Abstract
Aims
We assessed preoperative muscle wasting in patients undergoing left ventricular assist device (LVAD) implantations using abdominal skeletal muscle images on computed tomography (CT) and explored the associations between the preoperative muscle wasting and clinical outcomes after LVAD implantation.
Methods and results
We retrospectively examined the records of 111 patients who underwent continuous‐flow LVAD implantations as bridge‐to‐transplant therapy from January 2010 to December 2016 at our institution. After 33 patients were excluded, the study cohort consisted of 78 individuals. CT images used to calculate the skeletal muscle index (SMI) at the third lumbar vertebra level were obtained before the LVAD implantation procedures. Patients were classified as having muscle wasting if their SMI fell into the lowest gender‐based tertile. The median SMI for the study patients was 37.6 cm2/m2. The SMI cut‐off values for the lowest tertiles were 36.7 cm2/m2 for men and 28.2 cm2/m2 for women, resulting in 26 patients (33.3%) with muscle wasting in this study. During the mean follow‐up of 738 ± 379 days, there were 10 deaths (12.8% mortality). Seven of the 26 patients with muscle wasting (26.9%) died, and 3 of the 52 patients without muscle wasting (5.8%) died. The times to all‐cause mortality were significantly different between patients with and without muscle wasting (P = 0.0094). Muscle wasting was found to be associated with mortality in univariate and multivariate Cox analyses (hazard ratio: 4.32; 95% CI: 1.19–20.2).
Conclusions
Preoperative muscle wasting was associated with a higher mortality in patients with LVAD. Assessment of the abdominal skeletal muscle area on CT prior to LVAD implantation can help predict mortality.
Keywords: Heart failure, Left ventricular assist device, Sarcopenia, Skeletal muscle index, Computed tomography
Introduction
Heart failure is a major and growing public health problem associated with morbidity, hospitalization, and mortality worldwide.1 New therapeutic interventions for the treatment of heart failure have emerged, but many cases are still medically intractable. Left ventricular assist device (LVAD) is used as an effective therapeutic option in these advanced heart failure cases in which all other therapies have been exhausted.2, 3, 4 The use of LVAD improves the survival rate, the quality of life, and the functional capacity of patients with advanced heart failure.5, 6 The waiting period with LVAD for heart transplantation is extremely long in Japan because of donor shortage7; therefore, long‐term management is necessary, and morbidity and mortality during this period are a major concern in Japan.
Sarcopenia is a syndrome characterized by the generalized loss of skeletal muscle mass and a decrease in general strength.8 It is associated with poor outcomes and premature death in patients with various underlying diseases.9 In advanced heart failure, a loss of skeletal muscle mass contributes to reduced exercise capacity and frailty.10, 11, 12 However, it is unclear whether sarcopenia increases the likelihood of adverse clinical outcomes in advanced heart failure patients undergoing LVAD implantation. Therefore, we retrospectively screened a cohort of Japanese preoperative patients for muscle wasting using abdominal skeletal muscle measurements on computed tomography (CT) and explored the associations between the preoperative muscle wasting and survival rates after LVAD implantation.
Methods
We reviewed the records of 111 patients who underwent continuous‐flow LVAD implantation as bridge‐to‐transplant therapy at The University of Tokyo from January 2010 to December 2016. We excluded 5 patients who were younger than 18 years and 28 patients who had undergone conversions from extracorporeal LVAD to continuous‐flow LVAD. After these exclusions, the final study cohort consisted of 78 patients (Figure 1 ).
Figure 1.
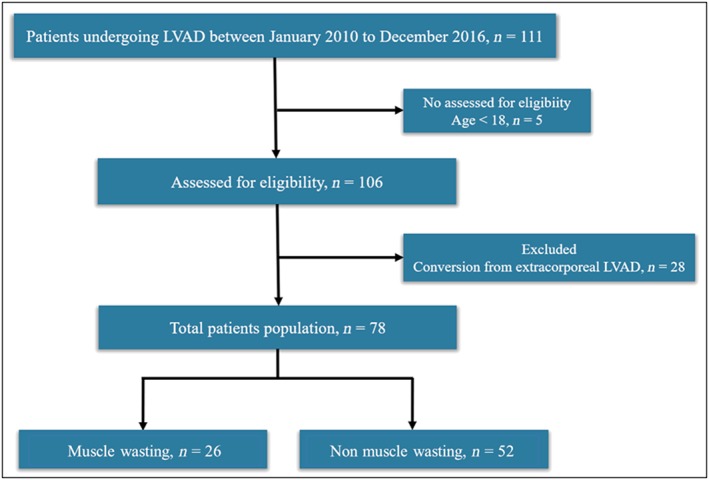
Flow chart of patients who met inclusion criteria for study population. LVAD, left ventricular assist device.
CTs were performed within 5 months before the LVAD implantations, and we found abdominal skeletal muscle measurements available in the medical record for all the patients. We blindly assessed the skeletal muscle area (SMA) at the third lumbar vertebra (L3) level. The muscles in the area included the psoas and paraspinal muscles, the erector spinae, the quadratus lumborum, the transversus abdominis, the obliquus externus abdominis, the obliquus internus abdominis, and the rectus abdominis. We analysed images using the ImageJ medical imaging software v1.42q (National Institutes of Health, http://rsb.info.nih.gov/ij). The cross‐sectional SMAs were measured according to attenuation thresholds of −29 to +150 Hounsfield units. We calculated skeletal muscle indexes (SMIs; cm2/m2) by normalizing the total skeletal muscle surface area by the height in square metres. Our intraobserver reliability analysis for the value of SMI yielded an intraclass correlation of 0.94. Patients were classified as having muscle wasting if their SMIs fell into the lowest, gender‐based tertile.
For laboratory data, fasting blood samples were collected, and each laboratory finding was assessed using the standard laboratory methods at The University of Tokyo Hospital (Tokyo, Japan). It was extracted within 2 weeks before the continuous‐flow LVAD implantation. Standard echocardiographic imaging was performed to evaluate the left ventricular ejection fraction, and haemodynamic data were evaluated using the Swan–Ganz catheter within 1 month before implantation. The study protocol conformed to the tenets of the Declaration of Helsinki, and the Institutional Review Board of The University of Tokyo in Japan reviewed and approved the protocol (11271).
Outcome
The primary outcome was all‐cause mortality with data censored at heart transplantation or LVAD explantation for myocardial recovery. Patients' survivals were followed up from the date of LVAD implantation until the time of death, heart transplantation, LVAD explantation, or survival with LVAD through the 31 August 2017. All causes of death were analysed. Among clinical outcomes, intracranial haemorrhage and driveline infection were also checked. Intracranial haemorrhage was diagnosed by a neurologist. Driveline infection was defined according to the Interagency Registry for Mechanically Assisted Circulatory Support definition of percutaneous site and pocket infection, which was treated with non‐prophylactic anti‐microbial agents.
Statistical analysis
Data are presented as mean ± standard deviation or as median (inter‐quartile range). We performed statistical analyses using JMP pro 13 (SAS Institute, Cary, NC, USA). A P value < 0.05 was considered statistically significant. We evaluated differences between groups using Student's t‐test or Mann–Whitney U‐test for continuous variables, and/or the χ 2 test for categorical variables, respectively.
We stratified the patients by tertiles according to their SMIs. Muscle wasting was defined in the categorical analyses as the lowest tertile (whose range is different between men and women). We used the Kaplan–Meier method to estimate the survival probability according to the muscle wasting status starting on the LVAD implantation date. Log‐rank tests were used to compare survival curves. And we performed univariate and multivariate analyses for survival using the Cox proportional hazards model. All continuous parameters were dichotomized at the 25th, 33rd, 50th, and 75th percentiles, and the percentile value with the lowest P value was chosen as the threshold for the analysis.13 Multivariate analyses were performed with univariate predictors determined by a value of P ≤ 0.05. Moreover, we generated receiver‐operating characteristic (ROC) curve analyses to determine the SMI cut‐offs for death. We chose values that maximized the sum of sensitivity and specificity as the cut‐off values to calculate the area under curve.
Results
Our study population consisted of 55 men (70.5%) and 23 women (29.5%). The mean age was 42 ± 12 years. Figure 2 presents a histogram distribution of SMIs. The median SMI for the study patients was 37.6 cm2/m2, whereas the mean SMI value was 37.5 cm2/m2. The SMI cut‐off values for the lowest tertiles were 36.7 cm2/m2 for men and 28.2 cm2/m2 for women, resulting in 26 patients (33.3%) with muscle wasting in this study. Table 1 shows baseline patient characteristics, and Figure 3 shows the type of LVAD in each group. SMA was 87.1 ± 22.3 cm2 in cases with muscle wasting and 113.0 ± 25.2 cm2 in cases without muscle wasting. Furthermore, the SMI was 30.4 ± 6.2 cm2/m2 in patients with muscle wasting and 40.9 ± 7.3 cm2/m2 in patients without muscle wasting. Patients with muscle wasting had lower body mass indexes (BMIs; P = 0.002). The duration from diagnosed heart failure to LVAD implantation was 8.9 ± 8.3 years in patients with muscle wasting and 7.4 ± 6.3 years without muscle wasting (P = 0.39). None of the patients in our cohort had neuromuscular diseases. We found the preoperative serum alanine aminotransferase (ALT) levels were significantly lower in patients with muscle wasting (P = 0.01). We also evaluated inflammation biomarkers, such as C‐reactive protein (CRP) and neutrophil lymphocyte ratios (NLRs). NLRs were calculated as the absolute neutrophil count divided by the absolute lymphocyte count. We found the mean CRP level and the mean NLR were significantly higher in patients with muscle wasting (P = 0.005 and P = 0.001, respectively; Figure 4 ). By contrast, the mean B‐type natriuretic peptide values were not significantly different between the groups.
Figure 2.
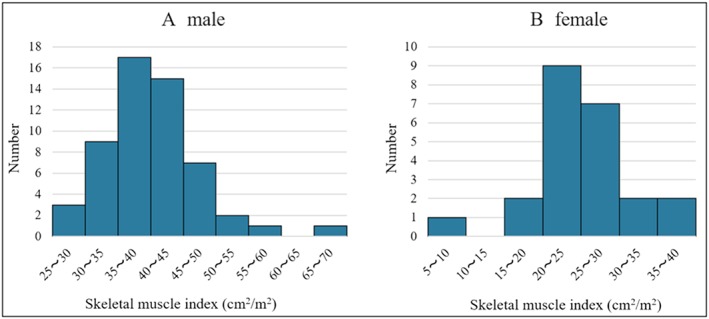
Distribution of skeletal muscle index in men (A) and women (B).
Table 1.
Comparison of baseline, clinical, laboratory findings, echocardiographic data, and right heart catheterization data between patients with and without muscle wasting
| Muscle wasting (n = 26) | Non‐muscle wasting (n = 52) | P value | |
|---|---|---|---|
| Demographic variables | |||
| Sex, male | 18 (69%) | 37 (71%) | 0.86 |
| Age (years) | 45.3 ± 12.0 | 40.0 ± 11.5 | 0.07 |
| Body weight (kg) | 52.6 ± 12.4 | 57.1 ± 9.6 | 0.08 |
| Body height (cm) | 168 ± 8.3 | 166 ± 8.2 | 0.24 |
| Body mass index (kg/m2) | 18.5 ± 3.2 | 20.8 ± 2.9 | 0.002 |
| Body surface area (m2) | 1.58 ± 0.20 | 1.62 ± 0.16 | 0.31 |
| Past medical history | |||
| Diabetes | 4 (15%) | 6 (12%) | 0.63 |
| Cerebrovascular disease | 4 (15%) | 7 (13%) | 0.82 |
| Atrial fibrillation | 4 (15%) | 11 (21%) | 0.54 |
| Smoking | 12 (46%) | 21 (40%) | 0.63 |
| Aetiology of heart failure | |||
| DCM | 19 (73%) | 37 (71%) | |
| d‐HCM | 2 (8%) | 7 (13%) | |
| ICM | 1 (4%) | 3 (6%) | |
| Others | 4 (15%) | 5 (10%) | |
| Medication | |||
| Beta‐blocker | 24 (92%) | 50 (96%) | 0.47 |
| ACE inhibitor/ARB | 20 (77%) | 43 (83%) | 0.54 |
| Aldosterone antagonist | 24 (92%) | 45 (87%) | 0.45 |
| Laboratory data | |||
| Hb (g/dL) | 11.5 ± 1.5 | 11.8 ± 1.9 | 0.46 |
| TP (g/dL) | 6.6 ± 0.5 | 6.6 ± 0.7 | 0.95 |
| Alb (g/dL) | 3.6 ± 0.49 | 3.7 ± 0.54 | 0.65 |
| AST (U/L) | 24 ± 13 | 31 ± 20 | 0.17 |
| ALT (U/L) | 19 ± 12 | 32 ± 24 | 0.01 |
| Na (mEq/L) | 134 ± 3.5 | 135 ± 3.9 | 0.31 |
| Bil (mg/dL) | 1.13 ± 0.5 | 1.25 ± 0.7 | 0.43 |
| BUN (mg/dL) | 21 ± 11 | 20 ± 9.4 | 0.82 |
| Cre (mg/dL) | 1.00 ± 0.35 | 1.10 ± 0.37 | 0.21 |
| eGFR (mL/min/1.73 m2) | 70.6 ± 30.8 | 63.5 ± 22.2 | 0.26 |
| PT‐INR | 1.5 ± 0.38 | 1.4 ± 0.30 | 0.18 |
| HbA1c (NGSP) (%) | 5.8 ± 0.6 | 6.0 ± 0.5 | 0.05 |
| BNP (pg/mL) | 722 (541–859) | 657 (366–1047) | 0.83 |
| Echocardiographic data | |||
| Diastolic dimension (mm) | 74.3 ± 11.1 | 72.6 ± 14.6 | 0.60 |
| Systolic dimension (mm) | 68.9 ± 11.5 | 66.3 ± 14.4 | 0.44 |
| Right heart catheterization data | |||
| Mean RAP (mmHg) | 7.8 ± 4.1 | 9.9 ± 4.6 | 0.07 |
| PCWP (mmHg) | 20.2 ± 7.0 | 22.8 ± 9.0 | 0.21 |
| RVSWI (g/m) | 6.6 ± 3.2 | 6.8 ± 3.8 | 0.86 |
| IABP | 10 | 19 | 0.87 |
| INTERMACS profile | 3 (2–3) | 3 (2–3) | 0.87 |
| HeartMate II Risk Score | 1.04 ± 0.76 | 0.81 ± 0.66 | 0.19 |
ACE, angiotensin‐converting enzyme; Alb, albumin; ALT, alanine aminotransferase; ARB, angiotensin II receptor blocker; AST, aspartate aminotransferase; Bil, bilirubin; BNP, B‐type natriuretic peptide; BUN, blood urea nitrogen; Cre, creatinine; DCM, dilated cardiomyopathy; d‐HCM, dilated phase of hypertrophic cardiomyopathy; eGFR, estimate glomerular filtration rate; Hb, haemoglobin; HbA1c, haemoglobin A1c; IABP, intra‐aortic balloon pumping; ICM, ischaemic cardiomyopathy; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; Na, sodium; NGSP, National Glycohemoglobin Standardization Program; PCWP, pulmonary capillary wedge pressure; PT‐INR, prothrombin time international normalized ratio; RAP, right atrial pressure; RVSWI, right ventricular stroke work index; TP, total protein.
Figure 3.
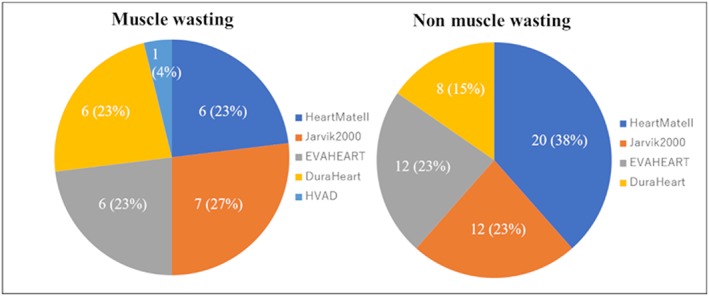
Type of left ventricular assist device in patients with and without muscle wasting.
Figure 4.
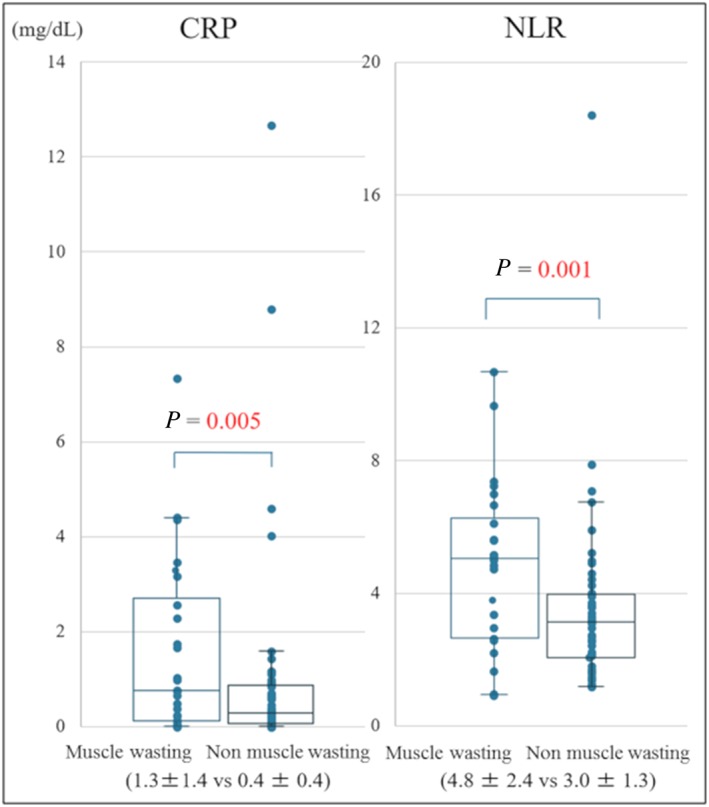
Bee swarm box plot of CRP and NLR in patients with and without muscle wasting. CRP, C‐reactive protein; NLR, neutrophil lymphocyte ratio.
We explored the relationship between SMIs and values of Swan–Ganz catheter data in order to reveal associations between muscle wasting and the heart failure statuses in the patients. However, we found no associations between the SMI values and right atrial pressure, pulmonary capillary wedge pressure, or right ventricle stroke work index (Table 1).
During the mean follow‐up of 738 ± 379 days, 10 deaths occurred in 78 patients with a mortality of 12.8%. While 26.9% of the patients with muscle wasting died (7 of the 26 patients), only 5.8% of those without muscle wasting died (3 of 52). Regarding the causes of death, five died of infections, four died owing to fatal bleeding, and one suffered from a fatal stroke. We found no significant difference in the mortality rate at 90 days after the LVAD operation (3.8% vs. 0%), whereas there was a marked difference in the long‐term survival rate between patients with and without muscle wasting (P = 0.0094; Figure 5 ). Our univariate and multivariate Cox analyses showed an association between muscle wasting and mortality (hazard ratio: 4.32; 95% CI: 1.19–20.2) (Table 2). On the other hand, the rate of intracranial haemorrhage and driveline infection was not different between patients with and without muscle wasting. Intracranial haemorrhage occurred in the four patients with sarcopenia and eight patients without muscle wasting [P = 0.97; Figure 6 A]. Driveline infection occurred in the 16 patients with muscle wasting and 38 patients without muscle wasting during the follow‐up period [P = 0.90; Figure 6 B].
Figure 5.
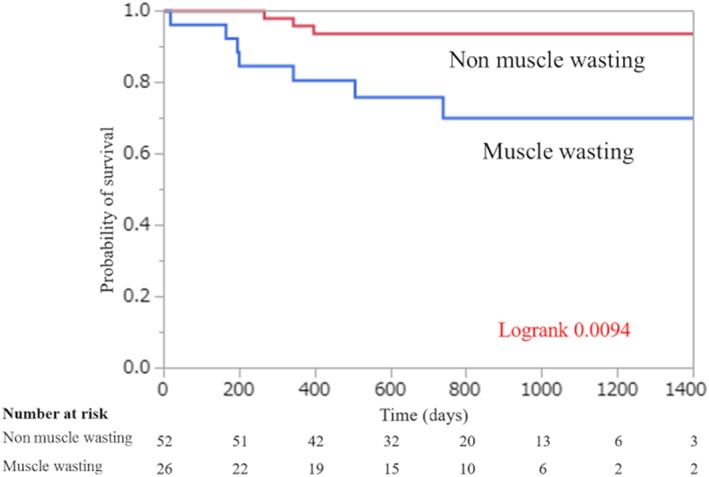
Probability of survival in patients with and without muscle wasting undergoing LVAD implantation.
Table 2.
Univariate and multivariate Cox proportional analysis of preoperative parameters predicting mortality after left ventricular assist device implantation
| Preoperative parameter | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Age (≧42 years) | 3.92 (0.97–26.1) | 0.05 | 3.16 (0.78–21.2) | 0.11 |
| Male | 1.56 (0.39–10.3) | 0.56 | ||
| Hb (≦11.2 g/dL) | 1.49 (0.43–5.84) | 0.53 | ||
| Alb (≦4.0 g/dL) | 3.55 (0.67–65.5) | 0.16 | ||
| Bil (≦0.7 mg/dL) | 1.97 (0.50–6.93) | 0.31 | ||
| Cre (≧1.2 mg/dL) | 3.03 (0.84–10.9) | 0.09 | ||
| PT‐INR (>1.6) | 3.12 (0.87–11.2) | 0.08 | ||
| CRP (≧0.16 mg/dL) | 2.13 (0.53–14.1) | 0.31 | ||
| NLR (≧2.7) | 2.14 (0.54–14.2) | 0.30 | ||
| IABP (+) | 2.04 (0.56–7.40) | 0.27 | ||
| INTERMACS 2 | 2.25 (0.64–8.84) | 0.20 | ||
| Muscle wasting | 5.01 (1.39–23.2) | 0.013 | 4.32 (1.19–20.2) | 0.03 |
Alb, albumin; Bil, bilirubin; Cre, creatinine; Hb, haemoglobin; IABP, intra‐aortic balloon pumping; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; PT‐INR, prothrombin time international normalized ratio.
Figure 6.
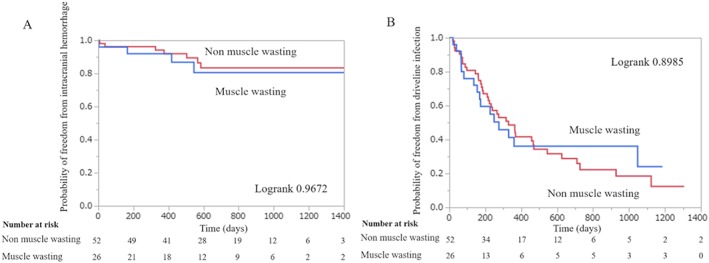
Probability of freedom from intracranial haemorrhage (A) and probability of freedom from driveline infection (B) in patients with and without muscle wasting undergoing LVAD implantation.
The ROC curve analysis identified the optimal SMI cut‐off values to predict all‐cause mortality to be 36.2 cm2/m2 in men and 28.0 cm2/m2 in women, and the areas under the curve were 0.61 in men and 0.86 in women. These cut‐off values had a sensitivity of 62.5% and a specificity of 76.6% in men and a sensitivity of 100% and a specificity of 76.2% in women.
Discussion
Sarcopenia is a co‐morbidity of elderly patients associated with increased mortality independent of age and other clinical and functional variables.14, 15, 16 The prevalence of sarcopenia in patients with heart failure is higher at 19.5% than that in healthy subjects of the same age.17 However, the reports on the prevalence of sarcopenia in heart failure include diverse backgrounds or differing methods for evaluating sarcopenia. For example, a report indicated sarcopenia in as much as 47% of young patients (<55 years of age) suffering from dilated cardiomyopathy.18 Evidence has been mounting on sarcopenia or other skeletal muscle parameters being a risk marker for adverse outcomes in heart failure.19 Our study demonstrated that muscle wasting was also a negative prognostic factor for long‐term survival in advanced heart failure patients undergoing LVAD implantation. This is the first report on muscle wasting in advanced heart failure patients undergoing LVAD implantation in Japan.
Various definitions and diagnostic criteria for sarcopenia exist.20, 21, 22, 23 We used SMIs as muscle wasting markers because the SMA at the L3 level has been shown to correlate strongly with the body muscle distribution24 and to be useful as a prognostic marker.25 We diagnosed muscle wasting in those with SMIs at the bottom tertile according to a publication in LVAD populations.26 These thresholds were found to be somewhat higher than those calculated by our ROC curve analysis for death. Therefore, there might be more accurate and appropriate threshold for muscle wasting. However, there was a report about subjects with hepatocellular carcinoma, which demonstrated very similar cut‐off value for muscle wasting with ours.25 The information on abdominal muscle mass calculated by CT has been reported to have less utility than the information on muscle function (such as the grip strength).27 On the other hand, muscle function is greatly affected by the inactivity level, which is derived from the status of heart failure.17, 18, 28, 29 By contrast, the abdominal muscle mass may have some different implications independent of the inactivity level; indeed, the parameters of heart failure status did not correlate with abdominal muscle mass in our study.
In our study, patients with muscle wasting had lower ALT levels than those without muscle wasting. Other studies have reported that low ALT is a biomarker for frailty, disability, sarcopenia, and malnutrition.30, 31 ALT is mainly derived from the liver; however, a small amount of ALT is derived from skeletal muscle.30 ALT is an enzyme in gluconeogenesis, which converts alanine into pyruvate for glucose production. We think that lower ALT in muscle wasting group is due to reduced release by skeletal muscle or decreased muscle proteins for gluconeogenesis. The BMI was also lower in patients with muscle wasting than in those without muscle wasting. This finding is consistent with the previous studies,17 and heart failure patients with higher BMIs have been shown to have better outcomes in terms of mortality and hospitalization rates (obesity paradox).32 However, the determining factors for the SMI in patients undergoing LVAD implantation were not identified in our study, and further robust investigations to identify them are warranted. Hypoalbuminaemia, renal dysfunction, and coagulopathy, which were reported to be associated with death within 90 days after the LVAD operation,33 did not have any relationships with SMI.
In a study similar to ours, Heberton et al. investigated about the relationship between overall mortality and low psoas muscle mass and demonstrated no difference in overall mortality according to psoas muscle area evaluated using CT images.26 This discrepancy with our results may be due to the fact that all LVADs were implanted as a bridge‐to‐transplant therapy and the HeartMate II risk scores33 were lower in our study. The patients' body surface area (BSA) was also low in our study. Lower BSA is an independent risk factor for mortality during the LVAD support34; therefore, these characteristics also affected the clinical course in the study patients. As a result, the effects of muscle wasting may have been more pronounced in our study as compared with their study.
One possible explanation of the poor prognosis in the presence of muscle wasting is that it exacerbated the severity of complications after LVAD implantation through an inflammatory pathway. We checked CRP and NLR as markers of inflammation, and these markers were increased in the presence of muscle wasting. Sarcopenia is known to be associated with inflammation.17, 35, 36 The CRP is a non‐specific serum acute‐phase inflammatory marker secreted by hepatocytes under the influence of interleukin‐6.37 NLR is thought to reflect ongoing inflammation that significantly modifies the immune responses.38 These inflammatory markers were reported to be higher in patients with sarcopenia.39 In the current study, the rate of complications such as cerebrovascular events and driveline infections was comparable between the patients with muscle wasting and those without it [Figure 6 (A,B)], whereas fatal cases with these complications were highly observed in the presence of muscle wasting. This inflammatory precondition may aggravate the response to physical stresses such as cerebrovascular events and infections, and patients with sarcopenia may have difficulty in tolerating these complications, resulting in higher mortality. However, there had been no report that demonstrated the interaction between preoperative inflammatory precondition and long‐term mortality after LVAD implantation. Clarifying the underlying mechanism for the relationship between preoperative sarcopenia and poor prognosis can help us optimize the therapeutic strategies against complications after LVAD implantation. It is expected that strategy, including exercise training and nutritional support, for prevention of muscle wasting should be established to improve sarcopenia in advanced heart failure.
Several limitations must be considered in our study. First, this is a retrospective study at a single centre with a small patient cohort. Further studies including large sample sizes and multiple institutions are needed. Second, in our cohort, the small number of events regarding patient deaths limited the statistical power of our analysis to detect weaker risk factors for survival. Third, the timing of CT varied for each patient and the median number of days from CT to LVAD implantation was 14 (7–41) days. Fourth, a single observer measured the muscle masses of all patients. Finally, we used only one component of sarcopenia other than measurements of muscle power and function using more specific tests.
In conclusion, muscle wasting is associated with higher mortality in patients with LVAD implantation. Assessment of the abdominal SMA using CT imaging prior to LVAD implantation is useful to predict mortality.
Conflict of interest
None declared.
Acknowledgement
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan through grant‐in‐aid (17K09488 to E.A.).
Tsuji, M. , Amiya, E. , Hatano, M. , Nitta, D. , Maki, H. , Bujo, C. , Saito, A. , Hosoya, Y. , Minatsuki, S. , Hara, T. , Nemoto, M. , Kagami, Y. , Endo, M. , Kimura, M. , Kinoshita, O. , Nawata, K. , Morita, H. , Ono, M. , and Komuro, I. (2019) Abdominal skeletal muscle mass as a predictor of mortality in Japanese patients undergoing left ventricular assist device implantation. ESC Heart Failure, 6: 526–535. 10.1002/ehf2.12429.
References
- 1. Writing Group Members , Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee , Stroke Statistics Subcommittee . Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016; 133: 447–454. [DOI] [PubMed] [Google Scholar]
- 2. Farrar DJ, Holman WR, McBride LR, Kormos RL, Icenogle TB, Hendry PJ, Moore CH, Loisance DY, El‐Banayosy A, Frazier H. Long‐term follow‐up of Thoratec ventricular assist device bridge‐to‐recovery patients successfully removed from support after recovery of ventricular function. J Heart Lung Transplant 2002; 21: 516–521. [DOI] [PubMed] [Google Scholar]
- 3. Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH. Use of a continuous‐flow device in patients awaiting heart transplantation. N Engl J Med 2007; 357: 885–896. [DOI] [PubMed] [Google Scholar]
- 4. Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH, Investigators HMII. Advanced heart failure treated with continuous‐flow left ventricular assist device. N Engl J Med 2009; 361: 2241–2251. [DOI] [PubMed] [Google Scholar]
- 5. Kiernan MS, Sundareswaran KS, Pham DT, Kapur NK, Pereira NL, Strueber M, Farrar DJ, DeNofrio D, Rogers JG. Preoperative determinants of quality of life and functional capacity response to left ventricular assist device therapy. J Card Fail 2016; 22: 797–805. [DOI] [PubMed] [Google Scholar]
- 6. Jakovljevic DG, McDiarmid A, Hallsworth SPM, Ninkovic VM, Parry G, Schueler S, Trenell MI, MacGowan GA. Effect of left ventricular assist device implantation and heart transplantation on habitual physical activity and quality of life. Am J Cardiol 2014; 114: 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fukushima N, Ono M, Saiki Y, Sawa Y, Nunoda S, Isobe M. Registry Report on Heart Transplantation in Japan (June 2016). Circ J 2017; 81: 298–303. [DOI] [PubMed] [Google Scholar]
- 8. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cruz‐Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, Chen LK, Fielding RA, Martin FC, Michel JP, Sieber C, Stout JR, Studenski SA, Vellas B, Woo J, Zamboni M, Cederholm T. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014; 43: 748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uchmanowicz I, Łoboz‐Rudnicka M, Szeląg P, Jankowska‐Polańska B, Łoboz‐Grudzień K. Frailty in heart failure. Curr Heart Fail Rep 2014; 11: 266–273. [DOI] [PubMed] [Google Scholar]
- 11. Kato A. Muscle wasting is associated with reduced exercise capacity and advanced disease in patients with chronic heart failure. Future Cardiol 2013; 9: 767–770. [DOI] [PubMed] [Google Scholar]
- 12. Szulc P, Feyt C, Chapurlat R. High risk of fall, poor physical function, and low grip strength in men with fracture‐the STRAMBO study. J Cachexia Sarcopenia Muscle 2016; 7: 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kormos RL, Teuteberg JJ, Pagani FD, Russell SD, John R, Miller LW, Massey T, Milano CA, Moazami N, Sundareswaran KS, Farrar DJ, HeartMate II Clinical Investigators . Right ventricular failure in patients with the HeartMate II continuous‐flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010; 139: 1316–1324. [DOI] [PubMed] [Google Scholar]
- 14. Genton L, Graf CE, Karsegard VL, Kyle UG, Pichard C. Low fat‐free mass as a marker of mortality in community‐dwelling healthy elderly subjects. Age Ageing 2013; 42: 33–39. [DOI] [PubMed] [Google Scholar]
- 15. Batsis JA, Mackenzie TA, Barre LK, Lopez‐Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr 2014; 68: 1001–1007. [DOI] [PubMed] [Google Scholar]
- 16. Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population‐based sample of community‐dwelling older adults. J Cachexia Sarcopenia Muscle 2016; 7: 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W, Anker SD, von Haehling S. Muscle wasting in patients with chronic heart failure: results from the studies investigating co‐morbidities aggravating heart failure (SICA‐HF). Eur Heart J 2013; 34: 512–519. [DOI] [PubMed] [Google Scholar]
- 18. Hajahmadi M, Shemshadi S, Khalilipur E, Amin A, Taghavi S, Maleki M, Malek H, Naderi N. Muscle wasting in young patients with dilated cardiomyopathy. J Cachexia Sarcopenia Muscle 2017; 8: 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamada S, Kamiya K, Kono Y. Frailty may be a risk marker for adverse outcome in patients with congestive heart failure. ESC Heart Fail 2015; 2: 168–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology—update 2014. J Cachexia Sarcopenia Muscle 2014; 5: 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC‐F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016; 7: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scherbakov N, Doehner W. Searching for a relevant definition of sarcopenia: results from the cross‐sectional EPIDOS study. J Cachexia Sarcopenia Muscle 2016; 7: 100–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dupuy C, Lauwers‐Cances V, Guyonnet S, Gentil C, Abellan Van Kan G, Beauchet O, Schott AM, Vellas B, Rolland Y. Searching for a relevant definition of sarcopenia: results from the cross‐sectional EPIDOS study. J Cachexia Sarcopenia Muscle 2015; 6: 144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011; 12: 489–495. [DOI] [PubMed] [Google Scholar]
- 25. Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami T, Sato M, Uchino K, Enooku K, Kondo Y, Asaoka Y, Tanaka Y, Ohtomo K, Shiina S, Koike K. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 2015; 63: 131–140. [DOI] [PubMed] [Google Scholar]
- 26. Heberton GA, Nassif M, Bierhals A, Novak E, LaRue SJ, Lima B, Hall S, Silvestry S, Joseph SM. Usefulness of psoas muscle area determined by computed tomography to predict mortality or prolonged length of hospital stay in patients undergoing left ventricular assist device implantation. Am J Cardiol 2016; 118: 1363–1367. [DOI] [PubMed] [Google Scholar]
- 27. Wang CW, Feng S, Covinsky KE, Hayssen H, Zhou LQ, Yeh BM, Lai JC. A comparison of muscle function, mass, and quality in liver transplant candidates: results from the functional assessment in liver transplantation study. Transplantation 2016; 100: 1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bekfani T, Pellicori P, Morris DA, Ebner N, Valentova M, Steinbeck L, Wachter R, Elsner S, Sliziuk V, Schefold JC, Sandek A, Doehner W, Cleland JG, Lainscak M, Anker SD, von Haehling S. Sarcopenia in patients with heart failure with preserved ejection fraction: impact on muscle strength, exercise capacity and quality of life. Int J Cardiol 2016; 222: 41–46. [DOI] [PubMed] [Google Scholar]
- 29. Dos Santos MR, Saitoh M, Ebner N, Valentova M, Konishi M, Ishida J, Emami A, Springer J, Sandek A, Doehner W, Anker SD, von Haehling S. Sarcopenia and endothelial function in patients with chronic heart failure: results from the Studies Investigating Comorbidities Aggravating Heart Failure (SICA‐HF). J Am Med Dir Assoc 2017; 18: 240–245. [DOI] [PubMed] [Google Scholar]
- 30. Le Couteur DG, Blyth FM, Creasey HM, Handelsman DJ, Naganathan V, Sambrook PN, Seibel MJ, Waite LM, Cumming RG. The association of alanine transaminase with aging, frailty, and mortality. J Gerontol A Biol Sci Med Sci 2010; 65: 712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramaty E, Maor E, Peltz‐Sinvani N, Brom A, Grinfeld A, Kivity S, Segev S, Sidi Y, Kessler T, Sela BA, Segal G. Low ALT blood levels predict long‐term all‐cause mortality among adults. A historical prospective cohort study. Eur J Intern Med 2014; 25: 919–921. [DOI] [PubMed] [Google Scholar]
- 32. von Haehling S, Lainscak M, Doehner W, Ponikowski P, Rosano G, Jordan J, Rozentryt P, Rauchhaus M, Karpov R, Tkachuk V, Parfyonova Y, Zaritskey AY, Shlyakhto EV, Cleland JG, Anker SD. Diabetes mellitus, cachexia and obesity in heart failure: rationale and design of the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). J Cachexia Sarcopenia Muscle 2010; 1: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cowger J, Sundareswaran K, Rogers JG, Park SJ, Pagani FD, Bhat G, Jaski B, Farrar DJ, Slaughter MS. Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. J Am Coll Cardiol 2013; 61: 313–321. [DOI] [PubMed] [Google Scholar]
- 34. Komoda T, Drews T, Hetzer R, Lehmkuhl HB. Lower body surface area is highly related to mortality due to stroke or systemic bleeding in patients receiving an axial flow blood pump as a left ventricular assist device. Eur J Cardiothorac Surg 2013; 43: 1036–1042. [DOI] [PubMed] [Google Scholar]
- 35. von Haehling S, Steinbeck L, Doehner W, Springer J, Anker SD. Muscle wasting in heart failure: an overview. Int J Biochem Cell Biol 2013; 45: 2257–2265. [DOI] [PubMed] [Google Scholar]
- 36. Josiak K, Jankowska EA, Piepoli MF, Banasiak W, Ponikowski P. Skeletal myopathy in patients with chronic heart failure: significance of anabolic‐androgenic hormones. J Cachexia Sarcopenia Muscle 2014; 5: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gabay C, Kushner I. Acute‐phase proteins and other systemic responses to inflammation. N Engl J Med 1999; 340: 448–454. [DOI] [PubMed] [Google Scholar]
- 38. Zhang X, Zhang W, Yuan X, Fu M, Qian H, Xu W. Neutrophils in cancer development and progression: roles, mechanisms, and implications (Review). Int J Oncol 2016; 49: 857–867. [DOI] [PubMed] [Google Scholar]
- 39. Malietzis G, Johns N, AI‐Hassi HO, Knight SC, Kennedy RH, Fearon KC, Aziz O, Jenkins JT. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann Surg 2016; 263: 320–325. [DOI] [PubMed] [Google Scholar]


