Abstract
Aims
Population data indicate that one in 25 persons of African ancestry has heart failure, a condition with relatively high mortality of around 50% in 5 years. Combined hydralazine and isosorbide dinitrate added to conventional therapy in African ancestry patients with heart failure and reduced ejection fraction improves quality of life and reduces the rate of first hospitalization for heart failure by 33% and annual mortality by 43%. The objectives of this study were to quantify the use of this guideline‐recommended therapy in Europe and the potential effect of implementation gaps on mortality.
Methods and results
Prescription drug registration and utilization databases and population statistics were analysed in a cross‐European survey without language restriction. Main outcomes were the number of unique patients prescribed the fixed combination hydralazine–isosorbide dinitrate (primary) or both drugs (secondary) in Europe in 2015, and the excess mortality related to prescribing practices was estimated. The survey indicates that around 12 million persons of African ancestry live in Europe. It is estimated that 480 000 persons of this population group have heart failure, with 120 000 eligible for hydralazine and isosorbide dinitrate therapy. However, single‐pill hydralazine–isosorbide dinitrate is not authorized and therefore not dispensed in Europe in 2015. Out of the 25 European nations surveyed, the UK and the Netherlands are the only countries with major African ancestry populations where both hydralazine and isosorbide dinitrate are available for oral use, aside Norway, Sweden, and Finland. Hydralazine and isosorbide dinitrate are prescribed to <500 European patients in 2015. Thus, despite the recommendations of the European Society of Cardiology, the large majority of African‐European patients with heart failure do not receive this drug combination, potentially resulting in 4800 to 5800 excess deaths yearly.
Conclusions
The life‐saving, guideline‐recommended, adjunctive therapy for heart failure in African ancestry patients with hydralazine and isosorbide dinitrate is rarely used in Europe. This major evidence‐practice gap should urgently be overcome to reduce excess mortality in African‐European patients with heart failure.
Keywords: Health inequities; Heart failure; African ancestry group; Mortality; Hydralazine (ATC C02DB02, PubChem CID: 3637); Isosorbide dinitrate (ATC C01DA08, PubChem CID: 6883); Hydralazine/isosorbide dinitrate combination (ATC C01DA58, PubChem CID: 9954530); Europe; Evidence‐practice gap
Introduction
Heart failure (HF) is a prevalent and deadly disease among persons of African ancestry (AA). According to the American National Heart, Lung and Blood Institute, one in 25 AA persons has HF.1 The 5 years' fatality rate after incident hospitalization for HF per 1000 person‐years is reported to be 52% in AA men and 46% in AA women.2 HF in AA is of predominantly non‐ischaemic aetiology, and the more severe natural history is largely explained by a greater risk factor burden of obesity, hypertension, diabetes, and chronic kidney disease.1, 2, 3, 4, 5, 6, 7 In addition, a distinct response to pharmacotherapy is noted, with low efficacy of angiotensin‐converting enzyme inhibitor therapy,4, 8, 9 which is thought to be associated with compromised bioavailability of nitric oxide (NO).5, 9 In line with this notion, a retrospective analysis of the Vasodilator‐Heart Failure Trial I indicated a significant survival benefit in AA but not European men when a combination of vasoactive drugs, hydralazine (H), an antioxidant that inhibits destruction of NO, and the NO donor isosorbide dinitrate (ISDN) was added to conventional therapy. In AA patients randomized to H + ISDN, the annual mortality ratio was 9.7% compared with 17.3% with placebo (−44%; P < 0.05), without significant difference in European patients.10 The subsequent African‐American Heart Failure Trial (A‐HeFT) in 1050 self‐identified AA patients with New York Heart Association Class III or IV HF with dilated ventricles confirmed these findings, with a 43% reduction in mortality with fixed‐dose, single‐pill H–ISDN vs. placebo during a mean follow‐up period of 10 months (6.2 vs. 10.2%; P < 0.05).11 In addition, a 33% relative reduction in the rate of first hospitalization for HF (16 vs. 24%; P < 0.05), a significant reduction in exacerbation of congestive HF (9 vs. 13%), and improvement in quality‐of‐life scores vs. placebo were reported. Headache (48 vs. 19%) and dizziness (29 vs. 12%) were the most common adverse effects reported vs. placebo.5, 11, 12 Although American as well as European guidelines advise H and ISDN for self‐described AA patients with New York Heart Association Class III–IV HF and reduced ejection fraction,2, 5, 13, 14, 15 recent American studies have indicated that doctors do not prescribe this life‐saving therapy to the majority of eligible hospitalized or outpatient AA patients.5 Therefore, this study analyses the implementation of this evidence in medical practice across Europe.
Methods
Design and definitions
For this survey, ‘Europe’ was defined as the European Union (EU) Member States and other European countries surveyed. To assess drug market authorization and drug use, electronic biomedical literature databases including PubMed and Embase were searched; and the European Medicines Agency (EMA) was contacted. The EMA works closely with the National Competent Authorities of the Member States of the EU and the European Economic Area responsible for human medicines. The National Competent Authorities are primarily responsible for the authorization of medicines available in the EU that do not pass through the centralized procedure.16 With a focus on European countries within the EU with relatively large African populations, European national bodies were requested for drug registration and utilization data, and demographic data of AA populations, excluding dependent territories, overseas departments, and collectivities (please view the Appendix for contact details). In line with existing literature,4, 5, 9, 10, 11, 12 AA is defined as sub‐Saharan African descent, either self‐defined or as defined by the consulted authorities. With a non‐response, at least one e‐mail reminder was sent, and with a second non‐response, bodies were contacted through the telephone. Data were requested of 2015 as most European bodies were able to provide these data at the time of the survey (2017 to 2018, with a pilot in December 2016). If not available, the nearest year before 2015 was requested. No language restriction was applied. Statistics are descriptive counts based on available data. The estimation of the point prevalence and mortality of HF is based on existing US evidence as explicated below. The STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guideline was used to report the outcome data.17
Outcomes
The primary outcome was the number of unique patients prescribed the fixed combination H–ISDN (as assessed in the A‐HeFT). The secondary outcome was the use of both H + ISDN in unique patients as a proxy for the use of the combination. Tertiary outcomes were estimated health effects associated with prescription practices.
Hydralazine and isosorbide dinitrate
The European Society of Cardiology (ESC) 2012 Guidelines for treatment of HF advise combination therapy with H and ISDN for AA patients, but the drug combination is also advised with angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker intolerance, to reduce the risk of hospitalization and risk of premature death in HF patients with ejection fraction ≤ 35% or ejection fraction ≤ 45% and a dilated left ventricle.14 The number needed to treat (36 months) is seven. All European cardiology societies endorse this ESC guideline,14 but there is no European performance measure for H and ISDN use. Although the guidelines mention combining H and ISDN, the FDA approved only the fixed combination H–ISDN as used in the A‐HeFT.13 Real‐world data indicate that mortality after 1 year of adherence is 41% higher with H + ISDN (17.0%), compared with fixed‐dose H–ISDN (12.1%), a difference partly attributed to consistent simultaneous intake of both drugs and better bioavailability with the fixed combination.18
Drug registration and utilization data
The Anatomical Therapeutic Chemical Classification (ATC) System codes for H and ISDN (Table 1)19 were used to search for the EU and European national data on the authorization and use of H and ISDN. The EMA and national bodies were requested whether the fixed combination H–ISDN (ATC C01DA58), H (ATC C02DB02), and (oral) ISDN (ATC C01DA08) had a market authorization for human use. Furthermore, European and national bodies were requested how many patients were prescribed the fixed combination ISDN and H or both H and ISDN in unique patients. If data on combined use in unique patient were not available, the number of patients prescribed H was used as a proxy for the maximum number of patients that could have received the combination. Data on H and ISDN use across Europe were also searched for in electronic bibliography databases including national registries, and PubMed and Embase from the inception to June 2018, using the ATC codes and the combination of key words ‘Isosorbide Dinitrate’ and ‘Hydrala*’ combined with ‘Europe’ and European country names, including the UK, England, Scotland, and (Northern) Ireland.
Table 1.
Relevant ATC codes
| Level 1 C Cardiovascular system | |||
|---|---|---|---|
| Level 2 | Level 3 | Level 4 | Level 5 |
| C01 Cardiac therapy | C01D Vasodilators used in cardiac diseases | C01DA Organic nitrates | C01DA08 Isosorbide dinitrate |
| C01DA58 Isosorbide dinitrate and hydralazine combinations | |||
| C02 Antihypertensive drugs | C02D Agents acting on arteriolar smooth muscle | C02DB Hydrazinophthalazine derivatives | C02DB02 Hydralazine |
Anatomical Therapeutic Chemical Classification (ATC) System codes of drugs included in this study. The ATC system is a World Health Organization Collaborating Centre for Drug Statistics Methodology used for the classification of active ingredients of drugs independent of the brand name. Each bottom‐level (level 5) ATC code stands for a pharmaceutically used substance, or a combination of substances, intended for a single medical indication.19
African populations in Europe
Data or estimations on the number of persons of AA living in Europe and in Europeans nations were requested at relevant global, European, and national statistics bureaus or other relevant organizations, as for 2015, or the nearest year before 2015. If data were not available or not complete, a similar request was made at non‐governmental organizations. Finally, the Internet was searched for data, mainly in online encyclopaedias. This study used the term ‘ancestry’ in contacts, but studies and other resources cited might have used ‘ethnicity’, or ‘race’, which are considered for this study to have a similar scope and meaning, referring to populations with (self‐defined) sub‐Saharan African heritage.
Estimation of heart failure prevalence and mortality in African‐Europeans
Heart failure prevalence increases with age and risk factor burden and may vary by ethnicity, sex, education level, and diagnostic criteria.1, 2, 3, 5, 7, 18, 20 In the absence of European data, the age‐adjusted population prevalence in AA is estimated to be 4% based on US data.1
Annual HF mortality rates vary considerably across populations, with an average of 17%.21 Cardiac and non‐cardiac factors, including sex, age, ethnicity, differences in risk factor burden, recent hospitalization for acute HF, and health care characteristics, may affect mortality.1, 2, 3, 5, 7, 10, 11, 12, 14, 15, 21, 22, 23, 24, 25, 26 The EU (national) data on mortality of AA with HF are not available. Data from the Duke Databank of Cardiovascular Disease26 indicated adjusted mortality rates for HF in AA of 12% at 1 year and 49% at 5 years. In the Atherosclerosis Risk in Communities study, 76% of the prospectively followed AA population members with HF were hospitalized within 1.7 years, with an age‐adjusted case fatality rate after the first hospitalization of 24% at 1 year in men and women, and 52% in men vs. 46% in women at 5 years.22 HF is a heterogeneous clinical entity, with a prominent distinction between patients with reduced ejection fraction (HFrEF, broadly corresponding to systolic HF) with usually a dilated left ventricular cavity size, occurring in around half of all HF patients, or preserved (HFpEF) left ventricular ejection fraction with usually a normal left ventricular cavity and concentric hypertrophy.7, 14, 15, 22, 23 Evidence indicates that HFpEF has a more benign prognosis than HFrEF, while H and ISDN typically improve outcomes in patients with HFrEF.2, 5, 7, 8, 10, 11, 12, 13, 14, 15, 18, 22, 23 After H + ISDN treatment in Vasodilator‐Heart Failure Trial I (only AA men), the annual mortality rate of HFrEF was 9.7 vs. 17.3% without receiving this therapy,10 and in A‐HeFT (AA men and women) using fixed combination H–ISDN, this was respectively 6.2 vs. 10.2%.11 Based on the available data, the HF population prevalence in European AA persons is estimated 4%, with 50% HFrEF, and around 25–30% of all AA HF patients to benefit from H and ISDN therapy.25 The number of eligible patients is assumed to be at steady state, with a linear benefit of therapy over time.25 Annual mortality rates are conservatively estimated as 6% with and 10% without H and ISDN use, rounded to 100 persons.
Results
Despite the implementation in the ESC guidelines, H and ISDN use in the European nations surveyed appears to be negligible. Figures 1, 2, 3, 4 and Table 2 summarize the estimated number of persons of AA and the registration and utilization of H–ISDN and its components from national drug registration and utilization databases in 25 European countries surveyed. In 2015, more than 10 years after A‐HeFT was published, the collected evidence indicates that no person in Europe had received fixed H–ISDN in that year and fewer than 500 received H + ISDN.
Figure 1.
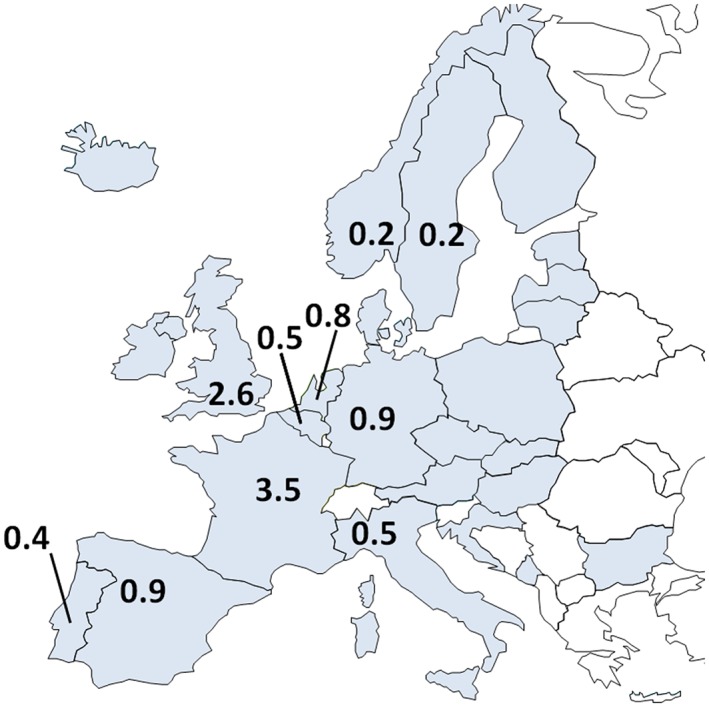
Estimation of sub‐Saharan African populations across Europe. With a focus on European Union (EU) countries, it is estimated that 9 to 15 million (averaged at 12 million) persons of sub‐Saharan African ancestry live in Europe. The countries surveyed are depicted in grey, 22 EU and 3 non‐EU nations (Norway, Iceland, and Monte Negro). All countries surveyed reported to have African ancestry citizens. Countries with an estimated African ancestry population of 0.1 million or more persons are labelled.
Figure 2.
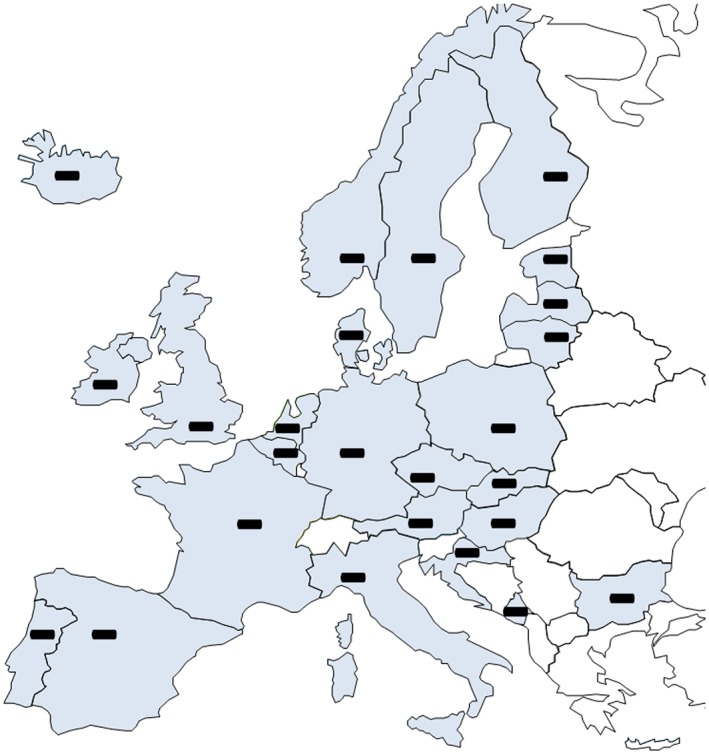
Market authorization for the FDA approved drug, fixed combination H–ISDN. The minus sign (−) depicts no market authorization. This study showed that there is neither a centralized EU market authorization nor a national authorization in any European country surveyed for the fixed combination H–ISDN (C01DA58).
Figure 3.
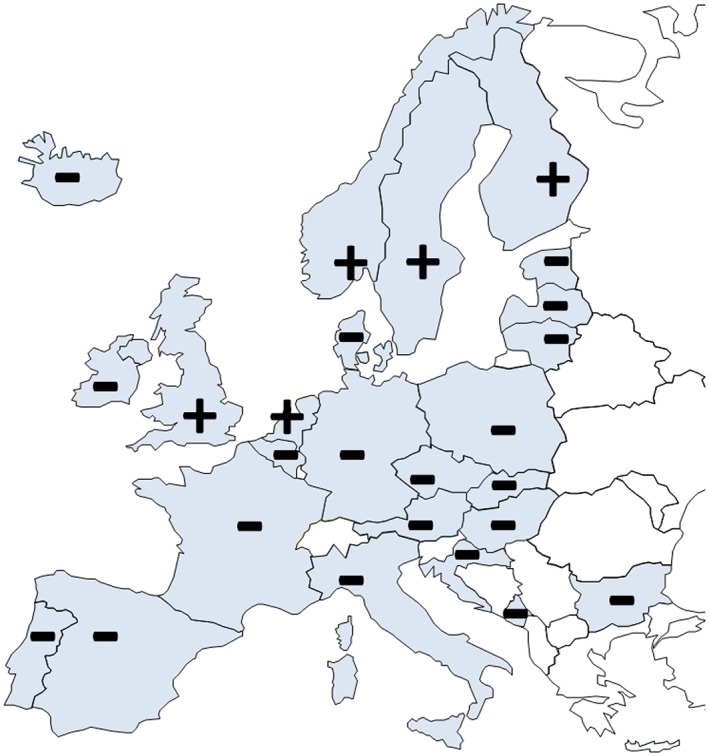
Market authorization for both components H and ISDN. Plus (+) and minus (−) signs depict national market authorization for both components of H–ISDN, H (ATC C02DB02), and ISDN (ATC C01DA08). There is no centralized EU authorization for either drug, and a national market authorization for both components in only 5 of the 25 European nations surveyed (the UK, the Netherlands, and three Nordic countries). Spain only has sublingual isosorbide dinitrate available (5 mg).
Figure 4.
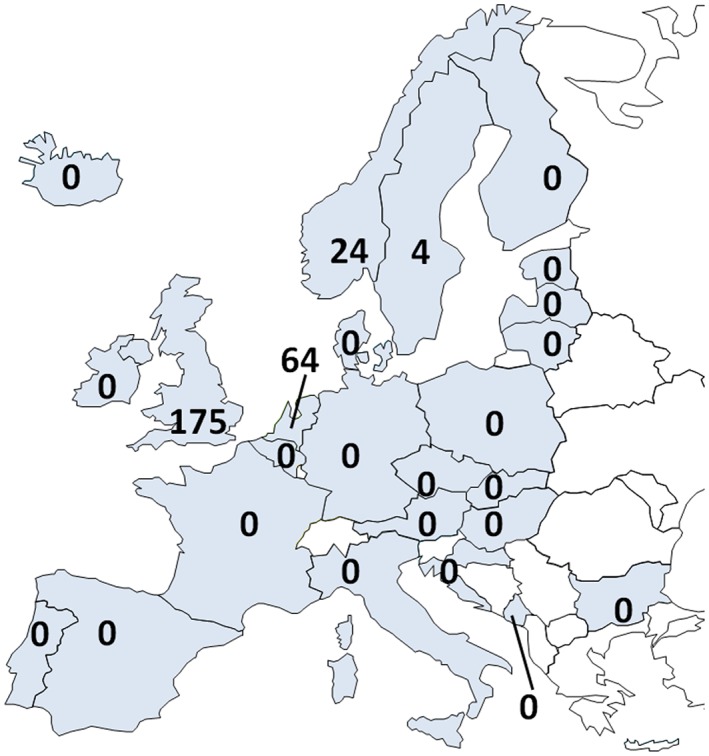
Unique persons receiving oral H and ISDN in 2015. The number of prescriptions of the trial drug, fixed‐dose H–ISDN was zero in all European nations surveyed. Depicted is the number of unique patients prescribed both single pharmaceutical drugs H and ISDN as at least once in 2015. With an estimated African ancestry population of 12 million, >100 000 prescriptions are expected, but <500 patients received these drugs. Data from the UK (extrapolated from a 20% population sample) and the Netherlands are reimbursed drugs prescribed to outpatients. Norway and Sweden include all pharmaceutical drug sales (Norway including hospital data); Finland reports all reimbursed prescription drugs.
Table 2.
The use of hydralazine (H) and isosorbide dinitrate (ISDN) combination therapy in Europe
| African ancestry populationa | Marketing authorizationc | H and ISDN useg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region | Data source | African ancestry (n) | Eligible HF patients | Authority | Fixed H–ISDN | H | ISDN | Registry | Coverage | Unique patients (n) |
| European Union | UN; ENAR | 9 000 000–15 000 000 | 144 000 | EMA | n.a. | n.a. | n.a. | Eurostat | EU | n.d. |
| France | ENAR; Insee; Cran | 2 000 000–5 000 000 | 42 000 | ANSM | n.a. | n.a. | + | Ameli | National | 0 |
| UK | ONS | 2 200 000–2 900 000 | 30 600 | MHRA | n.a. | + | + | THIN | 20% NP | 35h |
| Spain | ENAR; INE | 800 000–1 000 000 | 10 800 | AEMPS CIMA | n.a. | + | −e | Ministerio de Sanidad | National | 0 |
| Germany | UN; ENAR Destatis; ISD | 800 000–1 000 000 | 10 800 | Pharmnet BfArM | n.a. | n.a. | + | DAPI | 90% NP | 0 |
| The Netherlands | ENAR; CBS | 750 000–900 000 | 10 500 | CBG | n.a. | + | + | GIP/ZIN | National | 64h |
| Belgium | Wikipedia; Statbel; MECM | 500 000 | 6000 | FAGG | n.a. | n.a. | + | Farmanet/RIZIV | National | 0 |
| Italy | ENAR; ISTAT | 500 000 | 6000 | AIFA | n.a. | n.a. | + | AIFA | National | 0 |
| Portugal | Afropedia INE; SEF | 300 000–500 000 | 4800 | Infarmed | n.a. | n.a. | + | Infarmed | National | 0 |
| Norway | SSB | 210 000 | 2500 | NoMA | n.a. | + | + | NorPD | National | 24h |
| Sweden | SCB; ASR; MKC | 200 000 | 2400 | MPA | n.a. | + | + | Social‐styrelsen | National | 4h |
| Ireland | ENAR; CSO | 70 000 | 800 | HPRA | n.a. | + | −f | HSE‐PCRS | National | 0 |
| Denmark | DST; AEC | 65 000 | 800 | DMA | n.a. | n.a. | + | MEDSTAT | National | 0 |
| Austria | Wikipedia; SA; Sauer27, b | 40 000 | 500 | BASG AGES | n.a. | n.a. | n.a. | Haupt‐verband EKO | National | 0 |
| Finland | EAES; SF | 30 000 | 400 | Fimea | n.a. | + | + | KELA | National | 0h |
| Poland | SP; Zabek28 | <10 000 | <100 | URPL SCIOZd | n.a. | n.a. | + | NFZ | National | 0 |
| Bulgaria | EAES; NSI | <10 000 | <100 | BDA | n.a. | n.a. | + | BDA | National | 0 |
| Hungary | KSHb | <10 000 | <100 | OGYEI | n.a. | n.a. | n.a. | OGYEI | National | 0 |
| Croatia | DZS | <10 000 | <100 | HALMED | n.a. | n.a. | − | HALMED | National | 0 |
| Czech Republic | CZSO | <10 000 | <100 | Súkl | n.a. | n.a. | + | Súkl | National | 0 |
| Iceland | EAES; Statice | <1000 | <10 | IMA | n.a. | n.a. | + | Embætti landlæknis | National | 0 |
| Estonia | SEb | <1000 | <10 | SAM | n.a. | n.a. | + | SAM | National | 0 |
| Latvia | CSB | <1000 | <10 | ZVA | n.a. | n.a | + | ZVA | National | 0 |
| Lithuania | SL | <1000 | <10 | VVKT | n.a. | n.a. | + | VLK | National | 0 |
| Slovakia | SOSR | <1000 | <10 | ŠÚKL | n.a. | n.a. | + | ŠÚKL | National | 0 |
| Montenegro | Wikipedia; MONSTAT | <1000 | <10 | CALIMS | n.a. | n.a. | + | CALIMS | National | 0 |
Data are from 2015 (or nearest preceding year). A list of abbreviations and organizations' contact details is provided in the Appendix.
Data are estimates on the number of African ancestry (AA) persons living in the European Union (EU) and European nations (excluding dependencies).
Hungary and Estonia population data were from 2011 and Austria from 2005. The midpoint value of a range was used to estimate the number of persons with heart failure (HF), detailed in the Method section. Numbers were rounded to the nearest 100 (or 10 for <100).
Centralized (EU) and national data on market authorization of oral preparations of the fixed drug combination hydralazine and isosorbide dinitrate, H–ISDN, or its components. Plus sign (+), authorized drug; n.a, not authorized.
Authorization data from Poland include centralized (EU) and parallel import authorization.
Only 5 mg sublingual ISDN available.
Only intravenous ISDN available.
Number of unique persons, receiving both H and ISDN as pharmaceutical (prescription) drugs at least once in 2015; zero includes no market authorization for hydralazine and/or isosorbide dinitrate. Most registries provide data of reimbursed pharmaceutical H and ISDN, prescribed to non‐hospitalized patients.
The UK and the Netherlands report reimbursed drugs to outpatients, Norway and Sweden include all pharmaceutical drug sales (Norway including hospital data), and Finland reports all reimbursed prescription drugs. NP, national population; n.d. no data.
African populations
All European and national bodies approached replied to requests for data. Except for the UK, statistics bureaus stated not to collect governmental demographics by race/ethnicity. Main data sources to estimate the number of AA persons living in continental Europe at 12 million were national statistics bureaus' estimations based on country of birth (first and second generation migrant populations), data from the European Network Against Racism, non‐governmental organizations serving the AA population, scholarly papers, and online resources (Figure 1, Table 2 and the Appendix).27, 28, 29, 30
Market authorization, availability, and use of hydralazine and isosorbide dinitrate
No published reports were retrieved documenting the use of H and ISDN in AA patients with HF in Europe. The fixed combination H–ISDN was not authorized at the EMA or within any European nation (Figure 2). In addition, in many countries including France, commercially prepared oral H or oral ISDN was unavailable (Figure 3 and Table 2). In general, magistral preparations were not nationally registered, but some countries, such as Norway and Sweden, do file every prescription, while others, such as Denmark and Belgium, filed medicine sales. Both Denmark and Belgium indicated that pharmacies' production volumes for individual patients would by EU law be very low.31
The total number of persons using the fixed combination H–ISDN in Europe was nil (Table 2). As neither H nor ISDN has a central (EU) market authorization, the combined use in countries was considered to be nil if oral H or oral ISDN did not have a national market authorization or were unavailable, but confirmation was requested at national bodies that register drug use. Poland and Latvia did not have data to confirm that no unique patient used both H and ISDN, but there was no market authorization for H in either country.
Of the five countries where both oral H and oral ISDN were available (Figure 3 and Table 2), data from Norway and Sweden include all pharmaceutical drug sales (for Norway including hospital data), from Finland contain all reimbursed prescription drugs, while the UK and the Netherlands provided data on reimbursed drugs to outpatients only. With the focus on chronic care, >100 000 unique patients using these drugs were expected (Table 2). However, combined use of commercially available (pharmaceutical) H and ISDN in unique, non‐hospitalized patients across Europe was negligible at <500 in 2015 (Figure 4). Only Belgium reported the use of magistral preparations of H, in <250 patients in 2015.
Thus, less than 1% of European AA patients with HF could have received any combination of H and ISDN for chronic HF care (Table 2). At the expense of potential side effects, mainly headache and dizziness, the use of this drug combination might have substantially reduced premature mortality with 4800 to 5800 AA HF patients yearly.
Discussion
This study provides evidence of a significant care gap in best practice of HF treatment provided to persons of AA across Europe. Although there is evidence from randomized controlled trials and real‐world studies on the reduction of HF mortality in AA patients with H and ISDN adjunctive therapy,10, 11, 12, 13, 14, 15, 18, 25 the fixed combination is not authorized in the EU or in the European nations surveyed, and the availability of both components H and ISDN is very limited, to five countries. In the absence of a European performance measure for clinical practice, the use of this guideline‐recommended therapy14, 32 is negligible at less than 500 unique patients throughout Europe in 2015.
Because of this major discrepancy between best practice and care provided in usual clinical practice, 4800 to 5800 of African‐European HF patients may die prematurely each year. This estimation is conservatively based on a 40% reduction in mortality with H–ISDN from 10 to 6%, while real‐world data indicate that annual mortality rates in AA HF patients might be considerably higher,18, 21, 22, 26 in particular when the fixed combination is not used.18 These findings should have significant clinical and public health policy implications, to ensure that the ESC guideline‐recommended therapy with H and ISDN32 is applied and optimal care is provided to AA HF patients.
Strengths and limitations of this study
The study is to our knowledge the first to report the performance of European health care regarding H and ISDN, with negligible use as the main outcome. Although this study represents the best estimate of drug use, it required the gathering of data from heterogeneous sources that might have utilized different definitions and criteria for H and ISDN use and for being of ‘sub‐Saharan African ancestry’. Furthermore, there is a lack of data on drug dose and on prescription patterns in time or by age and sex, while data from European countries with very small AA populations were not exhaustively collected in this study. Also, many national bodies provided data on reimbursed drugs only. However, European countries have a universal health care system that provides health care and financial protection to >90% of the citizens.33 Because H is poorly available, it is highly unlikely that further data would change the magnitude of the main study outcome of negligible H and ISDN use in Europe. Regarding the tertiary outcome of health effects associated with this limited use, the assumptions might have underestimated or overestimated true effects in African‐European populations, as the estimation of HF epidemiology, effectiveness of H and ISDN, and the outcomes are based on US data. Significant East AA populations live in Europe, and there are no data whether the specific benefit of H and ISDN established in predominantly West African population is similar in these groups. Finally, because of the explorative nature of this study, only the impacts on reduction in deaths are estimated, not on hospitalizations, improvement in quality of life, cost‐effectiveness, or other important clinical or health economic outcomes.
Implications for policy and practice
Lack of availability of hydralazine–isosorbide dinitrate and its components in Europe
Both H and ISDN are on WHO Model List of Essential Medicines.34 The list includes H (25/50 mg) for hypertension during pregnancy and sublingual ISDN (5 mg) for angina pectoris but not oral ISDN. Pharmaceuticals with a priority health care need are intended to be available within the context of functioning health systems with stable and reliable stocks, in the appropriate dosage forms and with assured quality.34 However, H was not available in most EU countries. The question why drugs are not marketed or available was beyond the scope of this study, but with a free market, profitability is probably a factor.
Shortage of pharmaceuticals is an increasing problem within Europe. The health status of patients is put at risk if they are not receiving their prescribed medicines in a timely manner. The Heads of Medicines Agencies (HMA) is a network of the heads of the National Competent Authorities, whose organizations are responsible for the regulation of medicinal products in the European Economic Area. While throughout Europe there is no harmonized definition of ‘drug shortages’, the HMA has identified what types of drug shortages are most critical, and the definition includes pharmaceutical for which there is a medical need but that are not authorized or not marketed in a Member State.35 However, nor the joint Task Force on Availability of Authorised Medicines for human and veterinary use, created by the HMA and the EMA and dedicated to the availability of authorized medicinal products for human use, nor its different Network Members consider H–ISDN fixed combination or its components in their evaluation of shortages.35 This renders it nearly impossible for health workers in the majority of European nations to prescribe the fixed drug combination or its components other than magistral preparations, which are by law not intended for widespread use.31 Moreover, the use of the components of H–ISDN for HF might in some health systems be considered off‐label use.18 Thus, the use of the combination therapy should be properly registered, and the fixed combination H–ISDN and/or its components should become available across Europe for use for HF in AA patients.
Definition and statistical invisibility of African ancestry populations in Europe
A main issue regarding the evaluation of the use of H–ISDN is the ‘statistical invisibility’ of AA populations across Europe. Broadly, persons of sub‐Saharan African or their ancestors migrated to Western European countries from continental Africa or from the Americas. Refugees from Africa form a special category, having a marked impact on the size of African population in countries without long‐established African communities, such as Ireland, Switzerland, and the Nordic countries.36 Hence, Europe's African population is very diverse with members of established AA communities, such as in England, France, and the Netherlands, as well as recent migrants. However, except for the UK and Ireland, no national or European statistical or health data are collected on this population subgroup.36 As a consequence, despite (self‐)identification in the doctor–patient relationship, statistically speaking, AA persons in mainland Europe do not exist. This statistical ‘colour blindness’—meant to protect individuals—may impede the health of African populations. The EU Agency for Fundamental Rights has concluded that Africans ancestry populations belong to the most vulnerable groups in the EU.37 African populations may require special health care, such as screening for cardiovascular disease, adjusted guidelines, and performance measures.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 29 On the other hand, many scholars argue that ‘Blackness’ is a sociologically constructed identity that has historically been imposed on people.36 This matter is circumvented through self‐identification of AA,10, 11 but a biomarker to identify individuals who might benefit from H and ISDN therapy would be helpful. H has an antioxidant effect that enhances and sustains the efficacy of nitrates.10, 11 This suggests that patients with compromised NO generation might benefit from the therapy.9, 10, 11 Relatively low l‐arginine, associated with high creatine kinase activity and creatine synthesis, and glucose‐6‐phosphate dehydrogenase deficiency have been suggested to induce low NO bioavailability, and assessments of these enzyme systems might help predict responses to H and ISDN.9 Also, G‐protein beta‐3 subunit genotype was reported to predict the response to H and ISDN,38 but to date, no biomarker has been validated for clinical use, and self‐identified AA remains the best available predictor for benefit from H and ISDN therapy.4, 5, 8, 9, 10, 11 The lack of consensus on categories based on ancestry and inconsistent data collection are major hindrances for European health outcome assessments by AA. This difficulty in assessing data extends the problem of a significant evidence‐practice gap in the care of AA patients with HF.
Conclusions
This survey provides preliminary evidence that patients of AA in Europe with HF might experience excess mortality because of an unmet therapeutic need, as the guideline‐recommended, life‐saving adjunctive combination therapy with H and ISDN is not available or not prescribed. Aside primary prevention of HF—in particular the early detection and adequate treatment of hypertension—access to and use of this life‐prolonging therapy with H and ISDN are imperative to reduce premature mortality in AA patients with HF.
Conflict of interest
None declared.
Funding
This research was supported by the Creatine Kinase Foundation, a non‐profit organization in Amsterdam, the Netherlands. The funder had no role in study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
Author contributors
L.M.B. designed the study, collected, and analysed the data, drafted and revised the manuscript, and is the guarantor of the study. The corresponding author attests that no others meeting the criteria have been omitted.
Contact information of the organizations surveyed
Table A1.
Organizations for medicinal product authorization and pharmaceutical drug consumption data
| Location | Namea | Acronym | URL |
|---|---|---|---|
| EU | European Medicines Agency | EMA | http://www.ema.europa.eu |
| Eurostat | n.a. | http://ec.europa.eu/eurostat | |
| France | Agence Nationale de Sécurité du Médicament et des Produits de Santé, (National Agency for the Safety of Medicine and Health Products) | ANSM | http://ansm.sante.fr |
| http://agence‐prd.ansm.sante.fr | |||
| Assurance Maladie pour les médecins (French Health Insurance) | ameli | https://www.ameli.fr | |
| UK | Medicines and Healthcare Products Regulatory Agency | MHRA | http://www.mhra.gov.uk |
| The Health Improvement Network Database | THIN | http://www.ucl.ac.uk/pcph/research/thin‐database | |
| Spain | Agencia Española de Medicamentos y Productos Sanitarios (Spanish Agency for Medicines and Health Products) | AEMPS | https://www.aemps.gob.es |
| Centro de Información Online de Medicamentos de la AEMPS Medicine (Online Information Center of AEMPS) | CIMA | https://www.aemps.gob.es/cima | |
| Ministerio de Sanidad (Ministry of Health) | n.a. | https://www.msssi.gob.es | |
| Germany | Pharmnet.Bund | Pharmnet | https://www.pharmnet‐bund.de |
| Bundesinstitut für Arzneimittel und Medizinprodukte (Federal Institute for Drugs and Medical Devices) | BfArM | https://www.bfarm.de | |
| Deutsches Arzneipruefungsinstitut e.V. (German Institute for Drug Use Evaluation) | DAPI | http://www.dapi.de | |
| The Netherlands | College ter Beoordeling van Geneesmiddelen (Medicines Evaluation Board) | CBG | https://www.cbg‐meb.nl |
| Genees‐ en hulpmiddelen Informatie Project Zorginstituut Nederland (The Drug Information System of National Health Care Institute) | GIP/ZIN | https://www.geneesmiddeleninformatiebank.nl | |
| Belgium | Federaal Agentschap voor Geneesmiddelen en Gezondheidsproducten (FAGG) or Agence Fédérale des Médicaments et des Produits de Santé (AFMPS). (The Federal Agency for Medicines and Health Products; FAMHP) | FAGG | https://www.fagg‐afmps.be |
| Farmanet database, Rijksinstituut voor ziekte‐ en invaliditeitsverzekering | Farmanet RIZIV | https://www.riziv.fgov.be | |
| Italy | Agenzia Italiana del Farmaco (Italian Medicines Agency) | AIFA | http://www.aifa.gov.it |
| Portugal | Autoridade Nacional do Medicamento e Produtos de Saúde (National Authority for Medicines and Health Products) | Infarmed | http://www.infarmed.pt |
| Norway | Statens legemiddelverk (The Norwegian Medicines Agency) | NoMA | https://legemiddelverket.no |
| Norwegian Prescription Database | NorPD | http://www.norpd.no | |
| Sweden | Läkemedelsverket (Swedish Medical Products Agency) | MPA | https://lakemedelsverket.se |
| Socialstyrelsen (The National Board of Health and Welfare) | n.a. | https://www.socialstyrelsen.se | |
| Ireland | Health Products Regulatory Authority | HPRA | https://www.hpra.ie |
| Health Service Executive–Primary Care Reimbursement Service | HSE‐PCRS | https://www.hse.ie | |
| Denmark | Laegemiddelstyrelsen (Danish Medicines Agency) | DMA | https://laegemiddelstyrelsen.dk |
| The Danish Health Data Authority Health Analysis, Medicinal Product Statistics & Health Data Program | MEDSTAT | http://www.medstat.dk | |
| Austria | Bundesamt für Sicherheit im Gesundheitswesen (Austrian Federal Office for Safety in Health Care) | BASG | https://www.basg.gv.at |
| Österreichischen Agentur für Gesundheit und Ernährungssicherheit (Austrian Medicines and Medical Devices Agency), Arzneispezialitätenregister/PharmaIS Web | AGES | https://aspregister.basg.gv.at | |
| Der Hauptverband der Österreichischen Sozialversicherungsträger (The main association of Austrian social security institutions) | Hauptverband | http://www.hauptverband.at | |
| Erstattungskodex (Reimbursement Codex) | Eko |
https://www.sozialversicherung.at/oeko
http://oertl.at/eko |
|
| Finland | Finnish Medicines Agency | Fimea | http://www.fimea.fi |
| Kansaneläkelaitos, (The Social Insurance Institution) | KELA | http://www.kela.fi | |
| Poland | Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych (URPLWMiPB) (Office for Registration of Medicinal Products, Medical Devices and Biocidal Products) | URPL | http://urpl.gov.pl |
| Rejestry medyczne, Centrum Systemów Informacyjnych Ochrony Zdrowia (Center for Health Information Systems) | CSIOZ | http://pub.rejestrymedyczne.csioz.gov.pl | |
| Narodowy Fundusz Zdrowia (National Health Fund, NFZ) | NFZ | http://www.nfz.gov.pl/ | |
| Bulgaria | The Bulgarian Drug Agency | BDA | http://www.bda.bg |
| Hungary | Országos Gyógyszerészeti és Élelmezés‐egészségügyi Intézet (National Institute of Pharmacy and Nutrition) | OGYEI | https://www.ogyei.gov.hu |
| Croatia | Hrvatska agencija za lijekove i medicinske proizvode (Croatian Agency for Medicinal Products and Medical Devices) | HALMED | http://www.halmed.hr |
| Czech Republic | Státní ústav pro kontrolu léčiv (State Institute for Drug Control) | Súkl | http://www.sukl.cz |
| Iceland | The Icelandic Medicines Agency | IMA | https://www.ima.is |
| Embætti landlæknis (Directorate of Health) | n.a. | https://www.landlaeknir.is | |
| Estonia | Ravimiamet (State Agency of Medicines) | SAM | https://www.ravimiamet.ee |
| Latvia | Zāļu valsts aģentūra (State Agency of Medicines of the Republic of Latvia) | ZVA | https://www.zva.gov.lv |
| Lithuania | Valstybinė vaistų kontrolės tarnyba (State Medicines Control Agency) | VVKT | http://www.vvkt.lt/ |
| Valstybinė ligonių kasa (State Patient Fund) | VLK | http://www.vlk.lt | |
| Slovakia | Štátny ústav pre kontrolu liečiv (State Institute for Drug Control) | ŠÚKL | https://www.sukl.sk |
| Montenegro | Crne Gore Agencija za ljekove i medicinska sredstva (Agency for Medicines and Medical Devices of Montenegro) | CALIMS | https://www.calims.me |
Contact information of the organizations surveyed.
Official English nomenclature where applicable and available in round brackets. EU, European Union; n.a., not applicable; URL, uniform resource locator, valid as for 18 November 2018.
Table A2.
African ancestry population data
| Location | Namea | Acronym | URL |
|---|---|---|---|
| Global | United Nations | UN | https://www.un.org |
| EU | The European Network Against Racism | ENAR | https://www.enar‐eu.org |
| WWW | Encyclopedia of Afro‐European Studies | EAES | http://www.encyclopediaofafroeuropeanstudies.eu |
| Afropedia | n.a. | http://www.afropedea.org | |
| Wikipedia | n.a. |
http://www.wikipedia.org
https://en.wikipedia.org/wiki/African_immigration_to_Europe |
|
| France | Institut national de la statistique et des études économiques (National Institute of Statistics and Economic Studies) | Insee | https://www.insee.fr |
| Le Conseil représentatif des associations noires de France | CRAN | https://le‐cran.fr | |
| UK | Office for National Statistics | ONS | https://www.ons.gov.uk |
| Spain | Instituto Nacional de Estadistica (National Statistics Institute) | INE | http://www.ine.es |
| Germany | Statistisches Bundesamt | Destatis | https://www.destatis.de |
| Initiative Schwarze Menschen in Deutschland (Initiative Black People in Germany) | ISD | http://isdonline.de | |
| The Netherlands | Centraal Bureau voor de Statistiek (Statistics Netherlands) | CBS | https://www.cbs.nl |
| Belgium | Algemene Directie Statistiek (Statistics Belgium) | Statbel | https://statbel.fgov.be |
| Forum van Etnisch‐Culturele Minderheden, (Minority Forum) | MECM | http://www.minderhedenforum.be | |
| Italy | Istituto Nazionale di Statistica (National Institute of Statistics) | ISTAT | https://www.istat.it |
| Portugal | Instituto Nacional de Estatística (Statistics Portugal) | INE | https://www.ine.pt |
| Serviço de Estrangeiros e Fronteiras, Portal de Estatística. (Portuguese Immigration and Border Service, Statistics Portal) | SEF | http://sefstat.sef.pt | |
| Norway | Statistisk sentralbyrå (Statistics Norway) | SSB | https://www.ssb.no |
| Sweden | Statistiska centralbyrån (Statistics Sweden) | SCB | http://www.scb.se |
| Afrosvenskarnas riksförbund (The Afro‐Swedish National Association) | ASR | http://www.afrosvenskarna.se | |
| Mångkulturellt centrum (The Multicultural centre) | MKC | http://mkcentrum.se | |
| Ireland | Central Statistics Office Ireland | CSO | https://www.cso.ie |
| Danmark | Danmarks Statistik (Statistics Denmark) | DST | https://www.dst.dk |
| Afro Empowerment Center Denmark | AEC | http://www.aec‐cph.dk | |
| Austria | Bundesanstalt Statistik Österreich (Statistics Austria) | SA | http://www.statistik.at |
| Finland | Statistics Finland | SF | https://www.stat.fi |
| Poland | Główny Urząd Statystyczny (Statistics Poland) | SP | https://stat.gov.pl |
| Bulgaria | National Statistical Institute | NSI | http://www.nsi.bg |
| Hungary | Kosponti Statisztikai Hivatal (Hungarian Central Statistical Office) | KSH | https://www.ksh.hu |
| Croatia | Državni zavod za statistiku (Croatian Bureau of Statistics) | DZS | https://www.dzs.hr |
| Czech Republic | Český statistický úřad/Czech Statistical Office | CZSO | https://www.czso.cz |
| Iceland | Hagstofa Íslands (Statistics Iceland) | Statice | http://statice.is |
| Estonia | Statistikaamet (Statistics Estonia) | SE | http://pub.stat.ee |
| Latvia | Latvijas statistika (Central Statistical Bureau of Latvia) | CSB | http://www.csb.gov.lv |
| Lithuania | Statistics Lithuania | SL | https://www.stat.gov.lt/en |
| Slovakia | Statistical Office of the Slovak Republic | SOSR | https://slovak.statistics.sk |
| Montenegro | Zavod za statistiku Crne Gore (Statistical Office of Montenegro) | MONSTAT | http://www.monstat.org |
Contact information of the organizations surveyed.
Official English nomenclature where applicable and available in round brackets. EU, European Union; WWW, World Wide Web; n.a., not applicable; URL, uniform resource locator, valid as for 18 November 2018.
Brewster, L. M. (2019) Underuse of hydralazine and isosorbide dinitrate for heart failure in patients of African ancestry: a cross‐European survey. ESC Heart Failure, 6: 487–498. 10.1002/ehf2.12421.
References
- 1. National Heart, Lung and Blood Institute (NHLBI) . Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung and Blood Diseases. http://www.nhlbi.nih.gov/resources/docs/2012_ChartBook_508.pdf (18 December 2018).
- 2. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr, Willis M, Yancy CW, American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council . Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation 2017; 136: e393–e423. [DOI] [PubMed] [Google Scholar]
- 3. Bibbins‐Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med 2009; 360: 1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brewster LM, van Montfrans GA, Oehlers GP, Seedat YK. Systematic review: antihypertensive drug therapy in patients of African and South Asian ethnicity. Intern Emerg Med 2016; 11: 355–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferdinand KC, Elkayam U, Mancini D, Ofili E, Piña I, Anand I, Feldman AM, McNamara D, Leggett C. Use of isosorbide dinitrate and hydralazine in African‐Americans with heart failure 9 years after the African‐American Heart Failure Trial. Am J Cardiol 2014; 114: 151–159. [DOI] [PubMed] [Google Scholar]
- 6. Gu Q, Burt VL, Paulose‐Ram R, Yoon S, Gillum RF. High blood pressure and cardiovascular disease mortality risk among U.S. adults: the third National Health and Nutrition Examination Survey mortality follow‐up study. Ann Epidemiol 2008; 18: 302–309. [DOI] [PubMed] [Google Scholar]
- 7. Gupta DK, Shah AM, Castagno D, Takeuchi M, Loehr LR, Fox ER, Butler KR, Mosley TH, Kitzman DW, Solomon SD. Heart failure with preserved ejection fraction in African Americans: the ARIC (Atherosclerosis Risk in Communities) study. JACC Heart Fail 2013; 1: 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser response to angiotensin‐converting‐enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. N Engl J Med 2001; 344: 1351–1357. [DOI] [PubMed] [Google Scholar]
- 9. Brewster LM, Seedat YK. Why do hypertensive patients of African ancestry respond better to calcium blockers and diuretics than to ACE inhibitors and β‐adrenergic blockers? A systematic review. BMC Med 2013; 11: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carson P, Ziesche S, Johnson G, Cohn JN. Racial differences in response to therapy for heart failure: analysis of the vasodilator‐heart failure trials. Vasodilator‐Heart Failure Trial Study Group. J Card Fail 1999; 5: 178–187. [DOI] [PubMed] [Google Scholar]
- 11. Taylor AL, Ziesche S, Yancy C, Carson P, D'Agostino R Jr, Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, Cohn JN, African‐American Heart Failure Trial Investigators . Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351: 2049–2057. [DOI] [PubMed] [Google Scholar]
- 12. Anand IS, Win S, Rector TS, Cohn JN, Taylor AL. Effect of fixed‐dose combination of isosorbide dinitrate and hydralazine on all hospitalizations and on 30‐day readmission rates in patients with heart failure: results from the African‐American Heart Failure Trial. Circ Heart Fail 2014; 7: 759–765. [DOI] [PubMed] [Google Scholar]
- 13. US Food and Drug Administration . Drug Approval Package. Bidil (Isosorbide Dinitrate and Hydralazine Hydrochloride) Tablets. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/020727_s000_BidilTOC.cfm (18 December 2018).
- 14. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 15. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure. Circulation 2013; 128: e240–e327. [DOI] [PubMed] [Google Scholar]
- 16. European Medicines Agency . http://www.ema.europa.eu (18 December 2018).
- 17. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 18. Ofili E, Anand I, Williams RA, Akinboboye O, Xu L, Puckrein G. Fixed‐dose versus off‐label combination of isosorbide dinitrate plus hydralazine hydrochloride: retrospective propensity‐matched analysis in Black Medicare patients with heart failure. Adv Ther 2017; 34: 1976–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization . The Anatomical Therapeutic Chemical (ATC) Classification System. https://www.whocc.no/atc (18 December 2018).
- 20. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011; 8: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dokainish H, Teo K, Zhu J, Roy A, AlHabib KF, ElSayed A, Palileo‐Villaneuva L, Lopez‐Jaramillo P, Karaye K, Yusoff K, Orlandini A, Sliwa K, Mondo C, Lanas F, Prabhakaran D, Badr A, Elmaghawry M, Damasceno A, Tibazarwa K, Belley‐Cote E, Balasubramanian K, Islam S, Yacoub MH, Huffman MD, Harkness K, Grinvalds A, McKelvie R, Bangdiwala SI, Yusuf S, Investigators INTER‐CHF. Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER‐CHF) prospective cohort study. Lancet Glob Health 2017; 5: e665–e672. [DOI] [PubMed] [Google Scholar]
- 22. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008; 101: 1016–1022. [DOI] [PubMed] [Google Scholar]
- 23. Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation 2011; 123: 2006–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharma A, Colvin‐Adams M, Yancy CW. Heart failure in African Americans: disparities can be overcome. Cleve Clin J Med 2014; 81: 301–311. [DOI] [PubMed] [Google Scholar]
- 25. Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal implementation of evidence‐based heart failure therapies on mortality. Am Heart J 2011; 161: 1024–1030.e3. [DOI] [PubMed] [Google Scholar]
- 26. Thomas KL, East MA, Velazquez EJ, Tuttle RH, Shaw LK, O'Connor CM, Peterson ED. Outcomes by race and etiology of patients with left ventricular systolic dysfunction. Am J Cardiol 2005; 96: 956–963. [DOI] [PubMed] [Google Scholar]
- 27. Sauer W. Von Soliman zu Omofuma: Afrikanische Diaspora in Österreich 17. bis 20. Jahrhundert. Innsbruck: StudienVerlag; 2007. p 190. [Google Scholar]
- 28. Ząbek M, Bartoszyńska K. Africans in Poland: race relations in contemporary Polish society. Int J Sociol 2009; 39: 68–78. [Google Scholar]
- 29. Farkas L. Data Collection in the Field of Ethnicity: Analysis and Comparative Review of Equality Data Collection Practices in the European Union. Brussels, Belgium: European Commission; 2017. [Google Scholar]
- 30. Oppenheimer DB. Why France needs to collect data on racial identity—in a French way. Hastings Int'l & Comp L Rev 2008; 31: 735–752. [Google Scholar]
- 31. InfoCuria—Case‐law of the Court of Justice . http://curia.europa.eu/juris/liste.jsf?&num=C‐544/13 (18 December 2018).
- 32. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 33. World Health Organization . Universal Health Coverage. http://www.who.int/universal_health_coverage (18 December 2018).
- 34. World Health Organization . WHO Model Lists of Essential Medicines. http://www.who.int/medicines/publications/essentialmedicines/en/ (18 December 2018).
- 35. The Heads of Medicines Agencies . Task Force on Availability of Authorised Medicines for Human and Veterinary Use (TF AAM). http://www.hma.eu/598.html (18 December 2018).
- 36. Clarke A. People of African Descent in Europe. A UKREN Briefing Paper 2012. http://www.ukren.org (18 December 2018).
- 37. European Union Agency for Fundamental Rights . European Union Minorities and Discrimination Survey (EU‐MIDIS). Main Results Report; 2009: p 15. http://fra.europa.eu/eu‐midis (18 December 2018).
- 38. McNamara DM, Taylor AL, Tam SW, Worcel M, Yancy CW, Hanley-Yanez K, Cohn JN, Feldman AM. G‐protein beta‐3 subunit genotype predicts enhanced benefit of fixed‐dose isosorbide dinitrate and hydralazine: results of A‐HeFT. JACC Heart Fail 2014; 2: 551–557. [DOI] [PubMed] [Google Scholar]


