Abstract
Aim:
To describe the clinical picture of orbital relapse of retinoblastoma following uncomplicated enucleation.
Methods:
Case series of two patients with group E retinoblastoma with high-risk features on histopathology, namely, post-laminar optic nerve extension in one patient, and massive choroidal tumor infiltration in the other. Neither of them received adjuvant chemotherapy post enucleation.
Results:
Both patients had orbital relapse of tumor within 4 months from enucleation, manifesting as implant migration and unstable conformer. Systemic chemotherapy and external beam radiotherapy to the orbit resulted in complete tumor regression. Both patients were tumor free at the last follow up.
Conclusion:
Implant migration post enucleation should raise the suspicion for orbital relapse of retinoblastoma. High-risk histopathology features should increase the alert in an otherwise uncomplicated enucleation for retinoblastoma.
Keywords: eye, orbital tumors, retinoblastoma, tumor
Retinoblastoma (RB) is the most common primary intraocular tumor in the pediatric population.1 The recent advances in diagnosis and the new treatment protocols have contributed to improved outcomes in terms of survival, eye salvage, and potential visual recovery.2,3 Orbital extension of the tumor is one of the major contributors to RB mortality, with death rate ranging from 25% to 100%.3–5 Although its incidence is reducing, it is still a common diagnosis at presentation in the developing world.6,7
Orbital relapse of RB presents as an orbital mass arising within the socket weeks to years after the primary surgery; initial clinical signs may be subtle, and the diagnosis is often delayed. Unexplained displacement or extrusion of a previously well-fitting conformer or prosthetic implant in an eye enucleated for RB should raise the suspect of orbital tumor recurrence.8 We describe two cases of orbital relapse of RB presenting at the L V Prasad Eye Institute (Hyderabad, India) with implant displacement, following uncomplicated enucleation. Our study was approved by L V Prasad Eye Institute Ethics Committee (approval no. LEC 11-18-196). A statement of consent was obtained from the legal guardian of both patients for the use of clinical information and photographs in the manuscript.
Case presentation
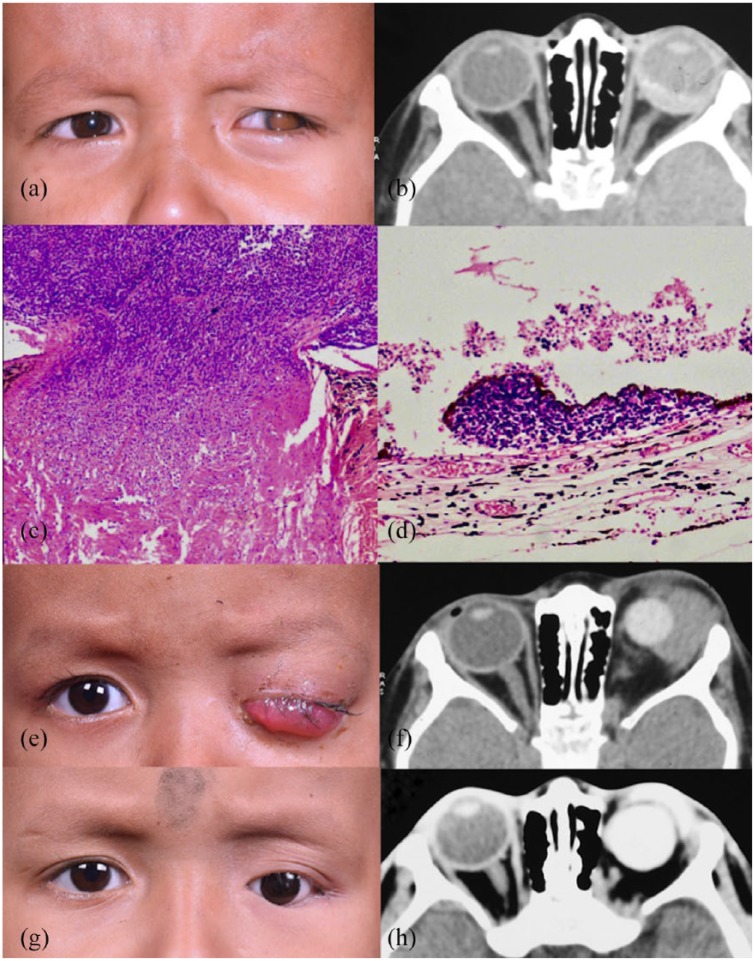
Patient 1 was a 2-year-old boy, who presented with white pupil in his left eye (OS) for 10 months, associated with pain and redness. Examination of the OS revealed iris neovascularization, ectropion uveae, increased intraocular pressure (IOP), leukocoria, and a diffuse infiltrating retinoblastoma. The right eye was normal. The child was diagnosed with OS Group E RB, according to the International Classification for Intraocular Retinoblastoma (ICRB)9 and cT3c according to 8th edition of American Joint Committee Classification (AJCC).10 The child subsequently underwent enucleation with a 19-mm implant. Histopathology revealed 1-mm post-laminar optic nerve tumor extension and minor choroidal tumor infiltration; cerebrospinal fluid (CSF) and bone marrow (BM) aspiration showed no tumor invasion. The child was lost to follow up; he returned 3 months after surgery, with parents complaining of ill-fitting conformer. Severe chemosis, diffuse congestion, and discharge from the socket were noted on ophthalmic examination; the implant was displaced nasally, and a firm mass was palpable in the lateral orbit. CSF cytology and BM aspiration showed no tumor invasion. Computed tomography (CT) of the orbit confirmed the diagnosis of orbital recurrence of RB. The child subsequently received 12 cycles of high-dose chemotherapy and external beam radiotherapy (EBRT) to the left orbit, with good regression of the tumor. At 2-year follow-up visit, the child had a grade 1 contracted socket, slightly migrated implant, shallow fornices, and a well-fitting prosthesis, with no evidence of persistent or recurrent tumor (Figure 1).
Figure 1.
Case 1: (a) a 2-year-old boy presented with leukocoria of the left eye (OS). (b) Computed tomography (CT) of the orbit showed diffuse infiltrating retinoblastoma OS. (c) Histopathology revealed post-laminar optic nerve tumor infiltration (hematoxylin and eosin stain, 10× magnification) and (d) minor choroidal tumor infiltration (hematoxylin and eosin stain, 40× magnification). (e) Orbital relapse of retinoblastoma with conjunctival chemosis, implant migration, and palpable orbital mass in the left socket. (f) The findings were confirmed by an isodense mass in the left lateral orbit on CT. (g) The tumor regressed with treatment, which is confirmed by CT orbit (h).
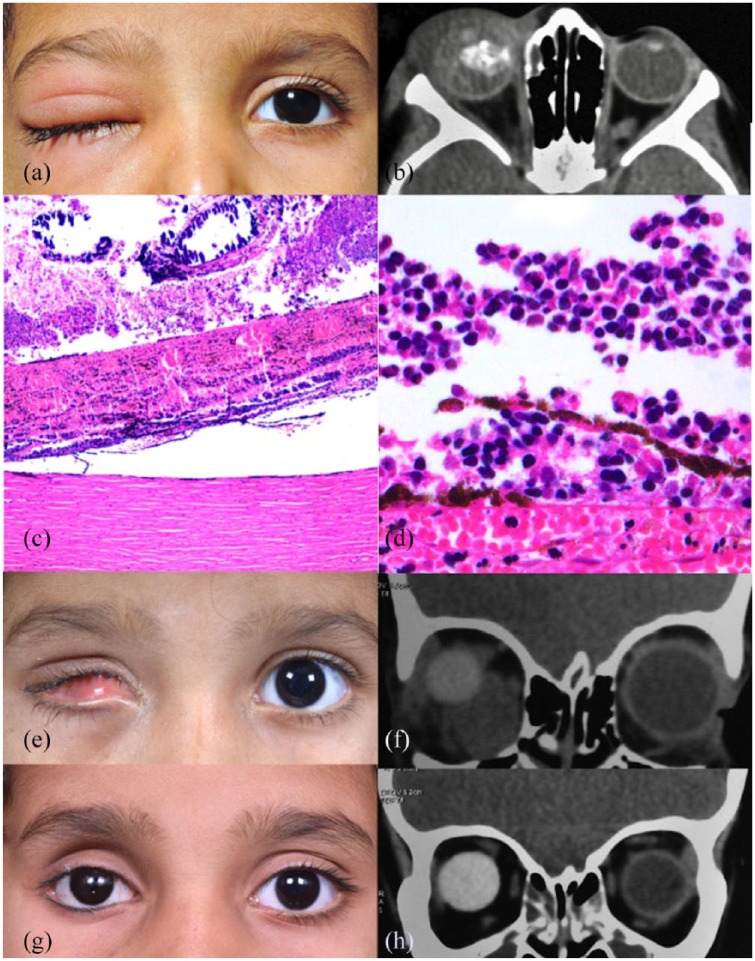
Patient 2 was a 2-year-old girl referred to our center for evaluation of right eye (OD) leukocoria and eyelid edema. Examination of the OD revealed preseptal edema, white pupillary reflex, focal iris neovascularization, and diffuse retinal detachment. The left eye was normal. B-scan OD showed a calcified lesion extending from the posterior pole to the retrolental space. A diagnosis of OD group E RB as per ICRB9 and cT3e10 as per AJCC was established. CSF cytology and BM aspiration were negative; CT of the orbit showed periocular edema with intraocular mass and no evidence of optic nerve, extraocular, or intracranial extension. The child received 48-h intravenous steroid (dexamethasone 4 mg, 3 times/day) and subsequently underwent enucleation with a 19-mm implant. Post surgery, the ocular prosthesis was fitting well and the implant was centered in the socket. Initial histopathology report revealed absence of high-risk features. Four months later, the child presented with complaints of poor fitting prosthesis for 2 days. Socket examination revealed superonasal implant migration and an inferomedial firm, hard, palpable mass. CSF cytology and BM aspiration showed no tumor invasion. CT scan confirmed orbital recurrence in the right socket. The histopathology slides were reviewed again, revealing presence of massive choroidal infiltration. The child received a total of 12 cycles of high-dose chemotherapy, followed by EBRT to the right orbit. The tumor regressed after treatment. At 6.5-year follow-up visit, the socket presented a good volume and surface, with a well-centered implant and mild superior sulcus deformity, with no evidence of tumor recurrence (Figure 2).
Figure 2.
Case 2: (a) a 2-year-old girl presented with preseptal cellulitis and leukocoria of the right eye (OD). (b) Computed tomography (CT) of the orbit showed tumor filling the globe with intratumor calcification. (c, d) Histopathology revealed massive choroidal tumor infiltration (hematoxylin and eosin stain, 10× and 40× magnification). (e) Orbital relapse of retinoblastoma with unstable conformer, shallow inferior fornix, implant migration, and palpable orbital mass in the right socket. (f) The findings were confirmed by an isodense mass in the right inferomedial orbit on CT. (g) The tumor regressed with treatment, which is confirmed by CT orbit (h).
Discussion
We presented two cases of orbital RB recurrence presenting as implant migration. A similar case has been reported by Karcioglu and colleagues11 in 1998. Orbital tumor recurrence following enucleation is a relatively rare complication, with incidence ranging from 4% to 23%.12,13 Wrong manipulation of the eyeball and optic nerve during enucleation, undetected orbital extension at the diagnosis, previous ocular surgery, or fine-needle aspiration biopsy are well-known predisposing factors.12,14,15
Clinically, the tumor usually presents as a subconjunctival mass in the socket, with a purplish hue, due to its prominent vascularity.16,17 Periocular swelling and ecchymosis, purulent discharge, or frank bleeding from the socket have also been reported.18 In patients wearing an artificial eye, problems with the ocular prosthesis, with extrusion or displacement, should raise the suspicion of orbital tumor recurrence. Our cases enhance the importance of prosthesis removal, followed by careful examination of the socket, at every periodic follow-up visit.
Advanced tumor stage at the diagnosis represents a significant clinical risk factor for orbital recurrence. In our study, both the cases presented with group E RB.13 There have also been efforts to demonstrate an association between high-risk histopathologic features of enucleated eyes and orbital relapse, although a consensus is lacking.19–21 In a study of 1674 consecutive patients who underwent enucleation for RB, orbital tumor recurrence was noted in 71 cases (4%). Of these 71 cases, all except 11 subjects had evidence of high-risk histopathology features.12 In both our cases, high-risk histopathologic features were present, including post-laminar optic nerve extension in case 1 and massive choroidal tumor infiltration in case 2. Case 2 also highlights the importance of critical study of calottes in addition to pupil–optic nerve section of the eyeball in RB cases.
The diagnosis of orbital relapse of RB is usually made within 2 years after enucleation, with approximately 97% of patients presenting within the initial 12 months.12,13 Indeed, our patients were diagnosed with tumor recurrence after 3 and 4 months after surgery, respectively.
Early identification and treatment of RB orbital relapses are crucial for the overall survival of the patients.4,12 Brain and orbital imaging, lumbar puncture, and BM aspiration or biopsy are mandatory to identify the extent of the disease.22 Magnetic resonance imaging (MRI) of the brain and orbit is preferred over CT imaging in cases of RB to minimize the radiation exposure. In this study, both cases underwent CT imaging as per the protocol at that time period. Our current practice is to perform MRI brain and orbit in all cases of RB.
Mortality rates of orbital recurrence are progressively reducing. In a series published in 1963, none of the 25 cases have survived, with most of the deaths occurring within 2 years.23 More recent studies have reported better long-term survival with aggressive multimodal treatment.8,12,14,24,25 Proposed protocol for orbital relapse of RB is high-dose chemotherapy (3–6 cycles) of vincristine, etoposide, and carboplatin, followed by orbital external beam radiotherapy.24,26 Surgical intervention in such cases may be limited to excision of the residual orbital mass or orbital exenteration post-systemic chemotherapy. Our cases showed no evidence of residual tumor after systemic chemotherapy, thus orbital exenteration was avoided. At 24- and 78-month follow-up visits, respectively, both were alive and healthy, with no evidence of metastatic disease or local tumor recurrence.
In conclusion, orbital relapse of RB is a dramatic complication of successful uncomplicated enucleation with high-risk histopathologic features. The new therapeutic approaches have increased the rate of survival for these patients; however, high mortality rates are still a concern. Poor histopathology reporting with missed findings on high-risk features, poor compliance for follow-up visits, and negligence of primary signs of tumor recurrence play a role in the overall prognosis. Clinicians should be aware that orbital recurrence of RB may be asymptomatic in the initial phase or present with subtle, non-localizing signs. Careful socket examination is mandatory in all cases post enucleation, even if the patient is asymptomatic. Caregivers should rigorously instruct the parents that enucleation for intraocular RB requires careful follow up for at least 2 years after surgery.
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our study was approved by L V Prasad Eye Institute Ethics Committee (approval no. LEC 11-18-196).
Funding: This work was supported by the Operation Eyesight Universal Institute for Eye Cancer (S.K.) and Hyderabad Eye Research Foundation (S.K.), Hyderabad, India.
Informed consent: A statement of consent was obtained from the legal guardian of both the patients for the use of clinical information and photographs in the manuscript.
ORCID iD: Swathi Kaliki  https://orcid.org/0000-0002-0800-9961
https://orcid.org/0000-0002-0800-9961
Contributor Information
Maria Vittoria Cicinelli, Operation Eyesight Universal Institute for Eye Cancer, L V Prasad Eye Institute, Hyderabad, India.
Swathi Kaliki, Operation Eyesight Universal Institute for Eye Cancer, L V Prasad Eye Institute, Hyderabad, India.
References
- 1. Dimaras H, Kimani K, Dimba EA, et al. Retinoblastoma. Lancet 2012; 379: 1436–1446. [DOI] [PubMed] [Google Scholar]
- 2. Tamboli A, Podgor MJ, Horm JW. The incidence of retinoblastoma in the United States: 1974 through 1985. Arch Ophthalmol 1990; 108: 128–132. [DOI] [PubMed] [Google Scholar]
- 3. Nyamori JM, Kimani K, Njuguna MW, et al. Retinoblastoma referral pattern in Kenya. Middle East Afr J Ophthalmol 2014; 21: 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Badhu B, Sah SP, Thakur SK, et al. Clinical presentation of retinoblastoma in Eastern Nepal. Clin Exp Ophthalmol 2005; 33: 386–389. [DOI] [PubMed] [Google Scholar]
- 5. Leal-Leal C, Flores-Rojo M, Medina-Sanson A, et al. . A multicentre report from the Mexican Retinoblastoma Group. Br J Ophthalmol 2004; 88: 1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stannard C, Lipper S, Sealy R, et al. Retinoblastoma: correlation of invasion of the optic nerve and choroid with prognosis and metastases. Br J Ophthalmol 1979; 63: 560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh AD, Shields CL, Shields JA. Prognostic factors in retinoblastoma. J Pediatr Ophthalmol Strabismus 2000; 37: 134–141. [DOI] [PubMed] [Google Scholar]
- 8. Ellsworth RM. Orbital retinoblastoma. Trans Am Ophthalmol Soc 1974; 72: 79–88. [PMC free article] [PubMed] [Google Scholar]
- 9. Linn Murphree A. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am 2005; 18: 41–53, viii. [DOI] [PubMed] [Google Scholar]
- 10. Mallipatna AC, Gallie BL, Chévez-Barrios P, et al. Retinoblastoma. In: Amin MB, Edge SB, Greene FL, et al. (eds) AJCC cancer staging manual. 8th ed Bern: Springer, 2017, pp. 819–831. [Google Scholar]
- 11. Karcioglu ZA, Mullaney PB, Millar LC. Extrusion of porous polyethylene orbital implant in recurrent retinoblastoma. Ophthalmic Plast Reconstr Surg 1998; 14: 37–44. [DOI] [PubMed] [Google Scholar]
- 12. Kim JW, Kathpalia V, Dunkel IJ, et al. Orbital recurrence of retinoblastoma following enucleation. Br J Ophthalmol 2009; 93: 463–467. [DOI] [PubMed] [Google Scholar]
- 13. Arif M, Islam Z. Retinoblastoma: postenucleation orbital recurrence. Can J Ophthalmol 2010; 45: 606–609. [DOI] [PubMed] [Google Scholar]
- 14. Hungerford J, Kingston J, Plowman N. Orbital recurrence of retinoblastoma. Ophthalmic Paediatr Genet 1987; 8: 63–68. [DOI] [PubMed] [Google Scholar]
- 15. Stevenson KE, Hungerford J, Garner A. Local extraocular extension of retinoblastoma following intraocular surgery. Br J Ophthalmol 1989; 73: 739–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee V, Subak-Sharpe I, Hungerford JL, et al. . Exposure of primary orbital implants in postenucleation retinoblastoma patients. Ophthalmology 2000; 107: 940–945, discussion 946. [DOI] [PubMed] [Google Scholar]
- 17. Grabowski EF, Abramson DH. Intraocular and extraocular retinoblastoma. Hematol Oncol Clin North Am 1987; 1: 721–735. [PubMed] [Google Scholar]
- 18. Mourits DL, Moll AC, Bosscha MI, et al. Orbital implants in retinoblastoma patients: 23 years of experience and a review of the literature. Acta Ophthalmol 2016; 94: 165–174. [DOI] [PubMed] [Google Scholar]
- 19. El Zomor H, Taha H, Aleieldin A, et al. High risk retinoblastoma: prevalence and success of treatment in developing countries. Ophthalmic Genet 2015; 36: 287–289. [DOI] [PubMed] [Google Scholar]
- 20. Chantada GL, Dunkel IJ, de Davila MT, et al. Retinoblastoma patients with high risk ocular pathological features: who needs adjuvant therapy. Br J Ophthalmol 2004; 88: 1069–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shields CL, Shields JA, Baez K, et al. Optic nerve invasion of retinoblastoma. Metastatic potential and clinical risk factors. Cancer 1994; 73: 692–698. [DOI] [PubMed] [Google Scholar]
- 22. Sirin S, deJong MC, de Graaf P, et al. High-resolution magnetic resonance imaging can reliably detect orbital tumor recurrence after enucleation in children with retinoblastoma. Ophthalmology 2016; 123: 635–645. [DOI] [PubMed] [Google Scholar]
- 23. Reese A. Tumors of the eye (ed. Ha R.). New York: Harper & Row, 1963, pp. 155–156. [Google Scholar]
- 24. Goble RR, McKenzie J, Kingston JE, et al. Orbital recurrence of retinoblastoma successfully treated by combined therapy. Br J Ophthalmol 1990; 74: 97–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antoneli CB, Steinhorst F, de Cassia Braga Ribeiro K, et al. Extraocular retinoblastoma: a 13-year experience. Cancer 2003; 98: 1292–1298. [DOI] [PubMed] [Google Scholar]
- 26. Shields CL, Shields JA. Retinoblastoma management: advances in enucleation, intravenous chemoreduction, and intra-arterial chemotherapy. Curr Opin Ophthalmol 2010; 21: 203–212. [DOI] [PubMed] [Google Scholar]