Abstract
Aims
Health data captured by commercially available smart devices may represent meaningful patient‐reported outcome measures (PROMs) in heart failure (HF) patients. The purpose of this study was to test this hypothesis by evaluating the feasibility of a new telemonitoring concept for patients following initial HF hospitalization.
Methods and results
We designed a cardio patient monitoring platform (CPMP) that comprised mobile iOS‐based applications for patients' smartphone/smartwatch and the equivalent application on a physicians' tablet. It allowed for safe and continuous data transmission of self‐measured physiological parameters, activity data, and patient‐reported symptoms. In a prospective feasibility trial with 692 patient days from 10 patients hospitalized for newly diagnosed HF with reduced ejection fraction (mean left ventricular ejection fraction (LVEF) 26.5 ± 9.8%), we examined the CPMP during the first 2 months following discharge (69 ± 15 observation days per patient). The mean daily step count recorded by the mobile devices emerged as a promising new PROM. Its 14 day average increased over the study period (3612 ± 3311 steps/day at study inclusion and 7069 ± 5006 steps/day at end of study; P < 0.0001). It is unique for continuously reflecting real‐life activity and correlated significantly with traditional surrogate parameters of cardiac performance including LVEF (r = 0.44; 95% CI 0.07–0.71; P = 0.0232), 6 min walk test (r = 0.67; 95% CI 0.38–0.84; P = 0.0002), and scores in health‐related quality of life questionnaires.
Conclusions
We provide the first patient monitoring platform for HF patients that relies on commercially available iOS/watchOS‐based devices. Our study suggests it is ready for implementation as a tool for recording meaningful PROMs in future HF trials and telemonitoring.
Keywords: Patient‐reported outcome measure, Mobile health, Heart failure, Mobile application, Telemonitoring, Daily step count
Introduction
Heart failure (HF) is a major healthcare problem with significant morbidity and mortality whose progression is characterized by frequent hospitalizations.1 Since 30–50% of the patients hospitalized for HF decease or experience readmission within the first 6 months after hospital discharge,2 close monitoring of patients during the time following inpatient stay is mandatory.
Telemonitoring (TM) is under consideration as an adjunctive component of outpatient care. It is based on the online transmission of physiological data, signs, and symptoms that are either self‐assessed by the patient or captured by supplementary equipment like mobile devices.3 Although current data on the benefit of TM in HF patients are heterogeneous,3, 4, 5, 6 specific TM concepts have been shown to reduce hospital readmissions/admissions and to improve further HF outcomes like all‐cause mortality3, 5 and health‐related quality of life (QoL).7, 8 This observation is further corroborated by the recently published results of the TIM‐HF II (Telemedical Interventional Management of Heart Failure II) trial by Koehler et al.9 showing that a structured remote patient management intervention reduces hospital admissions and all‐cause mortality in HF patients.
A promising possibility for TM of HF patients are commercially available mobile devices such as smartphones and smartwatches that are increasingly widespread in the general population.10 Mobile health (mHealth) applications proved useful to provide assistance in self‐management,11 symptom monitoring,12 and home‐based cardiac rehabilitation.13 In addition, smart devices have the potential to generate new patient‐reported outcome measures (PROMs) in HF patients. Their integrated sensor technologies allow not only for ambulatory data acquisition but also for more continuous monitoring of physiological data like heart rate or walking activity in the context of everyday life. Because of their regular and convenient usage throughout the day, smart device‐based assessment of patient‐reported outcomes and physiological data may therefore be more representative and more independent of short‐term alterations than office‐based methods.
Despite this enormous potential, medically engineered applications and correlations of device‐based sensor data with established parameters in HF populations are still missing. Thus, we developed the cardio patient monitoring platform (CPMP) for smart device‐based monitoring of HF patients and examined its performance towards both usability as mHealth concept and with regard to generating new PROMs in future HF trials. In a proof of principle study, we tested the feasibility of the CPMP and the validity of acquired data in patients hospitalized for newly diagnosed HF [Left ventricular ejection fraction (LVEF) ≤ 40% and New York Heart Association (NYHA) ≥ II] over a study period of 2 months beginning at the moment of discharge.
Methods
Study protocol
Following design and programming of the applications in iOS/watchOS, feasibility and usability of the CPMP were prospectively examined in patients hospitalized for newly diagnosed HF with reduced ejection fraction (HFrEF) at the University Medical Center Göttingen, Germany. We enrolled 10 patients for the duration of 2 months each in 2017. In addition to continuous monitoring via the CPMP for the duration of study, three monthly clinical visits were scheduled (time points t0 at the time of enrolment during the initial hospitalization, t1 at 1 month, and t2 at 2 months after enrolment) for comparison of CPMP data with traditional measures. Inclusion criteria were first hospitalization for newly diagnosed HFrEF with LVEF ≤ 40% and NYHA Class ≥ II (see the Supporting Information for more details). The study was carried out in accordance with the Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki and received approval from the local institutional ethics committee. All patients provided written informed consent.
Cardio patient monitoring platform
All participants were equipped with an identical combination of a smartphone (iPhone 6SE, Apple Inc., Cupertino, CA, USA, iOS Versions 10.2.1–11.2.1) and a smartwatch (Apple Watch 1st Gen., Apple Inc., watchOS Versions 3.1.1–4.2.2). Before discharge, study participants were briefly trained in the operation of the CPMP mobile application installed on both smartphone and smartwatch. For the collection of activity data, patients were advised to wear both devices at all times except while sleeping. Furthermore, participants were instructed to daily enter self‐measured blood pressure and body weight before breakfast. Additional app features including reminders/confirmation of medication intake or input of symptoms were offered to examine acceptance and functionality but were not mandatory. The CPMP ensured immediate worldwide Internet‐based transfer of the data via a secure transfer protocol from the patient device to the treating cardiologist who had access to transmitted data via the respective application on an iOS‐based tablet (iPad Pro A1584, Apple Inc., iOS Versions 10.2.1–11.2.1). Data were checked daily, and a response was initiated within 24 h when predefined margins in blood pressure were exceeded, increase in body weight, or new or worsening signs or symptoms occurred. Further information on the CPMP is available in the Supporting Information.
Device‐related health data
Health data from smartphone and smartwatch were accessed by the CPMP via the HealthKit interface. Compliance with daily data intake and daily usage of the devices was calculated as percentage of the whole. Wearing time of the devices was estimated based on the Clinical Document Architecture readout. The mean daily step count (MDSC) was calculated as arithmetic mean of 14 days. To validate the device‐based 6 min walk test (6MWT) integrated in the CPMP, it was carried out simultaneously to the execution of the standard 6MWT at all three clinical visits, allowing for direct comparison of the measured distance covered. For validation of smartwatch‐based heart rate measurements, data were extracted from the Clinical Document Architecture and statistically compared with results from 4 day Holter electrocardiograms (ECGs) at both t0 and t1. More details on device‐based methods are provided in the Supporting Information.
Procedures and questionnaires
At all three clinical visits, we conducted clinical examinations, echocardiography, cardiopulmonary exercise testing (CPET), and blood tests. Health‐related QoL was assessed by the Minnesota Living with Heart Failure Questionnaire (MLHFQ) and the Kansas City Cardiomyopathy Questionnaire (KCCQ). Depressive symptoms and heart‐focused anxiety were captured by the Patient Health Questionnaire Depression Scale (PHQ‐9) and the Cardiac Anxiety Questionnaire (CAQ). To assess patients' perception and adherence, we developed a questionnaire based on the Service User Technology Acceptability Questionnaire that was filled in by the patients at t2. Patients' eHealth literacy was captured by a short questionnaire similar to the eHealth Literacy Scale. More detailed information on procedures and questionnaires are available in the Supporting Information.
Statistical analysis
The statistical analyses were performed with GraphPad Prism_7 software (GraphPad Software, La Jolla, CA, USA). Continuous variables are presented as arithmetic mean ± standard deviation/standard error of the mean and categorical variables as numbers and percentages. Differences between clinical or device‐related parameters at the three time points were evaluated by paired t‐test (α = 0.05). To examine the association between conventional clinical parameters and mHealth data, the Pearson product–moment correlation coefficient (r) was calculated. The measurement accuracy of the smart devices as compared with criterion measurements was evaluated by quantifying the mean percentage error and the mean absolute percentage error.
Results
Design and performance of the cardio patient monitoring platform
In cooperation with Medopad Ltd, we designed a platform for iOS/watchOS to remotely monitor HF patients through collecting symptoms, medication adherence, activity data, vital signs, and additional PROMs. It consists of a patient‐facing app (on iPhone/Apple Watch) and a clinician web portal to view patient data (Figure 1 ). The platform is a CE (Conformité Européenne)‐marked Class I medical device registered with the Medicines and Healthcare products Regulatory Agency Information Governance Level 2 (Information Governance Statement of Compliance) and is compliant with the General Data Protection Regulation of the European Union. Data safety and communication was guaranteed using server‐side encryption and authenticated protocols are detailed in the Supporting Information Methods section.
Figure 1.
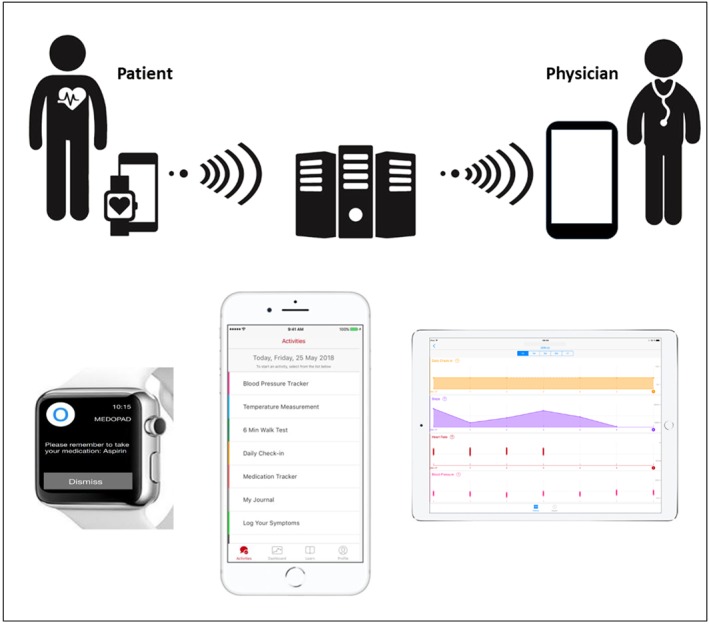
Cardio patient monitoring platform. Self‐measured physiological data and further patient‐reported outcome parameters are entered daily by the patient. Together with activity data from the Apple HealthKit, the information is transmitted via a secure immediate worldwide Internet‐based transfer to the treating physician.
Patient characteristics and clinical course
Ten patients were enrolled in the study (mean age 46.3 ± 7.8 years, six male and four female). In three participants, newly diagnosed HFrEF was due to ST‐segment elevation myocardial infarction, and in seven participants, it was caused by dilated cardiomyopathy. Paroxysmal or persistent atrial fibrillation (AF) was present in one patient at baseline and newly diagnosed in two participants during the study. One study participant received an implantable cardioverter defibrillator at the end of the inpatient stay as therapy of a symptomatic, intermittent grade 3 atrioventricular block. One patient developed an acute disabling cerebral ischaemia 7 weeks after inclusion, which prevented the t1 visit. Except for one patient, all participants were previous smartphone users (three iOS and six Android), and half of them were active users of at least one readily accessible mHealth application (pre‐installed fitness apps, nutrition apps, etc.).
Results of clinical examinations at baseline and during follow‐up are listed in Table 1. All patients exhibited severe HFrEF at the time of inclusion with markedly reduced LVEF, CPET capacity, and elevated N‐terminal pro‐brain natriuretic peptide (NT‐proBNP). Initial questionnaires reflected reduced health‐related QoL (MLHFQ and KCCQ) and pathological values in our screening tools for depression (PHQ‐9) and heart‐focused anxiety (CAQ). Treatment according to current guideline recommendations for HF14 was initiated in all patients, and an improvement in clinical status was observed during the study period. While NT‐proBNP improved numerically, LVEF significantly recovered from severe to moderately reduced. Likewise, physical performance as evaluated by the 6MWT improved significantly, as did health‐related QoL.
Table 1.
Clinical and laboratory parameters
| Value (valid n) | Baseline (t0) | 1 month follow‐up (t1) | End of study (t2) |
|---|---|---|---|
| Physical investigation | |||
| Body weight (kg) | 82.80 ± 9.810 | 81.79 ± 8.4610 | 82.74 ± 8.109 |
| RR systolic (mmHg) | 122 ± 17.9810 | 115 ± 14.5810 | 107.2 ± 13.499 |
| RR diastolic (mmHg) | 78 ± 8.810 | 76.11 ± 14.0910 | 69.44 ± 8.829 |
| Heart rate at rest (1/min) | 71.3 ± 15.010 | 71 ± 10.210 | 67.3 ± 8.09 |
| Transthoracic echocardiography | |||
| LVEF (%) | 26.5 ± 9.810 | 35.0 ± 11.2** , 10 | 36.8 ± 11.7** , 9 |
| Laboratory | |||
| NT‐proBNP i.S. (pg/mL) | |||
| All | 2518 ± 191310 | 1614 ± 152310 | 1278 ± 11599 |
| Without AF patients | 3022 ± 20447 | 1801 ± 17317 | 1331 ± 1325# , 6 |
| Creatinine i.S. (mg/dL) | 1.13 ± 0.5910 | 1.12 ± 0.5610 | 0.92 ± 0.169 |
| Standard 6MWT | |||
| Distance (m) | 433.9 ± 180.510 | 580.4 ± 148.3** , 10 | 643.2 ± 148.0** , ## , 9 |
| Cardiopulmonary exercise testing | |||
| Exercise time16 | 8.5 ± 3.95 | 8.5 ± 4.510 | 8.9 ± 4.48 |
| Peak watt | 112 ± 705 | 117 ± 5610 | 130 ± 568 |
| Peak VO2 (mL/min) | 1366 ± 6905 | 1598 ± 70210 | 1552 ± 6648 |
| Peak VO2 (mL/kg/min) | 15.74 ± 7.35 | 19.1 ± 7.110 | 18.43 ± 6.88 |
| VE/VO2 | 40.25 ± 3.25 | 35.9 ± 5.210 | 38.63 ± 7.18 |
| Questionnaires | |||
| MLHFQ | |||
| Global score | 47.1 ± 16.5410 | 30.56 ± 13.31* , 9 | 30.33 ± 20.019 |
| Physical subscore | 23.5 ± 7.5610 | 12.44 ± 5.92** , 9 | 11.33 ± 9.19* , 9 |
| Emotional subscore | 7.4 ± 5.6210 | 7.22 ± 6.049 | 7.11 ± 5.239 |
| KCCQ | |||
| Global score | 81.1 ± 16.7610 | 98.78 ± 12.92* , 9 | 105.8 ± 13.7* , 9 |
| Functional status | 48.1 ± 12.4410 | 61.44 ± 6.98* , 9 | 56.9 ± 20.899 |
| Clinical summary | 67.3 ± 13.0310 | 86.78 ± 12.16** , 9 | 83.4 ± 31.59 |
| PHQ9 | 16.8 ± 5.4110 | 13.9 ± 3.739 | 14.38 ± 4.219 |
| CAQ | 23.78 ± 9.5410 | 22.13 ± 9.859 | 19.67 ± 9.359 |
6MWT, 6 min walk test; AF, atrial fibrillation; CAQ, Cardiac Anxiety Questionnaire; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PHQ9, Patient Health Questionnaire Depression Scale; RR, Blood Pressure.
Values are presented as mean ± SEM.
P < 0.05 (compared with baseline).
PP < 0.01 (compared with baseline).
P < 0.05 (compared with t1).
PP < 0.01 (compared with t1).
Patients' acceptance and usability of the cardio patient monitoring platform
The CPMP performed technically as expected, with only minor bug fixes that could be implemented after the end of the trial. Patient adherence with daily intake of self‐measured data was satisfactory (82.95% for blood pressure and 78.18% for body weight). Questionnaires indicated a high degree of patient satisfaction with the CPMP (see the Results section in the Supporting Information with Figure S1 ). Likewise, from the physicians' perspective, the web‐based secure data transfer to the treating physicians' notepad and the usage of the app went without technical issues. In three study participants, medical therapy was modified in response to hypertensive/hypotensive blood pressure values based on transferred data. These were the only in‐between study interventions required in the cohort.
Device‐related measurement of steps
As indicator of everyday life physical activity (PA), we analysed the MDSC captured by built‐in pedometer functions of smartphone and smartwatch. Of the 692 patient days, a valid step count was available for 578 days (83.5%). As expected, the MDSC averaged over 14 days was low following hospital discharge (3612 ± 3311) and increased significantly to the first follow‐up (6927 ± 4871; P < 0.0001) and to the end of study (7069 ± 5006; P < 0.0001) (Figure 2 ).
Figure 2.
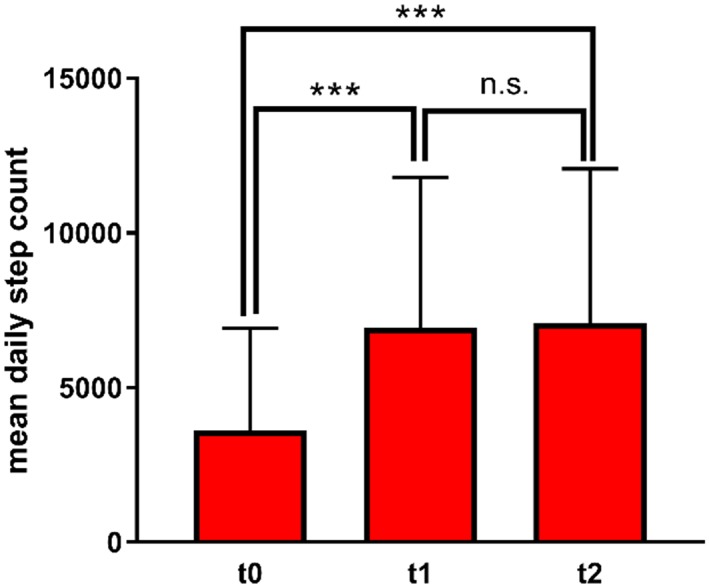
Mean daily step count (14 day average) at study inclusion (t0), at 1 month follow‐up (t1), and at the end of study (t2). Values are presented as mean + SEM. *** P < 0.001. n.s. not significant.
There was a significant correlation between the MDSC and several conventional clinical parameters (Table 2 and Figure 3 A). It correlated best with parameters of clinical exercise testing, that is the distance in the conventional 6MWT and peak VO2 in CPET. In addition, a strong association with patient‐reported outcomes in the MLHFQ and KCCQ, especially with the subscores representing health‐related QoL, HF symptoms, and PA, was observed. No association was seen between the MDSC and the results of the psychological surveys (PHQ9 and CAQ), suggesting the MDSC reflects motivational aspects much less compared with physical capability.
Table 2.
Correlation of the mean daily step count with clinical parameters
| Value | Pearson's correlation coefficient | Level of significance |
|---|---|---|
| NT‐proBNP (ng/mL) | r = −0.37 (95% CI −0.66, 0.03) | P = 0.0667 |
| MLHFQ | ||
| Global score | r = −0.52 (95% CI −0.76, −0.16) | P = 0.0077** |
| Physical subscore | r = −0.47 (95% CI −0.72, −0.09) | P = 0.0177* |
| Emotional subscore | r = −0.29 (95% CI −0.61, 0.12) | P = 0.1659 |
| KCCQ | ||
| Global score | r = 0.56 (95% CI 0.21, 0.78) | P = 0.0034** |
| Functional status | r = 0.51 (95% CI 0.14, 0.75) | P = 0.0099** |
| Clinical summary | r = 0.56 (95% CI 0.21, 0.78) | P = 0.0038** |
| PHQ9 | r = −0.27 (95% CI −0.60, −0.14) | P = 0.1878 |
| CAQ | r = −0.09 (95% CI −0.48, 0.34) | P = 0.6956 |
CAQ, Cardiac Anxiety Questionnaire; CI, confidence interval; MLHFQ, Minnesota Living with Heart Failure Questionnaire; KCCQ, Kansas City Cardiomyopathy Questionnaire; n.s. not significant; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PHQ9, Patient Health Questionnaire Depression Scale.
P < 0.05.
P < 0.01.
Figure 3.
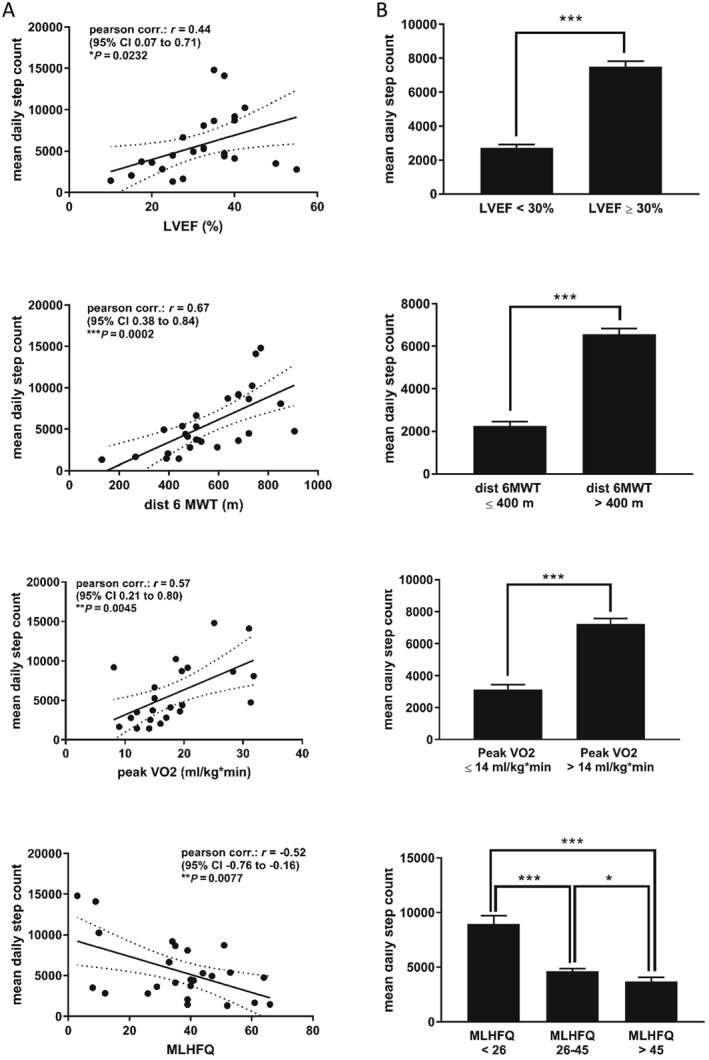
Correlation of the mean daily step count (14 day average) with traditional clinical parameters of heart failure severity. All available recordings were pooled for this analysis. (A) Pearson's correlation analysis. (B) Mean daily step count in subgroups classified after distinct cut‐offs. Values are presented as mean ± SEM. * P < 0.05, ** P < 0.01, and *** P < 0.001. CI, confidence interval; dist 6MWT: distance in 6 min walk test; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire.
The MDSC differed significantly between patients with severely (LVEF < 30%) and moderately reduced ejection fraction (LVEF ≥ 30%). Study participants with poor exercise performance (walking distance ≤400 m in the conventional 6MWT; peak VO2 ≤ 14 mL/kg/min in CPET) made significantly fewer steps per day than those with values above. With increasing health‐related QoL measured in the MLHFQ [categorized according to Behlouli et al.15 in good (<26), moderate (26–45), and poor QoL (>45)], the daily walking activity was higher as well (Figure 3 B).
Validation of the app‐based 6 min walk test
To allow for a more familiar measure of exercise capacity beyond the MDSC, we designed an app‐based 6MWT that instructs the patient to perform the test in analogy to the conventional supervised 6MWT. The device‐related mean measurement of the distance covered during the test showed acceptable divergence from standard measurement, as expressed in the low mean percentage error (7.4%) and mean absolute percentage error (14%) of all values (Figure 4 ). It exhibited a strong correlation (r = 0.93 with 95% CI 0.85–0.97; P < 0.0001) (Figure 4B ), although individual measurements differed up to 173 m. Measuring errors of the app resulted from underestimation and overestimation of very long or short distances, visible as a regression of measuring values towards the mean (Figure 4 A).
Figure 4.
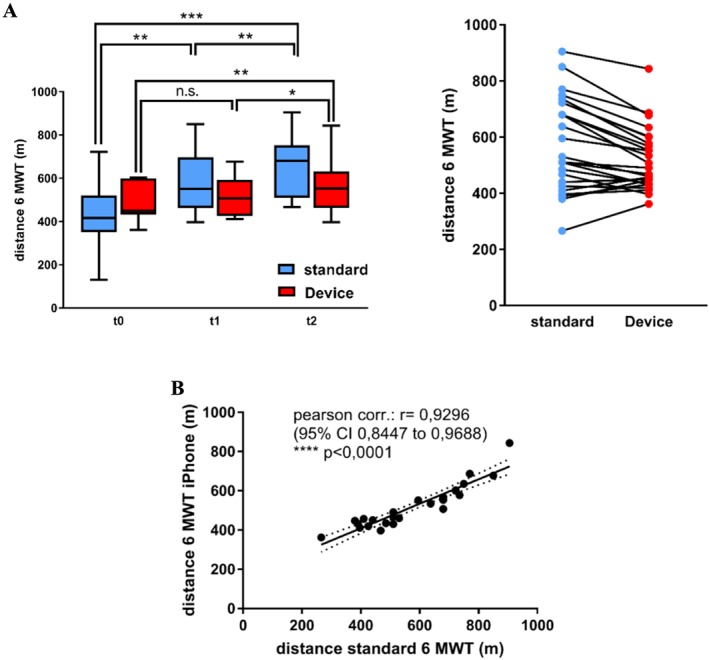
Validation of the app‐based 6MWT. (A) Mean ± SEM of distances measured in the standard 6 min walk test (6MWT) and the device‐based 6MWT at study inclusion (t0), the 1 month follow‐up (t1), and at the end of study (t2). *P < 0.05, **P < 0.01, and ***P < 0.001. (B) Pearson's correlation analysis. CI, confidence interval.
Smartwatch‐based heart rate measurement
The compliance with wearing the Apple Watch was acceptable with 587 valid wear days out of 692 (84.8%) and a mean daily wearing time of 12.71 ± 1.13 h. The arithmetic mean of all mean heart rates acquired by the CPMP and averaged over 7 days was 72.69 ± 8.35 b.p.m. at baseline, 74.45 ± 8.98 b.p.m. at t1, and 74.82 ± 8.01 b.p.m. at t2. To evaluate the measurement accuracy and clinical usability of heart rate data captured by the Apple Watch in free‐living conditions, we matched data with those of two Holter ECGs with 4 days of records each in six patients. The smartwatch measurements were punctual with varying intervals following a proprietary algorithm of watchOS (on average 10.8 ± 7.5 measuring events/h). We found a high level of consistency when comparing the mean heart rate measured by the Apple Watch (72.66 ± 7.40/min) and the Holter ECG (68.03 ± 8.4/min). Calculations yielded a high correlation coefficient (r = 0.87; 95% CI 0.76–0.93; P < 0.0001) and only a small mean percentage error (mean percentage error 7.26 ± 6.49%; mean absolute percentage error 7.56 ± 6.13%) so that the daily mean heart rate captured by the watch can be considered valid.
Discussion
Principal findings
To the best of our knowledge, this is the first study to evaluate a commercially available smartphone/smartwatch system for TM of HF patients. Moreover, for the first time, the usability of build‐in pedometers of iOS devices to assess PA in HF patients under real‐life conditions is investigated. The main findings are as follows: (i) the CPMP is the first fully functional iOS and consumer device‐based application for HF patients. It proved technically feasible with regard to functionality of hardware/software and patients' adherence. (ii) The MDSC exhibited significant correlations with established clinical parameters including 6MWT, peak VO2 in CPET, and scores in questionnaires for health‐related QoL (MLHFQ and KCCQ). (iii) Being more continuous and more robust towards motivational bias compared with traditional surrogate parameters of cardiac performance, the MDSC may therefore represent an excellent parameter of ambulatory PA in HF patients and a meaningful new PROM for TM and HF trials.
Patients' acceptance and feasibility of the cardio patient monitoring platform
We observed a high overall satisfaction with the CPMP together with good ratings for user‐friendliness and convenient handling of the mobile app. In line with this, patients' adherence to daily self‐measurement and input of vital signs was higher than expected and tended to range above average compared with previously reported trials.6, 7, 16, 17, 18 While more than half of the patients entered self‐measured data on over 85% days of the study, the majority of missing values could be attributed to few patients. In those, entering of data in the CPMP was impeded during cardiac rehabilitation (3–4 weeks) to a large extent because of missing Internet connectivity in rehabilitation clinics in Germany.
Validity of smart device‐based measurement of health data
In contrast to most step counters used in clinical trials,19, 20 smartphones and smartwatches with built‐in pedometers allow for more convenient and probably more accurate measurement of daily step count as marker of everyday PA. The observed compliance rate of >80% and mean wear time of 12.71 ± 1.13 h account for the validity of the measured step count. Still, in order to make smart device‐based step counting more meaningful for clinical research in future, the exact wear time should be known, for instance, by the use of sensor‐based algorithms as proposed by Choi et al.21
The comparison of the covered distance measured by the app‐based 6MWT with that of the standard 6MWT showed a small but significant systematic error of the smart device‐based method. The observed underestimation and overestimation of high and low distances may at least in part be explained by the fact that the used iOS/watchOS devices have the highest accuracy at a pace of 5 km/h walking speed that is less good with slower walking pace.22, 23
Apart from activity monitoring, wrist‐worn smart devices like the Apple Watch measure its wearer's heart rate via photoplethysmography. For the Apple Watch 1, several trials give evidence of its good or very good accuracy in measuring heart rate under laboratory conditions.24, 25 Our study is the first that validates heart rate measurements of the Apple Watch in HF patients against the standard criterion of a repeated long‐term ECG during a 4 day period in everyday life. Our results demonstrate that given a daily wear time >10 h/day, Apple Watch‐based heart rate measuring is appropriate to adequately assess the mean daily heart rate that is most relevant for both therapy modification and prognosis in HF,14 although a small systematic error results from the missing recording during night‐time charging.
Smart devices provide new patient‐reported outcome measures
In light of the recent debate on the limitations of endpoints in HF trials (LVEF, NT‐proBNP, and other surrogate parameters), there is a pressing need for meaningful PROMs. The study strongly suggests that the CPMP might address this need, providing continuous data from the patients' everyday life.
Daily walking activity quantified by pedometer‐based step count already represents an established marker for PA in cardiac patients.26 In our study, the MDSC was determined by multiple sensors with parallel acquisition as an averaged value over 14 days. Compared with the 6MWT and other clinical examinations, it is therefore more robust against short‐term performance variations and motivational bias. Still, we found strong correlations of the MDSC with exercise capacity (6MWT and peak VO2 in CPET) and LVEF. While the correlation of walking activity with functional class of the NYHA scale has already been demonstrated,27 our results also account for its correlation with health‐related QoL and symptom burden (MLHFQ and KCCQ) as well. The missing correlation of the MDSC with NT‐proBNP most likely points to high interindividual differences and known limitations including sensitivity towards AF of this biomarker.
We observed a low MDSC after hospital discharge that significantly increased during the first month and persisted at 2 months. Stable outpatients with chronic HF and severely reduced LVEF display similar low walking activity20 as in our trial. However, it should be mentioned that the degree of rise in daily step count together with the corresponding improvement of LVEF, NT‐proBNP, and 6MWT distance within 1 month after hospital discharge is higher than observed in previous trials. Because this was not an interventional study, and the cohort consisted of only 10 patients, the mechanisms behind the dramatic improvements remain obscure.
Apart from correlations with established clinical parameters, literature gives evidence of the predictive value of daily step count regarding HF outcomes like arrhythmias and death.28 Its significance for TM in the post‐discharge period is demonstrated by the finding that physical inactivity measured by step count predicts hospital readmissions after hospitalization for decompensated HF.29 While earlier‐cited previous studies used medical devices for step counting, we are the first to demonstrate the usability of build‐in pedometer technology of iOS devices to assess PA in HF patients under real‐life conditions. In addition, the CPMP collects both self‐measured physiological data and patient‐reported outcomes in everyday life conditions. Algorithms based on more than one parameter can predict worsening and readmission of HF patients. Accordingly, we speculate that parameter optimization by advanced machine learning algorithms may further improve predictability.
Study limitations
Some limitations of our study should be considered. Because of its design as a feasibility trial, we analysed only a small sample size without control group that was not sufficient to detect effects of the TM intervention on clinical outcomes like re‐hospitalization. Because patients were carefully screened for eligibility concerning their digital aptitude, a selection bias is obvious. Moreover, for the current being, we consider the interactive aspects of the technology only suitable for tech‐savvy patients. However, with rapidly increasing popularity of smart devices, the number of patients willing and capable to accept smart device‐based TM will rapidly increase.
Conclusions
Our study demonstrates the feasibility of a newly designed mobile application for TM of patients after HF hospitalization. The system allows for continuous and secure transmission of the patients' relevant health data to the treating physician. In our trial, we are the first to show that daily step count measured by iOS/watchOS devices in free‐living conditions is a valid surrogate parameter of daily PA and correlates to established clinical parameters. In future, the integration of self‐measured physiological data, patient‐reported outcomes, and health data captured by iOS devices in computer‐based algorithms may serve to predict worsening of clinical state and prevent hospital readmissions. Therefore, we suggest smart device‐based monitoring and in particular measurement of activity as new diagnostic parameter in TM concepts and a new endpoint in clinical HF trials.
Conflict of interest
Medopad Ltd received payment for the design of the CPMP and the technical support during the trial but had no role in the collection, analysis and interpretation of data, or the decision to submit for publication. We declare no competing interests.
Funding
The study was supported by the Department of Cardiology of the University Medical Center Göttingen.
Supporting information
Figure S1. Patient feedback questionnaire. Approval and disapproval of participants with statements regarding the use of the CPMP at the end of the clinical trial are depicted as mean ± SD on a 5 point Likert Scale.
Werhahn, S. M. , Dathe, H. , Rottmann, T. , Franke, T. , Vahdat, D. , Hasenfuß, G. , and Seidler, T. (2019) Designing meaningful outcome parameters using mobile technology: a new mobile application for telemonitoring of patients with heart failure. ESC Heart Failure, 6: 516–525. 10.1002/ehf2.12425.
References
- 1. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007; 93: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA 2011; 306: 1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dierckx R, Pellicori P, Cleland JG, Clark AL. Telemonitoring in heart failure: Big Brother watching over you. Heart Fail Rev 2015; 20: 107–116. [DOI] [PubMed] [Google Scholar]
- 4. Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, Phillips CO, Hodshon BV, Cooper LS, Krumholz HM. Telemonitoring in patients with heart failure. N Engl J Med 2010; 363: 2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inglis SC, Clark RA, McAlister FA, Stewart S, Cleland JG. Which components of heart failure programmes are effective? A systematic review and meta‐analysis of the outcomes of structured telephone support or telemonitoring as the primary component of chronic heart failure management in 8323 patients: Abridged Cochrane Review. Eur J Heart Fail 2011; 13: 1028–1040. [DOI] [PubMed] [Google Scholar]
- 6. Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Bohm M, Boll H, Baumann G, Honold M, Koehler K, Gelbrich G, Kirwan BA, Anker SD, Telemedical Interventional Monitoring in Heart Failure I . Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation 2011; 123: 1873–1880. [DOI] [PubMed] [Google Scholar]
- 7. Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C, Ross HJ. Mobile phone‐based telemonitoring for heart failure management: a randomized controlled trial. J Med Internet Res 2012; 14: e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smolis‐Bak E, Dabrowski R, Piotrowicz E, Chwyczko T, Dobraszkiewicz‐Wasilewska B, Kowalik I, Kazimierska B, Jedrzejczyk B, Smolis R, Gepner K, Maciag A, Sterlinski M, Szwed H. Hospital‐based and telemonitoring guided home‐based training programs: effects on exercise tolerance and quality of life in patients with heart failure (NYHA class III) and cardiac resynchronization therapy. A randomized, prospective observation. Int J Cardiol 2015; 199: 442–447. [DOI] [PubMed] [Google Scholar]
- 9. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C, Doerr G, Naegele H, Stork S, Butter C, Sechtem U, Angermann C, Gola G, Prondzinsky R, Edelmann F, Spethmann S, Schellong SM, Schulze PC, Bauersachs J, Wellge B, Schoebel C, Tajsic M, Dreger H, Anker SD, Stangl K. Efficacy of telemedical interventional management in patients with heart failure (TIM‐HF2): a randomised, controlled, parallel‐group, unmasked trial. Lancet 2018; 392: 1047–1057. [DOI] [PubMed] [Google Scholar]
- 10. Migo EM, Haynes BI, Harris L, Friedner K, Humphreys K, Kopelman MD. mHealth and memory aids: levels of smartphone ownership in patients. J Ment Health 2015; 24: 266–270. [DOI] [PubMed] [Google Scholar]
- 11. Johnston N, Bodegard J, Jerstrom S, Akesson J, Brorsson H, Alfredsson J, Albertsson PA, Karlsson JE, Varenhorst C. Effects of interactive patient smartphone support app on drug adherence and lifestyle changes in myocardial infarction patients: a randomized study. Am Heart J 2016; 178: 85–94. [DOI] [PubMed] [Google Scholar]
- 12. Athilingam P, Labrador MA, Remo EF, Mack L, San Juan AB, Elliott AF. Features and usability assessment of a patient‐centered mobile application (HeartMapp) for self‐management of heart failure. Appl Nurs Res 2016; 32: 156–163. [DOI] [PubMed] [Google Scholar]
- 13. Beatty AL, Magnusson SL, Fortney JC, Sayre GG, Whooley MA. VA FitHeart, a mobile app for cardiac rehabilitation: usability study. JMIR Hum Factors 2018; 5: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESCSD . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC): developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 15. Behlouli H, Feldman DE, Ducharme A, Frenette M, Giannetti N, Grondin F, Michel C, Sheppard R, Pilote L. Identifying relative cut‐off scores with neural networks for interpretation of the Minnesota Living with Heart Failure Questionnaire. Conf Proc IEEE Eng Med Biol Soc 2009; 2009: 6242–6246. [DOI] [PubMed] [Google Scholar]
- 16. Domingo M, Lupon J, Gonzalez B, Crespo E, Lopez R, Ramos A, Urrutia A, Pera G, Verdu JM, Bayes‐Genis A. Evaluation of a telemedicine system for heart failure patients: feasibility, acceptance rate, satisfaction and changes in patient behavior: results from the CARME (CAtalan Remote Management Evaluation) study. Eur J Cardiovasc Nurs 2012; 11: 410–418. [DOI] [PubMed] [Google Scholar]
- 17. Scherr D, Kastner P, Kollmann A, Hallas A, Auer J, Krappinger H, Schuchlenz H, Stark G, Grander W, Jakl G, Schreier G, Fruhwald FM, Investigators M . Effect of home‐based telemonitoring using mobile phone technology on the outcome of heart failure patients after an episode of acute decompensation: randomized controlled trial. Journal of Medical Internet Research 2009; 11: e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zan S, Agboola S, Moore SA, Parks KA, Kvedar JC, Jethwani K. Patient engagement with a mobile web‐based telemonitoring system for heart failure self‐management: a pilot study. JMIR Mhealth Uhealth 2015; 3: e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thorup C, Hansen J, Gronkjaer M, Andreasen JJ, Nielsen G, Sorensen EE, Dinesen BI. Cardiac patients' walking activity determined by a step counter in cardiac telerehabilitation: data from the intervention arm of a randomized controlled trial. J Med Internet Res 2016; 18: e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yavari M, Haykowsky MJF, Savu A, Kaul P, Dyck JRB, Haennel RG, Alberta HI. Volume and patterns of physical activity across the health and heart failure continuum. Can J Cardiol 2017; 33: 1465–1471. [DOI] [PubMed] [Google Scholar]
- 21. Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc 2011; 43: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duncan MJ, Wunderlich K, Zhao Y, Faulkner G. Walk this way: validity evidence of iPhone health application step count in laboratory and free‐living conditions. J Sports Sci 2017; 28: 1–10. [DOI] [PubMed] [Google Scholar]
- 23. Major MJ, Alford M. Validity of the iPhone M7 motion co‐processor as a pedometer for able‐bodied ambulation. J Sports Sci 2016; 34: 2160–2164. [DOI] [PubMed] [Google Scholar]
- 24. Dooley EE, Golaszewski NM, Bartholomew JB. Estimating accuracy at exercise intensities: a comparative study of self‐monitoring heart rate and physical activity wearable devices. JMIR Mhealth Uhealth. 2017; 5: e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wallen MP, Gomersall SR, Keating SE, Wisloff U, Coombes JS. Accuracy of heart rate watches: implications for weight management. PLoS One 2016; 11: e0154420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tudor‐Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij I, Ewald B, Gardner AW, Hatano Y, Lutes LD, Matsudo SM, Ramirez‐Marrero FA, Rogers LQ, Rowe DA, Schmidt MD, Tully MA, Blair SN. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act 2011; 8: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jehn M, Schmidt‐Trucksass A, Schuster T, Weis M, Hanssen H, Halle M, Koehler F. Daily walking performance as an independent predictor of advanced heart failure: prediction of exercise capacity in chronic heart failure. Am Heart J 2009; 157: 292–298. [DOI] [PubMed] [Google Scholar]
- 28. Palmisano P, Guerra F, Ammendola E, Ziacchi M, Luigi Pisano EC, Dell'Era G, Aspromonte V, Zaccaria M, Di Ubaldo F, Capucci A, Nigro G, Occhetta E, Maglia G, Ricci RP, Boriani G, Accogli M, Italian Association of Arrhythmology and Cardiac Pacing (AIAC) . Physical activity measured by implanted devices predicts atrial arrhythmias and patient outcome: results of IMPLANTED (Italian Multicentre Observational Registry on Patients With Implantable Devices Remotely Monitored). J Am Heart Assoc 2018; 7: e008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Waring T, Gross K, Soucier R, ZuWallack R. Measured physical activity and 30‐day rehospitalization in heart failure patients. J Cardiopulm Rehabil Prev 2017; 37: 124–129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patient feedback questionnaire. Approval and disapproval of participants with statements regarding the use of the CPMP at the end of the clinical trial are depicted as mean ± SD on a 5 point Likert Scale.


