Abstract
Aims
Understanding the pathophysiological background on haemodynamic changes in acute myocardial infarction and during its interventional treatment is important to adequately use mechanical circulatory support.
Methods and results
We describe haemodynamic simulations based on a real case scenario of infarct‐related ischaemia with beginning haemodynamic compromise illustrating the advantage of active haemodynamic support. The patient case used for computer simulation is that of an acute coronary syndrome, slightly hypotonic. The right coronary artery is chronically occluded, and both left main and a saphenous vein graft to the left anterior descending coronary artery (LAD) show subtotal stenosis. In this scenario used for computer modelling of haemodynamics, we illustrate how unprotected percutaneous coronary intervention would limit coronary blood flow and constantly reduce myocardial contractility until cardiac arrest occurs. The simulation demonstrates how an intra‐aortic balloon pump would delay but not prevent that compromise and how an Impella microaxial pump will actively support cardiac output and stabilize haemodynamics even when prolonged balloon inflations are performed, which will temporarily stop coronary perfusion.
Conclusions
The simulation illustrates how temporary circulatory support with an Impella microaxial pump can stabilize haemodynamics and allow for a safe procedure in an unstable patient. Using computer simulation of haemodynamics to understand changes in haemodynamics when performing interventions in unstable patients might help to properly select a suitable support device if needed.
Keywords: Cardiogenic shock, Impella, Mechanical circulatory support, Haemodynamics, LV function
Introduction
For haemodynamically unstable acute coronary syndrome (ACS), urgent percutaneous coronary intervention (PCI) is the recommended treatment strategy to prevent ongoing cardiac and systemic ischaemia. Persistent myocardial ischaemia can result in cardiogenic shock, an acute life‐threatening condition, which results in impaired end‐organ perfusion and oxygenation. Invasive ventilation and medical treatment with inotropes or vasopressors are necessary in many cases but are associated with worse long‐term prognosis.1, 2 Medical treatment frequently fails to sufficiently restore haemodynamics. Dedicated mechanical circulatory support devices that actively unload the left ventricle allow for myocardial recovery or save time as bridge to definitive treatment.2
An increasing number of haemodynamic support devices are currently available with differing individual theoretical advantages. These comprise intra‐aortic balloon pump (IABP), veno‐arterial extra‐corporeal membrane oxygenation, TandemHeart, and several percutaneous ventricular assist device (pVADs) that both support the circulation and provide left ventricular (LV) unloading such as Impella microaxial pumps (Abiomed), iVAC 2L (PulseCath), and HeartMate PHP (Thoratec/St. Jude/Abbott) (Table 1).3, 4
Table 1.
Overview of percutaneous ventricular support devices
| iVAC 2L | PHP | Impella 2.5 | Impella CP | Impella 5.0/LD | Tandem Heart | ECMO | |
|---|---|---|---|---|---|---|---|
| Catheter/sheath | 15/17 F | 13/14 F | 9 F | 9 F | 9 F | — | — |
| Canula size | 17 F | 24 F | 12 F | 14 F | 21 F | 21 F ven. 12–19 F art. | 17–21 F ven. 16–19 F art. |
| Max.flow (L/min) | 2.0 | 4.0 | 2.5 | 3.7–4.0 | 5.0 | 4.0 | 7.0 |
| Access | Percutaneous femoral | Percutaneous femoral | Percutaneous femoral | Percutaneous femoral | Surgical femoral/subclavian/transaortic (LD) | Percutaneous femoral | Percutaneous femoral |
| LV unloading | + | ++ | + | ++ | +++ | +++ | −− |
| Anticoagulation | + | − | + | + | + | + | + |
| CE/FDA | +/− | +/− | +/+ | +/+ | +/+ | +/+ | +/+ |
CE/FDA, approved by European (CE) and US‐American (FDA) authorities; ECMO, extra‐corporeal membrane oxygenation; F, catheter‐/sheath size in French; LV, left ventricular; PHP, percutaneous heart pump.
The pVADs, and particularly Impella CP, have been adopted in clinical practice more frequently, because the ubiquitously available IABP could not demonstrate an advantage over standard‐of‐care medical treatment in infarct‐related cardiogenic shock in a randomized trial.5 In experimental studies, LV unloading reduces wall tension even in territories of chronic occlusions and leads to improved myocardial perfusion without even revascularizing a critical last vessel stenosis.6 In large anterior myocardial infarctions, which are haemodynamically just borderline stable, decompensation can frequently occur within the first hours following reperfusion, and this could be prevented by actively unloading the LV and providing circulatory support, for example, by an Impella device.7 Our own experience in the HAnnover Cardiac Unloading REgistry (HACURE) as well as reports from other shock centres show that LV unloading using Impella CP in cardiogenic shock rapidly reduces the amount of infused vasopressors/inotropes in concordance with normalization of lactate levels representing improved systemic perfusion and subsequently preventing end‐organ ischaemia.8, 9
In this article, we illustrate haemodynamic changes in a borderline stable ACS patient, when PCI without active support would lead to deterioration of cardiac function, using a computer simulation of the cardiovascular system.10, 11, 12 We simulate PCI without support and with support by IABP or the Impella CP pVAD on the basis of an actual case that recently presented to our institution.
Methods
Mathematical modelling and simulation allow for an in‐depth examination of the cardiovascular system and provides the opportunity to develop deeper understanding. For the illustrations shown in this article, we used the Harvi‐Online simulation.10 The modelling is based on previous publications explaining the underlying simulations and validations in more detail.11, 13 Simulations are based on patient‐specific data [blood pressure, ejection fraction, heart rate (HR), cardiac outputs (COs), etc.] provided to the simulation, which adjusts parameter values to match specified patient conditions. The haemodynamic signals (pressure–volume loops, pressure, and coronary flow curves over time) are derived from the simulation and are not actual patient‐derived signals.
Results
Haemodynamics during coronary ischaemia
The basics of LV mechanics are displayed in pressure–volume loops (PV loops; Figure 1 , top). In brief, the four phases of the cardiac cycle define an area, which in its width reflects stroke volume and in its height represents blood pressure. The vertical parts of the PV loop delineate the end‐systolic (left border) and end‐diastolic (right border) LV volumes. Cardiac stroke work (SW) is clinically estimated as the product of stroke volume and mean arterial pressure (MAP) but is more accurately indexed by the area confined by the PV loop. Cardiac power is defined as the product of SW and HR, which is mathematically equivalent to the product of MAP and CO. The chemical energy (i.e. oxygen consumption) required to generate the illustrated work and power, however, is defined by the SW plus the area to the left of the loop bounded by LV end‐systolic and end‐diastolic pressure–volume relationships, which is referred to as the potential energy (PE) (Figure 1 , red‐shaded area). The sum of SW and PE is called the pressure–volume area (PVA), which correlates with oxygen consumption per beat.13
Figure 1.
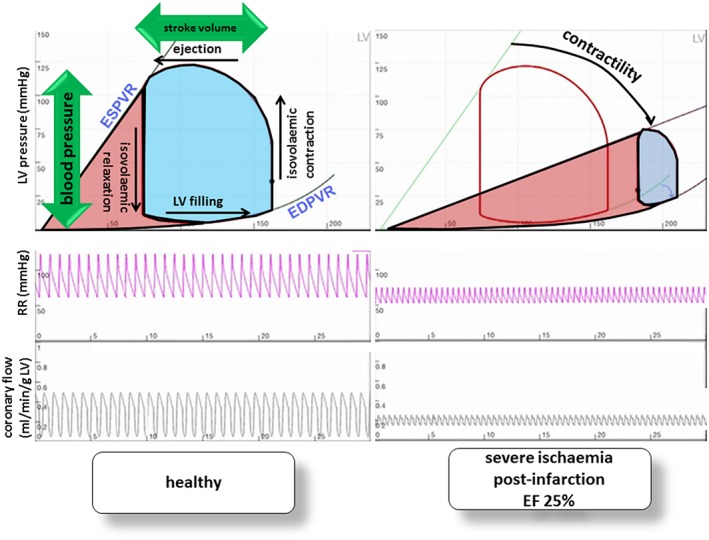
Simulation of pressure–volume loops under healthy conditions (left) and during ischaemia illustrating a patient with severe ischaemia and ejection fraction (EF) 25% after chronic myocardial infarction (right). Cardiac power is physiologically defined as the product of stroke volume and blood pressure, the area confined by the PV loop (blue). The work required to generate the illustrated cardiac power, however, is defined by the area of the PV loop (marked in blue) plus the area left to it bordered by LV end‐systolic (ESPVR) and end‐diastolic (EDPVR) pressure–volume relationships (marked in red). More ischaemia results in lower contractility and smoother ESPVR, and the PV loop is shorter, indicating reduced ejection and lower blood pressure; reduced ejection results in a higher LV volume at the beginning of the cardiac cycle, so PV loops move to the right; higher end‐systolic filling results in reduced filling at all, and PV loops become narrower, meaning reduced stroke volume as a consequence. Less stroke volume at lower systolic pressure and increased LV filling result in diminished coronary flow. ESPVR, end‐systolic pressure–volume relationship; EDPVR, end‐diastolic pressure–volume relationship.
If myocardial ischaemia occurs, contractility is reduced, blood pressure drops, and the PV loop becomes narrower and shifts to the right (Figure 1 , right side).13 The simulation illustrates how loss in contractility (curved arrow in the right part of Figure 1 ) leads to decreased coronary flow (Figure 1 , bottom right) and lower arterial blood pressure (Figure 1 , middle right) and finally results in LV overloading (Figure 1 , upper right). Because these haemodynamic changes lead to LV overloading in the sense of increased end‐diastolic volume and increase in overall PVA, strategies aiming for LV unloading to reduce myocardial oxygen demand have been shown to have beneficial effects in pre‐clinical models of myocardial infarction.14
Haemodynamic effects of coronary stenosis with normal left ventricular function conditions undergoing left main percutaneous coronary intervention
We first illustrate what happens when a 70% left main stenosis is suddenly introduced in an LV with normal LV function (Figure 2 , left). Upon creating the stenosis, coronary blood flow abruptly decreases but then gradually increases in response to coronary autoregulation [Figure 2 (C)]. Despite the decrease of coronary flow, there is no detectible change in LV contractility or arterial pressure because the degree of flow restriction is not sufficient to create an imbalance between energy supply and demand [Figure 2 (A) and 2 (B)]. This matches clinical conditions of patients with significant left main disease but in whom LV function is normal. The consequences of left main PCI are simulated by creating a further, critical reduction in coronary flow [to a degree that is comparable with the simulated non‐ST‐elevation myocardial infarction (NSTEMI) case shown later in this report; Figure 2 , right panel]. At the point of balloon inflation, flow is further reduced, and, over time, there is minimal rebound because the limits of autoregulation have been reached [Figure 2 (F)]. With this degree of flow restriction, a gradual decline in contractility [Figure 2 (D)] and in arterial pressure [Figure 2 (E)] is seen. However, under these conditions, the contractility reaches a new, lower but stable level within ~35 s; the decline in systolic blood pressure is only ~10 mmHg. These values and time courses are realistic when compared with those in clinical experience.
Figure 2.
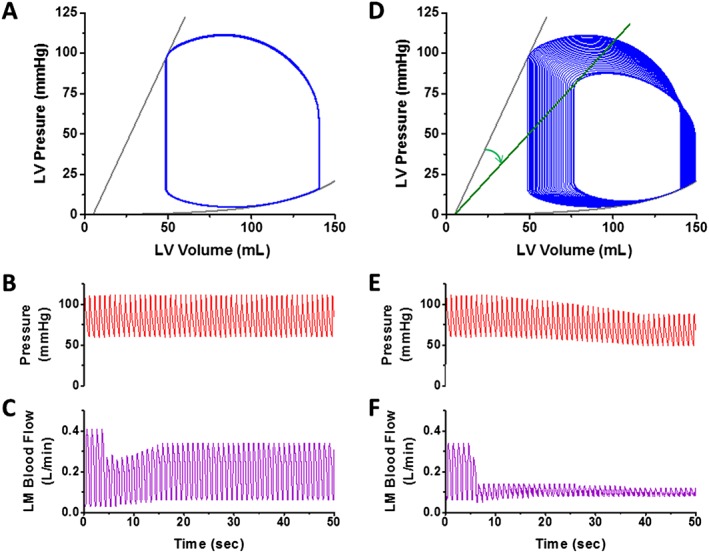
Hypothetical haemodynamic effects of an acutely developing 70% left main stenosis (left) and of prolonged PCI in that stenosis (right) on PV loops (A, D), aortic pressure (B, E), and left main blood flow (C, F) in a simulated uncompromised and haemodynamically stable patient. For detailed description, see main text. LM, left main stem; LV, left ventricle.
Haemodynamic effect of left ventricular unloading in a non‐ST‐elevation myocardial infarction undergoing urgent percutaneous coronary intervention
The haemodynamics of PCI in a compromised LV are illustrated with an example of a 74‐year‐old patient presenting urgently with NSTEMI to the catheterization laboratory, already receiving norepinephrine and dobutamine to achieve a blood pressure of 81/52 mmHg and an HR of 93 b.p.m. One year prior to the current presentation, the patient underwent aortic valve replacement with a biological prosthesis for severe aortic stenosis and coronary artery bypass surgery for left main stenosis using two venous bypasses, one to the LAD and one venous sequence to an obtuse marginal branch of the left circumflex artery and the distal right coronary artery (RCA). Urgent coronary angiography reveals an occluded RCA, a subtotal left main stenosis with proximal LAD occlusion, and contrast flow from the marginal branch into the sequential venous graft towards the RCA. The sequential venous graft is occluded at the aortic anastomosis; the second venous bypass graft to the LAD shows a subtotal stenosis at its aortic anastomosis resulting in perfusion of all vital myocardium through two subtotal stenoses (one in the left main coronary artery and one in the LAD bypass).
Haemodynamic simulation of the case (Figure 3 ) shows impressively what might occur at the moment of occluding one of two subtotal stenoses by balloon insertion during attempted PCI in this patient with compromised LV function and relative hypotension. In contrast to the simulation in a healthy patient [Figure 1 (D–F)], coronary obstruction rapidly caused complete loss of contractility within ~15–20 s, visualized by shortening and narrowing of PV loops until no ejection occurs (Figure 3 , top left, with straight arrow showing the trajectory over time of the end‐systolic pressure–volume point from the starting value to the point of no pressure generation); the corresponding time course of change of aortic pressure is shown in the tracings just below the PV loops. Higher‐resolution LV and aortic pressure tracings prior to PCI balloon inflation and during the final few seconds of the recording are shown in the bottom two panels.
Figure 3.
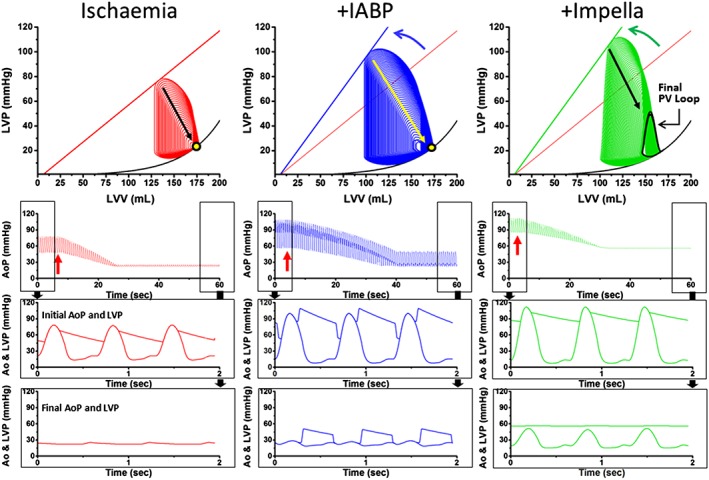
Simulation of haemodynamics in a NSTEMI case with all myocardium being perfused via two subtotal stenoses to demonstrate circulatory effects of ischaemia (left column), support by intra‐aortic balloon pump (IABP, middle column), or LV unloading using an Impella microaxial pump (right column). The simulation demonstrates the loss of contractility and cardiac output of an impaired left ventricle during balloon insertion in one of the subtotal stenoses without support (bottom left), on IABP (bottom middle), or on Impella CP (bottom right). The curved arrows in the upper panels indicate the initial improvement in contractility upon activation of either IABP or Impella before balloon insertion compared with ischaemia; the straight arrows indicate the decline of contractility following balloon‐mediated occlusion of one of the subtotal stenoses. The second panel from the top illustrates the consequence on aortic pressure over time. Aortic and left ventricular pressure are shown before (third panel from top) and after (bottom panel) coronary occlusion by balloon angioplasty. Clinical details are given in the main text. AoP, aortic pressure; IABP, intra‐aortic balloon pump; LVP, left ventricular pressure; LVV, left ventricular volume; PV loop, pressure–volume loop.
Using an IABP in this case (middle panel) has the potential to enhance LV contractility owing to increased diastolic blood pressure and coronary flow (higher and left‐shifted PV loops as illustrated by the curved arrow in Figure 3 , top, middle panel) improving haemodynamic conditions at the start of the procedure. However, during PCI, the simulation illustrates a slightly prolonged time to haemodynamic collapse, which also ultimately results in complete loss of ventricular function (straight arrow on the PV loop).
In contrast, when using active support and LV unloading, such as provided by an Impella microaxial pVAD, we see an improvement of the pre‐PCI haemodynamic conditions (higher and wider PV loops shifted to the left shown by the curved arrow in Figure 3 , top right) as was noted with the IABP simulation. We also note, in comparison with IABP, that the LV is relatively unloaded with a slightly lower end‐diastolic volume at the pre‐PCI stage and, therefore, lower PVA. However, notice the markedly different effect of active support during prolonged coronary occlusion during PCI. In this case, active haemodynamic support maintains sufficient MAP as LV contractility declines, which, in turn, can also maintain sufficient myocardial perfusion (through collateral flow) such that LV contractility can be maintained at a lower but stable level (bottom right). This is a condition referred to as myocardial hibernation.
In this case, although pulsatility can be lost during the procedure, such mechanical support by an Impella CP pVAD can provide ~3.4 L/min active support by the system, resulting in laminar blood flow (without pulsatility) at a mean pressure of ~60 mmHg, enabling the patient to feel comfortable without distress and while maintaining consciousness throughout the PCI (Figure 3 , bottom right). While pulsatile pressure changes are recordable in the LV itself, systemic blood flow is laminar (non‐pulsatile). A missing peripheral pulse should not alarm the interventional cardiologist as long as the patient feels comfortable; it is simply a sign of complete support.
Consistent with these fundamental principles, the patient was fully awake and felt comfortable during the actual case under discussion. Following successful complete revascularization, catecholamines were completely weaned and the Impella was uneventfully explanted.
Discussion
The haemodynamic simulations shown in this article provide the foundation for understanding pathophysiology of coronary ischaemia and hibernation during PCI in compromised patients and illustrate why circulatory support by a pVAD and LV unloading are a useful option in such an LV overload situation. A pVAD provides active haemodynamic support and prevents circulatory arrest, which would be prevented neither in unassisted PCI nor by IABP support.
An increasing number of haemodynamic support devices are currently available with differing theoretical advantages. Owing to lack of prospective data, current European and American guidelines only weakly recommend (based on expert opinion) consideration of the use of pVADs for refractory cardiogenic shock undergoing PCI (‘may be considered’; expert opinion),15, 16 in acute heart failure irrespective of ischaemic origin (‘Short‐term mechanical support … can be used in cardiogenic shock patients who are failing maximal medical therapy’; expert opinion),17 and in specific potentially reversible shock causes or severe heart failure (‘mechanical circulatory support may be used as a “bridge to decision” or longer term in selected patients’; expert opinion)18 without preference of a specific system. In elective high‐risk PCI, which potentially leads to haemodynamic compromise and shock due to impaired LV function and complex coronary anatomy, the PROTECT II trial demonstrated that support provided with an Impella 2.5 was superior to that provided by the IABP and in a per‐protocol analysis showed more complete revascularization and improvement in LV function at 90 days.19
The pVADs have been adopted in clinical practice more frequently, because the ubiquitously available IABP could not demonstrate an advantage over standard‐of‐care medical treatment in infarct‐related cardiogenic shock in a randomized trial.5 The major problem for IABP in infarct‐related cardiogenic shock is that the device cannot provide any support, if LV function is so severely compromised, that the aortic valve does not open anymore. The pathophysiological background explaining this lack of efficacy has been illustrated in our haemodynamic simulations. Computer simulations of haemodynamics may be an easy‐to‐use tool to illustrate advantages and disadvantages of different support devices or even of strategies without haemodynamic support in differing patient scenarios. Using simulation models might help to identify patients in whom available haemodynamic data is already suggestive for potential circulatory failure. Identification of those patients could lead to pre‐procedural comparison of available support devices, which can help to choose the appropriate tool for support during a procedure. Even though the haemodynamic simulations in our case presentations were made retrospectively, they help to understand why PCI without support might have been a bad choice for the patient as might also most probably have been the case if one had chosen an IABP. Using actual cases retrospectively for simulation can help to understand the underlying pathophysiological changes. The knowledge about the impact of different haemodynamic support devices in a given clinical scenario can help to more selectively and appropriately use haemodynamic support in future cases.
Conclusions
Coronary intervention in compromised and/or unstable patients often leads to further haemodynamic compromise. Passive haemodynamic support devices like IABP cannot provide sufficient haemodynamic support once contractility is severely impacted. Hence, they have failed to improve outcome in infarct‐related cardiogenic shock. More powerful and active haemodynamic pVADs such as the Impella microaxial pumps unload the left ventricle while providing haemodynamic support independent of intrinsic LV function. Therefore, they allow for more complex coronary interventions under stabilized haemodynamics. Understanding basic haemodynamics helps to foresee potential compromise in borderline stable ACS patients and builds the rationale for choosing appropriate haemodynamic support.
Conflict of interest
A.S., D.B., and J.B. have received lecture fees from Abiomed.
Funding
None.
Schäfer, A. , Burkhoff, D. , and Bauersachs, J. (2019) Haemodynamic simulation and the effect of early left ventricular unloading in pre‐shock acute coronary syndrome. ESC Heart Failure, 6: 457–463. 10.1002/ehf2.12417.
References
- 1. Samuels LE, Kaufman MS, Thomas MP, Holmes EC, Brockman SK, Wechsler AS. Pharmacological criteria for ventricular assist device insertion following postcardiotomy shock: experience with the Abiomed BVS system. J Card Surg 1999; 14: 288–293. [DOI] [PubMed] [Google Scholar]
- 2. Basir MB, Schreiber TL, Grines CL, Dixon SR, Moses JW, Maini BS, Khandelwal AK, Ohman EM, O'Neill WW. Effect of early initiation of mechanical circulatory support on survival in cardiogenic shock. Am J Cardiol 2017; 119: 845–851. [DOI] [PubMed] [Google Scholar]
- 3. Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, Tu T, Society for Cardiovascular Angiography and Interventions (SCAI) , Heart Failure Society of America (HFSA) , Society of Thoracic Surgeons (STS) , American Heart Association (AHA), and American College of Cardiology (ACC) . 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology‐Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol 2015; 65: e7–e26. [DOI] [PubMed] [Google Scholar]
- 4. Burkhoff D. Device therapy: where next in cardiogenic shock owing to myocardial infarction? Nat Rev Cardiol 2015; 12: 383–384. [DOI] [PubMed] [Google Scholar]
- 5. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K, IABP‐SHOCK II Trial Investigators . Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012; 367: 1287–1296. [DOI] [PubMed] [Google Scholar]
- 6. Aqel RA, Hage FG, Iskandrian AE. Improvement of myocardial perfusion with a percutaneously inserted left ventricular assist device. J Nucl Cardiol 2010; 17: 158–160. [DOI] [PubMed] [Google Scholar]
- 7. Kapur NK, Qiao X, Paruchuri V, Morine KJ, Syed W, Dow S, Shah N, Pandian N, Karas RH. Mechanical pre‐conditioning with acute circulatory support before reperfusion limits infarct size in acute myocardial infarction. JACC Heart Fail 2015; 3: 873–882. [DOI] [PubMed] [Google Scholar]
- 8. Sieweke JT, Berliner D, Tongers J, Napp LC, Flierl U, Zauner F, Bauersachs J, Schäfer A. Mortality in patients with cardiogenic shock treated with the Impella CP microaxial pump for isolated left ventricular failure. Eur Heart J Acute Cardiovasc Care 2018. [DOI] [PubMed] [Google Scholar]
- 9. Jensen PB, Kann SH, Veien KT, Møller‐Helgestad OK, Dahl JS, Rud CS, Jensen MK, Jensen LO, Schmidt H, Møller JE. Single‐centre experience with the Impella CP, 5.0 and RP in 109 consecutive patients with profound cardiogenic shock. Eur Heart J Acute Cardiovasc Care 2018; 7: 53–61. [DOI] [PubMed] [Google Scholar]
- 10. Burkhoff D, Dickstein ML, Schleicher T. Harvi—online. Retrieved from . 2017.
- 11. Doshi D, Burkhoff D. Cardiovascular simulation of heart failure pathophysiology and therapeutics. J Card Fail 2016; 22: 303–311. [DOI] [PubMed] [Google Scholar]
- 12. Verma S, Burkhoff D, O'Neill WW. Avoiding hemodynamic collapse during high‐risk percutaneous coronary intervention: advanced hemodynamics of Impella support. Catheter Cardiovasc Interv 2017; 89: 672–675. [DOI] [PubMed] [Google Scholar]
- 13. Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of mechanical circulatory support. J Am Coll Cardiol 2015; 66: 2663–2674. [DOI] [PubMed] [Google Scholar]
- 14. Meyns B, Stolinski J, Leunens V, Verbeken E, Flameng W. Left ventricular support by catheter‐mounted axial flow pump reduces infarct size. J Am Coll Cardiol 2003; 41: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 15. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127: e362–e425. [DOI] [PubMed] [Google Scholar]
- 16. Authors/Task Force m , Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014; 35: 2541–2619. [DOI] [PubMed] [Google Scholar]
- 17. Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, Ristic AD, Lambrinou E, Masip J, Riley JP, McDonagh T, Mueller C, deFilippi C, Harjola VP, Thiele H, Piepoli MF, Metra M, Maggioni A, McMurray JJ, Dickstein K, Damman K, Seferovic PM, Ruschitzka F, Leite‐Moreira AF, ,Bellou A, Anker SD, Filippatos G. Recommendations on pre‐hospital and early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine—short version. Eur Heart J 2015; 36: 1958–1966. [DOI] [PubMed] [Google Scholar]
- 18. Bauersachs J, Arrigo M, Hilfiker‐Kleiner D, Veltmann C, Coats AJS, Crespo‐Leiro MG, de Boer RA, van der Meer P, Maack C, Mouquet F, Petrie MC, Piepoli MF, Regitz‐Zagrosek V, Schaufelberger M, Seferovic P, Tavazzi L, Ruschitzka F, Mebazaa A, Sliwa K. Current management of patients with severe acute peripartum cardiomyopathy: practical guidance from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail 2016; 18: 1096–1105. [DOI] [PubMed] [Google Scholar]
- 19. O'Neill WW, Kleiman NS, Moses J, Henriques JPS, Dixon S, Massaro J, Palacios I, Maini B, Mulukutla S, Džavík V, Popma J, Douglas PS, Ohman M. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra‐aortic balloon pump in patients undergoing high‐risk percutaneous coronary intervention: the PROTECT II study. Circulation 2012; 126: 1717–1727. [DOI] [PubMed] [Google Scholar]


