Abstract
Possibly through its effects on glia, the peroxisome proliferator-activated gamma receptor (PPARγ) agonist pioglitazone (PIO) has been shown to alter the effects of heroin in preclinical models. Until now, these results have not been assessed in humans. Heroin-dependent participants were randomized to either active (45 mg, n = 14) or placebo (0 mg, n = 16) PIO maintenance for the duration of the three-week study. After stabilization on buprenorphine (8 mg), participants began a two-week testing period. On the first to fourth test days, participants could self-administer drug or money by making verbal choices for either option. On the fifth day, active heroin and money were administered and participants could work to receive heroin or money using a progressive ratio choice procedure. Test days 6–10 were identical to test days 1–5 with the exception that, during one of the test weeks, placebo was available on the first four days, and during the other week heroin was available. PIO failed to alter the reinforcing or positive subjective effects of heroin, but it did reduce heroin craving and overall anxiety. Although we were unable to replicate the robust effects found in preclinical models, these data provide an indication of drug effects that deserves further exploration.
Keywords: Abuse potential, glia, heroin, opioids, pioglitazone
Introduction
Pharmacotherapies for substance abuse represent opportunities to facilitate treatment and provide insight into the neuropharmacological mechanisms involved in the initiation and maintenance of addiction (Koob, Lloyd, and Mason 2009). Recent research on the interaction between drugs of abuse and immunocompetent (glia) cells indicates that the study of neuroimmunopharmacology may provide novel pharmacological targets to combat substance abuse (for reviews, see Bachtella et al. 2017; Cooper, Jones, and Comer 2012). One emerging area of research seeks to examine the relationship between glial cell activity and the behavioral effects of opioids and other drugs of abuse. Although previous research has shown that opioids are immunosuppressive (Eisenstein et al., 1996; Eisenstein 2011; Eisenstein et al. 2006), other studies have demonstrated that opioids activate glia, which modifies opioid-induced physiological and behavioral effects (Bland, Hutchinson, and Maier 2009; Hutchinson et al. 2007, 2009; Hutchinson and Watkins 2014; Watkins et al. 2005).
It is hypothesized that the interaction between opioids and glia occurs through the pattern recognition receptor, toll-like receptor 4 (TLR4), where opioids bind to the TLR4 co-receptor myeloid differentiation factor 2 (Hutchinson et al. 2010; Jacobsen, Watkins, and Hutchinson 2014; Liang et al. 2016; Wang et al. 2012). Opioid interactions with glial cells may contribute to opioid actions within the brain’s reward system, thereby mediating their abuse potential. There is also evidence of the importance of TLR4 in the development of tolerance and withdrawal (Eidson and Murphy 2013; Watkins et al. 2009).
Preclinical research into the behavioral effects of opioid-glia interactions has found that selectively increasing glial (astrocyte) activity in the nucleus accumbens (NAcc) and intracingulate cortex results in significantly greater preference for morphine-associated cues r elative to vehicle-associated cues [morphine-induced conditioned place preference (CPP)]. Investigators were able to attenuate this effect with a glial activity inhibitor (Narita et al. 2006). Preclinical research has shown that the glial inhibitors propentofylline [acting upon tumor necrosis factor alpha (TNFα), microglia and interleukin (IL)-1β] and minocycline (TNFα, IL1β, microglia) attenuated morphine-induced conditioned place preference (CPP), a measure of drug reward (Haney and Spealman 2008; Ledeboer et al. 2005; Narita et al. 2006; Sweitzer and De Leo 2011; Tikka et al. 2001; Yrjänheikki et al. 1998). Other preclinical studies showed that the microglia and astrocyte activity suppressor ibudilast (Suzumura et al. 1999) significantly reduced the magnitude of morphine-induced dopamine release in the NAcc (Bland, Hutchinson, and Maier 2009). Ibudilast also attenuated both antagonist-precipitated and deprivation-induced morphine withdrawal in rodents (Hutchinson et al. 2009; Ledeboer et al. 2006).
Preclinical research also supports the idea that the interaction between opioids and glial may mediate the effects of chronic opioid use (i.e., physiological dependence). Chronic antagonism of TLR4 during withdrawal has been shown to alter heroin craving resulting from prolonged abstinence (Theberge et al. 2013). These preclinical findings were corroborated by our own clinical laboratory assessments. In a recent investigation, we found that that opioid withdrawal symptoms (“anxious,” “perspiring,” “restless,” and “stomach cramps”) were significantly decreased by the glial inhibitor ibudilast in comparison to placebo (Cooper et al. 2016).
Another glial modulator under investigation is the Peroxisome proliferator-activated gamma receptor (PPARγ) agonist pioglitazone (PIO). PPARγ agonists inhibit the expression of cytokines by monocytes/macrophages and microglia (Kielian and Drew 2003). PPARγ agonists have shown promise in treating many forms of drug abuse; more specifically, in rodent models of alcohol abuse (Ferguson et al. 2014; Stopponi et al. 2011, 2013) and in human models of nicotine use (Jones et al. 2017). Concerning opioid abuse, preclinical research has also shown that PPARγ activation by PIO attenuated the development of analgesic tolerance to morphine (de Guglielmo et al. 2014), reduced heroin self-administration under a fixed-ratio and progressive-ratio schedule of reinforcement, and attenuated heroin-induced extracellular dopamine release in the NAcc (de Guglielmo et al. 2015).
The ability of PIO to alter the effects of heroin in humans has not been characterized in controlled, clinical laboratory settings. As such, the aim of the current study was to examine the subjective and reinforcing effects of heroin under PIO maintenance [0 (placebo), 45 mg] in opioid-dependent heroin users. Secondary aims were to examine the influence of PIO on the cognitive and physiological effects of heroin. We hypothesized that PIO would decrease indicators of the abuse potential of heroin, without altering its effects mediated through traditional opioid receptor mechanisms. If these hypotheses are supported, regulation of PPARγ may represent a new pharmacotherapeutic target for the treatment of opioid abuse and dependence.
Methods
Participant recruitment, selection, and compensation
Heroin users who were not seeking treatment for their drug use were recruited using print and online advertisements within the New York City (NYC) metropolitan area. Following a brief telephone interview, potential participants who met preliminary study criteria were scheduled for in-person screening visits at the New York State Psychiatric Institute (NYSPI). During screening visits, psychologists, nurses, and physicians assessed participants’ physical and mental health. Participants’ health was determined using an electrocardiogram, clinical laboratory tests (hematology, blood chemistry panel, liver and thyroid functioning, urinalysis, and syphilis serology), a clinical interview with a research psychologist, and a physical and psychiatric examination with a physician. At every screening visit, urine toxicology tests assessed for recent use of opioids, benzodiazepines, cocaine, and marijuana. Observable symptoms of withdrawal or response to an intramuscular injection of naloxone (0.2–0.8 mg) were used during screening to determine if individuals were currently physiologically dependent on opioids. In order to be enrolled, participants needed to meet DSM-IV criteria for opioid abuse and be physiologically dependent on them, be physically healthy, and be between the ages of 21 and 55 years.
Participants who had chronic pain, were dependent on any psychoactive substance other than opioids, nicotine, or caffeine, or met criteria for any Axis 1 diagnosis that may have interfered with their ability to provide informed consent were excluded. As compensation, participants were paid $25/day with a $25/day bonus for completing the study. All study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute (NYSPI IRB# 6255; ClinicalTrials.gov Identifier: NCT01395797).
Procedures
Opioid stabilization and maintenance
For the duration of the three-week study, participants resided on our secure inpatient unit, located on the fifth floor of the NYSPI. For the first 5–7 days following admission, participants were stabilized on the maintenance medication, a daily 8 mg sublingual (SL) dose of buprenorphine + 2 mg of naloxone. The maintenance medication was taken each evening at 8 pm. This dosing regimen was chosen to ensure that the maintenance medication had the least impact on study measures (as buprenorphine levels would be at their nadir during experimental testing sessions). During stabilization, participants were treated for emergent withdrawal symptoms with supplemental medications (e.g., clonazepam, clonidine, zolpidem). Self-reported and observer-rated withdrawal were assessed daily and experimental testing did not begin until withdrawal symptoms were no longer present.
For this investigation, an opioid-dependent sample, maintained on an opioid throughout the study (vs detoxified), was chosen based upon findings from Jones et al. (2016) in which no interaction between PIO and oxycodone was found. It was suggested that the results of this study may have varied if an opioid-dependent sample had been employed. According to the theory of opioid-induced glial activation and neuroinflammation, chronic opioid (ab)users may have higher tonic levels of glial activity upon which PIO could act (Hutchinson et al. 2009; Sweitzer and De Leo 2011; Woods et al. 2003).
Pioglitazone stabilization and maintenance
Participants were randomized to one of two PIO maintenance conditions [0 mg (placebo) or 45 mg]. The active PIO dose employed in this study is currently used clinically for the treatment of insulin resistance and type 2 diabetes (Gillies and Dunn 2000; Pfützner et al. 2006). PIO stabilization was initiated upon the day of admission onto the unit and participants were maintained on the same PIO dose for the duration of the study (a between-subjects design was used). Participants were maintained on PIO for approximately seven days prior to laboratory testing. Participants and study staff conducting laboratory sessions were blind to the PIO maintenance condition. The study MD was not blinded for safety purposes. Adverse events were assessed daily throughout the study with none determined to be “probably” or “definitely” related to PIO. Table 1 shows the overall study design with a schedule of laboratory sessions.
Table 1.
Representative study design.
| Week 1 | Week 2 | Week 3 | ||
|---|---|---|---|---|
| 5–7 Days | M T W Th | F | M T W Th | F |
| Stabilization | Verbal Choice Self-Admin: Heroin | Heroin PR Self-Admin & Cue Sessions | Verbal Choice Self-Admin: Placebo | Heroin PR Self-Admin & Cue Sessions |
| Bup/Nal 8/2 mg + PIO 0 mg or 45 mg | ||||
Experimental sessions
Following stabilization, participants began laboratory sessions, which took place from Monday to Friday. Participants completed three types of laboratory sessions over the course of two weeks. Monday through Thursday (Days 1–4), participants were given five opportunities each day (at 10 am, 11 am, 12 pm, 1 pm, and 2 pm) to choose between a dose of drug (heroin or placebo) and $10 using a simple “yes” or “no” verbal choice procedure. The same dose was tested in the procedure for the entire week (e.g., Week 1 = active heroin, Week 2 = placebo heroin). The order of active heroin and placebo weeks was randomly assigned.
Challenge doses of heroin or placebo were administered intravenously (IV: 10 mg) or intranasally (IN: 40 mg), depending on the participants’ preferred route of street heroin use. Our previous work with heroin has shown that this 1:4 IV-to-IN ratio produces a similar magnitude of reinforcing, subjective, and physiological effects (Comer et al. 1999). Completers in both PIO conditions consisted of roughly equal numbers of IV and IN users. Placebo consisted of a matching milliliter (ml) or milligram (mg) amount of dextrose solution or lactose powder, respectively.
Friday, or Day 5, laboratory sessions consisted of two parts. In the morning “sample” session (10 am), participants were administered an active dose of heroin followed by various assessments of subjective effects, performance tasks, and physiological measures (discussed later in this article). These data were collected at baseline and at various time points up to 90 minutes after drug administration. For safety, participants’ vital signs were continuously monitored during all drug administration sessions.
At approximately 2 pm, participants completed a drug “cue exposure” session. During the cue session, participants were shown a neutral stimulus (a water bottle) and asked to look at, hold and sniff it, and take a drink of the water inside (Neutral Cue). Participants were then asked to manipulate paraphernalia associated with intravenous or intranasal drug use (Active/Drug Cue). Neutral cues always preceded drug cue presentation to prevent carry-over effects from the active condition to the control condition.
Intranasal users were instructed to watch as a research nurse opened a wallet, removed a $1 bill, removed a packet of powder mimicking heroin, opened the packet, and rolled the dollar bill. Participants were then given the packet and dollar bill to hold for approximately 30 seconds. Intravenous users were instructed to watch as a research nurse opened a packet of heroin, poured its contents onto a spoon, added a few drops of water to the spoon, held an open flame froma lighter under the spoon, added cotton to the spoon, and drew the fluid into a syringe. Next, a tourniquet was placed on the participants’ arm and the participant was instructed to look for a vein. The participant was then given the syringe to hold for approximately 30 seconds. Because previous studies have shown that a physiological and subjective response can be induced by showing heroin users pictures or videos of drug paraphernalia, we believe that manipulating actual heroin paraphernalia should easily induce robust arousal (Preller et al. 2013; Zhao et al. 2012; Zijlstra et al. 2009).
Following the cue exposure session, participants completed a progressive ratio self-administration task to receive portions of the dose of drug or money they had sampled earlier in the day (0–100%, in increments of 10%). Participants were told that they could work for all or part of the sampled dose or the sampled money amount by choosing the drug or money option each time a choice was available. The alternative money value ($20) was chosen based on previous studies conducted in our laboratory (Comer, Collins, and Fischman 1997). Drug and money were available at each choice trial. For example, at each opportunity, intranasal users could respond for 4 mg (10% of the total dose of 40 mg) or $2 (10% of $20).
Completion of the ratio requirement for each choice trial was accompanied by a visual stimulus on the computer screen. After a choice was made for one option by clicking on its visual representation on the computer screen, responding for the other option was not possible until the ratio was completed and another trial was initiated. Responses to complete the ratio requirement consisted of finger presses on a computer mouse. The response requirement for each of the two options increased independently such that the initial ratio requirement for each option was 50 responses; the ratio increased progressively each time the option was selected (50, 100, 200, 400, 800, 1200, 1600, 2000, 2400, and 2800). At the end of the self-administration task (approximately 4 pm), the participant received whatever he or she had chosen: money (added to their study payment) and/or the IV or IN challenge drug.
Task and measures
Subjective effects
Six questionnaires were used to assess subjective drug effects and opioid withdrawal symptoms. A 26-item visual analog scale (VAS) was used to assess subjective and physiological drug effects, such as “I feel a good effect” and “I feel high.” Participants rated each item on the scale from “Not at all” (0 mm) to “Extremely” (100 mm). In addition, a six-item drug effects questionnaire (DEQ) was used to measure drug effects (strength of drug effects, good effects, bad effects, willingness to take the drug again, drug liking, and similarity to other drugs). Participants selected among a series of possible answers ranging from 0 (“No Effect”) to 4 (“Very Strong Effect:), except for the drug liking questionnaire, which ranged from −4 (“Dislike Very Much”) to 4 (“Like Very Much”). Craving questionnaires for heroin, cocaine, and cigarettes (individualized for each participant) were also utilized (Tiffany and Drobes 1991; Tiffany et al. 1993). Finally, the Subjective and Clinical Opioid Withdrawal Scales (SOWS and COWS, respectively) were used to identify the presence and severity of opioid withdrawal symptoms (Handelsman et al. 1987). During the cue session, the Spielberger’s Situational Anxiety Scale (SSAS) asked participants to rate, on a 5-point Likert scale, the degree to which they experienced events such as “salivating” and “trembling” while manipulating the drug paraphernalia (Spielberger et al. 1983).
Physiological measures
Miosis was assessed as a physiological indicator of mu agonist effects using a NeurOptics™ Pupillometer under ambient lighting conditions. For safety, oxygen saturation (%SpO2), respiration (breaths per minute), heart rate, and blood pressure (systolic and diastolic) were continuously monitored during sessions and recorded every five minutes. Supplemental oxygen also was provided throughout the session.
During the cue-exposure sessions, galvanic skin response (GSR), skin temperature, and heart rate were assessed immediately before and after active and neutral drug cue presentation. These measures were used as indicators of autonomic arousal (Mendes 2009).
Performance effects
The performance battery consisted of two tasks: a 10-min divided attention task (DAT) and a 3-min digit-symbol substitution task (DSST). Custom-made software was used for these performance tasks (see Comer et al. 1999 for details). The divided attention task consisted of concurrent pursuit-tracking and vigilance components. Participants tracked a moving stimulus on the video screen using the mouse and also signaled when a small black square appeared at any of the four corners of the video screen. Accurate recognition of the brief flash of a black square is considered a “Hit,” and failure to respond is considered a “Miss.” If the participant indicates that the square appeared when it does not, this is considered a “False Alarm.” The distance between the cursor and moving stimulus was measured, as was the speed of the moving stimulus (with greater accuracy, the stimulus moved at a faster rate). The digit-symbol substitution task consisted of nine three-row by three-column squares (with one black square per row) displayed across the top of the computer screen. A randomly generated number indicated which of the nine patterns should be emulated on a keypad by the participant on a particular trial. Participants were required to emulate as many patterns as possible by entering the pattern associated with randomly generated numbers appearing on the bottom of the screen. Prior to testing for the main study, participants were trained in how to complete the performance tasks during a practice session performed while screening.
Drugs
Naloxone HCl (Narcan) for IM injection was obtained from the International Medication System Limited Amphastar (South Elmonte, CA). PIO tablets (15 mg) were provided by the OMEROS Corporation (Seattle, WA), and were overencapsulated by the New York State Psychiatric Institute Pharmacy. Each daily dose consisted of three capsules of active drug and/or lactose-filled placebo, depending on the final target dose (e.g., 45 mg = 3 ° 15 mg capsules; 0 mg = 3 ° 0 mg capsules). The three capsules were administered to participants by a research nurse.
Buprenorphine/naloxone tablets for SL administration were provided by Reckitt Benckiser Pharmaceuticals, Inc. (Richmond, VA), while heroin HCl powder was obtained from the Research Triangle Institute (Research Park Triangle, NC). Placebo IN heroin doses consisted of lactose monohydrate powder, purchased from Spectrum Chemicals (Gardena, CA). For intravenous users, placebo consisted of sterile 5% dextrose solution. All IN doses were insufflated through a plastic straw within 5–10 seconds, and IV doses were injected through a catheter by a research physician within 30 seconds. Both IV and IN users were recruited in order to improve participant flow into the study. Heroin was administered by both routes of administration in order to maximize the salience of the drug cue during the cue-exposure sessions. All study drug blinding and packaging was performed by the New York State Psychiatric Institute Pharmacy.
Statistical analyses
An a priori power analysis was conducted to ensure that effects on the outcome measures of interest could be detected. Estimates for our primary measure of heroin’s reinforcing effects were obtained from a similar trial assessing the reinforcing effects of heroin in buprenorphine/naloxone-maintained heroin abusers (Comer et al. 2005). The targeted sample size of 20 per arm was calculated to provide 80% power to detect a 26% between-group difference in average percentage of drug choice, which is an effect size of 0.65. This assumes a standard deviation of 28%. The sample size goal was also estimated to provide 80% power to detect a 500-point between-group difference in progressive ratio breakpoint values, which is an effect size of 0.64. This assumes a standard deviation of 594 and a between-level correlation of 0.60. Estimates of positive subjective effects were also considered. Twenty completers per arm in each study calculated to provide 83% power to detect a 15-mm difference in ratings of “Liking” as measured by the VAS. This analysis assumes a common standard deviation of differences of 16 mm (e.g., effect size of 0.66).
Continuous and categorical demographics variables were summarized descriptively and compared between the 0 mg and 45 mg PIO conditions using the chi-square (X2) or t-tests. The primary statistical analyses were comparisons of acute drug effects and self-administration (from Day 5/Friday Session) between placebo (0 mg) and active (45 mg) PIO maintenance. These comparisons were made using analysis of variance (ANOVA) evaluating the time course of drug effects. F-tests were also used to compare PR heroin breakpoint values between active and placebo PIO groups. This same method was used to compare mean autonomic arousal following presentation of active drug cues.
ANOVA was employed to compare heroin choices between active and placebo PIO groups during the preceding four days (Monday–Thursday). Tukey’s HSD post-hoc tests were used to identify significant differences between PIO groups. In order to determine whether the results differed between individuals who received IN heroin versus IV heroin, independent-samples t-tests compared these measures between these two groups. Because no significant differences were found, the data presented as follows were collapsed across this condition. For all analyses, the significance level of α was set at <.05, with an α of .05–.10 considered as approaching statistical significance. All data analyses were performed using SPSS version 18 (SPSS 2009) and SuperANOVA (Gagnon et al. 1990).
Results
Participants
A total of 46 heroin users (without chronic pain) were enrolled in the current study. Sixteen participants from the PIO 0 mg group completed while the 45 mg group had 14 completers. Ten participants either discontinued or were withdrawn from the protocol (five participants withdrew because of personal issues or because they could not tolerate the boredom or confinement of the inpatient unit, two participants discontinued due to significant weight gain, one from elevated liver function tests, one from an unreported leg injury sustained prior to admission, which began to worsen, and one because an intravenous catheter could not be placed for laboratory sessions). Six enrolled participants were included in a 15 mg PIO condition that was eventually dropped from the study due to limitations of the grant timeline. Table 2 displays the major demographic and drug use variables for completers of both groups.
Table 2.
Sample demographics.
| Participants (%) or Median (Std. Dev.) | |||
|---|---|---|---|
| PIO 0 mg (n = 16) | PIO 45 mg (n = 14) | p-value | |
| Age | 44.5 (8.1) | 42.4 (8.6) | NS |
| Sex | |||
| Male | 15 (94) | 28 (93) | NS |
| Female | 1 (6) | 1 (7) | |
| Ethnic/Racial Category | |||
| Asian | – | 1 (7) | |
| African American | 4 (25) | 3 (21) | NS |
| Caucasian | 6 (38) | 3 (21) | |
| Hispanic/Latino | 5 (31) | 4 (29) | |
| More than One Race/Unreported | 1 (6) | 3 (21) | |
| Heroin Use | |||
| Heroin Use (bags/day) | 6.5 (2.7) | 6.9 (4.3) | NS |
| Years of Use | 18.5 (9.4) | 12.0 (9.1) | .06 |
| Route of Administration | |||
| Preference | |||
| Intranasal | 9 (56) | 7 (50) | NS |
| Intravenous | 7 (54) | 7 (50) | NS |
| Concomitant Substance Use | |||
| Nicotine (Yes) | 13 (81) | 13 (92) | NS |
| Cigarettes per Day | 12.9 (11.5) | 9.3 (4.4) | NS |
| Cocaine (Yes) | 7 (43.7) | 5 (35.7) | NS |
| Alcohol (Yes) | 7 (43.7) | 12 (41) | NS |
| Marijuana (Yes) | 2 (12.5) | 5 (35.7) | NS |
The 0 mg and 45 mg conditions were similarly matched on most demographic variables of interest. Completers in both groups consisted of roughly equal numbers of IV and IN users, who were using 6–7 bags of heroin daily. Nicotine, cocaine, and alcohol use was common (> 50%) among both groups, while the use of marijuana, sedatives (benzodiazepines/barbiturates), hallucinogens, and prescription opioids was rare.
Reinforcing effects
During the first four days of testing, active doses of heroin were chosen significantly more than placebo heroin (PBO Heroin vs Active Heroin: p < .001) using the simple verbal choice self-administration procedure. However, the percentage of heroin choices (Figure 1, upper panel) did not vary as a function of PIO maintenance dose (PIO 0 mg vs PIO 45 mg: p = NS). On Day 5, when active heroin versus money was available and participants responded under a progressive ratio schedule of reinforcement, robust drug breakpoints (>1500) were observed under all conditions tested. The reinforcing effects of heroin did not vary as a function of the availability of heroin the preceding four days (i.e., active heroin or placebo heroin), or as a function of PIO maintenance condition (Figure 1, lower panel) (PIO 0 mg vs PIO 45 mg: p = NS).
Figure 1.
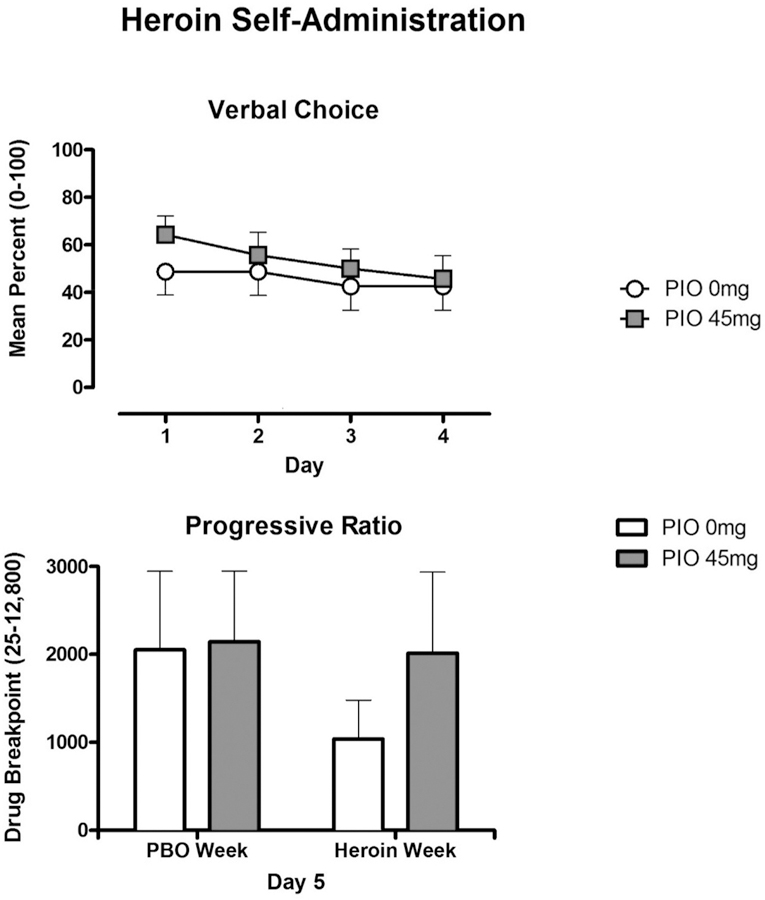
Self-administration (± SEM) of heroin for the PIO 0mg (n=16) and PIO 45 mg (n=14) conditions, assessed usingthe verbal choice procedure during Days 1–4 (upper panel). Self-administration (± SEM) of heroin using a progressive-ratio procedure following 4 days of access to placebo or active heroin (lower panel).
Positive subjective effects
The subjective effects of heroin were measured at baseline on Days 1–4 and on Day 5 following an experimenter-administered dose of heroin (IV: 10 mg; IN 40 mg). In comparison to the pre-dose baseline (−45 min), post-dose ratings (5, 15, 45, and 60 min) of “Good Effect” (p < .05), “High Quality” (p < .01), “Liking” (p < .01), “High” (p < .05), “Would Take Again” (p < .05), and “Would Pay” (p < .05) were significantly elevated. Similar results were observed on the more general descriptors of “Strong” (p < .05), “Sedated” (p < .01), and “Potent” (p < .01). These measures did not vary as a function of the availability of heroin the preceding week (i.e., active heroin or placebo heroin). Overall, no main effect of PIO maintenance condition or interactions was observed. An effect of active PIO to decrease “Good Effect” approached significance, but only following active heroin availability (PIO 0 mg vs PIO 45 mg: p < .10).
Aversive subjective effects
Heroin did not produce any notable increases in assessments of “Bad” drug effect, “Depressed,” “Irritable,” “Nauseous,” or “Nervous.” These ratings were also not affected by PIO. VAS ratings of “Anxious” were slightly lower during week-long active heroin self-administration. Only under this condition did we observe an effect of PIO to further attenuate anxiety. PIO’s effect approached statistical significance during Days 1–4 (PIO 0 mg vs PIO 45 mg: p < .10; Figure 2, upper panel), and was significant on Day 5 (PIO 0 mg vs PIO 45 mg: p < .05).
Figure 2.
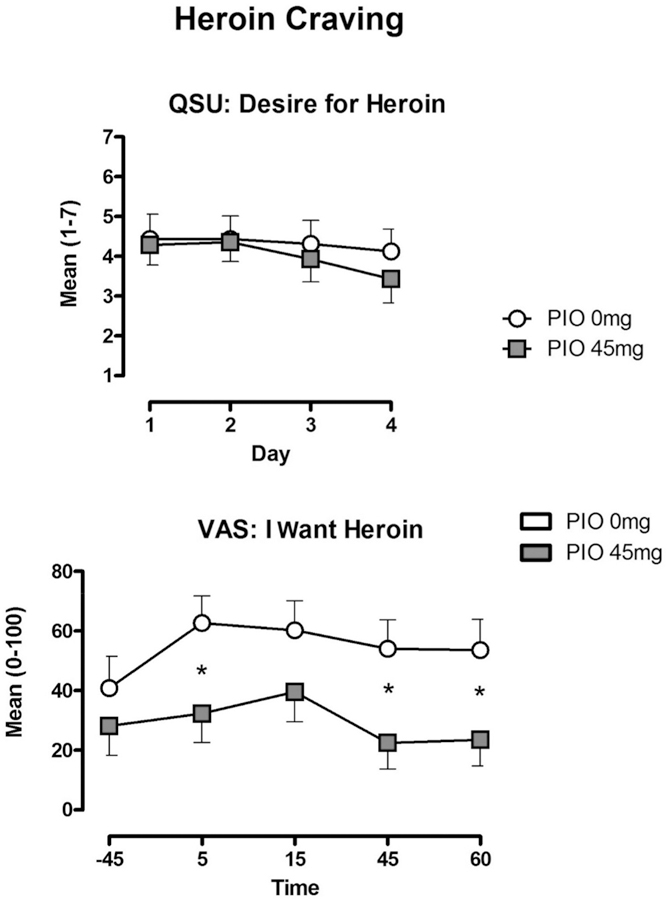
Mean (± SEM) subjective ratings of heroin “Desire” during Days 1–4 (upper panel), and Day 5 “Want” of heroin (lower panel) for the PIO 0 mg and PIO 45 conditions. * Indicates significance at p<0.05.
Craving
Throughout the study, VAS ratings of “Wanting” for cocaine and alcohol were generally low (<10 of 100), with the exception of tobacco (scores ranged between 35 and 60 mm). We observed no effect of heroin or PIO dose on these measures. However, measures of heroin craving assessed using the VAS (“I Want Heroin”), SOWS (“I Feeling Like Shooting Up”), and craving scales (“Desire” and “Urge” for heroin) were generally lower under the active PIO maintenance condition (Figure 2, upper panel), though this difference did not meet statistical significance. The ability of PIO to reduce craving was only statistically significant (PIO 0 mg vs PIO 45 mg: p < .05) on the VAS measure of “I Want Heroin” measures during the Day 5 sample session (Figure 2, lower panel).
Physiological and performance effects
On Day 5, heroin significantly decreased pupil diameter, heart rate, and breaths per minute in comparison to baseline (p < .05). On the cognitive performance tasks, heroin significantly decreased the total number of correct responses on the DSST (PBO Heroin vs Active Heroin: p < .05) and increased the number of “misses” on the DAT. The total tracking distance on the DAT, and the number of “hits” and “false alarms,” were not affected. No moderating effect of PIO was found on these measures.
Response to drug cues
During the cue sessions, presentation of active drug cues (versus neutral cues) failed to alter the participants’ assessments of their own “Sweating Hands,” “Salivating,” “Faster Heart Rate,” and “Trembling” while manipulating drug paraphernalia, though a significant increase in reports of “Heavy Stomach” were reported (p < .05). These findings were not altered by PIO and were consistent, regardless of whether the participant received active or placebo heroin the preceding week. Assessments of “Desire for Heroin,” “I Want Heroin,” and “Anxious” were increased following active drug cue presentation. These findings only approached statistical significance (Active Cue vs Neutral Cue: p < .10) and were not affected by any other study conditions. Assessment of autonomic arousal during the cue session revealed that active drug cues increased skin conductance to a degree that approached statistical significance (Active Cue vs Neutral Cue: p < .10), but this effect was not altered by PIO or heroin availability in the days prior to the session. Heart rate and skin temperature were not significantly altered by any of our study conditions.
Discussion
The current study sought to determine whether PIO, a PPARγ agonist and glial modulator, would alter the subjective and reinforcing effects of heroin. In our sample of opioid-dependent participants, heroin produced typical mu opioid agonist effects, including miosis, respiratory depression, and mild cognitive impairment (Comer, Collins, and Fischman 1997; Comer et al. 1999; Rook et al. 2006). Heroin was also self-administered significantly more than placebo under all conditions tested, producing increases in reports of positive subjective effects with relatively minimal aversive effects. These data demonstrate the abuse potential common to most opioid drugs. However, the current study failed to find evidence that PIO altered the reinforcing effects of heroin. We also did not observe an effect of PIO on direct assessments of the positive or negative subjective effects of heroin. However, PIO did significantly reduce craving for heroin, an effect that is often associated with relapse to heroin use in patients who are in treatment (Tsui et al. 2014).
The results of this first clinical assessment of PIO for treating opioid use disorder are inconsistent with preclinical findings showing significant reductions in heroin self-administration in rats (de Guglielmo et al. 2015). Such robust preclinical data suggest that more clinical testing is needed, as parametric differences between preclinical and clinical studies often affect replicability (Ahmed 2005; Foltin et al. 2015; Haney and Spealman 2008; Mello and Negus 1996). However, as we did not meet the recruitment goals outlined in our power analysis, this conclusion is difficult to make, as we may have been underpowered to detect differences in these measures.
Consistent with de Guglielmo and colleagues (2015), the current investigation did observe a reduction in heroin craving during maintenance on active PIO. Although PIO did not alter drug self-administration among our non-treatment-seeking sample, the attenuated opioid craving in treatment-seeking samples may be important in maintaining abstinence from illicit opioids (Moore et al. 2013; Weiss et al. 2003). Similar to another clinical assessment we conducted using another glial inhibitor, we also found evidence of reduced anxiety under active medication (vs. placebo) conditions (Cooper et al. 2016).
We also attempted to corroborate preclinical findings showing that PIO can reduce cue-induced heroin seeking (de Guglielmo et al. 2017). However, the cue exposure procedure we employed produced only marginal psychological and physiological arousal. One parametric condition likely responsible for this finding is the dosing of heroin less than two hours before the cue session. We believe a greater period of drug abstinence preceding the session would increase the salience of the drug cues. Therefore, the investigators feel that we did not produce a sufficient cue effect upon which PIO could act. Based on the ability of PIO to alter craving during other laboratory sessions, its effects on cue-induced craving should be reassessed, independently of opioid administration.
We were unable to ascertain the mechanism(s) underlying the effects that were observed. With regard to our hypothesis about PIO-induced inhibition of glial activation, the most relevant measurements of inflammation would come from cerebrospinal fluid (CSF), but performing spinal taps on our participants would have increased risks to participants, and would have not been well-tolerated (Hopkins et al. 2012). Although measurements of inflammatory markers can also be found in plasma samples, previous research has demonstrated that plasma levels of inflammatory markers cannot be used to identify relative changes in the CSF (Watkins et al. 2009).
Some researchers have questioned the proposed mechanism underlying the clinical utility of glial inhibitors for opioid abuse, citing that opioids are immunosuppressive (Eisenstein 2011). However, the nature of the interaction between opioids and glia is most likely multifaceted. The robust nature of the preclinical findings, combined with the current clinical data, suggests that there is a need for further clinical assessment with glial modulators. Opioid agonist maintenance and antagonism are effective clinical tools used to manage opioid abuse. However, concerns regarding abuse potential and compliance stress the need for continued development of non-opioid pharmacotherapies (Substance Abuse and Mental Health Services Administration 2014; Wright et al. 2016). Medications acting through glial mechanisms may meet this need.
Acknowledgments
The medical assistance of Janet Murray and Claudia Tindall, along with the technical assistance of Rachel Luba, Jonathan Vogelman, Andrew Segoshi, and Brian Wade, is gratefully acknowledged.
Funding
Financial support for this study was provided by the National Institute on Drug Abuse grant R01DA031022 to SDC and AB. Additional support for the manuscript preparation was provided by grant K01DA030446 to JDJ. Study medication (pioglitazone) was provided by the OMEROS Corporation. The authors would like to thank the National Institute on Drug Abuse and the OMEROS Corporation for supporting this research. Only the authors are responsible for the content and preparation of this manuscript. The funding source played no role in the collection, analysis, and interpretation of data, in the writing of the article, or in the decision to submit it for publication.
Footnotes
Disclosure statement
Drs. Comer, Mogali, and Manubay have received compensation (in the form of partial salary support) from studies supported by Alkermes, Braeburn Pharmaceuticals, Cerecor Inc., Endo Pharmaceuticals, Indivior PLC/Reckitt-Benckiser Pharmaceuticals, Johnson & Johnson Pharmaceutical Research & Development, MediciNova, Omeros, and Schering-Plough Corporation. In addition, Dr. Comer has served as a consultant to the following companies: Analgesic Solutions, AstraZeneca, BioDelivery Sciences International, Cephalon, Clinilabs, Daiichi Sankyo, Egalet, Endo, Inflexxion, Innovative Science Solutions, Janssen, KemPharm, King, Lightlake (now Opiant), Neuromed, Pfizer, and Salix. Dr. Bisaga served as an unpaid consultant to Alkermes and received an honorarium from Indivior for an unbranded educational activity.
References
- Ahmed SH 2005. Imbalance between drug and non-drug reward availability: A major risk factor for addiction. European Journal of Pharmacology 526:9–20. doi: 10.1016/j.ejphar.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Bachtella RK, Jones JD, Heinzerling CG, Beardsley PM, and Comer SD. 2017. Glial and neuroinflammatory targets for treating substance use disorders. Drug and Alcohol Dependence 180:156–70. doi: 10.1016/j.drugalcdep.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Hutchinson MR, and Maier SF. 2009. The glial activation inhibitor AV411 reduces morphine-induced nucleus accumbens dopamine release. Brain, Behavior, and Immunity 23:492–97. doi: 10.1016/j.bbi.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Collins ED, and Fischman MW. 1997. Choice between money and intranasal heroin in morphine-maintained humans. Behavioural Pharmacology 8 (8):677–90. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, MacArthur RB, and Fischman MW. 1999. Comparison of intravenous and intranasal heroin self-administration by morphine-maintained humans. Psychopharmacol 143:327–38. doi: 10.1007/s002130050956. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, and Walker EA. 2005. Comparison of intravenous buprenorphine and methadone self-administration by recently detoxified heroin-dependent individuals. Journal Of Pharmacology and Experimental Therapeutics 315:1320–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Jones JD, and Comer SD. 2012. Glial Inhibitors: A novel pharmacological approach to modulating the behavioral effects of abused substances. Expert Opinion Investigations Drugs 21:169–78. doi: 10.1517/13543784.2012.651123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Johnson KW, Pavlicova M, Glass A, Vosburg SK, Sullivan MA, Manubay JM, Martinez DM, Jones JD, Saccone PA, and Comer SD. 2016. The effects of ibudilast, a glial activation inhibitor, on opioid withdrawal symptoms in opioid-dependent volunteers. Addiction Biology 21 (4):895–903. doi: 10.1111/adb.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Scuppa G, Demopulos G, Gaitanaris, and Ciccocioppo R. 2017. Pioglitazone attenuates the opioid withdrawal syndrome and reduces the vulnerability to relapse to heroin seeking. Psychopharmacology 234:223–34. doi: 10.1007/s00213-016-4452-1. [DOI] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Scuppa G, Stopponi S, Demopulos G, Gaitanaris G, and Ciccocioppo R. 2014. Analgesic tolerance to morphine is regulated by PPARγ. British Journal of Pharmacology 171 (23):5407–16. doi: 10.1111/bph.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Melis M, de Luca MA, Kallupi M, Li HW, Niswender K, and Giordano A. 2015. PPARγ activation attenuates opioid consumption and modulates mesolimbic dopamine transmission. Neuropsychopharmacol 40:927–37. doi: 10.1038/npp.2014.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson LN, and Murphy AZ. 2013. Blockade of Toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. The Journal of Neuroscience 33:15952–63. doi: 10.1523/JNEUROSCI.1609-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein TK 2011. Opioids and the immune system: What is their mechanism of action? British Journal of Pharmacology 164:1826–28. doi: 10.1111/j.1476-5381.2011.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein TK, Hilburger ME, and Lawrence DMP. 1996. Immunomodulation by morphine and other opioids. In Drugs of abuse, immunity and infections, ed. Friedman H, Klein TW, and Specter S, 103–20. Boca Raton: CRC Press. [Google Scholar]
- Eisenstein TK, Rahim RT, Feng P, Thingalaya NK, and Meissler JJ. 2006. Effects of opioid tolerance and withdrawal on the immune system. Journal of Neuroimmune Pharmacology 1:237–49. doi: 10.1007/s11481-006-9019-1. [DOI] [PubMed] [Google Scholar]
- Ferguson LB, Most D, Blednov YA, and Harris RA. 2014. PPAR agonists regulate brain gene expression: Relationship to their effects on ethanol consumption. Neuropharmacology 86:397–407. doi: 10.1016/j.neuropharm.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Haney M, Rubin E, Reed SC, Vadhan N, Balter R, and Evans SM. 2015. Development of translational preclinical models in substance abuse: Effects of cocaine administration on cocaine choice in humans and non-human primates. Pharmacology, Biochemistry, and Behavior 134:12–21. doi: 10.1016/j.pbb.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon J, Roth JM, Carroll M, Haycock KA, Plamondon J, Feldman DS, and Simpson J. 1990. Superanova accessible general linear modeling. Yale Journal of Biology and Medicine 63:191–92. [Google Scholar]
- Gillies PS, and Dunn CJ. 2000. Pioglitazone. Drugs 60 (2):333–43. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, and Kanof PD. 1987. Two new rating scales for opiate withdrawal. The American Journal of Drug and Alcohol Abuse 13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Haney M, and Spealman R. 2008. Controversies in translational research: Drug self-administration. Psychopharmacology (Berl) 199 (3):403–19. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins SJ, McMahon CJ, Singh N, Galea J, Hoadley M, Scarth S, Patel H, Vail A, Hulme S, Rothwell NJ, King AT, and Tyrrell PJ. 2012. Cerebrospinal fluid and plasma cytokines after subarachnoid haemorrhage: CSF interleukin-6 may be an early marker of infection. Journal of Neuroinflammation 9:255–64. doi: 10.1186/1742-2094-9-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, and Watkins LR. 2014. Why is neuroimmunopharmacology crucial for the future of addiction research? Neuropharmacolo 76:218–27. doi: 10.1016/j.neuropharm.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, Maier SF, Watkins LR, and Johnson KW. 2009. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain, Behavior, and Immunity 23:240–50. doi: 10.1016/j.bbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Rezvani N, Zhang Y, Wieseler JL, Somogyi AA, Yin H, Maier SF, Rice KC, and Watkins LR. 2010. Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience 167:880–93. doi: 10.1016/j.neuroscience.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, and Watkins LR. 2007. Opioid-induced glial activation: Mechanisms of activation and implications for opioid analgesia, dependence and reward. Scientific World Journal 7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JHW, Watkins LR, and Hutchinson MR. 2014. Discovery of a novel site of opioid action at the innate immune pattern-recognition receptor TLR4 and its role in addiction. International Review of Neurobiology 118:129–63. doi: 10.1016/B978-0-12-801284-0.00006-3. [DOI] [PubMed] [Google Scholar]
- Jones JD, Sullivan MA, Manubay J, Metz V, Mogali S, and Comer SD. 2016. The effects of pioglitazone, a PPARγ receptor agonist, on the abuse liability of oxycodone. Physiology and Behavior 159:33–39. doi: 10.1016/j.physbeh.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Comer SD, Metz VE, Manubay JM, Mogali S, Ciccocioppo R, Martinez S, Mumtaz M, and Bisaga A. 2017. Pioglitazone, a PPARγ agonist, reduces nicotine craving in humans, with marginal effects on abuse potential. Pharmacology, Biochemistry & Behavior 163:90–100. doi: 10.1016/j.pbb.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T, and Drew PD. 2003. Effects of PPARy agonists on central nervous system inflammation. Journal of Neuroscience Research 71:315–25. doi: 10.1002/jnr.10494. [DOI] [PubMed] [Google Scholar]
- Koob GF, Lloyd K, and Mason B. 2009. Development of pharmacotherapies for drug addiction: A Rosetta Stone approach. Nature Reviews. Drug Discovery 8 (6):500–15. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, and Watkins LR. 2005. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Liu T, Shumilla JA, Mahoney JH, Vijay S, Gross MI, Vargas JA, Sultzbaugh L, Claypool MD, Sanftner LM, Watkins LR, and Johnson KW. 2006. The glial modulatory drug AV411 attenuates mechanical allodynia in rat models of neuropathic pain. Neuron Glia Biology 2:279–91. doi: 10.1017/S1740925X0700035X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Chu H, Jiang Y, and Yuan L. 2016. Morphine enhances IL-1β release through toll-like receptor 4-mediated endocytic pathway in microglia. Purinergic Signalling 12:637–45. doi: 10.1007/s11302-016-9525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello MK, and Negus SS. 1996. Preclinical evaluation of pharmocotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacol 14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Mendes WB 2009. Assessing autonomic nervous system activity. In Methods in social neuroscience, ed. Harmon-Jones E, and Beer J New York, NY: Guilford Press. [Google Scholar]
- Moore TM, Seavey A, Ritter K, McNulty JK, Gordon KC, and Stuart GL. 2013. Eco-logical momentary assessment of the effects of craving and affect on risk for relapse during substance abuse treatment. Psychology of Addictive Behaviors 28:619–24. doi: 10.1037/a0034127. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Narita M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y, and Suzuki T. 2006. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacol 31:2476–88. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- Pfützner A, Schneider CA, and Forst T. 2006. Pioglitazone: an antidiabetic drug with cardiovascular therapeutic effects. Expert Review Of Cardiovascular Therapy 4:445–459. [DOI] [PubMed] [Google Scholar]
- Preller KH, Wagner M, Sulzbach C, Hoenig K, Neubauer J, Franke PE, Petrovsky N, Frommann I, Rehme AK, and Quednow BB. 2013. Sustained incentive value of heroin-related cues in short- and long-term abstinent heroin users. European Neuropsychopharmacology 23 (10):1270–79. doi: 10.1016/j.euroneuro.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Rook EJ, van Ree JM, van den Brink W, Hillebrand MJ, Huitema AD, Hendriks VM, and Beijnen JH. 2006. Pharmacokinetics and pharmacodynamics of high doses of pharmaceutically prepared heroin, by intravenous or by inhalation route in opioid-dependent patients. Basic & Clinical Pharmacology & Toxicology 98 (1):86–96. doi: 10.1111/j.1742-7843.2006.pto_233.x. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Jacobs G, Russel F, and Crane R. 1983. Assessment of anger: The State-Trait Anger Scale. In Advances in personality assessment, ed. Butcher JN, and Spielberger CD, vol. 3, 112–34. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- SPSS I. 2009. SPSS 18.0.0 for windows Chicago, IL, SPSS Inc. [Google Scholar]
- Stopponi S, de Guglielmo G, Somaini L, Cippitelli A, Cannella N, Kallupi M, Ubaldi M, Heilig M, Demopulos G, Gaitanaris G, and Ciccocioppo R. 2013. Activation of PPARγ by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcoholism, Clinical and Experimental Research 37:1351–60. doi: 10.1111/acer.12091. [DOI] [PubMed] [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, Ruggeri B, Heilig M, Demopulos G, Gaitanaris G, Massi M, and Ciccocioppo R. 2011. Activation of nuclear PPARγ receptors by the anti-diabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biological Psychiatry 69:642–49. doi: 10.1016/j.biopsych.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. 2014. National survey of substance abuse treatment services. Data on substance abuse treatment facilities Rockville MD: US Department of Health and Human Services, DHHS Publication SMA; 05–4112. [Google Scholar]
- Suzumura A, Ito A, Yoshikawa M, and Sawada M. 1999. Ibudilast suppresses tnf-alpha production by glial cells functioning mainly as type iii phosphodiesterase inhibitor in the cns. Brain Research 837:203–12. doi: 10.1016/S0006-8993(99)01666-2. [DOI] [PubMed] [Google Scholar]
- Sweitzer S, and De Leo J. 2011. Propentofylline: Glial modulation, neuroprotection, and alleviation of chronic pain. Handbook of Experimental Pharmacology 200:235–50. [DOI] [PubMed] [Google Scholar]
- Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM, Baumann MH, Hutchinson MR, Rice KC, Watkins LR, and Shaham Y. 2013. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biological Psychiatry 73:729–37. doi: 10.1016/j.biopsych.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, and Drobes DJ. 1991. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction 86:1467–76. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, and Henningfield JE. 1993. The development of a cocaine craving questionnaire. Drug and Alcohol Dependence 34:19–28. [DOI] [PubMed] [Google Scholar]
- Tikka T, Fiebich BL, Goldsteins G, Keinanen R, and Koistinaho J. 2001. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. The Journal of Neuroscience 21 (8):2580–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui JI, Anderson BJ, Strong DR, and Stein MD. 2014. Craving predicts opioid use in opioid-dependent patients initiating buprenorphine treatment: A longitudinal study. The American Journal of Drug and Alcohol Abuse 40 (2):163–69. doi: 10.3109/00952990.2013.848875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, and Yin H. 2012. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proceedings National Academic Sciences USA 109:6325–30. doi: 10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, and Maier SF. 2009. The “toll” of opioid-induced glial activation: Improving the clinical efficacy of opioids by targeting glia. Trends in Pharmacological Sciences 30:581–91. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Johnston IN, and Maier SF. 2005. Glia: novel counter-regulators of opioid analgesia. Trends Neuroscience 28:661–669. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, Mazurick C, Berkman B, Gastfriend DR, Frank A, Barber JP, Blaine J, Salloum I, and Moras K. 2003. The relationship between cocaine craving, psychosocial treatment, and subsequent cocaine use. The American Journal of Psychiatry 160:1320–25. doi: 10.1176/appi.ajp.160.7.1320. [DOI] [PubMed] [Google Scholar]
- Woods JW, Tanen M, Figueroa DJ, Biswas C, Zycband E, Moller DE, Austin CP, and Berger JP. 2003. Localization of PPARdelta in murine central nervous system: Expression in oligodendrocytes and neurons. Brain Research 975:10–21. doi: 10.1016/S0006-8993(03)02515-0. [DOI] [PubMed] [Google Scholar]
- Wright N, D’Agnone O, Krajci P, Littlewood R, Alho H, Reimer J, Roncero C, Somaini L, and Maremmani I. 2016. Addressing misuse and diversion of opioid substitution medication: Guidance based on systematic evidence review and real-world experience. Journal of Public Health (Oxford) 38 (3):e368–74. doi: 10.1093/pubmed/fdv150. [DOI] [PubMed] [Google Scholar]
- Yrjänheikki J, Keinänen R, Pellikka M, Hökfelt T, and Koistinaho J. 1998. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proceedings National Academic Sciences USA 95 (26):15769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Fan C, Du J, Jiang H, Chen H, and Sun H. 2012. Cue-induced craving and physiological reactions in recently and long-abstinent heroin-dependent patients. Addictive Behaviors 37 (4):393–98. doi: 10.1016/j.addbeh.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra F, Veltman DJ, Booij J, van den Brink W, and Franken IH. 2009. Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug and Alcohol Dependence 99:183–92. doi: 10.1016/j.drugalcdep.2008.07.012. [DOI] [PubMed] [Google Scholar]


