Modelling predicts that drought tolerance during reproductive development is important for higher yield potentials (up to 37%) and greater yield stability for wheat under future climate change in Europe.
Keywords: Climate change, drought stress, drought tolerance, ideotype optimization, reproductive development, wheat yield potential, yield stability
Abstract
Drought stress during reproductive development could drastically reduce wheat grain number and yield, but quantitative evaluation of such an effect is unknown under climate change. The objectives of this study were to evaluate potential yield benefits of drought tolerance during reproductive development for wheat ideotypes under climate change in Europe, and to identify potential cultivar parameters for improvement. We used the Sirius wheat model to optimize drought-tolerant (DT) and drought-sensitive (DS) wheat ideotypes under a future 2050 climate scenario at 13 contrasting sites, representing major wheat growing regions in Europe. Averaged over the sites, DT ideotypes achieved 13.4% greater yield compared with DS, with higher yield stability. However, the performances of the ideotypes were site dependent. Mean yield of DT was 28–37% greater compared with DS in southern Europe. In contrast, no yield difference (≤1%) between ideotypes was found in north-western Europe. An intermediate yield benefit of 10–23% was found due to drought tolerance in central and eastern Europe. We conclude that tolerance to drought stress during reproductive development is important for high yield potentials and greater yield stability of wheat under climate change in Europe.
Introduction
Wheat (Triticum aestivum L.) is one of the key staple crops for global food security, providing about 20% of the total dietary calories and protein needs, with about 730 million tons of annual production from around 2.1 million km2 harvested area globally (Shiferaw et al., 2013; FAO, 2016). In Europe, wheat is the most widely grown food crop, contributing 34% to global wheat production from about 27% of the global wheat area (FAOSTAT, 2014). The ongoing climate changes, characterized by increase in frequency and severity of climatic extreme events and adverse weather conditions, threaten global wheat production including Europe (Asseng et al., 2015; Stratonovitch and Semenov, 2015; Zampieri et al., 2017). Among different adverse weather conditions and climatic extreme events, drought is one of the major abiotic stresses that limit crop production (Lipiec et al., 2013; Basu et al., 2016; Fahad et al., 2017). The frequency and intensity of drought stresses are predicted to increase under future climate change in Europe, particularly in central and southern Europe (Dai, 2013; Kovats et al., 2014). Thus, the risk of yield losses and even crop failure will increase in Europe under the future climatic conditions (Trnka et al., 2014, 2015).
Drought affects both source and sink strengths, leading to source- and sink-limited yield reduction of up to 92% in wheat, depending on the crop growth stage, duration, and intensity of drought stress (Farooq et al., 2014; Semenov et al., 2014). The drought stress, particularly during reproductive development, reduces grain number in wheat (Dolferus et al., 2011; Dong et al., 2017; Ma et al., 2017). Reproductive development includes development of floral and reproductive structures, formation of the male and female gametophytes, fertilization, and primary grain setting. The potential grain number is set first by the number of flower initials that are formed on the spike. Potential grain number could be reduced by premature abortion of florets due to drought stress (Dolferus et al., 2011, 2013). The potential grain number in wheat can be reduced considerably further even by a short spell of drought during meiosis and gametogenesis due to male and female sterility (Lalonde et al., 1997; Ji et al., 2010; Dolferus et al., 2011; Barber et al., 2015; Onyemaobi et al., 2017). The young microspore stage is the most vulnerable to drought stress in wheat, leading to reproductive sterility (Ji et al., 2010; Dolferus et al., 2013). Malfunction and irreversible abortion of male and female reproductive organs and gametophytes are the main reasons for drought-induced male and female infertility in wheat (Saini, 1997; Ji et al., 2010; de Storme and Geelen, 2014; Dong et al., 2017; Onyemaobi et al., 2017), and reduced viability of gametophytes due to drought stress decreases the final fertile grain number (Lalonde et al., 1997; Saini, 1997; Ma et al., 2017).
Developing wheat cultivars tolerant to drought stress during reproductive development is currently a big challenge for wheat breeders (Cattivelli et al., 2008; Mwadzingeni et al., 2016). Drought tolerance is not a qualitative trait, but a complex quantitative plant trait, which is controlled by numerous genes and other plant traits, with minor individual contributions (Blum, 2010; Dolferus et al., 2013; Hu and Xiong, 2014; Serba and Yadav, 2016). Breeding of drought-tolerant wheat cultivars suffers from complex multi-trait and polygenic control of drought tolerance, high genotype and environment (G×E) interactions, low heritability, and difficulty in mass screening of plant traits and genes (Cattivelli et al., 2008; Fleury et al., 2010; Hu and Xiong, 2014). Experimental analysis of potential yield benefits of drought tolerance during reproductive development under future climate change and identification of the target traits for improvement are difficult due to the complexity in designing such experiments in the real world for climates that can only be predicted with a high degree of uncertainty.
Donald (1968) proposed an alternative breeding approach, ‘breeding of crop ideotypes’, in which breeders select plant ideotypes based on knowledge of crop physiology for improvement of plant traits under the target environment and then breed for them, rather than breeding for ‘defect elimination’ and ‘selection for yield’. A crop ideotype is a virtual idealized crop, or a crop model, which is expected to produce a greater quality and quantity of grain when developed as a cultivar. Process-based ecophysiological crop models are the most powerful tools that help to (i) deconvolute a complex trait, such as drought tolerance, into a list of simpler component traits suitable for further analyses and linking with phenotype and genotype, and then breeding; (ii) evaluate the performance of different plant traits under future climatic conditions and thus assist in discovering and prioritizing target traits for improvement; (iii) search optimal combinations and trade-offs between target traits; (iv) design ideotypes optimized for target environments and guide plant breeders towards the most relevant targets (Hammer et al., 2005; Semenov and Halford, 2009; Reynolds et al., 2011; Sylvester-Bradley et al., 2012; Martre et al., 2015; Rötter et al., 2015; Gouache et al., 2017). In our study, we used Sirius, which is a process-based wheat model coupled with an ideotype optimization framework. Sirius was calibrated and validated for different modern wheat varieties, and performed well under diverse climatic conditions across Europe, USA, Australia and New Zealand, including free-air CO2 enrichment experiments (Jamieson et al., 1998, 2000; Lawless and Semenov, 2005; Martre et al., 2006; Stratonovitch and Semenov, 2010; He et al., 2012; Semenov et al., 2014; Asseng et al., 2015). Although a few studies have projected the effects of common water limitation and drought stresses on wheat yield potentials under future climate change in Europe, the quantitative effects of reproductive stage drought stress on grain number and subsequently on grain yield have not been considered before (Semenov and Stratonovitch, 2010; Semenov and Shewry, 2011; Stratonovitch and Semenov, 2015; Trnka et al., 2014, 2015). A few experimental studies have reported the performance of reproductive stage drought-tolerant and drought-sensitive wheat germplasms under current climate, but quantitative potential yield benefits from drought tolerance during reproductive development under future climatic conditions are mostly unknown (Ji et al., 2010; Dong et al., 2017; Ma et al., 2017). In the present study, the important mechanism for the effect of drought stress on grain number during reproductive development was incorporated into Sirius for evaluation of yield benefits of drought tolerance during reproductive development under future climate change in Europe.
The main objectives of the present modelling study were (i) to assess potential yield benefits of drought tolerance during reproductive development for wheat ideotypes under future climate change in Europe, and (ii) to identify cultivar parameters that could be related to wheat traits to achieve high yield potential under climatic change.
Materials and methods
Target site and future 2050 climate
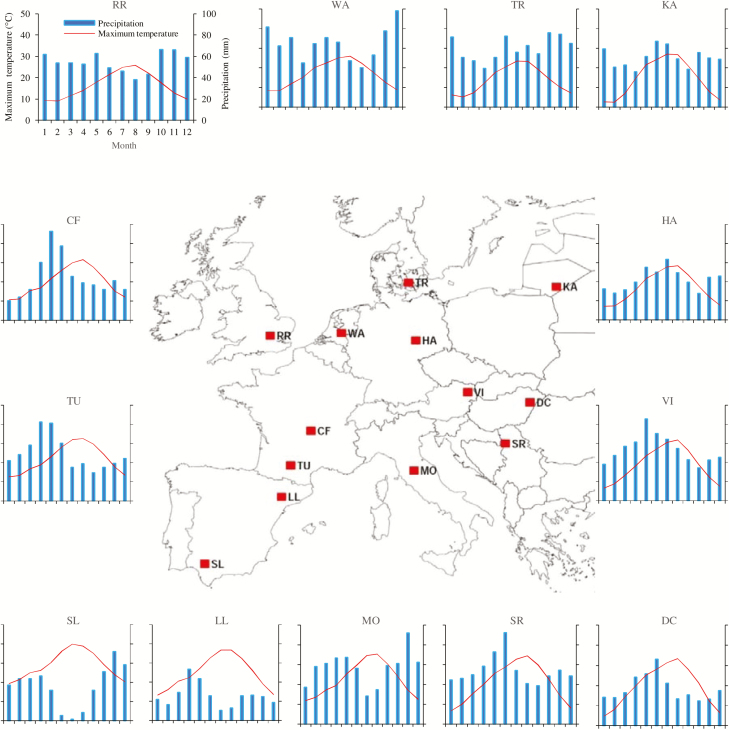
For the present study, 13 sites across Europe were selected, representing the major and contrasting wheat growing regions in Europe, from Spain in the south to Denmark in the north, and Hungary in the east to the UK in the west (Fig. 1). Table 1 shows the detailed site characteristics, typical locally cultivated wheat varieties, and the sowing dates. Out of six wheat cultivars, Cartaya and Creso are spring wheat, whereas the others are winter wheat cultivars. The future climate in 2050 was based on a global climate model (GCM), HadGEM2, from the CMIP5 ensemble (Taylor et al., 2012) for the Representative Concentration Pathway 8.5 (RCP8.5). The RCP8.5 combines assumptions about high population and modest technological improvements, leading to high energy demand with the highest greenhouse gas (GHG) concentration and a radiative forcing of +8.5 W m−2 (Riahi et al., 2011). Climate projections from HadGEM2 were downscaled to the local daily weather by using the LARS-WG 6.0 weather generator (Semenov and Stratonovitch, 2010, 2015). The atmospheric CO2 concentration in 2050 was increased to 541 ppm following the RCP8.5 scenario. For each site, the climatic scenario contains 100 years of site-specific daily weather, which was used as input for optimization and evaluation of ideotype performances (Fig. 1; Table 1).
Fig. 1.
Locations of 13 selected study sites, representing major wheat growing regions across Europe. Mean maximum temperature and mean monthly precipitation are shown for the future 2050 climate scenario (based on HadGEM2 and RCP8.5). (This figure is available in colour at JXB online.)
Table 1.
Site characteristics of the selected wheat growing regions across Europe
| No. | ID | Site | Country | Latitude (°) | Longitude (°) | Average air temperature (°C)a | Precipitation (mm year−1)a | Cultivarb | Sowing dateb |
|---|---|---|---|---|---|---|---|---|---|
| 1 | SL | Seville | Spain | 37.42 | −5.88 | 22.1 | 434 | Cartaya | 30 December |
| 2 | LL | Lleida | Spain | 41.63 | 0.60 | 18.0 | 311 | Creso | 25 November |
| 3 | MO | Montagnano | Italy | 43.30 | 11.80 | 16.0 | 686 | Creso | 25 November |
| 4 | TU | Toulouse | France | 43.62 | 1.38 | 16.7 | 595 | Thesee | 20 November |
| 5 | SR | Sremska | Serbia | 45.00 | 19.51 | 15.2 | 649 | Thesee | 15 November |
| 6 | CF | Clermont-Ferrand | France | 45.80 | 3.10 | 14.7 | 536 | Thesee | 15 November |
| 7 | DC | Debrecen | Hungary | 47.60 | 21.60 | 14.2 | 441 | Thesee | 18 October |
| 8 | VI | Vienna | Austria | 48.23 | 16.35 | 14.5 | 643 | Thesee | 20 October |
| 9 | HA | Halle | Germany | 51.51 | 11.95 | 12.7 | 509 | Claire | 20 October |
| 10 | RR | Rothamsted | UK | 51.80 | −0.35 | 12.2 | 653 | Mercia | 10 October |
| 11 | WA | Wageningen | Netherlands | 51.97 | 5.67 | 12.3 | 779 | Claire | 20 October |
| 12 | KA | Kaunas | Lithuania | 54.88 | 23.83 | 10.5 | 605 | Avalon | 25 October |
| 13 | TR | Tylstrup | Denmark | 57.20 | 9.90 | 10.6 | 721 | Avalon | 18 October |
a Future 2050 climate scenario (based on HadGEM2 and RCP8.5).
b Typical local cultivated wheat varieties and the sowing dates.
Sirius model
Sirius is a process-based wheat model with an optimization framework based on evolutionary algorithms with self-adaptation. This framework allows designing ideotypes and optimization of cultivar parameters for target environments. A detailed description of the Sirius model can be found elsewhere (Jamieson et al., 1998, 2000; Jamieson and Semenov, 2000; Brooks et al., 2001; Lawless et al., 2005; Martre et al., 2006; Semenov and Halford, 2009; Semenov et al., 2009, 2014; Stratonovitch and Semenov, 2010). In brief, Sirius consists of submodels that describe soils, water and nitrogen (N) uptake, photosynthesis, biomass accumulation and partitioning (leaf, stem, grain, and root), phenological development, including responses to limitation of N supply, along with adverse climatic effects such as heat and drought stress.
Photosynthesis and biomass accumulation
Photosynthesis and biomass production are calculated on a daily basis as the product of intercepted photosynthetically active radiation (PAR) and radiation use efficiency (RUE), limited by temperature and water stress. Radiation interception is related to leaf area index (LAI) via the Lambert–Beer law, with a default extinction coefficient of 0.45. In Sirius, RUE is proportional to atmospheric CO2 concentration, with an increase of 30% for a doubling in CO2 concentration for a C3 crop (e.g. wheat) (Vanuytrecht et al., 2012). The shortage of N limits leaf area, and hence light interception and biomass production.
Canopy development
Canopy development is described as a series of leaf layers associated with individual mainstem leaves. Leaf area development in each layer is simulated by a thermal time submodel, and actual leaf area is calculated using a simple limitation rule. Phenological development is calculated from the mainstem leaf appearance rate and final leaf numbers, with the latter determined by responses to daylength and vernalization. Maximum area of flag leaf (A) influences the rate of canopy expansion and the maximum achievable LAI. The duration of leaf senescence is expressed in thermal time and linked to the rank of the leaf in the canopy. Total canopy senescence synchronizes with the end of grain filling. Leaf senescence could be accelerated by shortage of N to sustain green leaves and grain filling, or by abiotic stress, viz. temperature or water stress. One of the strategies to increase grain yield is to extend the duration of leaf senescence and maintain green leaf area longer after anthesis, termed ‘stay green’ (SG).
Phenology and grain development
Phyllochron (Ph), daylength response (Pp) and duration of grain filling (Gf) are directly related to phenological development of wheat. Ph is the thermal time required for the appearance of successive leaves, and is a major driver of phenological development. Ph and Pp together determine the rate of crop development and the date of flowering and maturity. Gf is defined as a cultivar-specific amount of thermal time that needs to be accumulated to complete grain filling. During grain filling, assimilates for the grain are available from two sources viz. (i) new biomass produced from intercepted radiation after anthesis, and (ii) water-soluble or labile carbohydrates stored mostly in the stem before anthesis.
Root growth and soil water uptake
Soil is described as a cascade of 5-cm layers up to a user-defined depth. Roots continue to grow until reaching a soil-dependent maximum depth or until anthesis, whichever occurs first. Each soil layer contains root-available (water potential <−1.5 MPa) and -unavailable (water potential >−1.5 MPa) water, depending on its water retention characteristics. Only a proportion of available soil water can be extracted by plants from each layer of the root zone on any day depending on efficiency of water extraction (λ) and rate of root water uptake (Ru).
Impact of water limitation on biomass production and grain yield
Water limitation adversely affects both source (carbohydrate production) and sink strength (grain filling) in the plant. Photosynthesis and biomass production are reduced by water limitation. New biomass production decreases proportionally to the response of photosynthesis to water stress (Wsa), defined as Wsa=SFβ, where SF is a stress factor and β is a cultivar-independent constant. The rate of leaf senescence increases under water limitation by a factor, maximum acceleration of leaf senescence (Wss), that modifies daily increment of thermal time. Earlier leaf senescence will reduce grain yield by reducing grain size due not only to reduction in intercepted radiation and photosynthesis, but also to reduction in translocation of the labile plant reserve carbohydrate to the grain due to premature termination of grain filling driven by early leaf senescence.
Impact of drought stress on grain number during reproductive development
A simple mechanism was implemented in the current version of Sirius to account for the effect of drought stress on grain number during reproductive development by using a drought stress factor (DSF). The DSF was calculated as a ratio of actual transpiration (Ta) to potential transpiration (Tp) during reproductive development. However, linking different reproductive development stages (floral formation and development, meiosis and gametogenesis, fertilization, etc.) to corresponding plant growth stages for modelling is difficult as they often happen within a short period and vary depending on the climatic conditions and abiotic stresses. For example, meiosis in wheat often coincides with booting stage, but meiosis within a single floret can last only for 1–2 d, whereas meiosis within an ear and plant could be extended by 3–5 d (Bennett et al., 1972; Saini and Aspinall, 1982; Barber et al., 2015; Onyemaobi et al., 2017). In general, around 10 d before the flowering date until 5 d after the flowering date is the most critical period for reproductive development including fertilization and vulnerability to drought stress in wheat (Dolferus et al., 2011; Wise et al., 2011; Barber et al., 2015). In the present study, the impact of drought stress on reproductive development was implemented in Sirius for an average of 15 d, viz. 10 d before the flowering date and 5 d after the flowering date.
In the absence of drought stress, the sink capacity of the grains (Ypot, g m−2) is set as the product of the potential number of grains and the potential weight of an individual grain:
where DMear (g m−2) is the dry matter accumulated in ears prior to anthesis, Npot (grains g−1) is the maximum number of grains per unit of ear dry mass, and Wpot (g grain−1) is the potential weight of a single grain. In the absence of abiotic stress, the default parameter values of Npot=100 grains g−1 and Wpot=50 mg are large enough to provide sufficient sink capacity to accommodate newly produced and translocated biomass. Therefore, in the absence of drought stress, grain yield will be determined by the source capacity of the crop.
To account for the effect of drought stress during reproductive development, the number of fertile grains produced per unit of ear dry matter is reduced when DSF falls below a threshold, DSGNT. The reduction factor of grain number (R, dimensionless) is calculated as:
where DSGNRMax is maximum drought stress grain number reduction, DSGNS is drought stress grain number reduction saturation, DSGNT is drought stress grain number reduction threshold, and S is the slope of the grain number reduction, and S=(1−DSGNRMax)/(DSGNT−DSGNS). The value of parameters for drought-sensitive cultivars were selected as DSGNT=0.9, DSGNS=0.3 and DSGNRMax=0.2. The actual number N (grains g−1) of grains per unit of ear dry matter is the product of the potential number of grains and the drought reduction factor:
Target traits for improvement under future climate change
Plants deploy many strategies and adaptations for survival and complete the life cycle in different ways under water stress, viz. drought escape, avoidance, and tolerance (Farooq et al., 2014; Yadav and Sharma, 2016). Drought tolerance is a complex trait controlled by many individual plant traits with small contributions (Semenov and Halford, 2009; Fleury et al., 2010; Hu and Xiong, 2014). A total of eight cultivar parameters related to drought escape, avoidance, and tolerance traits were selected for improvement to maximize yield potential of future wheat cultivars under targeted climatic conditions (Table 2). By adjusting Ph and Pp, the rate of crop development could be increased, which could shorten the duration of the vegetative growth phase and help to escape terminal drought by early flowering and maturity. Early flowering is an important trait for drought escape while maintaining potential yield (Shavrukov et al., 2017). Gf has been suggested as a possible trait for increasing grain yield in wheat (Evans and Fischer, 1999). However, increasing Gf could be in conflict with yield improvement due to water stress under terminal drought. The rate of canopy expansion and the maximum LAI could be adjusted by altering the cultivar parameter A. This in turn will change the pattern and quantity of light interception and transpiration, and therefore, will affect crop growth, water use efficiency, and finally grain yield. A decrease in A could help to avoid drought stress by reducing transpiration and root water uptake. Ru is an important root trait affecting temporal patterns and total amount of water uptake in water-limited environments (Manschadi et al., 2006). A faster root water uptake reduces current water stress experienced by the plant, but could be risky for successful completion of the life cycle under terminal drought. On the other hand, slower water uptake with a likely drought at the end of the growing season is less risky for drought avoidance and may achieve on average higher yields. SG is a drought tolerance trait that enables plants to retain more green leaves longer after anthesis and improve potential yield under drought stress (Silva et al., 2001; Triboi and Triboi-Blondel, 2002; Luche et al., 2015; Christopher et al., 2016). Wsa and Wss are two additional drought tolerance traits that could help in increasing yield potential under drought stress (Semenov and Halford, 2009; Semenov et al., 2014).
Table 2.
Sirius cultivar parameters used for designing wheat ideotypes under the future 2050 climate scenario (based on HAdGEM2 and RCP8.5), and genetical variation observed in those parameters
| No. | Parameters | Symbol | Unit | Range used in model optimization | Genetical variation observed for wheat | Reference |
|---|---|---|---|---|---|---|
| 1 | Phyllochron | Ph | °C day | 80–130 | ≤20% | Ishag et al. (1998), Mosaad et al. (1995) |
| 2 | Day length response | Pp | Leaf h−1 day length | 0.05–0.70 | 9.74–107.40a | Kosner and Zurkova (1996) |
| 3 | Duration of grain filling | Gf | °C day | 500–900 | ≤40% | Akkaya et al. (2006), Charmet et al. (2005), Robert et al. (2001) |
| 4 | Maximum area of flag leaf | A | m2 leaf m−2 soil | 0.003–0.01 | ≤40% | Fischer et al. (1998), Shearman et al. (2005) |
| 5 | Stay green | SG | — | 0.0–1.5 | ||
| 6 | Rate of root water uptake | Ru | % | 1.0–7.0 | Large variation | Asseng et al. (1998), Manschadi et al. (2006) |
| 7 | Response of photosynthesis to water stress | Wsa | — | 0.1–2.1 | ||
| 8 | Maximum acceleration of leaf senescence due to water stress | Wss | — | 1.2–1.9 |
a Varietal difference in number of days till heading under long and short day conditions varied between 9.74 and 107.40 in a photoperiodic response experiment.
Designing wheat ideotypes under future climate change
In the present study, a crop ideotype was defined as a set of cultivar parameters that would deliver optimal yield performance in a target environment. The ideotype will produce maximum possible yield when developed as a cultivar. A wheat ideotype was characterized by eight cultivar parameters, controlling crop growth, development, and responses to drought stresses, which are summarized in Table 2 and described in the section ‘Target traits for improvement under future climate change’. We designed a drought-sensitive (DS) and a drought-tolerant (DT) ideotype for each site separately (Tables 2 and 3). The DS ideotype is sensitive to drought stress during reproductive development, in which grain number could be reduced depending on the severity of drought stress. In contrast, a DT ideotype is tolerant, or insensitive to any such drought stress during reproductive development. A total of eight cultivar parameters were optimized during the ideotype design, where initial cultivar parameters were the same for both DS and DT ideotypes at a given site (Tables 2, 3).
Table 3.
Optimized parameter values of drought-tolerant (DT) and drought-sensitive (DS) wheat ideotypes under the future 2050 climate scenario (based on HadGEM2 and RCP8.5) at 13 sites across major wheat growing regions in Europe
| Ideotype | Optimized parameter | |||||||
|---|---|---|---|---|---|---|---|---|
| Ph | Pp | Gf | A | SG | Ru | Wsa | Wss | |
| (°C day) | (leaf h−1 day length) | (°C day) | (m2 leaf m−2 soil) | (%) | ||||
| SL (Seville, Spain) | ||||||||
| Initiala | 105.0 | 0.2000 | 550.0 | 0.0065 | 0.5000 | 3.00 | 0.50 | 1.27 |
| DS final | 97.4 | 0.1152 | 827.1 | 0.0069 | 0.1244 | 7.00 | 0.10 | 1.28 |
| DT final | 129.9 | 0.1247 | 900.0 | 0.0100 | 1.3461 | 3.07 | 0.10 | 1.20 |
| LL (Lleida, Spain) | ||||||||
| Initial | 90.0 | 0.6000 | 650.0 | 0.0030 | 0.5000 | 3.00 | 0.50 | 1.27 |
| DS final | 102.9 | 0.1046 | 761.9 | 0.0044 | 0.8404 | 7.00 | 0.10 | 1.20 |
| DT final | 127.6 | 0.1157 | 900.0 | 0.0100 | 1.2294 | 2.15 | 0.10 | 1.20 |
| MO (Montagnano, Italy) | ||||||||
| Initial | 90.0 | 0.6000 | 650.0 | 0.0030 | 0.5000 | 3.00 | 0.50 | 1.27 |
| DS final | 109.3 | 0.1165 | 839.0 | 0.0089 | 1.0216 | 6.33 | 0.10 | 1.20 |
| DT final | 129.7 | 0.1378 | 900.0 | 0.0100 | 1.3384 | 3.97 | 0.10 | 1.20 |
| TU (Toulouse, France) | ||||||||
| Initial | 94.0 | 0.4000 | 650.0 | 0.0040 | 0.5000 | 3.00 | 0.50 | 1.27 |
| DS final | 129.2 | 0.0500 | 900.0 | 0.0100 | 1.0971 | 7.00 | 0.10 | 1.20 |
| DT final | 130.0 | 0.0500 | 900.0 | 0.0100 | 0.9760 | 6.79 | 0.10 | 1.20 |
| SR (Sremska, Serbia) | ||||||||
| Initial | 94.0 | 0.4000 | 650.0 | 0.0040 | 0.5000 | 3.00 | 0.50 | 1.27 |
| DS final | 120.5 | 0.0500 | 899.4 | 0.0049 | 1.3993 | 7.00 | 0.10 | 1.26 |
| DT final | 129.7 | 0.0500 | 900.0 | 0.0100 | 1.3865 | 5.88 | 0.10 | 1.20 |
| CF (Clermont-Ferrand, France) | ||||||||
| Initial | 94.0 | 0.4000 | 650.0 | 0.0040 | 0.5000 | 3.00 | 0.50 | 1.27 |
| DS final | 115.9 | 0.0500 | 829.4 | 0.0100 | 0.9802 | 7.00 | 0.10 | 1.20 |
| DT final | 129.8 | 0.0500 | 900.0 | 0.0100 | 1.1370 | 6.08 | 0.10 | 1.20 |
| DC (Debrecen, Hungary) | ||||||||
| Initial | 94.0 | 0.4000 | 650.0 | 0.0040 | 0.5000 | 3.00 | 0.50 | 1.27 |
| DS final | 115.1 | 0.0500 | 803.2 | 0.0060 | 1.3279 | 7.00 | 0.10 | 1.20 |
| DT final | 129.9 | 0.0500 | 900.0 | 0.0100 | 0.9615 | 5.12 | 0.10 | 1.20 |
| VI (Vienna, Austria) | ||||||||
| Initial | 94.0 | 0.4000 | 650.0 | 0.0040 | 0.5000 | 3.00 | 0.50 | 1.27 |
| DS final | 129.7 | 0.0500 | 900.0 | 0.0100 | 1.0178 | 7.00 | 0.10 | 1.20 |
| DT final | 130.0 | 0.0500 | 900.0 | 0.0100 | 1.5000 | 5.94 | 0.10 | 1.20 |
| HA (Halle, Germany) | ||||||||
| Initial | 110.0 | 0.5000 | 650.0 | 0.0070 | 0.5000 | 3.00 | 0.50 | 1.27 |
| DS final | 110.3 | 0.0500 | 859.8 | 0.0044 | 0.8355 | 7.00 | 0.10 | 1.20 |
| DT final | 130.0 | 0.0503 | 899.5 | 0.0100 | 1.0085 | 4.16 | 0.10 | 1.20 |
| RR (Rothamsted, UK) | ||||||||
| Initial | 107.0 | 0.5300 | 650.0 | 0.0075 | 0.5000 | 3.00 | 0.50 | 1.27 |
| DS final | 130.0 | 0.0500 | 899.9 | 0.0100 | 0.8288 | 7.00 | 0.10 | 1.20 |
| DT final | 130.0 | 0.0890 | 900.0 | 0.0100 | 0.9612 | 5.91 | 0.10 | 1.20 |
| WA (Wageningen, Netherlands) | ||||||||
| Initial | 110.0 | 0.5000 | 650.0 | 0.0070 | 0.5000 | 3.00 | 0.50 | 1.27 |
| DS final | 129.9 | 0.0500 | 900.0 | 0.0100 | 0.8039 | 7.00 | 0.10 | 1.20 |
| DT final | 129.9 | 0.0500 | 900.0 | 0.0099 | 0.9955 | 6.81 | 0.10 | 1.20 |
| KA (Kaunas, Lithuania) | ||||||||
| Initial | 90.0 | 0.6500 | 650.0 | 0.0065 | 0.5000 | 3.00 | 0.50 | 1.27 |
| DS final | 130.0 | 0.0968 | 715.9 | 0.0061 | 1.4786 | 6.37 | 0.10 | 1.20 |
| DT final | 130.0 | 0.0595 | 900.0 | 0.0100 | 1.3428 | 4.89 | 0.10 | 1.20 |
| TR (Tylstrup, Denmark) | ||||||||
| Initial | 90.0 | 0.6500 | 650.0 | 0.0065 | 0.5000 | 3.00 | 0.50 | 1.27 |
| DS final | 126.1 | 0.0678 | 792.6 | 0.0039 | 1.4974 | 5.32 | 0.10 | 1.39 |
| DT final | 129.9 | 0.0874 | 900.0 | 0.0100 | 1.3877 | 3.09 | 0.10 | 1.20 |
a Initial values represent the parameter values of the current wheat varieties in a given site.
A, maximum area of flag leaf; Gf, duration of grain filling; Ph, phyllochron; Pp, day length response; Ru, rate of root water uptake; SG, stay green; Wsa, response of photosynthesis to water stress; Wss, maximum acceleration of leaf senescence.
Ideotype optimization
Both ideotypes, viz. DS and DT, were optimized to achieve high yield potentials in target environments. An evolutionary search algorithm with self-adaptation (EASA) was used in Sirius to optimize cultivar parameters in a high-dimensional parameter space with a complex fitness function for the best performance of crop ideotypes (Schwefel and Rudolph, 1995; Stratonovitch and Semenov, 2010). The EASA is considered as a universal search optimization method. The main advantage of EASA compared with genetic algorithms is that it does not require tuning control parameters during the search, where predefined heuristic rules are unavailable or difficult to formulate (Beyer, 1995; Bäck, 1998; Semenov and Terkel, 2003). The EASA optimized cultivar parameters by randomly perturbing (mutating) their values and testing their performance in the target environment. At each step of optimization, 16 new candidate ideotypes were generated from a ‘parent’ by perturbing its cultivar parameters randomly within the predefined parameters’ limits (Table 2). The parameter ranges were based on calibration of Sirius for modern cultivars allowing for variations reported in the literature for existing wheat germplasms (Semenov and Halford, 2009; He et al., 2012; Semenov and Stratonovitch, 2013; Semenov et al., 2014). Yield was calculated for each of 16 candidates for 100 years under future climatic conditions. Ideotypes with the coefficient of variation (CV) of yield exceeding 10% were excluded from the selection process and the candidate with the highest mean yield over 100 years was selected as a ‘parent’ for the next step of optimization. The stopping rules of optimization process were: (i) no further improvement in yield potential is possible, and (ii) parameter convergence and parameter optimal state are achieved.
In an additional set of simulations, we relaxed the criteria of yield variance during the optimization process, excluding ideotypes with CV exceeding 15% from the selection process. The purpose of increasing CV was to evaluate the effect of increased yield variance during optimization on the performance of ideotypes.
Simulation set-up
We used Sirius version 2015, which is available from https://sites.google.com/view/sirius-wheat/. A single soil-water profile (Hafren) with a total available water capacity of 177 mm was used at all sites to eliminate site-specific soil effects from the analysis. The soil profile was filled with the maximum available water capacity at sowing. An increase of 10% in RUE was assumed for wheat by 2050 (Zhu et al., 2010). Both the wheat ideotypes (DS and DT) were optimized for the maximum possible yield under future climatic conditions (2050) for the selected sites. All simulations were assumed to be water-limited, but no N limitation was considered. Additionally, the effects of an adverse weather event of heat stress around flowering on grain number and size (Stratonovitch and Semenov, 2015) were excluded in the present study to simulate only the effect of drought stress under future climate change.
Results
Simulated yield potential of wheat ideotypes under target future 2050 climate
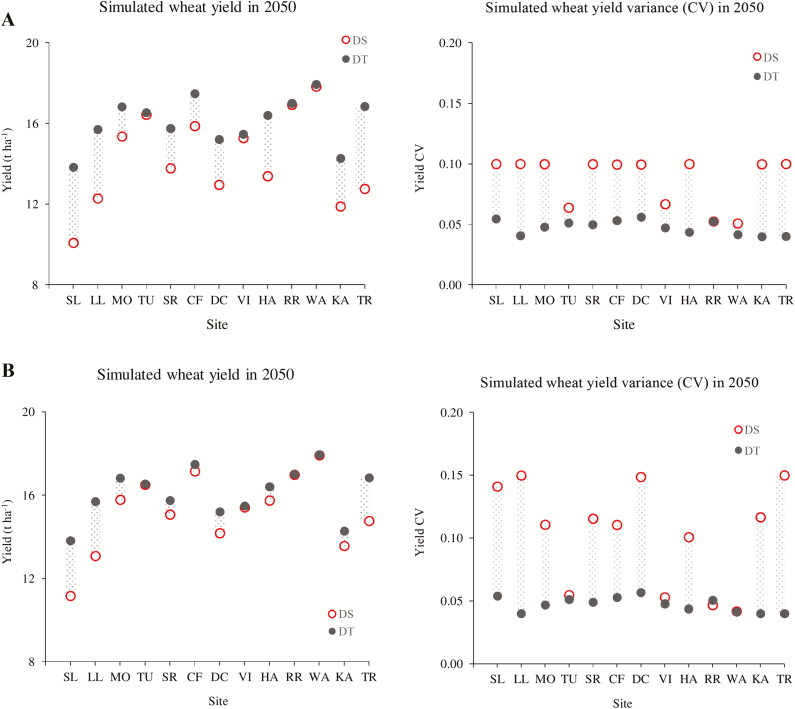
The upper two panels in Fig. 2 show simulated wheat yield and yield variance (coefficient of variation; CV) for DT and DS ideotypes under the future climate change in 2050 at 13 sites across major wheat growing regions in Europe, when yield CV was limited to 10% during model optimization. Averaged over the sites, the DT ideotype achieved 13% higher mean yield (16.1 t ha−1) compared with DS (14.2 t ha−1). Averaged over both ideotypes, the highest wheat yield was found at WA (17.9 t ha−1), followed by RR, CF, and TU, whereas the lowest yield was observed at SL (12.0 t ha−1), followed by KA. All the other sites had a yield in the range of 14–16 t ha−1. However, there were strong site effects on ideotype performances at individual sites.
Fig. 2.
Wheat yield and yield coefficient of variance (CV) of drought-sensitive (DS) and drought-tolerant (DT) ideotypes optimized under the future 2050 climate scenario (based on HadGEM2 and RCP8.5) at 13 sites, representing major wheat growing regions in Europe. The yield CV during model optimization was limited to 10% (A) and 15% (B). (This figure is available in colour at JXB online.)
When the site-specific performance of individual wheat ideotypes was compared, the highest yields (17–18 t ha−1) were achieved for the DT ideotype at WA, RR, TR, CF, TU, and MO, whereas lowest yields (~14 t ha−1) were obtained at SL and KA. Intermediate yields of 15–16 t ha−1 were found at the rest of the sites. On the other hand, maximum yields (16–18 t ha−1) for DS were obtained at WA, RR, TU, and CF, whereas minimum yield was found at SL (~10 t ha−1), followed by KA and LL (~12 t ha−1). A medium wheat yield of around 13–15 t ha−1 was observed at other sites. When the site-specific performance of DT was compared with DS, simulated wheat yields of DT were 28–37% greater compared with DS at SL, LL, and TR, with the highest potential yield benefit of 37% for DT at SL. In contrast, a similar magnitude of wheat yield was obtained for both DT and DS ideotypes in the range 15–18 t ha−1 at WA, RR, VI, and TU, where the difference in yield potentials between two ideotypes was ≤1%. The mean wheat yield of DT was 10–23% greater compared with DS at the other six sites.
Stability of simulated wheat yield potential under future climate
Averaged over sites, mean yield variance (CV) over 100 years’ simulations was 0.05 (CV=0.04–0.06) and 0.09 (CV=0.05–0.10) for DT and DS ideotypes, respectively (Fig. 2). The result indicates 44% smaller yield variance for DT compared with the DS ideotype. Among individual sites, four out of 13 sites had almost equal yield variance (CV~0.05) for both the ideotypes, viz. TU, VI, RR, and WA. In the rest of the sites, CV of wheat yield potential for DT was 50% smaller compared with DS.
Effect of limiting yield variance during optimization process on simulated yield potentials and variability
The lower two panels in Fig. 2 show simulated wheat yields and yield CV for DT and DS ideotypes across 13 European sites when yield CV over 100 years was limited to 15% during model optimization. When yield CV was increased from 10% to 15% during the optimization process, no effect was found on the results (yields and CV) for the DT ideotype across different sites as reported above. Averaged over the sites, the minimum and mean wheat yields of DS increased by 11 and 7%, respectively, whereas maximum yield did not increase either at individual sites or averaged over different sites. At the same time, the maximum and mean CV of wheat yield for the DS ideotype increased by 50 and 18%, respectively.
Optimization of wheat ideotype parameters under target future 2050 climate
Averaged over the sites and ideotypes, optimized values for Ph, Gf, A, and SG were found to be increased from their initial values by 29, 36, 87, and 122%, in which optimized values were 10, 8, 54, and 87% greater, respectively for the DT ideotype compared with DS (Table 3). In contrast, the optimized value of Ru increased by 95% compared with its initial value, but optimized values were 54% greater for the DS ideotype compared with DT. An equally high optimized Ru value (~7%) was obtained at almost all the sites for the DS ideotype. Optimized values of the rest of the three parameters, viz. Pp, Wsa and Wss, decreased by 83, 80, and 4%, respectively, where no difference (≤2%) was found between ideotypes, except for Pp. Similar to the simulated wheat yields, strong site effects were found on the optimized parameter values and between ideotypes in an individual site.
The highest differences in the optimized parameter values between ideotypes were found for Ph (33%), Pp (63%), Gf, (26%), A (156%), SG (982%), and Ru (226%) at SL, LL, KA, and TR (Table 3). For all these parameters, optimized values were greater for DT compared with DS, except for Pp and Ru, where both the values were greater for DS compared with DT. An overall minimum or almost no difference in these parameter values between ideotypes was found at WA, RR, VI, and TU. An intermediate ideotype effect was obtained for the same parameters at the rest of the sites, with greater improved values for DT compared with DS. There were no ideotype and site effects for the other two parameters, viz. Wsa and Wss, where almost constant model optimization effects were obtained (80% for Wsa and ~4% for Wss).
Discussion
Overall, higher yield potential of the DT compared with the DS ideotype under future climatic condition could be linked to a greater number of grains for the DT ideotype. The number of fertile grains setting during reproductive development was reduced by drought stress for the DS ideotype, depending on the level of drought stress at different sites, whereas the primary grain setting number remained unaffected for DT due to drought tolerance during reproductive development. Reduction in grain number decreased the total sink capacity resulting in reduced yield potentials for DS. Adverse effects of drought stress on the primary grain setting number during reproductive development have been reported by different experimental studies for common wheat germplasms/lines/cultivars (Lalonde et al., 1997; Ji et al., 2010; Dong et al., 2017; Ma et al., 2017; Onyemaobi et al., 2017) and reviewed for different cereals including wheat (Saini, 1997; Dolferus et al., 2011; de Storme and Geelen, 2014). These studies also found some wheat germplasms tolerant to drought stress during reproductive development, indicating the possibility of improvement in drought tolerance through genetical adaptation. Our study shows that drought tolerance during reproductive development is an important trait for achieving high wheat yield potential under future climate change in Europe.
Another reason for higher yield potential of the DT ideotype compared with DS could be overall better optimized cultivar parameters contributing to a greater source and sink capacity under drought stress, and a direct and indirect contribution to drought tolerance. For example, SG, Gf, and A were greater in the DT ideotype compared with DS. A high SG value implies delaying leaf senescence and greater plant capacity to maintain more active photosynthetic tissues longer under water stress during anthesis and grain filling, and is thus considered one of the most important traits for drought tolerance (Cattivelli et al., 2008; Farooq et al., 2014; Luche et al., 2015; Christopher et al., 2016). Greater number of fertile grains per ear, increased average grain weight, and high total yield were reported for different crop cultivars, including wheat, with the stay green trait or genotypes (Silva et al., 2003; Foulkes et al., 2007; Luche et al., 2013). The use of the stay green character in future wheat breeding programmes would result in significant genetic progress for tolerance to terminal drought stress and high yield (Luche et al., 2015). An extended grain filling period (a high Gf value) increases grain yield by increasing light interception for more photosynthesis and production of more carbohydrate to be translocated directly to the developing grains. It also increases the possibility of completion of re-translocation of labile carbohydrate reserves to the grains (Semenov and Halford, 2009; Semenov and Stratonovitch, 2013). Duration of the grain filling period is an important trait to improve through breeding for increasing wheat yield potentials under future climate change (Evans and Fischer, 1999; Semenov et al., 2014). Greater A increases the potential LAI, which in turn increases light interception, photosynthesis, carbohydrate production, and finally grain yield (Semenov and Stratonovitch, 2013). Increasing both leaf area and grain filling period could increase yield potential further under future climatic conditions for drought-tolerant wheat cultivars. However, greater leaf area and longer grain filling period could conflict with drought avoidance for drought-sensitive cultivars. Our results of relatively smaller increase in optimized values of A and Gf for DS show that increasing A and Gf is a trade-off between avoiding drought by reducing transpiration and achieving higher yield potential for the DS ideotype. Ph was smaller in the DS ideotype compared with DT, indicating a relatively higher chance for DS to escape any terminal drought by early flowering and maturity due to the shorter vegetative stage. Early flowering and maturity are important drought escape traits for many crops including wheat (Yadav and Sharma, 2016; Shavrukov et al., 2017). Greater Ru values for DS compared with DT indicate that drought-sensitive cultivars could take up soil water faster to avoid current drought stress. But, faster water uptake could be risky for DS to complete the life cycle successfully under severe drought at the end of the growing season. On the other hand, drought-tolerant cultivars would take up soil water slower in favour of successful completion of the life cycle with a likely terminal drought. Drought stress generally reduces crop yield through decreased photosynthesis and increased leaf senescence (Yadav and Sharma, 2016; Fahad et al., 2017). Decreased response of photosynthesis to water stress (Wsa) and reduced maximum acceleration of leaf senescence (Wss) are important traits for drought tolerance (Semenov and Halford, 2009; Semenov and Stratonovitch, 2013). We found reduced (4–80%) optimized values for both Wsa and Wss, but the respective values were almost equal across sites and ideotypes. Our result indicates that both the cultivar parameters/traits are important for high yield potentials under future climatic conditions, irrespective of tolerance or sensitivity to reproductive stage drought stress. A high genetic variation in Ph (Mosaad et al., 1995; Ishag et al., 1998), Pp (Kosner and Zurkova, 1996), and Ru (Asseng et al., 1998; Manschadi et al., 2006) was found in wheat, whereas up to 40% variation in genotypes was observed in Gf (Robert et al., 2001; Charmet et al., 2005; Akkaya et al., 2006) and A (Fischer et al., 1998; Shearman et al., 2005) for wheat, indicating the possibility of future improvements through wheat breeding.
The overall high yield potentials of DT and DS ideotypes and their differences under future climatic conditions could be considered as the interactive effects between drought tolerance/sensitivity during reproductive development and eight cultivar parameters optimized for high yield potential under the target climatic condition. Our results demonstrate the importance of the drought tolerance trait during reproductive development in achieving higher yield potentials for the DT ideotype, along with an extra beneficial effect of improved cultivar parameters, linked with high yield and drought tolerance directly and indirectly, whereas results for the DS ideotype, where selected cultivar parameters were not able to be optimized to the same extent as for DT, show that drought sensitivity during reproductive development limits optimization of those parameters. However, optimized cultivar parameters in the DS ideotype compensated for the potential yield loss due to drought sensitivity during reproductive development at least to some extent. Thus, the present study reveals that improved cultivar parameters, such as decreased leaf senescence, reduced response of photosynthesis to water stress and increased root water uptake, may increase yield potentials also for drought-sensitive cultivars. Different studies reported similar importance of drought tolerance during reproductive development and different improved plant traits for high yield potentials of different crops including wheat (Manschadi et al., 2006; Cattivelli et al., 2008; Ji et al., 2010; Farooq et al., 2014; Semenov et al., 2014; Yadav and Sharma, 2016; Shavrukov et al., 2017).
The highest yield benefits (28–37%) due to drought tolerance were obtained mainly in southern Europe (e.g. SL and LL). This result could be explained by the high probability of drought stress during reproductive development under future climate change, and the highest differences in the optimized parameter values (e.g. SG, Gf, A, Ph, Pp, and Ru) between the ideotypes at those sites. Similarly, a medium yield benefit of 10–23% for DT in most of the central and eastern European sites (e.g. MO, SR, DC, and HA) could be due to a most likely medium drought stress during reproductive development under future climatic conditions and an intermediate difference in optimized parameter values in favour of the DT ideotype. In contrast, a minimum or no yield benefit for DT compared with DS was found only at a few sites in north-western and central-western Europe (e.g. RR, WA, and TU), characterized with a low probability of, or no, severe drought stress during reproductive development under future climate change. In these sites, almost equal optimized cultivar values were obtained for both the DT and DS ideotypes. This is in accordance with the minimum or no difference in wheat yield potentials due to ideotypes at those sites. It is worthy of note that we found the highest yield benefits (28–37%) due to drought tolerance at one additional site, TR (Denmark), but it was characterized by a good annual precipitation (721 mm year−1) (Figs 1, 2; Table 1). We also found no yield benefit due to drought tolerance at one extra site, VI (Austria), but it was characterized by a medium annual precipitation (643 mm year−1) (Figs 1, 2; Table 1). Our results demonstrate that the adverse impact of drought stress on yield potential during reproductive development could happen locally at some sites even with good annual precipitation, for example in Denmark. Drought stress during reproductive development is a dynamic, but a short-term, event (~10–15 d), which depends on many abiotic and biotic factors at that time. Thus, a drought-tolerant wheat cultivar would be important for high yield potential under climate change even in northern Europe (e.g. Denmark). The results for the DS ideotype on decreased wheat yield potentials under future climate change in most of the European sites are in line with Trnka et al. (2014, 2015), who predicted that there would be less adaptation options for wheat under future climate change in Europe, with a high risk of loss of yield due to increased adverse extreme events including drought.
Another important finding of the present study was that drought-tolerant wheat ideotypes would have greater yield stability under future climatic conditions, whereas yield stability of drought-sensitive ideotypes would be substantially lower. These results were further supported by the fact that the yield potentials of the DT ideotype were not affected across different sites even when the selection criteria of yield variance (CV) was increased to 15% during the optimization process. On the other hand, mean yield potential of the DS ideotype increased (7%) at the cost of reducing yield stability (18%) further. This indicates that there would be a trade-off between yield potential and yield stability for drought-sensitive wheat cultivars.
We did not account for a detailed mechanism for the adverse effects of drought stress on grain number during reproductive development for wheat at a molecular level; for example, change in hormonal composition and gene expression (Ji et al., 2010, 2011; de Storme and Geelen, 2014; Farooq et al., 2014; Dong et al., 2017; Onyemaobi et al., 2017). However, introduction of a relatively simple description in Sirius for a reduction in grain number due to drought stress during reproductive development was found to be effective for quantitative evaluation of the potential yield benefits from drought tolerance in wheat under future climatic conditions. We conclude that drought tolerance during reproductive development is important for high yield potentials with high yield stability of wheat under future climate change in most of the wheat growing regions in Europe, with predicted maximum potential yield benefits of 28–37% in southern Europe and 10–23% in central and eastern Europe. Introduction of drought tolerance during reproductive development, along with improvements in cultivar parameters linked with increased yield and drought tolerance directly or indirectly, is the key to achieving higher wheat yield potentials along with higher yield stability under future climatic conditions in Europe. Our results identified the important cultivar parameters and their optimal combination for future improvement to achieve high yield potentials under climate change in Europe, viz. grain filling duration, phyllochron, leaf area, stay green, rate of root water uptake, photosynthetic response to water stress, and maximum acceleration of leaf senescence due to water stress.
Acknowledgements
Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council (BBSRC) Designing Future Wheat programme (BB/P016855/1).
References
- Akkaya A , Dokuyucu T , Kara R , Akçura M. 2006. Harmonization ratio of post- to pre-anthesis durations by thermal times for durum wheat cultivars in a Mediterranean environment. European Journal of Agronomy 24, 404–408. [Google Scholar]
- Asseng S , Ewert F , Martre P , et al. 2015 Rising temperatures reduce global wheat production. Nature Climate Change 5, 143–147. [Google Scholar]
- Asseng S , Ritchie JT , Smucker AJM , Robertson MJ. 1998 Root growth and water uptake during water deficit and recovering in wheat. Plant and Soil 201, 265–273. [Google Scholar]
- Bäck T. 1998 An overview of parameter control methods by self-adaptation in evolutionary algorithms. Fundamenta Informaticae 35, 51–66. [Google Scholar]
- Barber HM , Carney J , Alghabari F , Gooding MJ. 2015 Decimal growth stages for precision wheat production in changing environments? Annals of Applied Biology 166, 355–371. [Google Scholar]
- Basu S , Ramegowda V , Kumar A , Pereira A. 2016 Plant adaptation to drought stress. F1000Research 5, F1000 Faculty Rev-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MD , Smith JB , Kemble R. 1972. The effect of temperature on meiosis and pollen development in wheat and rye. Canadian Journal of Genetics and Cytology 14, 615–624. [Google Scholar]
- Beyer HG. 1995. Toward a theory of evolution strategies: self-adaptation. Evolutionary Computation 3, 311–347. [Google Scholar]
- Blum A. 2010. Plant breeding for water limited environments. New York: Springer. [DOI] [PubMed] [Google Scholar]
- Brooks RJ , Semenov MA , Jamieson PD. 2001. Simplifying Sirius: sensitivity analysis and development of a meta-model for wheat yield prediction. European Journal of Agronomy 14, 43–60. [Google Scholar]
- Cattivelli L , Rizza F , Badeck FW , et al. 2008. Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field Crops Research 105, 1–14. [Google Scholar]
- Charmet G , Robert N , Branlard G , Linossier L , Martre P , Triboï E. 2005. Genetic analysis of dry matter and nitrogen accumulation and protein composition in wheat kernels. Theoretical and Applied Genetics 111, 540–550. [DOI] [PubMed] [Google Scholar]
- Christopher JT , Christopher MJ , Borrell AK , Fletcher S , Chenu K. 2016. Stay-green traits to improve wheat adaptation in well-watered and water-limited environments. Journal of Experimental Botany 67, 5159–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai AG. 2013. Increasing drought under global warming in observations and models. Nature Climate Change 3, 52–58. [Google Scholar]
- de Storme N , Geelen D. 2014. The impact of environmental stress on male reproductive development in plants: biological processes and molecular mechanisms. Plant, Cell & Environment 37, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolferus R , Ji X , Richards RA. 2011. Abiotic stress and control of grain number in cereals. Plant Science 181, 331–341. [DOI] [PubMed] [Google Scholar]
- Dolferus R , Powell N , JI X , Ravash R , Edlington J , Oliver S , Van Dongen J , Shiran B. 2013. The physiology of reproductive-stage abiotic stress tolerance in cereals. In: Rout GR , Das AB, eds. Molecular stress physiology of plants. New Delhi, India: Springer, 193–216. [Google Scholar]
- Donald CM. 1968. The breeding of crop ideotypes. Euphytica 17, 385–403. [Google Scholar]
- Dong B , Zheng X , Liu H , Able JA , Yang H , Zhao H , Zhang M , Qiao Y , Wang Y , Liu M. 2017. Effects of drought stress on pollen sterility, grain yield, abscisic acid and protective enzymes in two winter wheat cultivars. Frontiers in Plant Science 8, 1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LT , Fischer RA. 1999. Yield potential: its definition, measurement, and significance. Crop Science 39, 1544–1551. [Google Scholar]
- Fahad S , Bajwa AA , Nazir U , et al. 2017. Crop production under drought and heat stress: plant responses and management options. Frontiers in Plant Science 8, 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO. 2016. Global Information and Early Warning System. Crop prospects and food situation. Rome, Italy: Food and Agriculture Organization of the United Nations. [Google Scholar]
- FAOSTAT. 2014. FAOSTAT. Rome, Italy: Food and Agriculture Organization of the United Nations; http://faostat.fao.org/site/567/default.aspx#ancor. [Google Scholar]
- Farooq M , Hussain M , Siddique KHM. 2014. Drought stress in wheat during flowering and grain-filling periods. Critical Reviews in Plant Sciences 33, 331–349. [Google Scholar]
- Fischer RA , Rees D , Sayre KD , Lu ZM , Condon AG , Saavedra AL. 1998. Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Science 38, 1467–1475. [Google Scholar]
- Fleury D , Jefferies S , Kuchel H , Langridge P. 2010. Genetic and genomic tools to improve drought tolerance in wheat. Journal of Experimental Botany 61, 3211–3222. [DOI] [PubMed] [Google Scholar]
- Foulkes MJ , Sylvester-Bradley R , Weightman R , Snape JW. 2007. Identifying physiological traits associated with improved drought resistance in winter wheat. Field Crops Research 103, 11–24. [Google Scholar]
- Gouache D , Bogard M , Pegard M , et al. 2017. Bridging the gap between ideotype and genotype: challenges and prospects for modelling as exemplified by the case of adapting wheat (Triticum aestivum L.) phenology to climate change in France. Field Crops Research 202, 108–121. [Google Scholar]
- Hammer GL , Chapman S , van Oosterom E , Podlich DW. 2005. Trait physiology and crop modelling as a framework to link phenotypic complexity to underlying genetic systems. Australian Journal of Agricultural Research 56, 947–960. [Google Scholar]
- He J , Le Gouis J , Stratonovitch P , et al. 2012. Simulation of environmental and genotypic variations of final leaf number and anthesis date for wheat. European Journal of Agronomy 42, 22–33. [Google Scholar]
- Hu H , Xiong L. 2014. Genetic engineering and breeding of drought-resistant crops. Annual Review of Plant Biology 65, 715–741. [DOI] [PubMed] [Google Scholar]
- Ishag HM , Mohamed BA , Ishag KHM. 1998. Leaf development of spring wheat cultivars in an irrigated heat-stressed environment. Field Crops Research 58, 167–175. [Google Scholar]
- Jamieson PD , Berntsen J , Ewert F , et al. 2000. Modelling CO2 effects on wheat with varying nitrogen supplies. Agriculture Ecosystems and Environment 82, 27–37. [Google Scholar]
- Jamieson PD , Semenov MA. 2000. Modelling nitrogen uptake and redistribution in wheat. Field Crops Research 68, 21–29. [Google Scholar]
- Jamieson PD , Semenov MA , Brooking IR , Francis GS. 1998. Sirius: a mechanistic model of wheat response to environmental variation. European Journal of Agronomy 8, 161–179. [Google Scholar]
- Ji X , Dong B , Shiran B , Talbot MJ , Edlington JE , Hughes T , White RG , Gubler F , Dolferus R. 2011. Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiology 156, 647–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X , Shiran B , Wan J , Lewis DC , Jenkins CL , Condon AG , Richards RA , Dolferus R. 2010. Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant, Cell & Environment 33, 926–942. [DOI] [PubMed] [Google Scholar]
- Kosner J , Zurkova D. 1996. Photoperiodic response and its relation to earliness in wheat. Euphytica 89, 59–64. [Google Scholar]
- Kovats RS , Valentini R , Bouwer LM , Georgopoulou E , Jacob D , Martin E , Rounsevell M , Soussana JF. 2014. Europe. In: Barros VR , Field CB , Dokken DJ, et al. , eds. Climate change 2014: impacts, adaptation, and vulnerability. Part B: regional aspects. Contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change. New York: Cambridge University Press, 1267–1326. [Google Scholar]
- Lalonde S , Beebe DU , Saini HS. 1997. Early signs of disruption of wheat anther development associated with the induction of male sterility by meiotic-stage water deficit. Sexual Plant Reproduction 10, 40–48. [Google Scholar]
- Lawless C , Semenov MA. 2005. Assessing lead-time for predicting wheat growth using a crop simulation model. Agricultural and Forest Meteorology 135, 302–313. [Google Scholar]
- Lawless C , Semenov MA , Jamieson PD. 2005. A wheat canopy model linking leaf area and phenology. European Journal of Agronomy 22, 19–32. [Google Scholar]
- Lipiec J , Doussan C , Nosalewicz A , Kondracka K. 2013. Effect of drought and heat stresses on plant growth and yield: a review. International Agrophysics, 27, 463–477. [Google Scholar]
- Luche HD , da Silva JAG , da Maia LC , de Oliveira AC. 2015. Stay-green: a potentiality in plant breeding. Ciencia Rural 45, 1755–1760. [Google Scholar]
- Luche HD , Gonzalez Da Silva JA , Nornberg R , da Silveira SFS , Baretta D , Groli EL , da Maiato LC , de Oliveira AC. 2013. Per se performance and genetic parameters of wheat lines expressing the ‘stay-green’ character. Pesquisa Agropecuaria Brasileira 48, 167–173. [Google Scholar]
- Ma J , Li R , Wang H , Li D , Wang X , Zhang Y , Zhen W , Duan H , Yan G , Li Y. 2017. Transcriptomics analyses reveal wheat responses to drought stress during reproductive stages under field conditions. Frontiers in Plant Science 8, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschadi AM , Christopher J , deVoil P , Hammer GL. 2006. The role of root architectural traits in adaptation of wheat to water-limited environments. Functional Plant Biology 33, 823–837. [DOI] [PubMed] [Google Scholar]
- Martre P , Jamieson PD , Semenov MA , Zyskowski RF , Porter JR , Triboi E. 2006. Modelling protein content and composition in relation to crop nitrogen dynamics for wheat. European Journal of Agronomy 25, 138–154. [Google Scholar]
- Martre P , Quilot-Turion B , Luquet D , Memmah M-MO-S , Chenu K , Debaeke P. 2015. Model-assisted phenotyping and ideotype design. In: Calderini DF, ed. Crop physiology, 2nd edn, San Diego: Academic Press, 349–373. [Google Scholar]
- Mosaad MG , Ortizferrara G , Mahalakshmi V , Fischer RA. 1995. Phyllochron response to vernalization and photoperiod in spring wheat. Crop Science 35, 168–171. [Google Scholar]
- Mwadzingeni L , Shimelis H , Dube E , Laing MD , Tsilo TJ. 2016. Breeding wheat for drought tolerance: progress and technologies. Journal of Integrative Agriculture 15, 935–943. [Google Scholar]
- Onyemaobi I , Liu H , Siddique KH , Yan G. 2017. Both male and female malfunction contributes to yield reduction under water stress during meiosis in bread wheat. Frontiers in Plant Science 7, 2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds M , Bonnett D , Chapman SC , Furbank RT , Manès Y , Mather DE , Parry MA. 2011. Raising yield potential of wheat. I. Overview of a consortium approach and breeding strategies. Journal of Experimental Botany 62, 439–452. [DOI] [PubMed] [Google Scholar]
- Riahi K , Rao S , Krey V , Cho C , Chirkov V , Fischer G , Kindermann G , Nakicenovic N , Rafaj P. 2011. RCP 8.5—A scenario of comparatively high greenhouse gas emissions. Climatic Change 109, 33. [Google Scholar]
- Robert N , Berard P , Hennequet C. 2001. Dry matter and nitrogen accumulation in wheat kernel. Genetic variation in rate and duration of grain filling [Triticum aestivum L.]. Journal of Genetics and Breeding 55, 297–305. [Google Scholar]
- Rötter RP , Tao F , Höhn JG , Palosuo T. 2015. Use of crop simulation modelling to aid ideotype design of future cereal cultivars. Journal of Experimental Botany 66, 3463–3476. [DOI] [PubMed] [Google Scholar]
- Saini HS. 1997. Effects of water stress on male gametophyte development in plants. Sexual Plant Reproduction 10, 67–73. [Google Scholar]
- Saini HS , Aspinall D. 1982. Abnormal sporogenesis in wheat (Triticum-aestivum L.) induced by short period of high-temperature. Annals of Botany 49, 835–846. [Google Scholar]
- Schwefel HP , Rudolph G. 1995. Contemporary evolution strategies. In: Morán F , Moreno A , Merelo JJ , Chacón P, eds. Advances in artificial life. Third European conference on Artificial life, Granada, Spain, June 4–6, 1995 Proceedings Berlin, Heidelberg: Springer, 891–907. [Google Scholar]
- Semenov MA , Halford NG. 2009. Identifying target traits and molecular mechanisms for wheat breeding under a changing climate. Journal of Experimental Botany 60, 2791–2804. [DOI] [PubMed] [Google Scholar]
- Semenov MA , Martre P , Jamieson PD. 2009. Quantifying effects of simple wheat traits on yield in water-limited environments using a modelling approach. Agricultural and Forest Meteorology 149, 1095–1104. [Google Scholar]
- Semenov MA , Shewry PR. 2011. Modelling predicts that heat stress, not drought, will increase vulnerability of wheat in Europe. Scientific Reports 1, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov MA , Stratonovitch P. 2010. Use of multi-model ensembles from global climate models for assessment of climate change impacts. Climate Research 41, 1–14. [Google Scholar]
- Semenov MA , Stratonovitch P. 2013. Designing high-yielding wheat ideotypes for a changing climate. Food and Energy Security 2, 185–196. [Google Scholar]
- Semenov MA , Stratonovitch P. 2015. Adapting wheat ideotypes for climate change: accounting for uncertainties in CMIP5 climate projections. Climate Research 65, 123–139. [Google Scholar]
- Semenov MA , Stratonovitch P , Alghabari F , Gooding MJ. 2014. Adapting wheat in Europe for climate change. Journal of Cereal Science 59, 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov MA , Terkel DA. 2003. Analysis of convergence of an evolutionary algorithm with self-adaptation using a stochastic Lyapunov function. Evolutionary Computation 11, 363–379. [DOI] [PubMed] [Google Scholar]
- Serba DD , Yadav RS. 2016. Genomic tools in pearl millet breeding for drought tolerance: status and prospects. Frontiers in Plant Science 7, 1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavrukov Y , Kurishbayev A , Jatayev S , Shvidchenko V , Zotova L , Koekemoer F , de Groot S , Soole K , Langridge P. 2017. Early flowering as a drought escape mechanism in plants: how can it aid wheat production? Frontiers in Plant Science 8, 1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman VJ , Sylvester-Bradley R , Scott RK , Foulkes MJ. 2005. Physiological processes associated with wheat yield progress in the UK. Crop Science 45, 175–185. [Google Scholar]
- Shiferaw B , Smale M , Braun H-J , Duveiller E , Reynolds M , Muricho G. 2013. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Security 5, 291–317. [Google Scholar]
- Silva SA , de Carvalho FIF , Caetano VdaR , de Oliveira AC , de Coimbra JLM , de Vasconcellos NJS , Lorencetti C. 2001. Genetic basis of stay-green trait in bread wheat. Journal of New Seeds 2, 55–68. [Google Scholar]
- Silva SA , de Carvalho FIF , Nedel JL , Cruz PJ , Peske ST , Simioni D , Cargnin A. 2003. Seed filling in near-isogenic lines of wheat with presence and absence of the stay-green trait. Pesquisa Agropecuaria Brasileira 38, 613–618. [Google Scholar]
- Stratonovitch P , Semenov MA. 2010. Calibration of a crop simulation model using an evolutionary algorithm with self-adaptation. Procedia Social and Behavioral Sciences 2, 7749–7750. [Google Scholar]
- Stratonovitch P , Semenov MA. 2015. Heat tolerance around flowering in wheat identified as a key trait for increased yield potential in Europe under climate change. Journal of Experimental Botany 66, 3599–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester-Bradley R , Riffkin P , O’Leary G. 2012. Designing resource-efficient ideotypes for new cropping conditions: wheat (Triticum aestivum L.) in the high rainfall zone of southern Australia. Field Crops Research 125, 69–82. [Google Scholar]
- Taylor KE , Stouffer R J , Meehl GA. 2012. An overview of CMIP5 and the experiment design. Bulletin of the American Meteorological Society 93, 485–498. [Google Scholar]
- Triboi E , Triboi-Blondel AM. 2002. Productivity and grain or seed composition: a new approach to an old problem—invited paper. European Journal of Agronomy 16, 163–186. [Google Scholar]
- Trnka M , Hlavinka P , Semenov MA. 2015. Adaptation options for wheat in Europe will be limited by increased adverse weather events under climate change. Journal of the Royal Society Interface 12, 20150721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trnka M , Rotter RP , Ruiz-Ramos M , Kersebaum KC , Olesen JE , Zalud Z , Semenov MA. 2014. Adverse weather conditions for European wheat production will become more frequent with climate change. Nature Climate Change 4, 637–643. [Google Scholar]
- Vanuytrecht E , Raes D , Willems P , Geerts S. 2012. Quantifying field-scale effects of elevated carbon dioxide concentration on crops. Climate Research 54, 35–47. [Google Scholar]
- Wise K , Johnson B , Mansfield C , Krupke C. 2011. Managing wheat by growth stage. Purdue Extension ID-422. Purdue, IN, USA: Purdue University. [Google Scholar]
- Yadav S , Sharma KD. 2016. Molecular and morphophysiological analysis of drought stress in plants. In: Rigobelo EC, ed. Plant growth. Rijeka: InTech, 149–173. [Google Scholar]
- Zampieri M , Ceglar A , Dentener F , Toreti A. 2017. Wheat yield loss attributable to heat waves, drought and water excess at the global, national and subnational scales. Environmental Research Letters 12, 064008. [Google Scholar]
- Zhu XG , Long SP , Ort DR. 2010. Improving photosynthetic efficiency for greater yield. Annual Review of Plant Biology 61, 235–261. [DOI] [PubMed] [Google Scholar]