Abstract
This paper presents evidence that plant brassinosteroid (BR) hormones play a role in promoting germination. It has long been recognized that seed dormancy and germination are regulated by the plant hormones abscisic acid (ABA) and gibberellin (GA). These two hormones act antagonistically with each other. ABA induces seed dormancy in maturing embryos and inhibits germination of seeds. GA breaks seed dormancy and promotes germination. Severe mutations in GA biosynthetic genes in Arabidopsis, such as ga1-3, result in a requirement for GA application to germinate. Whereas previous work has shown that BRs play a critical role in controlling cell elongation, cell division, and skotomorphogenesis, no germination phenotypes have been reported in BR mutants. We show that BR rescues the germination phenotype of severe GA biosynthetic mutants and of the GA-insensitive mutant sleepy1. This result shows that BR stimulates germination and raises the possibility that BR is needed for normal germination. If true, we would expect to detect a germination phenotype in BR mutants. We found that BR mutants exhibit a germination phenotype in the presence of ABA. Germination of both the BR biosynthetic mutant det2-1 and the BR-insensitive mutant bri1-1 is more strongly inhibited by ABA than is germination of wild type. Thus, the BR signal is needed to overcome inhibition of germination by ABA. Taken together, these results point to a role for BRs in stimulating germination.
Because plants are not motile, the choice between continued dormancy and germination in the seed is of critical importance to plant survival. The establishment of seed dormancy in higher plants is influenced by environmental cues such as moisture, light, and temperature. Although little is known about how these cues are transduced into a physiological seed response, at least two hormones, abscisic acid (ABA) and gibberellins (GA) have been implicated (for review, see Koornneef and Karssen, 1994). Broadly speaking, ABA and GA play antagonistic roles in regulating seed dormancy and germination. ABA establishes dormancy during embryo maturation, whereas GA is needed to break ABA-induced dormancy.
Much of our understanding of the balancing control by these hormones in determining the developmental state of the seed comes from studies involving hormone biosynthetic and response mutants in Arabidopsis. ABA-biosynthetic (aba) and ABA-insensitive mutants (abi) exhibit reduced seed dormancy with the latter showing insensitivity to ABA in germination assays (Koornneef et al., 1982, 1984; Finkelstein and Somerville, 1990; Finkelstein, 1994; Léon-Kloosterziel et al., 1996). Mutants with an enhanced response to ABA (era), show increased sensitivity to ABA in germination compared to wild type. In contrast, severe mutations in genes that act early in GA biosynthesis including GA1, GA2, and GA3 cause failure to germinate (Koornneef and van der Veen, 1980). The germination phenotype of GA biosynthetic mutants can be rescued by application of GA, by introduction of a transparent testa mutation (tt) or by the introduction of an ABA biosynthetic or ABA-insensitive mutation (Karssen and Lacka, 1986; Nambara et al., 1992; Léon-Kloosterziel et al., 1996; Steber et al., 1998; Debeaujon and Koornneef, 2000). Although the relationship of GA and ABA in seed development is well established, the contribution of other hormones to these processes is unclear. Ethylene has been reported to stimulate germination and exposure of non-germinating GA auxotrophs to this gas does rescue the GA-dependent germination defect of these mutants (Karssen et al., 1989; Koornneef and Karssen, 1994). Whether ethylene can reverse ABA-induced dormancy or stimulate germination in a GA-independent manner has not been determined. In this paper we show the plant hormone brassinosteroid (BR) is also involved in the control of germination in Arabidopsis. Furthermore, the role of BR seems to be similar to GA in that it helps to break ABA-induced dormancy and stimulate germination.
BRs are a family of over 40 naturally occurring plant steroid hormones found in a wide variety of plant species (for review, see Mandava, 1988; Arteca, 1995; Yokota, 1997; Clouse and Sasse, 1998). BRs have been recovered from virtually every plant tissue but are most plentiful in the pollen and seeds (Arteca, 1995; Schmidt et al., 1997). The chemical purification of brassinolide (BL), the active component of BR, has allowed the assignment of this hormone to a diverse variety of physiological responses including cell elongation, reduced root elongation, leaf bending and unrolling (epinasty), pollen tube growth, thermotolerance, and induction of ethylene biosynthesis (Mandava, 1988; Arteca, 1995; Salchert et al., 1998; Dhaubhadel et al., 1999). Recent genetic evidence has further uncovered a role for BRs in control of skotomorphogenesis, the process by which etiolated seedlings grow a long hypocotyl, develop an apical hook, and fail to expand their cotyledons in the dark. Mutants deficient in BR biosynthesis or response fail to establish skotomorphogenesis and, therefore, develop similarly to light-grown plants (for review, see Clouse and Sasse, 1998; Schumacher and Chory, 2000). In the light, BR mutants share a number of phenotypes with GA mutants in that they are dwarfed and show reduced male fertility. These shared phenotypes led to the misclassification of two pea BR mutants as GA insensitive (Reid and Ross, 1989; Nomura et al., 1997). This study makes use of mutants in two BR genes, DET2 and BRI1. DET2 encodes a steroid 5 α-reductase required for BR biosynthesis (Li et al., 1996; Noguchi et al., 1999). BRI1 encodes a Leu-rich repeat receptor kinase and is a good candidate for a BR receptor (Li and Chory, 1997; Friedrichsen et al., 2000; He et al., 2000).
This study reports the observation that BRs stimulate germination. This observation was made while determining whether the GA-insensitive mutant sleepy1 (sly1) was involved in any aspect of BR-regulated development. We previously determined that sly1 mutants do not show a de-etiolation response in the dark and that their dwarf phenotype is not BR-rescued (Steber et al., 1998). This is compelling evidence that sly1 is not a BR mutant. In the course of this study, we found that BR stimulates the germination and hypocotyl elongation of sly1 and of GA biosynthetic mutants. Moreover, BR can be used to identify sly1 mutants in a screen for BR-dependent germination. Although no previous BR mutants have been shown to have a germination phenotype, this observation raised the question of whether BRs play a role in the Arabidopsis germination response. Here we present evidence that the BR biosynthetic mutant det2-1 and the BR-response mutant bri1-1 are more susceptible to inhibition of germination by ABA than wild type. The discovery of this germination phenotype in BR mutants suggests that the BR signal is required to reverse ABA-induced dormancy and stimulate germination in Arabidopsis.
RESULTS
BRs Rescue the Germination of GA Mutants
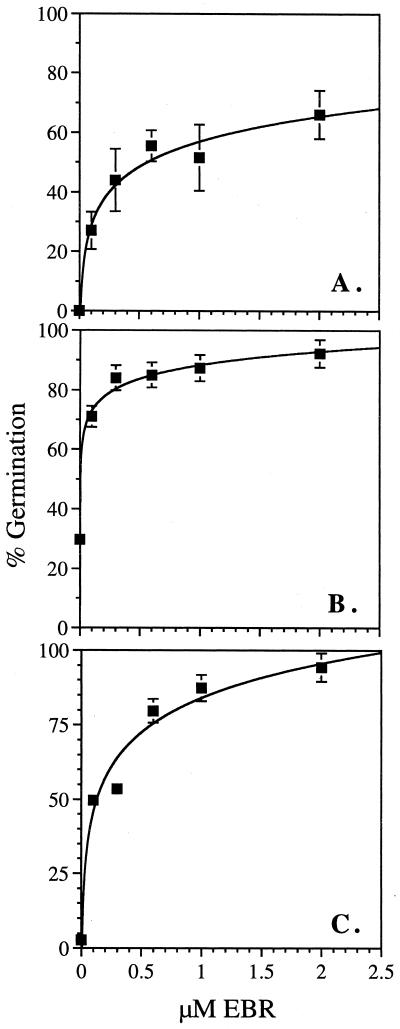
Severe GA biosynthetic mutants fail to germinate in the absence of exogenous GA hormone. To test if BR can stimulate the germination of these mutants, we measured the germination of ga1-3, ga2-1, and ga3-1 on increasing concentrations of the BR hormone 24-epibrassinolide (EBR). All seed were sterilized in a single tube so that all data points received the same treatment. Seeds were plated on Murashige and Skoog medium lacking hormone, on 10 μm GA3, and on a different concentrations of EBR ranging from 0.1 to 2 μm. Germination was scored after 5 d. As expected, 100% germination was observed on the 10-μm GA3 plate. It is interesting that EBR showed a concentration-dependent rescue of the germination phenotype (Fig. 1). At 2 μm EBR an average germination rate of 66% was observed in ga1-3, 92% in ga2-1, and 91% in ga3-1. This can be compared with an average germination rate of 0%, 30%, and 2% in the absence of hormone, respectively. To confirm that rescue by EBR is not due to impurities, the experiment was repeated for ga1-3 with ultrapure BL (a gift from T. Yokota). BL also rescues ga1-3 germination. For a single seed lot, 0.1 μm BL resulted in 30% germination, whereas 0.1 μm EBR resulted in 24% germination.
Figure 1.
Rescue of germination in GA biosynthetic mutants by EBR. Percent germination of ga1-3 (A), ga2-1 (B), and ga3-1 (C) on increasing concentrations of EBR. Germination was scored for 30 to 60 seeds per hormone concentration after 4 d at 4°C followed by 5 d at 22°C. Error bars indicate se for average of four independent experiments for A, and three independent experiments for B and C.
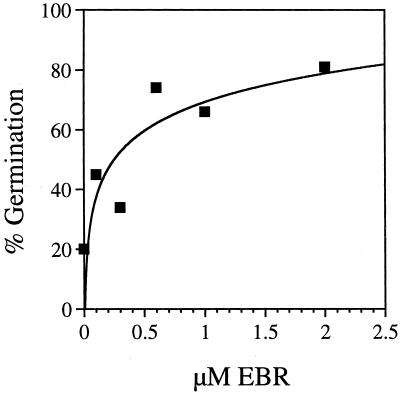
To test if EBR also rescues the germination of the GA-insensitive mutant sleepy1-2 (sly1-2), sly1-2 seeds were plated on an EBR concentration gradient. In the experiment shown in Figure 2, sly1-2 germinated at 81% on 2 μm EBR, as opposed to 20% in the absence of hormone. The observation that BR promotes germination of GA mutants suggests that BRs play a role in the decision to germinate.
Figure 2.
Rescue of sly1-2 germination by EBR. Percent germination is shown on increasing concentrations of EBR. Germination was scored for 30 to 60 seeds per hormone concentration.
This result predicts that GA-insensitive mutants may be recovered based on EBR-dependent germination. To test this notion, a total of approximately 9,000 fast-neutron mutagenized M2 seed from nine pools were plated on Murashige and Skoog medium. After 5 d under lights un-germinated seed were transferred to medium containing 1 μm EBR. Of 40 M2 candidates that germinated on EBR, a single mutant retested for the EBR-dependent germination phenotype in the M3. This candidate resembled ga1-3 in that it had flat dark green leaves and was partly infertile. However, none of these phenotypes was rescued by GA4 or by EBR. Subsequent segregation and complementation test data indicated that this was a new allele of SLY1, designated sly1-10 (data not shown). Thus, EBR provides a new method for identifying GA-insensitive mutants causing failure to germinate.
BRs Stimulate Hypocotyl Elongation of GA Mutants But Do Not Rescue Dwarfism
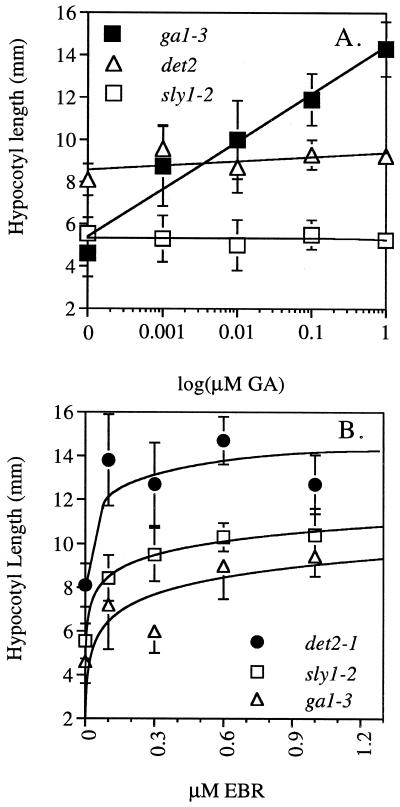
The observation that BRs rescue the germination phenotype of GA mutants raises the question of whether BRs do so by breaking dormancy, or by stimulating cell elongation. The capacity of BR to stimulate cell elongation can be quantified by assaying hypocotyl elongation in the dark (Li et al., 1996; Szekeres et al., 1996). Dark-grown hypocotyls are used to avoid the inhibition of cell elongation by light. We examined the effect of EBR and of GA4 on elongation of dark-grown hypocotyls of det2-1, ga1-3, and sly1-2. The BR biosynthetic mutant det2-1 was included in this experiment as a positive control since EBR has been demonstrated to cause elongation of det2 hypocotyls in the dark (Li et al., 1996). Wild-type Arabidopsis ecotypes Landsburg erecta (Ler) and Columbia (Col), det2-1, ga1-3, and sly1-2 seeds were sterilized and plated on medium without hormone and on increasing concentrations of EBR and GA4. After 4 d of incubation at 4°C followed by 10 d at 22°C in the dark, the average length of hypocotyls was determined (Fig. 3).
Figure 3.
Dose response of hypocotyl elongation of dark-grown seedlings to increasing concentrations of GA (A) or EBR (B). Length of hypocotyls in millimeters is given as a function of hormone concentration. Shown are averages of 10 random hypocotyl measurements. Error bars indicate sd.
On GA4, ga1-3 cell elongation was rescued, but det2-1 and sly1-2 elongation was not. On average, wild-type Ler reached 14.9 mm in length in the absence of hormone (data not shown). A concentration of 1 μm GA4 rescued ga1-3 hypocotyl elongation giving an average length of 14.3 mm, whereas det2-1 and sly1-2 hypocotyl length reached 9.25 and 5.25 mm, respectively (Fig. 3A). This is consistent with previously published reports that BR biosynthetic mutant det2-1 is unaffected by GA (Li et al., 1996) and that sly1-2 is GA insensitive (Steber et al., 1998).
Elongation of ga1-3 and sly1-2 hypocotyls was partly rescued by EBR. As expected for the positive control, the average length of det2-1 hypocotyls was fully rescued to 14.7 mm at 0.6 μm EBR (Fig. 3B). This is comparable with the length of 16.1 mm of wild-type Col hypocotyls in the absence of hormone (data not shown). The average length of ga1-3 hypocotyls increased from 4.6 mm without hormone to 9.4 mm at 1 μm EBR. The average length of sly1-2 hypocotyls increased from 5.5 mm without hormone to 10.4 mm at 1 μm EBR. These lengths are 64% and 71%, respectively, of the maximum length seen in EBR-rescued det2-1 hypocotyls. Hence EBR can stimulate hypocotyl elongation of GA mutants but does not fully rescue hypocotyl elongation. This suggests that stimulation of cell elongation may be one mechanism by which EBR stimulates germination of GA mutants.
The result above was unexpected because BRs do not rescue dwarfism of light-grown GA mutants. We previously reported that BRs do not rescue the dwarfism of light-grown sly1-2 mutants (Steber et al., 1998). To confirm that BRs do not rescue the dwarfism of light-grown ga1-3 plants, we compared the effect of BL on det2-1 and ga1-3 plants. Four plants of each genotype were sprayed with water or with an aqueous solution of 1 μm BL every 3 d for 1 month. As expected, application of BL did not rescue the dwarfism of ga1-3, but did rescue elongation and unrolling of det2-1 leaves and petioles. Thus, BRs do not rescue dwarfism of light-grown GA mutant plants. Hence, the effect of EBR on the GA mutant appears to be specific to promotion of hypocotyl elongation.
BR Mutants Show Increased Sensitivity to ABA in Germination
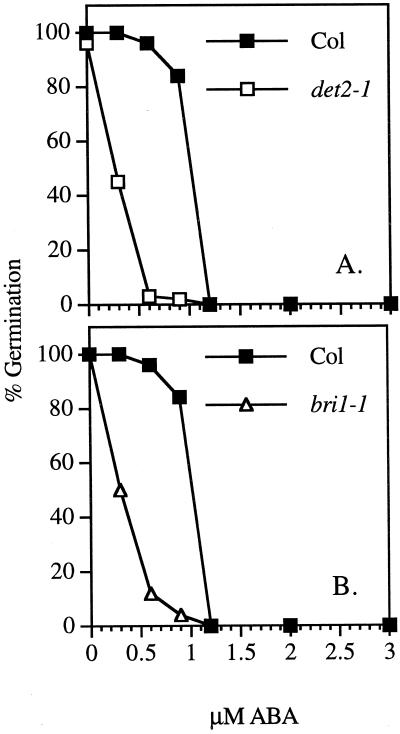
If BRs play a role in germination, one would expect BR mutants to show a germination phenotype. Paradoxically, all known BR mutants germinate well (for review, see Clouse and Sasse, 1998). We reasoned that if BR is needed to stimulate germination, BR mutant seed germination might be inhibited by lower concentrations of ABA than wild-type seed. To test this hypothesis, we examined the germination of det2-1 and bri1-1 on increasing concentrations of ABA. The results shown in Figure 4 indicate that both det2-1 and bri1-1 have increased sensitivity to ABA in germination compared with wild-type Col. Both det2-1 and bri1-1 germination were significantly inhibited at 0.6 μm ABA, whereas Col germination was not significantly inhibited until 1.2 μm ABA. After 5 d at 0.6 μm ABA, det2-1 showed 3% germination, bri1-1 showed 12% germination, and wild-type Col showed 95% germination. Thus, BR biosynthesis and sensitivity is needed to antagonize inhibition of germination by ABA.
Figure 4.
Dose-response of germination to ABA. Compares percent germination of Col wild-type (▪) to det2-1 (□) (A) and of Col wild-type to bri1-1 (B). Percent germination was determined for over 50 seeds for det2-1 and for over 30 seeds for bri1-1.
DISCUSSION
Are BRs Required for Germination?
This paper presents evidence that BRs play a role in germination. In Arabidopsis, ABA is required to set up seed dormancy during embryo maturation, whereas GA is required to break dormancy and to germinate (Koornneef and Karssen, 1994). We propose that BRs are also needed to antagonize seed dormancy and stimulate germination. This is based on two observations: BR partly rescues the germination of GA biosynthetic and GA insensitive mutants (Figs. 1 and 2), and BR biosynthesis and response mutants show increased sensitivity to ABA in germination (Fig. 4).
While highly suggestive, the observation that BR stimulates the germination of GA mutants does not prove a role for BRs in germination. For example, EBR-stimulated germination may be a response to BR overdose since we do not know if BR application results in physiological hormone levels. Further, none of the mutants in BR biosynthesis or response have a germination defect. It is possible that the BR mutants germinate because they are leaky, or because the plant embryo compensates for reduced BR signaling either by reducing ABA biosynthesis or by increasing GA biosynthesis or sensitivity. Measuring ABA and GA levels in seeds might detect such scenarios. The strongest evidence for a role of BR in germination is the increased ABA sensitivity caused by det2-1 and bri1-1 mutations (Fig. 4). This demonstrates that decreased BR biosynthesis or response increases sensitivity to ABA in germination. Application of exogenous ABA to these seeds detects the reduced germination potential of BR mutants. This phenotype resembles that of era1. Although era1 is more sensitive to exogenous ABA than det2-1 or bri1-1, in principle, BR mutants could be identified in a screen for enhanced response to ABA (Cutler et al., 1996). The hypothesis that BR is involved in germination is supported by the presence of the BRs castasterone and 24-EBR in Arabidopsis seeds (Schmidt et al., 1997).
The observation that BR plays a role in germination has implications for the study of dormancy and germination. GA alone cannot account for all of the environmental effects on seed dormancy and germination. For example, sensitivity to GA in germination is increased by light and chilling, even in a ga1-2 mutant background (Derkx and Karssen, 1993). This stimulation of GA sensitivity in germination may be related to BR. In addition, BR gives a tool for the study of severe GA-insensitive mutants like sly1. Germination of sly1 is partly rescued by exogenous EBR, but not by GA (Fig. 2). In fact, we identified a new allele, sly1-10, in a screen for EBR-dependent germination. Finally, stimulation of seed germination by BR may be useful in efforts to preserve germplasm (Yamaguchi et al., 1987; Takeuchi et al., 1997).
Possible Mechanisms for BR Stimulation of GA Mutant Seed Germination
Germination results from breakdown of the seed coat and expansion of the embryo. Initially, BR seemed likely to act through seed coat breakdown, since EBR does not stimulate hypocotyl elongation of the GA biosynthetic mutant ga5-1 (Szekeres et al., 1996). Our data, however, show that EBR stimulates hypocotyl elongation of ga1-3 and sly1-2 in the dark (Fig. 3). Thus, partial rescue of seed germination in these mutants might be due to stimulated hypocotyl elongation. Auxin also stimulates cell elongation but does not to rescue germination of ga1-3 (Koornneef and Karssen, 1994). Thus, if BR stimulates germination via embryo expansion, this effect is likely specific to germination.
A previous study showed that ethylene gas rescues germination of ga1-1 (Karssen et al., 1989; Koornneef and Karssen, 1994). That BR stimulates ethylene production in stem tissue (Arteca, 1995) raises the question of whether BR stimulates ga1-3 germination via ethylene production. There are several arguments against this theory. First, light inhibits BR-induced ethylene production in mung bean (Mandava, 1988; Arteca, 1995). Our study shows stimulation of germination under constant light. Second, ethylene levels were not elevated in cress seedlings following BR treatment of seeds (Jones-Held and VanDoren, 1996). Finally, ethylene completely rescues ga1-1 germination, and the resulting seedlings exhibit the triple response (Karssen et al., 1989). In contrast, BR partially rescues ga1-3 germination (66%, Fig. 1), and the resulting seedlings do not show the triple response (data not shown).
Interactions between Hormone Signaling Pathways
This paper raises the possibility that BR signaling interacts with ABA or GA signaling during germination. Previous studies suggest that the BR signal transduction pathway interacts with auxin, GA, and ABA signaling (Mandava, 1988; Clouse and Sasse, 1998; Ephritikhine et al., 1999). GA antagonizes ABA in part by controlling gene expression. For example, the GA-induced gene GASTA1 is repressed by ABA in Arabidopsis (Raventos et al., 2000). A similar antagonism may exist between ABA and BR in germination. If so, this antagonism is specific to germination as ABA and BR do not have opposing effects on root elongation. Both ABA and BR inhibit root elongation. The bri1-1 mutant was previously shown to have increased sensitivity to ABA-inhibition of root growth (Clouse et al., 1996).
Partial rescue of the germination and dark hypocotyl elongation phenotypes of GA mutants by EBR raises the possibility that GA and BR interact in these physiological processes. If the GA and BR signaling pathways modulate one another, they may do so through effects on one another's biosynthesis or response. Our data suggest that BRs act in parallel or downstream of GA to regulate cell elongation and germination. BR application only partly rescues GA mutant seed germination. Thus, stimulation of germination by GA cannot result solely from increased BR biosynthesis or sensitivity. Since BR stimulates the germination of a GA-insensitive mutant sly1, it is unlikely that BR acts by increasing GA sensitivity. It is possible that BR acts by stimulating GA biosynthesis. If so, then GA should rescue the germination phenotype of BR mutants.
MATERIALS AND METHODS
Plant Material
Arabidopsis ecotypes Landsburg erecta (Ler) and Columbia (Col) were used in these experiments. The ga1-3, ga2-1, ga3-1, bri1-1, and det2-1 mutant seed were obtained from the Arabidopsis Biological Resource Center. Fast neutron-mutagenized Ler M2 seed were obtained from Lehle Seeds.
Growth Conditions and Germination Experiments
Most plants were grown under continuous fluorescent or halide light (100–150 μE m−2 s−1) at 22°C. The bri1-1 mutant was grown under a 16-h photoperiod to improve seed set. Seeds used for comparison were as close as possible in age and were between 2 weeks and 6 months old. Plants containing GA biosynthetic mutations were sprayed weekly with 10 μm GA3 to rescue fertility. Before plating, seeds were surface sterilized by incubation in 10% (v/v) bleach/0.01% (v/v) SDS for 10 min, followed by four to six washes with sterile water. Seeds were imbibed for 4 d at 4°C to encourage synchronous germination, then moved to constant fluorescent lighting (50 μE m−2 s−1) at 22°C. Percent germination was determined after 5 d. Seeds with emerging cotyledons were scored as germinated. Seeds were germinated on 0.8% (g/v) agar containing 0.5× Murashige and Skoog basal salt mixture (Sigma, St. Louis), buffered to pH 5.7 with 5 mm 2-[N-morpholino] ethanesulfonic acid (MES). Stock solutions of GA (Sigma), 24-EBR (Sigma), and BL (a gift from T. Yokota) were in ethanol. Stock solutions of ABA (mixed isoschizimers, Gibco-BRL, Cleveland) were in methanol. Plant hormones were added to autoclaved media after cooling to approximately 55°C.
Hypocotyl Elongation Assay
Seeds were sterilized as described above with the exception that seeds were treated with 20% (v/v) bleach/0.01% (v/v) SDS for 20 min to reduce fungal contamination. Germination of ga1-3 seeds in the dark was stimulated by cutting the seed coat (Telfer et al., 1997). Sterilized seeds were plated in medium containing the indicated concentration of phytohormones. Plates were wrapped in three layers of aluminum foil and kept in an opaque box in the same 22°C incubator used for the germination experiments. All plates were imbibed for 4 d at 4°C and then transferred to 22°C. After 10 d the length of 10 hypocotyls was determined for each hormone concentration, and an average calculated. Due to poor germination frequency, five to 10 hypocotyls were used for the ga1-3 in the absence of hormone. It should be noted that higher concentrations of EBR cause the hypocotyls to kink. Hence, hypocotyls were pulled straight during measurement using forceps.
ACKNOWLEDGMENTS
We would like to thank Y. Kamiya, T. Yokota, and D. Friedrichsen for helpful discussions. We would like to thank K. McGinnis and members of the McCourt laboratory for critical reading of the manuscript and T. Yokota for the kind gift of ultrapure BL.
Footnotes
This work was supported by the National Science Foundation (grant no. INT–9704233), the U.S. Department of Agriculture National Research Initiative Competitive Grants Program (award no. 1999–01809 to C.M.S.), and by a Natural Sciences and Engineering Research Council of Canada grant (to P.M.).
LITERATURE CITED
- Arteca RN. Brassinosteroids. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry, and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 206–213. [Google Scholar]
- Clouse SD, Langford M, McMorris TC. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996;111:671–678. doi: 10.1104/pp.111.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Sasse JM. BRASSINOSTEROIDS: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:427–451. doi: 10.1146/annurev.arplant.49.1.427. [DOI] [PubMed] [Google Scholar]
- Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P. A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science. 1996;273:1239–1241. doi: 10.1126/science.273.5279.1239. [DOI] [PubMed] [Google Scholar]
- Debeaujon I, Koornneef M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol. 2000;122:415–424. doi: 10.1104/pp.122.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkx MPM, Karssen CM. Effects of light and temperature on seed dormancy and gibberellin-stimulated germination in Arabidopsis thaliana: studies with gibberellin-deficient and -insensitive mutants. Physiol Plant. 1993;89:360–368. [Google Scholar]
- Dhaubhadel S, Chaudhary S, Dobinson KF, Krishna P. Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol Biol. 1999;40:333–342. doi: 10.1023/a:1006283015582. [DOI] [PubMed] [Google Scholar]
- Ephritikhine G, Fellner M, Vannini C, Lapous D, Barbier-Brygoo H. The sax1 dwarf mutant of Arabidopsis thaliana shows altered sensitivity of growth responses to abscisic acid, auxin, gibberellins and ethylene and is partially rescued by exogenous brassinosteroid. Plant J. 1999;18:303–314. doi: 10.1046/j.1365-313x.1999.00454.x. [DOI] [PubMed] [Google Scholar]
- Finkelstein RR. Mutations at two new Arabidopsis ABA response loci are similar to abi3 mutations. Plant J. 1994;5:765–771. [Google Scholar]
- Finkelstein RR, Somerville CR. Three classes of abscisic acid (ABA)-insensitive mutations of Arabidopsis define genes that control overlapping subsets of ABA responses. Plant Physiol. 1990;94:1172–1179. doi: 10.1104/pp.94.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichsen DM, Joazeiro CA, Li J, Hunter T, Chory J. Brassinosteroid-insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor Serine/Threonine kinase. Plant Physiol. 2000;123:1247–1256. doi: 10.1104/pp.123.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Wang ZY, Li J, Zhu Q, Lamb C, Ronald P, Chory J. Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science. 2000;288:2360–2363. doi: 10.1126/science.288.5475.2360. [DOI] [PubMed] [Google Scholar]
- Jones-Held S, VanDoren M. Brassinolide application to Lepidum sativum seeds and the effects on seedling growth. J Plant Growth Reg. 1996;15:63–67. [Google Scholar]
- Karssen CM, Lacka E. A revision of the hormone balance theory of seed dormancy: studies on gibberellin and/or abscisic acid-deficient mutants of Arabidopsis thaliana. In: Bopp M, editor. Plant Growth Substances 1985. Heidelberg: Springer-Verlag; 1986. pp. 315–323. [Google Scholar]
- Karssen CM, Zagorski S, Kepczynski J, Groot SPC. Key role for endogenous gibberellins in the control of seed germination. Ann Bot. 1989;63:71–80. [Google Scholar]
- Koornneef M, Jorna ML, Brinkhorst-van der Swan DLC, Karssen CM. The isolation and analysis of abscisic acid (ABA)-deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) Heynh. Theor Appl Genet. 1982;61:385–393. doi: 10.1007/BF00272861. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Karssen CM. Seed dormancy and germination. In: Somerville CR, Meyerowitz EM, editors. Arabidopsis. Cold Spring Harbor , NY: Cold Spring Harbor Laboratory Press; 1994. pp. 313–334. [Google Scholar]
- Koornneef M, Reuling G, Karssen CM. The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant. 1984;61:377–383. [Google Scholar]
- Koornneef M, van der Veen JH. Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) Heynh. Theor Appl Genet. 1980;58:257–263. doi: 10.1007/BF00265176. [DOI] [PubMed] [Google Scholar]
- Léon-Kloosterziel KM, Alvarez Gil M, Ruijs GJ, Jacobsen SE, Olszewski NE, Schwartz SH, Zeevaart JAD, Koornneef M. Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J. 1996;10:655–661. doi: 10.1046/j.1365-313x.1996.10040655.x. [DOI] [PubMed] [Google Scholar]
- Li J, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/s0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- Mandava NB. Plant growth-promoting brassinosteroids. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:23–52. [Google Scholar]
- Nambara E, Satoshi N, McCourt P. A mutant of Arabidopsis which is defective in seed development and storage protein accumulation is a new abi3 allele. Plant J. 1992;2:435–441. [Google Scholar]
- Noguchi T, Fujioka S, Takatsuto S, Sakurai A, Yoshida S, Li J, Chory J. Arabidopsis det2 is defective in the conversion of (24R)-24-methylcholest-4-En-3-one to (24R)-24-methyl-5α-cholestan-3-one in brassinosteroid biosynthesis. Plant Physiol. 1999;120:833–840. doi: 10.1104/pp.120.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Nakayama M, Reid JB, Yasutomo T, Yokota T. Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in garden pea. Plant Physiol. 1997;113:31–37. doi: 10.1104/pp.113.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raventos D, Meier C, Mattsson O, Jensen AB, Mundy J. Fusion genetic analysis of gibberellin signaling mutants. Plant J. 2000;22:427–438. doi: 10.1046/j.1365-313x.2000.00759.x. [DOI] [PubMed] [Google Scholar]
- Reid JB, Ross JJ. Internode length in Pisum: two further gibberellin-insensitivity genes, lka and lkb. Physiol Plant. 1989;75:81–88. [Google Scholar]
- Salchert K, Bhalerao R, Koncz-Kalman Z, Koncz C. Control of cell elongation and stress responses by steroid hormones and carbon catabolic repression in plants. Philos Trans R Soc Lond B Biol Sci. 1998;353:1517–1520. doi: 10.1098/rstb.1998.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J, Altmann T, Adam G. Brassinosteroids from seeds of Arabidopsis thaliana. Phytochemistry. 1997;45:1325–1327. doi: 10.1016/s0031-9422(97)00177-5. [DOI] [PubMed] [Google Scholar]
- Schumacher K, Chory J. Brassinosteroid signal transduction: still casting the actors. Curr Opin Plant Biol. 2000;3:79–84. doi: 10.1016/s1369-5266(99)00038-2. [DOI] [PubMed] [Google Scholar]
- Steber CM, Cooney SE, McCourt P. Isolation of the GA-response mutant sly1 as a suppressor of ABI1–1 in Arabidopsis thaliana. Genetics. 1998;149:509–521. doi: 10.1093/genetics/149.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres M, Nemeth K, Koncz-Kalman Z, Altmann T, Redei GP, Nagy F, Schell J, Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Omigawa Y, Ogasawara M, Yoneyama K, Konnai M, Worsham AD. Effects of brassinosteroids on conditioning and germination of clover broomrape seeds. Plant Growth Reg. 1997;16:153–160. [Google Scholar]
- Telfer A, Bolman KM, Poethig RS. Phase change and the regulation of trichome distribution in Arabidopsis. Development. 1997;124:645–654. doi: 10.1242/dev.124.3.645. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Wakizuka T, Hirai K, Fujii S, Fujita A. Stimulation of Germination in Aged Rice Seed by Pre-Treatment with Brassinolide. Proceedings of the 14th Annual Plant Growth Regulator Society of America Meeting, Hawaii. 1987. pp. 26–27. [Google Scholar]
- Yokota T. The structure, biosynthesis and function of brassinosteroids. Trends Plant Sci. 1997;2:137–143. [Google Scholar]