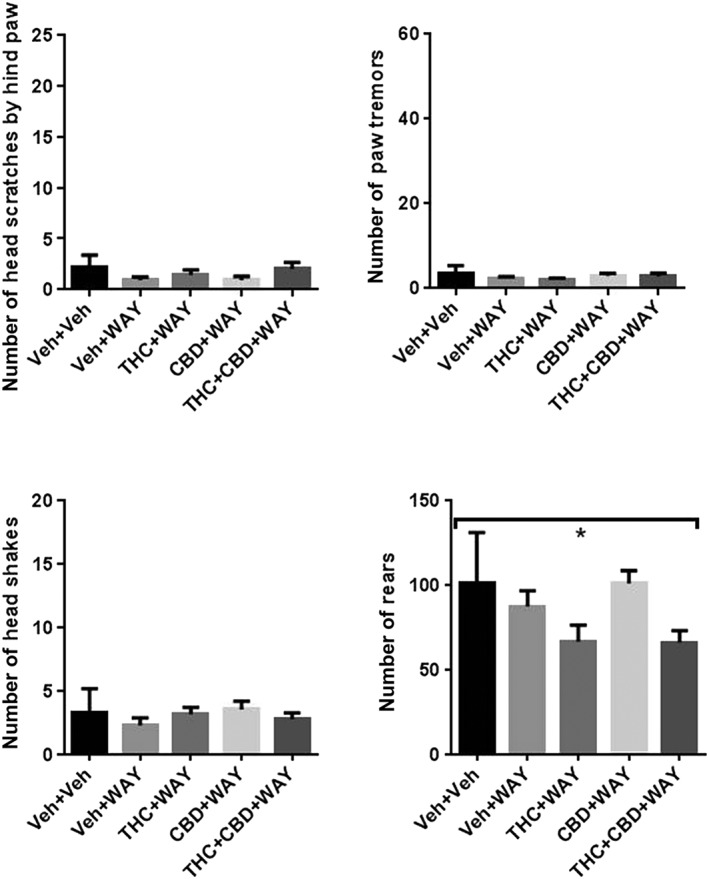
Effects of WAY100135‐precipitated withdrawal from chronic administration of THC, CBD or their combination. Acute administration of WAY100135 produced no effect on scratching, paw tremors, head shakes or rearing and did not precipitate these behaviours in THC, CBD or combination‐treated mice. N = 8 per group. Underneath overarching bracket, * P < 0.05, significantly different overall; one‐way ANOVA.
5‐HT1A receptor antagonist precipitated withdrawal – WIN55212 and CBD
One‐way ANOVA showed no significant effects of chronic WIN and CBD cannabinoid treatment and acute injection of the 5‐HT1A receptor antagonist WAY 100635 on scratching, head shakes or paw tremors. There was a significant overall effect of treatment on rearing behaviour [F(4,35) = 10.13, P = 0.0001], and several post hoc significant differences. The following treatment groups were significantly different from vehicle‐treated mice: WIN+WAY and WIN+CBD + WAY showed significant decreases in rearing behaviour. These groups were also significantly different from WAY only‐treated mice (Figure 6).
Figure 6.
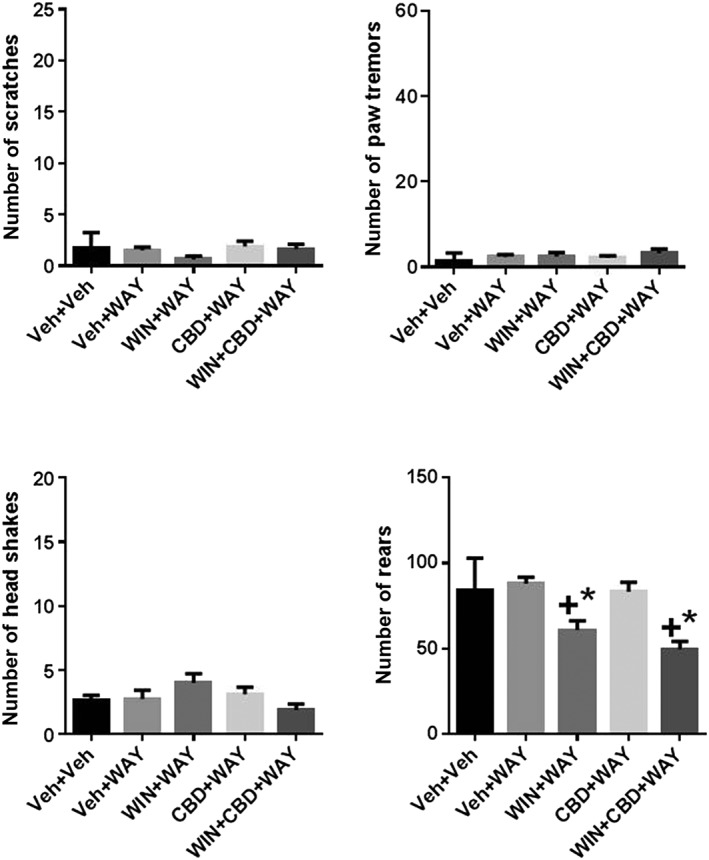
Effects of WAY100135‐precipitated withdrawal from chronic administration of WIN, CBD or their combination. Acute administration of WAY100135 produced no effect on scratching, paw tremors, head shakes or rearing and did not precipitate these behaviours in WIN, CBD or combination‐treated mice. N = 8 per group. + P < 0.05, significantly different from Veh + Veh; * P < 0.05, significantly different from Veh + SR; # P < 0.05, significantly different from WIN+CBD; one‐way ANOVA.
Elevated plus maze
% time spent in open arm
The ability of the 5‐HT1A receptor antagonist WAY100635, the TRPV1 receptor antagonist capsazepine or the adenosine A2A receptor antagonist SCH 58261 to precipitate withdrawal associated anxiogenic behaviour in chronically treated CBD mice was assessed using the EPM. In chronic vehicle‐treated mice, only acute administration of WAY 100635 produced a significant increase in % time in the open arm. Compared with chronic vehicle treatment, chronic CBD treatment also increased % time spent on the open arms. This significant difference was observed in mice pretreated prior to EPM with vehicle, capsazepine or SCH 58261. No significant difference was observed between vehicle and CBD‐treated mice given WAY 100635 prior to EPM (Figure 7).
Figure 7.
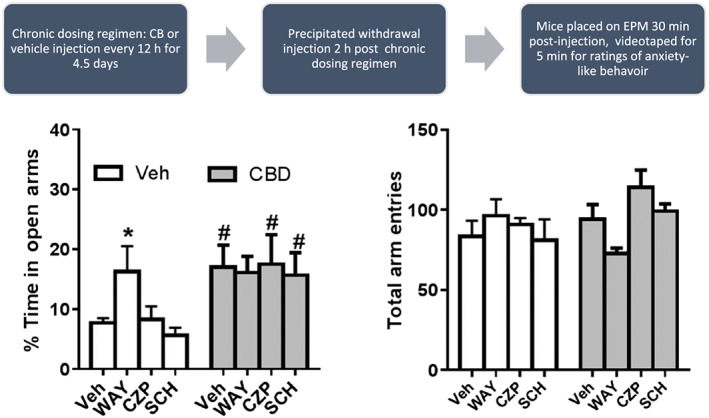
Effect of acute antagonist treatment on anxiety‐like behaviour in chronically CBD‐treated mice. (A) Chronic administration of CBD (shaded bars produced significant increases in time spent in the open arm compared to pretreatment matched controls (open bars) with the exception of the WAY pretreatment mice]. (B) WAY and capsazepine pretreatment produced trends toward alterations, but no significant changes, in total arm entries in chronic CBD versus chronic vehicle treated groups. N = 8 per group. * P < 0.05, significantly different from Veh; one‐way ANOVA.
Total arm entries
None of the acute antagonist treatments altered total arm entries on the EPM in chronic vehicle‐treated mice. Chronic CBD treatment also did not alter total arm entries as compared to chronic vehicle treatment (Figure 7B). In chronic CBD‐treated mice administered acute antagonists, WAY 100635 produced a non‐significant decrease (P=0.06) in total arm entries as compared to chronic CBD/acute vehicle treatment as well as chronic vehicle/acute WAY treatment. Also, acute administration of capsazepine produced a non‐significant increase in total arm entries in chronic CBD treated compared with chronic vehicle treated mice.
Discussion
The present studies were designed to test the hypothesis that chronic CBD treatment does not produce cognitive deficits or precipitated withdrawal signs and does not mitigate the cognitive deficits or precipitated withdrawal signs associated with cessation of CB1 receptor agonist use. Several animal studies have been conducted to determine the learning and memory and dependence‐associated effects of the primary phytocannabinoid THC and other mixed CB1/CB2 receptor agonists, but fewer studies have addressed the effects of the second most abundant phytocannabinoid CBD alone and in combination with THC. In the present set of experiments, we observed mild effects of acute THC or WIN‐55212 administration on learning and memory, no cognitive impairing effects of CBD treatment alone and little to no evidence that CBD attenuates the learning and memory deficits associated with acute cannabinoid agonist treatment. Similarly, we observed that chronic administration of THC or WIN‐55212 was associated with behavioural signs indicative of precipitated withdrawal following acute administration of the CB1 receptor antagonist SR‐141716. Chronic administration of CBD was not associated with any behavioural signs indicative of precipitated withdrawal following acute administration of SR141716 or the 5‐HT1A receptor selective antagonist WAY100635. Chronic co‐administration of CBD with THC or WIN55212 did not alter tolerance to head scratching behaviour seen with CB agonist alone, but did appear to attenuate chronic CB agonist‐associated paw tremors. Taken together, our data suggest that CBD by and large did not attenuate retention deficits or withdrawal behaviours when combined with THC. Additionally, it did not produce these effects on its own. These results are important because Sativex (1:1 THC to CBD ratio) is already in use in many countries and many researchers and lay people have assumed that CBD mitigates some of the adverse effects caused by THC.
We tested the effects of cannabinoid agonists alone and in combination with CBD in an auditory conditional discrimination task with a reversal component and an appetitively motivated spatial reference memory task. Results from our auditory discrimination task revealed that THC at doses of 2.5–10 mg·kg−1 i.p. did not disrupt auditory discrimination following extensive training, nor did it disrupt reversal learning of the task. CBD alone and in combination with THC were also without effect. Previously, Sokolic et al. (2011) reported that THC administration (0.3–10 mg·kg−1 i.p.) disrupted a well‐learned auditory discrimination in Wistar rats, while Mallet and Beninger (1998) reported that visual/auditory discrimination was not affected by 2.0–4.0 mg·kg−1 i.p. THC in Wistar rats (however, subsequent performance on a delayed non‐match to sample reversal of the task was significantly impacted). One explanation for the lack of THC effect on performance in the present task could be related to dose or strain, as THC appears to be relatively less potent in C57Bl6 mice than other rodent models. For example, Varvel et al. (2001) reported that 100 mg·kg−1 THC i.p. was necessary to impair reference memory in male C57Bl/6 mice. This dose of THC also produced significant hypolocomotion and catalepsy which makes it difficult to separate out these effects. One explanation for this relative tolerance could be CB1 receptor density, as it was shown in one study that CB1 receptor densities were 25% lower in C57Bl6 mice compared with that of DBA/2 mice. Significant differences in affinity were also observed between the two lines (Hungund and Basavarajappa, 2000). WIN55212 produced a non‐significant trend to disrupt auditory discrimination following extensive training, and this trend appeared to be attenuated by co‐administration of CBD at 2.0 but not 3.0 mg·kg−1 WIN55212. Previous experiments from our laboratory as well as others ( Fan et al., 1994) have shown that this higher dose of WIN55212 produces significant hypolocomotion, so it is unclear whether the trend observed in the discrimination task was specific to cognition or related to motor impairment.
Significant performance‐decreasing effects of THC were observed in the Barnes maze assessing spatial reference memory. THC administration significantly attenuated latency to first target zone entry, the total time spent in the target zone and the number of entries into the target zone. Co‐administration of CBD did not attenuate these effects; the THC + CBD‐treated group looked identical to the THC alone group on all three measures. In addition to these indicators of spatial learning performance, mean speed and total distance travelled were also measured to control for more general changes in behaviour. THC administration at the doses tested did not lead to significant effects on total distance travelled or average speed, and as previously mentioned, significant hypolocomotion was not observed in C57Bl6 mice until 30 mg·kg−1 i.p. in the Varvel et al. (2001) study. CBD administered alone produced a significant increase in total distance travelled and average speed on the Barnes maze. As CBD did not produce a cognitive‐enhancing effect in the maze, we believe that the most likely explanation for this increased locomotor behaviour could be anxiolysis, a behavioural effect that has been associated with CBD treatment in several rodent (see Guimaraes et al., 1990; Moreira et al., 2006; Fogaca et al., 2014) and human (Zuardi et al., 1982; Bergamaschi et al., 2011; Crippa et al., 2011) studies. At the lower dose combinations, THC + CBD tracked the effects of CBD alone, but at the highest combination, THC + CBD tracked the effects of THC alone. In fact for average speed, 20THC + 20CBD showed a non‐significant trend toward decreasing average speed to below baseline and 20 mg·kg−1 THC alone levels. Interestingly, another recent study in rats showed that CBD alone increased locomotor activity but enhanced THC‐induced hypolocomotion (Britch et al., 2017). One explanation for the potentiating effects of CBD on THC is that CBD decreased THC metabolism (Klein et al., 2011; Britch et al., 2017).
Taking together the cognitive performance measurements with the motor performance measurements in the present study, THC produced dose‐dependent deficits in reference spatial learning in the Barnes maze at doses that did not produce overt motor effects. These effects on hippocampal‐dependent spatial learning are similar to those previously observed with the effects of THC in the radial arm maze (Lichtman et al., 1995; Lichtman and Martin, 1996; Wise et al., 2009) and Morris water maze (Da Silva and Takahashi, 2002; Varvel et al., 2001). The combination of THC + CBD did not attenuate these cognitive effects and showed a trend toward producing more performance deficits at the highest combination tested. Hayakawa et al. (2008) reported that combination ratios higher in CBD than THC exacerbated THC‐induced memory deficits on the eight arm radial maze in ddY mice, while Fadda et al. (2004) reported no effect of CBD‐rich extract of Cannabis on the spatial working memory deficits induced by a THC‐rich extract in Lister rats. Wright Jr. et al. (2013) demonstrated in rhesus monkeys that CBD (5.0 mg·kg−1) attenuated the effect of THC (0.5 mg·kg−1) on a visuospatial learning task but not THC‐induced deficits in other behavioural tasks. Lastly, Englund et al. (2013) reported that in healthy human volunteers, pretreatment with 600 mg CBD significantly attenuated episodic memory deficits associated with i.v. administration of 1.5 mg THC. These data suggest that potentiation or antagonism of THC‐induced memory deficits is dependent on several factors including but not limited to (i) the ratio between THC and CBD, (ii) the cognitive domain being assessed, and (iii) the species or strain tested. As has been shown in these studies and others, CBD alone showed no effect on cognitive performance and may show an anxiolytic profile in the task. An additional possibility in previous studies where CBD enhanced effects of THC is that the CBD extract used may contain a small percentage of THC, which at high CBD concentrations may contribute to combination effects. The CBD used in the present study was obtained through the NIDA Drug Supply Program and is >99% pure CBD.
The withdrawal effects following cessation of chronic THC exposure using animal models have been studied since the 1970's (see Wikler, 1976). However, the long half‐life of cannabinoids such as THC and the consequent delay of withdrawal effects make quantifying these effects a challenge. Not surprisingly, the results of abstinence withdrawal studies in laboratory animals have been mixed. The availability of SR141716 was a major advancement toward this research as it allowed researchers to turn from a spontaneous withdrawal model to one of precipitated withdrawal. Determining whether chronic CBD treatment leads to dependence shares this challenge as it shares the pharmacokinetic profile of THC, and an additional challenge is introduced as well. In the precipitated withdrawal model, an appropriate receptor antagonist is administered to a drug‐dependent animal and the antagonist displaces the agonist from the receptor, immediately eliciting withdrawal effects. In the unique case of CBD, direct and indirect effects upon CB1 receptors are yet to be clearly defined, while direct or indirect effects upon other receptor targets have been more conclusively demonstrated. Therefore in the present study, we sought to determine whether withdrawal signs would be precipitated in chronically treated CBD mice following acute injection of selective antagonists for the CB1 receptor, 5‐HT1A receptor, TRPV1 receptor or adenosine A2A receptor. A second goal was to determine whether chronic co‐administration of CBD potentiated or attenuated withdrawal signs associated with precipitated withdrawal from THC or WIN55212.
Out of the four withdrawal variables measured, changes in head scratching, paw tremor and head shakes were the most consistent behaviours associated with precipitated withdrawal from chronic cannabinoid exposure. Suppression of rearing behaviour appeared to be more indicative of hypolocomotive effects of chronic cannabinoid administration than of precipitated withdrawal. Head scratching is elicited by the CB1 receptor antagonist SR141716, possibly through interactions with the 5‐HT system (Ward et al., 2009), and the absence of this effect on chronically CB1 receptor agonist‐treated rodents is believed to reflect tolerance and therefore insensitivity of the CB1 receptor to the head‐scratch eliciting effects of SR141716 (Cook et al., 1998). In the present study, acute administration of SR141716 failed to elicit head‐scratching behaviour in mice chronically treated with THC, THC + CBD, WIN‐55212 or WIN+CBD. Chronic treatment with CBD alone had no effect on SR41716A‐elicited head scratching behaviour. Acute administration of SR141716 elicited paw tremors in chronic THC, but not chronic WIN55212‐treated mice. This effect was also observed by Cook et al. (1998) and Lichtman et al. (2001). Co‐administration of THC + CBD attenuated this effect in that the combination‐treated group did not show a significant increase in paw tremors, compared with control groups. Acute administration of SR141716 produced a significant number of head shakes, also potentially through its interactions with the 5‐HT system. Chronic administration of THC, CBD or their combination did not lead to tolerance to this effect (THC effect in agreement with Cook et al., 1998). However, chronic administration of WIN55212 or WIN+CBD did lead to tolerance to the acute effect of SR141716 on head shake behaviour. Significant decreases in rearing were observed in mice chronically treated with THC + CBD, WIN55212 and WIN+CBD. As mentioned, because CBD is known to act as a direct agonist at 5‐HT1A receptors, we also tested the hypothesis that chronic CBD treatment could lead to precipitated withdrawal following acute injection of the 5‐HT1A receptor antagonist WAY 100635. Acute administration of WAY 100635 produced no overt behavioural effects in mice chronically treated with CBD, THC, WIN55212 or their combinations. Taken together, to our knowledge, this is the first report of the effects of chronic CBD administration alone or in combination with CB receptor agonists on precipitated withdrawal, and the results suggest that dependence to CBD does not occur following chronic treatment and that co‐administration of equal ratios of CBD and THC does not lead to a substantial attenuation of THC dependence.
Because CBD administration is associated with anxiolysis, a final precipitated withdrawal experiment was performed on the EPM with CBD and a range of antagonists for receptors associated with the pharmacological effects of CBD to determine whether withdrawal from chronic CBD exposure would elicit anxiogenic behaviour. This approach has been taken with THC, and Huang et al. (2010) demonstrated that acute administration of SR141716 precipitated an anxiety‐like phenotype on the maze in mice chronically treated with THC. Chronic CBD administration produced significant anxiolysis in mice treated acutely with vehicle, the TRPV1 antagonist capsazepine or the adenosine A2A receptor antagonist SCH 58261. Chronic CBD followed by administration of WAY 100635 did not produce open arm activity that was significantly different from chronic vehicle/WAY 100635‐treated mice, suggesting that pretreatment with the 5‐HT1A receptor antagonist blocked the anxiolytic effect CBD. However, quite surprisingly, WAY 100635 produced an anxiolytic profile in the vehicle‐treated mice, so it is difficult to interpret this result. Overall, these results also demonstrate that chronic CBD administration does not lead to dependence and withdrawal.
The present results are important because they add to a growing literature documenting potential antagonistic or additive interactions between the two most abundant phytocannabinoids in the Cannabis plant. The present results are important because they specifically and extensively test the hypothesis that the 1:1 THC‐CBD combination does not produce fewer adverse cognitive or drug withdrawal effects as compared to THC alone. As mentioned above, Sativex is already in use in many countries and is fast‐tracked for late stage clinical trials in the US, based to a large extent on the assumption that CBD mitigates some of the adverse effects caused by THC. While the present results demonstrate that CBD may not increase the safety profile of THC when given in combination, they do support other studies showing a lack of adverse behavioural effects of CBD alone. Therefore, it is also critical to determine the efficacy of CBD alone compared with THC and the CBD + THC combination, to determine optimal cannabinoid‐based treatment strategies for the future. Recently, we have demonstrated, for example, that while both CBD and THC alone prevent development of mechanical sensitivity in a mouse model of chemotherapy‐induced peripheral neuropathic pain, a 1:1 ratio of CBD to THC acted synergistically, leading to an approximate 10‐fold increase in potency in the model (King et al., 2017). Thus, CBD as a monotherapy may represent a safe and effective treatment strategy for neuropathic pain, while co‐administration of equivalent THC + CBD doses may lead to lower doses of each agent being necessary, thereby attaining the goal of decreasing THC‐associated adverse effects.
Author contributions
A.M.M. performed behavioural testing and was involved in statistical analysis and critical review of the manuscript. P.B.S. performed behavioural testing and was involved in statistical analysis and critical review of the manuscript. J.D.F. assisted in the writing of the manuscript. R.F.T. assisted in the conception and design of the work and in critical review of the manuscript. S.J.W. was responsible for the conception and design of the work. She also was responsible for the majority of the statistical analyses and the drafting of the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This work was supported by NIDA/NIH grants R03DA034761 (SJW) and P30 DA013429 (EMU). We thank Ellen A. Walker for editorial assistance.
Myers, A. M. , Siegele, P. B. , Foss, J. D. , Tuma, R. F. , and Ward, S. J. (2019) Single and combined effects of plant‐derived and synthetic cannabinoids on cognition and cannabinoid‐associated withdrawal signs in mice. British Journal of Pharmacology, 176: 1552–1567. 10.1111/bph.14147.
References
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Striessnig J, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. Br J Pharmacol 174: S160–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ (2011). The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend 119: 123–129. [DOI] [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F et al (2011). Cannabidiol reduces the anxiety induced by simulated public speaking in treatment‐naive social phobia patients. Neuropsychopharmacology 36: 1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisen‐Hersh EB, Hineline PN, Walker EA (2013). Effects of early chemotherapeutic treatment on learning in adolescent mice: implications for cognitive impairment and remediation in childhood cancer survivors. Clin Cancer Res 19: 3008–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I et al (2001). Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 134: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong MG, Jansma JM, van Hell HH, Jager G, Oudman E, Saliasi E et al (2012). Effects of delta9‐tetrahydrocannabinol on human working memory function. Biol Psychiatry 71: 693–699. [DOI] [PubMed] [Google Scholar]
- Britch SC, Wiley JL, Yu Z, Clowers BH, Craft RM (2017). Cannabidiol‐delta9‐tetrahydrocannabinol interactions on acute pain and locomotor activity. Drug Alcohol Depend 175: 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodkin J, Moerschbaecher JM (1997). SR141716A antagonizes the disruptive effects of cannabinoid ligands on learning in rats. J Pharmacol Exp Ther 282: 1526–1532. [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R (2004). Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry 161: 1967–1977. [DOI] [PubMed] [Google Scholar]
- Campos AC, Fogaca MV, Sonego AB, Guimaraes FS (2016). Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res 112: 119–127. [DOI] [PubMed] [Google Scholar]
- Carrier EJ, Auchampach JA, Hillard CJ (2006). Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A 103: 7895–7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarotto PC, Gomes FV, Resstel LB, Guimaraes FS (2010). Cannabidiol inhibitory effect on marble‐burying behaviour: involvement of CB1 receptors. Behav Pharmacol 21: 353–358. [DOI] [PubMed] [Google Scholar]
- Castane A, Maldonado R, Valverde O (2004). Role of different brain structures in the behavioural expression of WIN 55, 212‐2 withdrawal in mice. Br J Pharmacol 142: 1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SA, Lowe JA, Martin BR (1998). CB1 receptor antagonist precipitates withdrawal in mice exposed to delta9‐tetrahydrocannabinol. J Pharmacol Exp Ther 285: 1150–1156. [PubMed] [Google Scholar]
- Cooper ZD, Haney M (2008). Cannabis reinforcement and dependence: role of the cannabinoid CB1 receptor. Addict Biol 13: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Giagnoni G, Franke C, Trovato AE, Colleoni M (2004). Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol 143: 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar‐Poli P, Gonzalez R (2013). Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol Rev 23: 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa JA, Derenusson GN, Ferrari TB, Wichert‐Ana L, Duran FL, Martin‐Santos R et al (2011). Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol 25: 121–130. [DOI] [PubMed] [Google Scholar]
- Cunha GM, Canas PM, Melo CS, Hockemeyer J, Muller CE, Oliveira CR et al (2008). Adenosine A2A receptor blockade prevents memory dysfunction caused by beta‐amyloid peptides but not by scopolamine or MK‐801. Exp Neurol 210: 776–781. [DOI] [PubMed] [Google Scholar]
- Curran HV, Brignell C, Fletcher S, Middleton P, Henry J (2002). Cognitive and subjective dose‐response effects of acute oral delta 9‐tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 164: 61–70. [DOI] [PubMed] [Google Scholar]
- Curran HV, Freeman TP, Mokrysz C, Lewis DA, Morgan CJ, Parsons LH (2016). Keep off the grass? Cannabis, cognition and addiction. Nat Rev Neurosci 17: 293–306. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva GE, Takahashi RN (2002). SR 141716A prevents delta 9‐tetrahydrocannabinol‐induced spatial learning deficit in a Morris‐type water maze in mice. Prog Neuropsychopharmacol Biol Psychiatry 26: 321–325. [DOI] [PubMed] [Google Scholar]
- El‐Remessy AB, Tang Y, Zhu G, Matragoon S, Khalifa Y, Liu EK et al (2008). Neuroprotective effects of cannabidiol in endotoxin‐induced uveitis: critical role of p38 MAPK activation. Mol Vis 14: 2190–2203. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S et al (2013). Cannabidiol inhibits THC‐elicited paranoid symptoms and hippocampal‐dependent memory impairment. J Psychopharmacol 27: 19–27. [DOI] [PubMed] [Google Scholar]
- Fadda P, Robinson L, Fratta W, Pertwee RG, Riedel G (2004). Differential effects of THC‐ or CBD‐rich cannabis extracts on working memory in rats. Neuropharmacology 47: 1170–1179. [DOI] [PubMed] [Google Scholar]
- Fan F, Compton DR, Ward S, Melvin L, Martin BR (1994). Development of cross‐tolerance between delta 9‐tetrahydrocannabinol, CP 55,940 and WIN 55,212. J Pharmacol Exp Ther 271: 1383–1390. [PubMed] [Google Scholar]
- Ferrari F, Ottani A, Vivoli R, Giuliani D (1999). Learning impairment produced in rats by the cannabinoid agonist HU 210 in a water‐maze task. Pharmacol Biochem Behav 64: 555–561. [DOI] [PubMed] [Google Scholar]
- Fogaca MV, Reis FM, Campos AC, Guimaraes FS (2014). Effects of intra‐prelimbic prefrontal cortex injection of cannabidiol on anxiety‐like behavior: involvement of 5HT1A receptors and previous stressful experience. Eur Neuropsychopharmacol 24: 410–419. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Resstel LB, Guimaraes FS (2011). The anxiolytic‐like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5‐HT1A receptors. Psychopharmacology (Berl) 213: 465–473. [DOI] [PubMed] [Google Scholar]
- Guimaraes FS, Chiaretti TM, Graeff FG, Zuardi AW (1990). Antianxiety effect of cannabidiol in the elevated plus‐maze. Psychopharmacology (Berl) 100: 558–559. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Hazekawa M, Sano K, Irie K, Orito K et al (2008). Cannabidiol potentiates pharmacological effects of Delta(9)‐tetrahydrocannabinol via CB(1) receptor‐dependent mechanism. Brain Res 1188: 157–164. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC (1991). Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11: 563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Hampson RE, Deadwyler SA (1993). Effects of delta‐9‐tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short‐term memory associated with changes in task specific firing of hippocampal cells. J Pharmacol Exp Ther 264: 294–307. [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Yang R, Lichtman AH, Lupica CR (2007). Opposing actions of chronic Delta9‐tetrahydrocannabinol and cannabinoid antagonists on hippocampal long‐term potentiation. Learn Mem 14: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Liu‐Chen LY, Kirby LG (2010). Anxiety‐like effects of SR141716‐precipitated delta9‐tetrahydrocannabinol withdrawal in mice in the elevated plus‐maze. Neurosci Lett 475: 165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Liu‐Chen LY, Unterwald EM, Cowan A (2009). Hyperlocomotion and paw tremors are two highly quantifiable signs of SR141716‐precipitated withdrawal from delta9‐tetrahydrocannabinol in C57BL/6 mice. Neurosci Lett 465: 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungund BL, Basavarajappa BS (2000). Distinct differences in the cannabinoid receptor binding in the brain of C57BL/6 and DBA/2 mice, selected for their differences in voluntary ethanol consumption. J Neurosci Res 60: 122–128. [DOI] [PubMed] [Google Scholar]
- Jones RT (1983). Cannabis and health. Annu Rev Med 34: 247–258. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KM, Myers AM, Soroka‐Monzo AJ, Tuma RF, Tallarida RJ, Walker EA et al (2017). Single and combined effects of Δ9‐tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy‐induced neuropathic pain. Br J Pharmacol 174: 2832–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T et al (2011). Cannabidiol potentiates Delta(9)‐tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology (Berl) 218: 443–457. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR (1995). Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl) 119: 282–290. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Fisher J, Martin BR (2001). Precipitated cannabinoid withdrawal is reversed by Delta(9)‐tetrahydrocannabinol or clonidine. Pharmacol Biochem Behav 69: 181–188. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR (1996). Delta 9‐tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology (Berl) 126: 125–131. [DOI] [PubMed] [Google Scholar]
- Liou GI, Auchampach JA, Hillard CJ, Zhu G, Yousufzai B, Mian S et al (2008). Mediation of cannabidiol anti‐inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Invest Ophthalmol Vis Sci 49: 5526–5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ (1998). The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta9‐tetrahydrocannabinol or anandamide. Psychopharmacology (Berl) 140: 11–19. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Antonazzo KR, Craft RM, Wiley JL (2014). Evaluation of sex differences in cannabinoid dependence. Drug Alcohol Depend 137: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ (1993). Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol 327: 535–550. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misner DL, Sullivan JM (1999). Mechanism of cannabinoid effects on long‐term potentiation and depression in hippocampal CA1 neurons. J Neurosci 19: 6795–6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Guimaraes FS (2006). Anxiolytic‐like effect of cannabidiol in the rat Vogel conflict test. Prog Neuropsychopharmacol Biol Psychiatry 30: 1466–1471. [DOI] [PubMed] [Google Scholar]
- Nakamura EM, da Silva EA, Concilio GV, Wilkinson DA, Masur J (1991). Reversible effects of acute and long‐term administration of delta‐9‐tetrahydrocannabinol (THC) on memory in the rat. Drug Alcohol Depend 28: 167–175. [DOI] [PubMed] [Google Scholar]
- Pandolfo P, Silveirinha V, dos Santos‐Rodrigues A, Venance L, Ledent C, Takahashi RN et al (2011). Cannabinoids inhibit the synaptic uptake of adenosine and dopamine in the rat and mouse striatum. Eur J Pharmacol 655: 38–45. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Thomas A, Stevenson LA, Maor Y, Mechoulam R (2005). Evidence that (−)‐7‐hydroxy‐4′‐dimethylheptyl‐cannabidiol activates a non‐CB(1), non‐CB(2), non‐TRPV1 target in the mouse vas deferens. Neuropharmacology 48: 1139–1146. [DOI] [PubMed] [Google Scholar]
- Ramesh D, Schlosburg JE, Wiebelhaus JM, Lichtman AH (2011). Marijuana dependence, not just smoke and mirrors. ILAR J 52: 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock EM, Limebeer CL, Petrie GN, Williams LA, Mechoulam R, Parker LA (2017). Effect of prior foot shock stress and Δ9‐tetrahydrocannabinol, cannabidiolic acid, and cannabidiol on anxiety‐like responding in the light‐dark emergence test in rats. Psychopharmacology 234: 2207–2217. [DOI] [PubMed] [Google Scholar]
- Rubino T, Patrini G, Massi P, Fuzio D, Vigano D, Giagnoni G et al (1998). Cannabinoid‐precipitated withdrawal: a time‐course study of the behavioral aspect and its correlation with cannabinoid receptors and G protein expression. J Pharmacol Exp Ther 285: 813–819. [PubMed] [Google Scholar]
- Russo EB (2011). Taming THC: potential cannabis synergy and phytocannabinoid‐terpenoid entourage effects. Br J Pharmacol 163: 1344–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK (2005). Agonistic properties of cannabidiol at 5‐HT1a receptors. Neurochem Res 30: 1037–1043. [DOI] [PubMed] [Google Scholar]
- Sartim AG, Guimaraes FS, Joca SR (2016). Antidepressant‐like effect of cannabidiol injection into the ventral medial prefrontal cortex – possible involvement of 5‐HT1A and CB1 receptors. Behav Brain Res 303: 218–227. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME (1996). Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther 278: 989–999. [PubMed] [Google Scholar]
- Sokolic L, Long LE, Hunt GE, Arnold JC, McGregor IS (2011). Disruptive effects of the prototypical cannabinoid delta(9)‐tetrahydrocannabinol and the fatty acid amide inhibitor URB‐597 on go/no‐go auditory discrimination performance and olfactory reversal learning in rats. Behav Pharmacol 22: 191–202. [DOI] [PubMed] [Google Scholar]
- Thomas A, Ross RA, Saha B, Mahadevan A, Razdan RK, Pertwee RG (2004). 6″‐Azidohex‐2″‐yne‐cannabidiol: a potential neutral, competitive cannabinoid CB1 receptor antagonist. Eur J Pharmacol 487: 213–221. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Hamm RJ, Martin BR, Lichtman AH (2001). Differential effects of delta 9‐THC on spatial reference and working memory in mice. Psychopharmacology (Berl) 157: 142–150. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Lefever TW, Rawls SM, Whiteside GT, Walker EA (2009). Age‐dependent effects of the cannabinoid CB1 antagonist SR141716A on food intake, body weight change, and pruritus in rats. Psychopharmacology (Berl) 206: 155–165. [DOI] [PubMed] [Google Scholar]
- Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, Walker EA (2014). Cannabidiol inhibits paclitaxel‐induced neuropathic pain through 5‐HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol 171: 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A (1976). Aspects of tolerance to and dependence on cannabis. Ann N Y Acad Sci 282: 126–147. [DOI] [PubMed] [Google Scholar]
- Wise LE, Thorpe AJ, Lichtman AH (2009). Hippocampal CB(1) receptors mediate the memory impairing effects of delta(9)‐tetrahydrocannabinol. Neuropsychopharmacology 34: 2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ Jr, Vandewater SA, Parsons LH, Taffe MA (2013). Delta(9)tetrahydrocannabinol impairs reversal learning but not extra‐dimensional shifts in rhesus macaques. Neuroscience 235: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG (1982). Action of cannabidiol on the anxiety and other effects produced by delta 9‐THC in normal subjects. Psychopharmacology (Berl) 76: 245–250. [DOI] [PubMed] [Google Scholar]


