Abstract
This article provides a description of the rationale and processes adopted by the Gulf Coast Health Alliance: Health Risks related to the Macondo Spill consortium to evaluate and communicate the risk of exposure to polycyclic aromatic hydrocarbons (PAHs) in seafood over several years following the Deepwater Horizon disaster and subsequent oil spill. We examined gaps in knowledge associated with PAH toxicity following exposure to petrogenic (oil-derived) PAHs by studying the metabolic fate of PAHs and their potential toxicity using sophisticated analytical methods. Using the data generated, we developed a risk communication strategy designed to meet the needs of the stakeholder communities including a consumption guideline calculator, a web-based tool to reconcile seafood consumption with risk of adverse health effects.
Keywords: risk communication, Deepwater Horizon, seafood safety
Introduction
The Gulf of Mexico is a rich resource not only for the petrochemical industry but also for both commercial and recreational fishing. Oil and gas extraction represent about $39.8 billion in annual economic activity,1 while in 2012 alone, commercial fishermen operating in the Gulf Region landed 1.7 billion pounds of finfish and shellfish, representing $763 million in revenue.2 Likewise, recreational fishing contributes billions of dollars to the Gulf coast state economies. A major oil spill highlights the potential for conflict between these respective economic drivers—as illustrated by the aftermath of the Macondo Deepwater Horizon (DWH) oil spill in 2010, which shut down fishing for weeks to months, depending on location, due to concerns about seafood safety for human consumption.3,4
Polycyclic aromatic hydrocarbon
Prior to the DWH disaster, few studies documented the effects of oil spills on human health, and those that exist primarily examined the acute effects and/or mental health symptoms. A review by Aguilera et al.5 provides an overview of relevant studies. Studies also focused on exposure through consumption of contaminated seafood; for example, studies with mussels contaminated by oil from the tanker Erika in 1999 demonstrated that polycyclic aromatic hydrocarbons (PAHs) could bioaccumulate in seafood and also elicit toxic responses in human cell lines.6 The PAHs are widespread organic pollutants containing two or more aromatic rings with varying toxicities among the individual PAH compounds (Figure 1), although increased toxicity correlates with the more persistent high molecular weight species (those with three or more benzene rings). The PAHs represent between 0.2% and 7% (depending on location) of crude oil,7 and estimates of the duration of elevated PAH levels in seafood following previous oil spills have ranged from a few weeks to several years. Unsubstituted PAHs alone compromise over 100 different compounds, but many known chemical substitutions on the PAHs substantially increase this number, in particular the oil-derived (petrogenic) PAHs that are heavily alkylated (contain one or more alkyl groups), or become oxygenated due to the weathering of oil.
Figure 1.
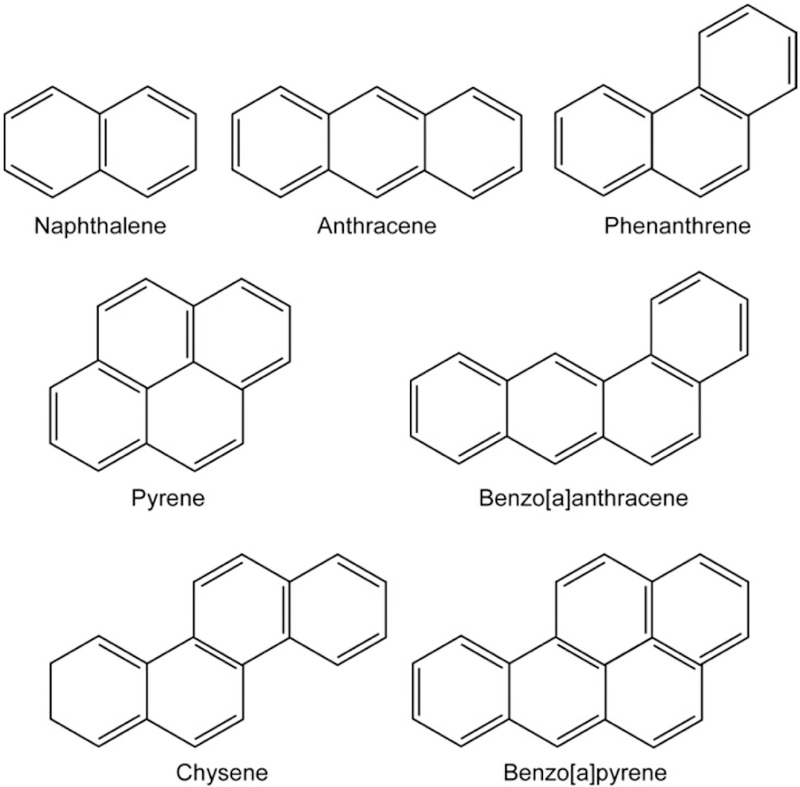
Polycyclic aromatic hydrocarbons are aromatic hydrocarbons—organic compounds consisting of only carbon and hydrogen—composed of multiple aromatic (benzene) rings, ranging from as few as two rings (Naphthalene), to larger multi-ring structures such as Benzo[a]pyrene (B[a]P). The examples presented constitute unsubstituted PAHs.
A major identifiable health risk associated with oil spills is exposure to the petrogenic PAHs. This concern exists because pyrogenic PAHs derived from incomplete combustion of fossil fuels, for example, benzo[a]pyrene (B[a]P), are ranked as Group 1 or known human carcinogens by the International Agency for Research on Cancer.8 Pyrogenic PAHs also have health effects unrelated to cancer end points and include reproductive and developmental toxicity and immunosuppressive effects.9 Petrogenic PAHs differ in structure from pyrogenic PAHs due to alkylation and oxygenation and may confer different toxicities. Exposure to petrogenic PAHs can occur occupationally, for example, oil industry workers, clean-up workers, first responders, and fisher folk including those involved in the Vessels of Opportunity program during the DWH disaster; and through entry into the food chain and subsequent consumption of contaminated seafood.
Limits of existing risk assessment methods
A university-community consortium was established that co-created Gulf Coast Health Alliance: health Risks related to the Macondo Spill (GC-HARMS) using community-based participatory research (CBPR) principles to address seafood safety after the DWH oil spill. At that time, regulatory agencies assessed seafood safety by a combination of sensory and chemical methods. Seafood samples that smelled of petroleum were considered unsafe, while batches of seafood that passed this smell test were analyzed further by gas chromatography/mass spectrometry after being spiked with internal standards to correct for losses during sample preparation and analysis. Compounds measured included seven carcinogenic PAHs and five noncarcinogenic PAHs and incorporated representative alkylated derivatives found in the oil.10 Levels were then summed using a Toxic Equivalency Quotient based on B[a]P (e.g., chrysene which has 1/1000th of the carcinogenicity of B[a]P is given a value of 0.001).11 When these summed levels dropped below the level-of-concern for B[a]P food safety, fisheries were reopened. There are several weaknesses of the approach. First, the known carcinogenic PAHs are predominately pyrogenic and not present in the oil-spill to begin with. Second, there is substantial disagreement between the level-of-concern for B[a]P in food between U.S. regulatory agencies (U.S. Food and Drug Administration [FDA] limit 35–132 μg B[a]P/Kg for finfish and shrimp) and the European Economic Community (2.0–10 μg B[a]P/Kg for finfish and shrimp).12–15 Third, the Toxic Equivalency Quotient for petrogenic PAHs is largely unknown because their toxic properties have not been elucidated. Fourth, many of the alkylated PAHs were not measured as single entities raising concerns about the reliability of the analytical method, and the potential masking of individual PAH congeners thus precluding their detection. Fifth, no oxygenated PAHs were measured as part of the risk assessment. Hence, the current approach, which relies on detecting every PAH congener in a complex mixture and factoring in the Toxic Equivalency Quotient of each congener (if even known) to determine the Level-of-Concern, is apt to underestimate the actual toxicity of PAH mixtures.16–18
Petrogenic PAH metabolism
To improve the risk assessment and improve hazard characterization, we measured the metabolism of alkylated and oxygenated petrogenic PAHs present in the oil using commercially available (ATCC) human liver cells (HepG2) and human small intestine cells (CaCO2) to mimic exposure to oil contaminated seafood through ingestion. The alkylated petrogenic PAHs studied were compounds that contained either three rings: 1-methyl-phenanthrene (C1) and 9-ethyl-phenanthrene (C2)19; 1-methyl-7-isopropyl-phenanthrene also known as retene (C4)20; or four rings: 5-methyl-chyrsene (C1)21; 6-ethyl-chrysene (C2, where C1 to C4 refers to the number of alkyl groups present)22; and phenanthrene-9,10-dione (an oxygenated petrogenic PAH).23 Studies were performed using concentrations of petrogenic PAHs detected in the seafood. Profiles of metabolites were detected using high-performance liquid chromatography with photodiode array and fluorescence detection. The identity of metabolites was further established by liquid chromatography-ion trap mass spectrometry; and in some instances with liquid chromatography-Q-Exactive hybrid quadrupole high-resolution mass spectrometry which gave exact mass to 2 ppm. Compounds were further identified by tandem mass spectrometry and/or by reference to authentic synthetic standards.
These studies showed that the petrogenic PAHs were rapidly metabolized (changed into other chemicals), since no starting material could be detected after 24 h. Thus, risk assessment based on the measurement of petrogenic PAHs in human biospecimens (urine or serum) to determine levels of oil exposure in population-based studies represent a flawed strategy. For each of the aforementioned petrogenic PAHs studied, we found evidence for the formation of reactive metabolites that could damage DNA and water soluble metabolites that could be excreted. We also identified unique bifunctional reactive metabolites that have the potential to cross-link protein and DNA. Studies with retene—one of the most abundant PAHs in the Macondo well oil (Figure 2)—were most compelling since this petrogenic PAH forms these bifunctional reactive metabolites (Figure 3). The presence of reactive metabolites and their downstream water soluble—and thus readily excretable—metabolites suggests that health effects of petrogenic PAH exposure represents a balance between metabolite toxicity and detoxification processes. Whichever pathway predominates can be influenced by genetic variation in the pathway genes and environmental factors that may affect expression of those genes in the human population. Consequently, petrogenic PAH toxicity may vary considerably between individuals. However, unique petrogenic PAH metabolites identified in our cell-based studies could be used as potential human biomarkers of exposure to oil spills on an individual basis and be correlated with health effects measured longitudinally and thus inform future risk assessments.
Figure 2.
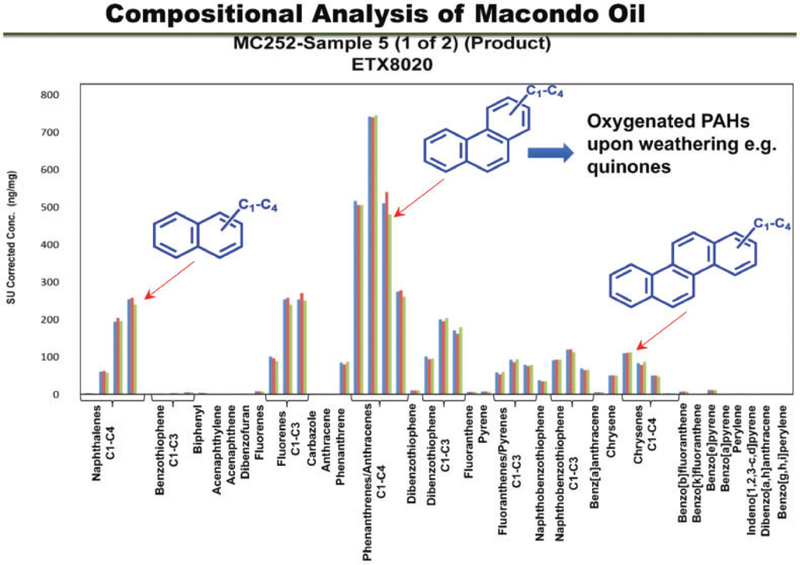
Compositional analysis of the macondo oil. From left to right, PAHs of increasing ring number; C1–C4 refer to the number of alkyl groups present on an individual PAH in ascending order; bars represent concentration of a C1–C4 PAH within that ring series. Representative compound structures are shown in the inset. Notice the low levels of carcinogenic PAH measured in the original safety assessment: benzo[a]pyrene, dibenz[a,h] anthracene, indeno[1,2,3]-dipyrene, benzo[b]flouranthene, benzo[k]flouranthene, and chrysene.
Figure 3.
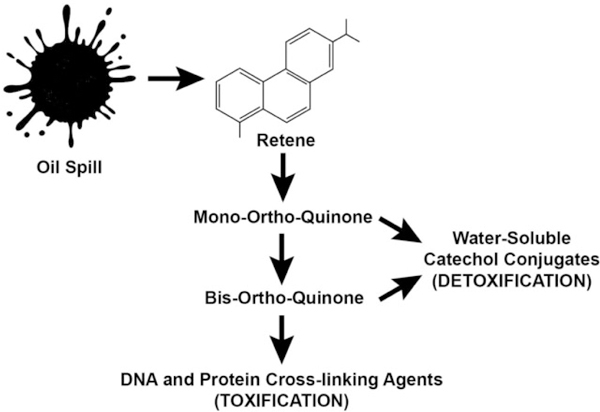
Schematic showing the conversion of retene. Retene is a C4-phenanthrene converted to reactive mono-ortho quinones and bis-ortho-quinones observed in human liver (HepG2) cells. The bis-ortho-quinones have the potential to act as DNA and protein crosslinking agents (toxic); and formation of water-soluble catechol conjugates (detoxification). Adapted from Huang et al.20
Evidence-based risk assessment biomarker development
To this end, investigators at the University of Texas Medical Branch collected human urine samples from four cohorts that were estimated to have markedly different levels of exposure to contaminated seafood and hence petrogenic PAHs. Cohort 1: Gulfport, Mississippi (Vietnamese American Community Partner) is the high-exposure group where the Vietnamese fisher folk not only consume the seafood they catch as part of their stable diet, they eat all portions of the organisms including the soft organs known to bioaccumulate petrogenic PAHs. Cohort 2: Biloxi, Mississippi (Center for Environmental & Economic Justice) represents a predominantly African-American community that also consumes the harvested seafood but refrains from eating the potentially contaminated soft organs. This community shares the geographic locale occupied by the Vietnamese fisher folk. Cohort 3, Southeast Louisiana (United Houma Nation) constitutes a low-exposure group since incursion of the oil along this part of the Gulf coast was proportionally less; and Cohort 4, Galveston, Texas, was originally chosen as a control comparison group since this region experienced no direct impact of the DWH oil spill. Each cohort consisted of up to 100 individuals: 50 adults 20 to 55 years of age, 25 > 55 years of age and 25, between the ages of 5 to 19 years. Each participant was followed for three years.
In a convenience sample of 10 individuals from these cohorts, we found the presence of three petrogenic PAH metabolites identified through our cell-based work, in the urine of donors. These biomarkers were absent from the urine of 10 archival age- and gender-matched subjects from Philadelphia who had no history of exposure to contaminated seafood. It is worth noting that the Philadelphia (control) samples included only male samples, hence we used male (gender-matched) samples from our Gulf community cohorts. We have no evidence to suggest that the metabolic fate of the PAHs differ in females. Analysis suggested that these biomarkers had greater than 90% specificity (i.e., identify only those individuals exposed to oil) and 90% sensitivity (i.e., identify all individuals exposed to oil) and could discriminate between exposed and non-exposed groups. These studies are currently undergoing a validation study using 40 individuals from the GC-HARMS study and 40 individuals from the archival set. If the validation study is positive, these biomarkers could be used to specifically assess exposure to petrogenic PAHs in future epidemiological studies.
Measuring PAH bioactivity in seafood extracts
Given the complexity of the petrogenic PAH profile in crude oil (Figure 2), their metabolic fates following uptake by marine organisms ultimately consumed as seafood, and the lack of toxicity data, including Toxic Equivalency Quotient values for the petrogenic PAHs, we opted to use an effect-directed analysis involving the Chemical Activated LUciferase gene expression (CALUX) bioassay. The CALUX assay is based on a well-characterized biochemical pathway that responds to bioactive PAHs frequently associated with multiple toxic responses,18,24 the CALUX assay itself does not directly assess toxicity. The CALUX bioassay is an Environmental Protection Agency-approved low-cost, high-throughput method for detecting the presence of halogenated aromatic hydrocarbon and PAH contaminants in myriad different sample types.25 Assay measurements compare readouts generated with PAH extracted from seafood samples to a standard curve of the reference compound B[a]P. The advantage of the CALUX assay over the Toxic Equivalency Quotient-based chemical analysis is that the former integrates the action of every bioactive PAH in an extract without depending on an a priori knowledge of the Toxic Equivalency Quotient for each PAH in the mixture.
Using the CALUX bioassay, we obtained relative potency values—measured as B[a]P equivalents—for PAH mixtures extracted from 432 seafood samples caught at 208 distinct locations along the Gulf coast over a four-year period (Table 1). The assessment of the B[a]P equivalents using the seafood PAH extracts revealed that PAH contamination was increased in the immediate period following capping of the well head and decreased to a sustained basal level by early 2011 (Figure 4), with oysters exhibiting the highest PAH levels (Figure 5), consistent with previous observations.26 The absence of pre-spill baseline information precludes comparison of the pre-and post-spill PAH contamination in seafood, but the levels detected after August 2011 are below the Level-of-Concern for cancer risk based on consumption patterns for the 90th percentile seafood consumers using the 2005–2006 National Health and Nutrition Examination Survey study. Using the cancer risk calculation and the parameter values described by Gohlke et al.,4 we determined a maximum daily B[a]P equivalents oral exposure value (ng B[a]P equivalents/kg body weight/day) for Gulf of Mexico near-shore seafood. This maximum B[a]P equivalents oral exposure value was used to develop a proposed seafood consumption guideline calculator (https://www.utmb.edu/scg/) that integrates several variables, including empirically derived seafood B[a]P equivalents (in ng/g seafood for finfish and shellfish), body weight (in pounds), duration of exposure (in years), risk level (ranging from a high of one-in-ten-thousand to a low of one-in-a-million increase in cancer incidence), and frequency of seafood consumption (in days/week). Conceptually, the consumption guideline calculator was designed for use as a personal seafood consumption guideline based on an individual’s risk tolerance, consistent with messaging expectations of our community partners. As with any risk assessment, it is important to note these consumption guidelines do not account for other sources of PAH contamination, which may be considerable depending on individual diet, tobacco use, local environment, and a plethora of other lifestyle choices. For example, there are reports that grilled and smoked food, in particular meats, can contain myriad PAHs; this includes commercially available smoked meat products.7,27,28 Studies have also shown that cigarette smoke is another substantial source of PAH contamination.29 Consequently, these other sources of PAH exposure should ideally be factored in when addressing an individual’s seafood consumption. The consumption guideline calculator focused exclusively on cancer risk, not other potential PAH-induced adverse health outcomes.
Table 1.
Complete information for each site used in the study, including the number of samples for each species and location, and date of collection.
| No. of samples | |
|---|---|
| Date | |
| 8/3/10–2/2/11 | 26 |
| 8/3/11–2/2/12 | 67 |
| 2/3/12–8/2/12 | 78 |
| 8/3/12–2/2/13 | 159 |
| 2/3/13–8/2/13 | 67 |
| 8/3/13–2/3/14 | 35 |
| Species | |
| Crab | 64 |
| Fin fish | 43 |
| Oyster | 121 |
| Shrimp | 204 |
| Location | |
| Alabama | 89 |
| Galveston | 32 |
| Louisiana | 238 |
| Mississippi | 73 |
| Total samples | 432 |
Figure 4.
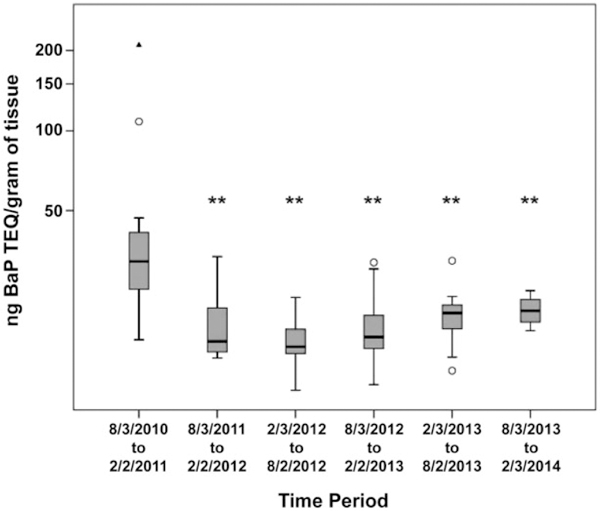
PAH contamination in Gulf seafood over time. Box plots of PAH levels as B[a]P Toxic Equivalency Quotients in nanograms per gram of tissue in all species, regardless of collection location, over time. Horizontal line in the box denotes the median, and the upper and lower limits of the box represent the third and first quartiles, respectively. Asterisks indicate statistically significant differences (**p <.01) in PAH levels compared to the first collection period (8/3/2010–2/2/2011). Open circles denote extreme values that were included in the statistical analyses. The solid triangle represents an outlier not used in the analyses.
Figure 5.
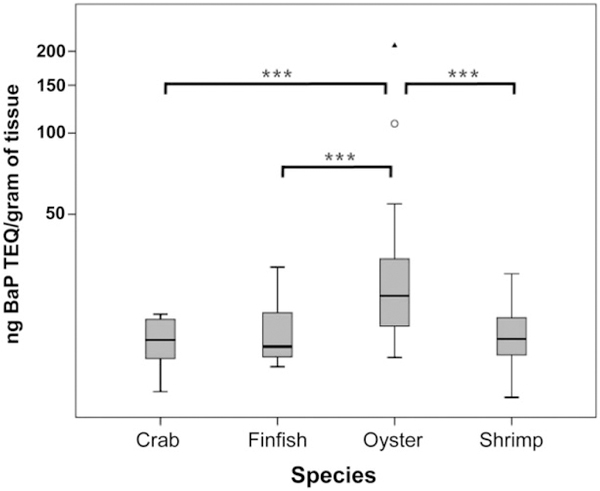
PAH levels in Gulf seafood by species. Box plots of PAH levels as B[a]P Toxic Equivalency Quotients in nanograms per gram of tissue in Gulf seafood by species compiled from all the samples collected across all locations and times. Horizontal line in the box denotes the median, and the upper and lower limits of the box represent the third and first quartiles, respectively. Open circles denote extreme values that were included in the statistical analyses. The solid triangle represents an outlier not used in the analyses. Significance is indicted by asterisk, ***p <.005.
Communicating risk assessment
Risk assessment as described by the National Academies red book has four elements: risk identification, risk characterization; risk management; and risk communication. In subsequent iterations, it also included vulnerable populations, for example, women and children.30 Using this rubric, we can ask where do we stand in risk assessment of large oil spills to human health. Risk characterization requires estimates of the Margin-of-Exposure to petrogenic PAHs. The Margin-of-Exposure is calculated using the Level-of-Concern (the level which if exceeded would cause harm in humans) divided by the estimates of exposure. The larger the Margin-of-Exposure, the greater the safety factor. The Level-of-Concern, and thus risk, of an adverse health outcome can vary depending on the end point chosen, be it cancer or a non-cancer end point.
Risk perception is a subjective evaluation of potential for harm that aids people in negotiating the uncertainties and potential dangers of life.30 Communicating risk must incorporate risk perception including both cognitive and emotional components in order to be effective. Communicating risk in the context of the GC-HARMS project posed challenges in that the science was evolving and included a fair degree of uncertainty. In addition, the target audiences spanned a large geographic area covering Louisiana, Mississippi, and Alabama. In our case, the affected Gulf communities included Vietnamese, native American, African American, and white communities. These communities represented substantially different demographics with distinct fish consumption and preparation habits that were informed by cultural values and practices. Clearly, a single method of risk communication would not suffice for all groups.
Risk communication strategies needed to be tailored, and we recognized that we did not inherently possess the skills and cultural understanding to accomplish this alone. From the outset, a CBPR process was used. We engaged key community leaders as partners in the entirety of the research process.31 We recognized that the success of the project required bi-directional engagement by all stakeholders. Together with the community partners, we established goals, processes, outcomes, and communication strategies. Just as we hoped to become more culturally literate, we recognized that we needed to develop the communities’ environmental health literacy. We engaged the fishermen from each community and taught them how to process seafood samples for study from their catch and secure a chain of custody of the samples. In so doing, they learned about the scientific method, the toxicological principles, and developed trust in the process. The fishermen received results of their specific catch and became well informed and trusted local science resources for their communities.
From the beginning, our community partners had specific questions in mind. Is the seafood safe to eat and sell? What are the risks of exposure to petrogenic PAHs? One of the challenges for our research project was the dearth of information regarding the toxicity of petrogenic PAHs. The GC-HARMS program was unique among the National Institute of Environmental Health Sciences DWH consortia components by studying the bioactivity of the petrogenic PAH mixtures extracted from seafood samples, and the metabolic fate of the PAHs including the potential toxicity of the metabolites. As a consequence, our findings produced a more nuanced risk assessment message distinct from that voiced by others who, based solely on seafood PAH levels in the absence of Toxic Equivalency Quotient data, determined that the seafood was safe for consumption. Communicating this difference and the rationale for the distinction was critical. With partners in place, key principles of risk communication were employed to design the risk communication messages. Nevertheless, the message needed to incorporate scientific uncertainties, including unknown individual PAH body burdens due to seafood consumption and the potential for PAH exposures from other sources, remained one of the most important challenges to our efforts. People dislike uncertainty, so the risk communication needed to account for this. It is not that people are unwilling to accept risk, sometimes they are willing to accept great risk because they understand it and they recognize great value and benefit from the risky endeavor. However, when the risk is less well understood or the benefit is less clear, people become more concerned about the risk.32 Risk perception of unfamiliar risk can lead to a variety of actions depending on the individual. People may ignore unfamiliar risks, feel apathetic about them and take no action on their own behalf. Conversely, people may worry excessively about an unfamiliar risk and adopt counter-productive responses.33
Our goal in risk communication was to encourage positive action by providing an understanding of risk of harm to health without engendering fear that they might abandon fish consumption. The challenge for effective risk communication is to move risk perception out of the realm of apathy or panic to a level of interest and understanding that promotes a more constructive action. There are a number of characteristics of risk that can lead individuals to overestimate the risk by engaging the emotional aspects of risk perception. These include sources of risk that are industrial, controlled by others, handled in an unreliable way, or handled by individuals or an organization that has already proven to be unreliable. They are often risks that are unknown or involuntary. The perception of risk by residents of the Gulf coast affected by the DWH oil spill was influenced by the characteristics of risk that would tend to enhance the emotional aspects of risk perception. The spill was industrial, clean up would be managed by a large foreign company under the guidance of government agencies, and conflicting information was being provided to the population through a variety of sources regarding recommendations and risk estimates (FDA, EPA, state, and local health agencies). The individual’s value system, including respect for others’ welfare and feelings, a sense of fairness, and responsibility that are communicated by both words and actions drive risk perception. Hence, discrepancies in the information, a lack of communication or inadequate communication, and communication deemed untrustworthy tended to offend values. As a result, public perception that risk is not managed in a responsible manner may result in outrage. In our stakeholder Gulf coast communities, a number of factors tended to increase the likelihood of outrage. Many of the communities were marginalized due to race, ethnicity, language, education, or socioeconomic status. They were already environmentally overburdened by heavy industry, recent natural disasters (hurricanes Katrina, Rita, and Gustav), and they often lacked access to physicians with environmental exposure expertise to help them differentiate symptoms associated with chemical exposures from symptoms due to other causes.34
When designing the content for the risk communication message for our seafood study, we needed to clarify that the information we were providing was specific for one group of contaminants—the seafood-derived petrogenic PAHs. No other contaminants were tested in our study, although individuals should consider other contaminants—most notably heavy metals such as mercury—when making a determination about safe seafood consumption practices. We became aware that there were very specific cultural and regional patterns of seafood consumption that fluctuated seasonally and often exceeded recommended portion sizes. Our goal was to avoid unnecessary fear or unrealistic reassurance while still considering vulnerable populations, such as pregnant women, young children, and people with medical problems who may be impacted. At the same time, it was very important to consider the economic burden on individuals whose livelihood was dramatically affected by the oil spill. The cultural impact of consumption restrictions often needed to be considered given the heavy reliance on seafood for some cultures. One of the harms that we sought to avoid was instilling excessive concerns leading to cessation of all seafood consumption, which is known to be harmful for pregnant women in particular. We identified the following key messages:
Some petrogenic PAHs in oil are hazardous to people.
Some petrogenic PAHs change into other chemicals that are hazardous to people.
Some people may be more susceptible than others to the harmful effects.
Standard tests used for petrogenic PAHs do not detect the metabolites that may be harmful.
Seafood testing over several years after the spill detected a stable level of petrogenic PAHs that is reflective of the pre-spill PAH levels detected through the NOAA Mussel Watch program.
Oil continues to be released into the Gulf through natural events (oil seeps) and from anthropogenic activities including oil industry transportation processes and urban runoff.
Conclusion
We endeavored to provide our community partners with the insights that would allow them to identify safe seafood consumption practices. We reinforced the understanding that seafood is an important part of a healthy diet, and we generated a Web-based consumption guideline calculator to help individuals determine a safe amount of seafood based on a calculable cancer risk. Several principles of toxicology were incorporated into the calculator, including body weight, frequency and duration of consumption, and the self-selected risk level. We used the Paling Perspective Scale35 to help communities conceptualize the risk of eating seafood with lifetime risks associated with consuming other familiar items such as alcohol, peanut butter, and hamburgers. In addition, the calculator uses empirically derived toxic equivalences in the various shellfish and finfish in the Gulf of Mexico, hence its use is only applicable to seafood harvested in the Gulf coast region. Moreover, the seafood calculator was described to community members as a dynamic tool that will evolve and be revised as our toxicological understanding of other adverse health effects due to petrogenic PAH exposure matures.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by the Center for Environmental Toxicology and the Sealy Center for Environmental Health and Medicine at the University of Texas Medical Branch, and the Center of Excellence in Environmental Toxicology, Perelman School of Medicine, University of Pennsylvania, funded in part by a U19 Award (5 U19 ES020676) and P30 Core Center Awards (P30 ES006676 and P30 ES013508) from the National Institute of Environmental Health Sciences, National Institutes of Health.
Author Biographies
Daniel Jackson is a graduate from Wittenberg University and completed his postgraduate training at the University of Texas Medical Branch in the Department of Pharmacology and Toxicology. He is a postdoctoral fellow at the University of Pennsylvania studying neurodegenerative disorders.
Meng Huang is a senior scientist in Enanta Pharmaceuticals. He was a research associate at the Center of Excellence in Environmental Toxicology, Perelman School of Medicine, University of Pennsylvania. His research interests are focused on the metabolism of small molecule xenobiotics (including environmental pollutants and drugs) using liquid chromatography-tandem mass spectrometry as a tool.
Harshica Fernando is an assistant professor at Prairie View A&M University (PVAMU). Before joining PVAMU, he was a scientist at the University at Texas Medical Branch. His research interest is environmental toxicology, lipidomics, and computational chemistry.
Ghulam Ansari is a professor in the Departments of Pathology and Biochemistry & Molecular Biology, University of Texas Medical Branch, and has an active and successful research career in toxicology. His research has been funded by agencies such as the National Institute of Environmental Health Sciences, Environmental Protection Agency, National Institute of Occupational Safety and Health, World Health Organization, and the National Institute on Alcohol Abuse and Alcoholism. In this project, his expertise in analytical toxicology was utilized to analyze petrogenic polycyclic aromatic hydrocarbons in marine and plasma samples.
Marilyn Howarth is an occupational and environmental medicine physician who directs the Community Engagement Core of the Center of Excellence in Environmental Toxicology, a National Institute of Environmental Health Sciences-funded P30 Environmental Health Core Center at the University of Pennsylvania. Her work through the center includes community-engaged research and outreach aimed at improving environmental health, policy, and environmental health literacy.
Clementina Mesaros is the technical director of the Biomarker Core Facility in the Center of Excellence in Environmental Toxicology, a P30 Environmental Health Sciences Core, since 2008. She is an expert in bioorganic synthesis, liquid chromatography, mass spectrometry, and biomarker method development.
Trevor Penning is the Thelma-Brown and Henry Charles Molinoff Professor of Pharmacology and Director of the Center of Excellence in Environmental Toxicology at the Perelman School of Medicine at the University of Pennsylvania. He is an international authority on hormonal and chemical carcinogenesis. He is a Fellow of the American Chemical Society and Senior Editor for Population and Prevention Science, Cancer Research.
Cornelis Elferink graduated with a PhD from the University of Adelaide, Australia, in 1988. After postdoctoral training at Stanford University, California, he joined the faculty at Wayne State University, Michigan, in 1993 until 2001. He is currently a professor in the Department of Pharmacology and Toxicology at the University of Texas Medical Branch, Texas.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Yoskowitz DW and Montagna PA. Socio-economic factors that impact the desire to protect freshwater flow in the Rio Grande, USA. Wit Trans Ecol Environ 2009; 122: 547–558. [Google Scholar]
- 2.Lowther A (ed). National marine fisheries service, office of science and technology. Silver Springs: Fisheries of the United States, 2012, National Oceanic and Atmospheric Administration, US Department of Commerce. [Google Scholar]
- 3.Keim ME. The public health impact of industrial disasters. Am J Disaster Med 2011; 6: 265–272. [DOI] [PubMed] [Google Scholar]
- 4.Gohlke JM, Doke D, Tipre M, et al. A review of seafood safety after the Deepwater Horizon blowout. Environ Health Perspect 2011; 119: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguilera F, Mendez J, Pasaro E, et al. Review on the effects of exposure to spilled oils on human health. J Appl Toxicol 2010; 30: 291–301. [DOI] [PubMed] [Google Scholar]
- 6.Lemiere S, Cossu-Leguille C, Bispo A, et al. DNA damage measured by the single-cell gel electrophoresis (Comet) assay in mammals fed with mussels contaminated by the ‘Erika’ oil-spill. Mutat Res 2005; 581: 11–21. [DOI] [PubMed] [Google Scholar]
- 7.Xia K, Hagood G, Childers C, et al. Polycyclic aromatic hydrocarbons (PAHs) in Mississippi seafood from areas affected by the Deepwater Horizon Oil Spill. Environ Sci Technol 2012; 46: 5310–5318. [DOI] [PubMed] [Google Scholar]
- 8.International Agency for Research on Cancer Working Group. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures In IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 92 Lyon: World Health Organization IARC, 2010. [PMC free article] [PubMed] [Google Scholar]
- 9.IRIS. Toxicological review of benzo[a]pyrene. EPA/635/R-17/003Fa,cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0136tr.pdf, 2017.
- 10.Ylitalo GM, Krahn MM, Dickhoff WW, et al. Federal seafood safety response to the Deepwater Horizon oil spill. Proc Natl Acad Sci USA 2012; 109: 20274–20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delistraty D Toxic equivalency factor approach for risk assessment of polycyclic aromatic hydrocarbons. Toxicol Environ Chem 1997; 64: 81–108. [Google Scholar]
- 12.European Union Commission Regulation No 208/2005. Amending regulation (EC) No 466/2001 as regards polycyclic aromatic hydrocarbons. J Eur Union, http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=0J:L:2005:034:0003:0005:EN:PDF [Google Scholar]
- 13.FDA/ORA/DFS Laboratory Information Bulletin No 4475 screen for the presence of polycyclic aromatic hydrocarbons in select sea foods using LC-fluorescence, https://www.fda.gov/downloads/scienceresearch/ucm220209.pdf
- 14.Park J-H and Penning TM. Polyaromatic hydrocarbons In Stadler RH and Lineback ER (eds) Process-induced food toxicants; occurrence, formation, mitigation & health risks. Chap. 28, pp. 243–282. Hoboken: Wiley & Sons. [Google Scholar]
- 15.Huang M and Penning TM. Processing contaminants: polycyclic aromatic hydrocarbons (PAHs) In Motarjemi Y (ed) Encyclopedia of food safety, 2014, pp. 416–423. Waltham, MA: Academic Press. [Google Scholar]
- 16.Goldstein LS, Weyand EH, Safe S, et al. Tumors and DNA adducts in mice exposed to benzo[a]pyrene and coal tars: implications for risk assessment. Environ Health Perspect 1998; 106: 1325–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider K, Roller M, Kalberlah F, et al. Cancer risk assessment for oral exposure to PAH mixtures. J Appl Toxicol 2002; 22: 73–83. [DOI] [PubMed] [Google Scholar]
- 18.Pieterse B, Felzel E, Winter R, et al. PAH-CALUX, an optimized bioassay for AhR-mediated hazard identification of polycyclic aromatic hydrocarbons (PAHs) as individual compounds and in complex mixtures. Environ Sci Technol 2013; 47: 11651–11659. [DOI] [PubMed] [Google Scholar]
- 19.Huang M, Mesaros C, Hackfeld LC, et al. Potential metabolic activation of representative alkylated polycyclic aromatic hydrocarbons 1-methylphenanthrene and 9-ethylphenanthrene associated with the deepwater horizon oil spill in human hepatoma (HepG2) cells. Chem Res Toxicol 2017; 30: 2140–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang M, Mesaros C, Hackfeld LC, et al. Potential metabolic activation of a representative C4-alkylated polycyclic aromatic hydrocarbon retene (1-methyl-7-isopro-pyl-phenanthrene) associated with the deepwater horizon oil spill in human hepatoma (HepG2) cells. Chem Res Toxicol 2017; 30: 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang M, Zhang L, Mesaros C, et al. Metabolism of an alkylated polycyclic aromatic hydrocarbon 5-methylchrysene in human hepatoma (HepG2) cells. Chem Res Toxicol 2015; 28: 2045–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang M, Mesaros C, Zhang S, et al. Potential metabolic activation of a representative C2-alkylated polycyclic aromatic hydrocarbon 6-ethylchrysene associated with the deepwater horizon oil spill in human hepatoma (HepG2) cells. Chem Res Toxicol 2016; 29: 991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang M, Zhang L, Mesaros C, et al. Metabolism of a representative oxygenated polycyclic aromatic hydrocarbon (PAH) phenanthrene-9,10-quinone in human hepatoma (HepG2) cells. Chem Res Toxicol 2014; 27: 852–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrison PM, Tullis K, Aarts JM, et al. Species-specific recombinant cell lines as bioassay systems for the detection of 2,3,7,8-tetrachlorodibenzo-p-dioxin-like chemicals. Toxicol Sci 1996; 30: 194–203. [DOI] [PubMed] [Google Scholar]
- 25.Smith S, Schecter A, Papke O, et al. Quantitation of the extracellular domain of epidermal growth factor receptor in the plasma of dioxin-exposed individuals. Am J Indust Med 1998; 34: 1–5. [DOI] [PubMed] [Google Scholar]
- 26.Meador JP, Stein JE, Reichert WL, et al. Bioaccumulation of polycyclic aromatic hydrocarbons by marine organisms. Rev Environ Contam Toxicol 1995; 143: 79–165. [DOI] [PubMed] [Google Scholar]
- 27.Hitzel A, Pohlmann M, Schwagele F, et al. Polycyclic aromatic hydrocarbons (PAH) and phenolic substances in meat products smoked with different types of wood and smoking spices. Food Chem 2013; 139: 955–962. [DOI] [PubMed] [Google Scholar]
- 28.Chen S, Kao TH, Chen CJ, et al. Reduction of carcinogenic polycyclic aromatic hydrocarbons in meat by sugar-smoking and dietary exposure assessment in Taiwan. J Agric Food Chem 2013; 61: 7645–7653. [DOI] [PubMed] [Google Scholar]
- 29.Ding YS, Ashley DL and Watson CH. Determination of 10 carcinogenic polycyclic aromatic hydrocarbons in mainstream cigarette smoke. J Agric Food Chem 2007; 55: 5966–5973. [DOI] [PubMed] [Google Scholar]
- 30.Trust Slovic P., emotion, sex, politics, and science: surveying the risk-assessment battlefield. Risk Anal 1999; 19: 689–701. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan AAJ, Croisant S, Howarth M, et al. Building and maintaining a citizen science network with fishermen and fishing communities post deepwater horizon oil disaster using a CBPR approach. New Solut 2018; 28: 416–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slovic P Perception of risk. Science 1987; 236: 280–285. [DOI] [PubMed] [Google Scholar]
- 33.Peters E, Slovic P, Hibbard JH, et al. Why worry? Worry, risk perceptions, and willingness to act to reduce medical errors. Health Psychol 2006; 25: 144–152. [DOI] [PubMed] [Google Scholar]
- 34.Meirs KW and Howarth MV. Environmentally overburdened gulf state residents lack access to environmental specialty care. New Solut 2018; 28: 448–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paling J Strategies to help patients understand risks. BMJ 2003; 327: 745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]


