Short abstract
Background
Memantine is one of the important clinical medications in treating moderate to severe Alzheimer disease. The effect of memantine on preventing or treating punctate allodynia has been thoroughly studied but not on the induction of dynamic allodynia. The aim of this study is to investigate whether memantine could prevent the induction of dynamic allodynia and its underlying spinal mechanisms.
Results
(1) In in vivo spared nerve injury pain model, pretreatment with memantine at a lower dose (10 nmol, intrathecal; memantine-10) selectively prevented the induction of dynamic allodynia but not the punctate allodynia. (2) Pretreatment with either MK801-10 (MK801-10 nmol, intrathecal) or higher dose of memantine (30 nmol, intrathecal; memantine-30) prevented the induction of both dynamic and punctate allodynia. (3) Memantine-10 showed significant effect on the inhibition of the spared nerve injury-induced overactivation of microglia in spinal dorsal horn. (4) In contrast, in complete freund′s adjuvant (CFA) model, memantine-10 neither affected the CFA injection-induced activation of microglia in spinal dorsal horn nor the induction of dynamic allodynia. (5) Immunohistological studies showed Kir2.1 channel distributed widely and co-localized with microglia in the spinal dorsal horn of mice. (6) Pretreatment with either minocycline, a microglia inhibitor, or ML133, a Kir2.1 inhibitor, both selectively prevented the overactivation of microglia in spinal dorsal horn and the induction of dynamic allodynia following spared nerve injury.
Conclusion
The selective inhibitory effect on the induction of dynamic allodynia in spared nerve injury model by low dose of the memantine (memantine-10) was tightly correlated with the blockade of microglia Kir2.1 channel to suppress the microglia activation.
Keywords: Memantine, microglia, dynamic allodynia, spared nerve injury, Kir2.1 channel
Introduction
Herpes zoster is characterized by clustered vesicles accompanied by severe pain in human subjects.1 After healing from the skin lesions, postherpetic neuralgia established for long days which is often resistant to conventional analgesics2,3 even morphine. Among various types of pain (burning pain, aching pain, periodic piercing pain, allodynia, etc.), previous study revealed that appropriately 90% of the patients with postherpetic neuralgia suffered from dynamic allodynia, a painful sensation elicited by gentle stroking stimulation.4 Different from punctate, different neural circuits were found to mediate the development of dynamic allodynia including primary afferent fibers, interneurons in spinal cord, and so forth.5,6 Although many research works have focused more attention on the establishing procedure of dynamic allodynia, no precise mechanisms and effective therapies documented clearly yet. In our previous studies, we proved that dynamic mechanical hypersensitivity induced by nerve injury or inflammation was compromised in mice with ablation of spinal VT3Lbx1 neurons, and preserved somatostatin lineage neurons were still largely sufficient to mediate punctate mechanical hypersensitivity.5 In this study, we investigated the clinical drug—memantine’s (MEM) effect on preventing the induction of dynamic allodynia after nerve injury.
MEM is approved to treat moderate to severe Alzheimer’s disease because of its ability of blocking excessive N-methyl-D-aspartate receptor (NMDAR) activity in central nervous system7. NMDAR also plays pivotal role in central sensitization and pain-related hyperexcitability during the development of neuropathic pain. In some animal neuropathic pain models, MEM alleviated8–10 or did not change11,12 punctate allodynia. Clinical efficacy of MEM on painful patients also has not been proven, clearly targeting various types of pain.13 Previous studies mentioned earlier mainly studied the MEM’s effect on punctate mechanical allodynia, and the results remained somewhat contentious. However, the efficacy of MEM on the induction of dynamic mechanical allodynia was less studied at present.
Microglia was strongly activated by neuropathic pain, and treatment with MEM could suppress the activation of microglia.10 Kir channel family has been found in a wide variety of cells and show inward rectification which not only orchestrate the passive and active electrical properties of cells but also link cellular metabolic state and membrane excitability.14 Kir2.1 channel was especially abundant in the lamina I and II of the spinal dorsal horn.15 Another research proved that MEM could block Kir2.1 channels which further affected the functional activities of microglia.16 In this study, we explored whether MEM prevented the induction of dynamic allodynia after spared nerve injury (SNI) and whether the underlying mechanism was tightly related with microglia and Kir2.1 channel in the dorsal spinal cord. Our results demonstrated that the selective prevention of dynamic allodynia by low-dose MEM (10 nmol, intrathecal (i.t.); MEM-10) was conducted by blocking of the Kir2.1 channel and inhibiting the activation of the microglia in spinal dorsal horn of mice.
Methods
Animals
Male C57BL/6J mice (6w-8w) were purchased from Shanghai Slac Laboratory Animal Co. Ltd and housed in the animal facilities of Institutes of Brain Science, Fudan University for this study. The animal cages were under controlled conditions of room temperature (25°C) and humidity (65–70%), on a 12-h light–dark cycle (light: 7:00 am–19:00 pm; dark: 19:00 pm–7:00 am) and allowed free access to food and water. After one week in the animal cages, the mice were injected with drugs and then operated.
Drug application
Drug administration was performed 30 min before the surgery. Normal saline (10 µl), MEM (10 nmol and 30 nmol), MK801 (3 nmol and 10 nmol), and minocycline (30 nmol) were dissolved in 10 µl normal saline and directly injected into spinal cavity by i.t. injection method.17 ML133 (Selleck) was dissolved in dimethyl sulfoxide (at concentration of 200 mM) and diluted with normal saline (final concentration 3 mM) before i.t. administration (10 µL) to mice. In the CFA injection experiment, MEM-10 was dissolved in artificial cerebrospinal fluid (ACSF). Either normal saline or ACSF was used as vehicle control. MEM, MK801, and minocycline were purchased from Sigma-Aldrich (USA).
SNI model
SNI model was performed as described by Decosterd and Woolf.18 Briefly, anesthesia was applied with isoflurane (0.2%), followed by 1-cm skin incision exposing the femur left thigh. Next, left tibial and common peroneal branches of sciatic nerve were ligated and transected distally, while the sural nerve was left intact. In the sham controls, the sciatic nerve and its branches were exposed without any ligation or transection. Following all procedures, the mice were returned to recovery cage and observed till they were fully ambulatory and able to take food and water. All experimental mice were handled gently to ensure that animal distress is minimized or eliminated during examination.
CFA model
Chronic inflammatory pain was induced by subplantar injection of complete Freund’s adjuvant (CFA, 10 µl; Sigma-Aldrich, St. Louis, MO) into the left hind paw under anesthetic conditions with isoflurane according to previous studies.5 The subplantar of mice in control group was injected with normal saline (10 µl). Mice were checked in behavior test at one and three days after CFA injection.
Behavior test
Mice were placed on the apparatus for consecutive three days before SNI surgery or CFA injection in order to acclimatize the new environment. After habituation and baseline sensitivity measurements, mice were examined punctate and dynamic allodynia in days 1 and 3 post-SNI surgery and CFA injection. The interval was at least 15 min for two different tests.
Punctate allodynia
Von Frey filaments were used to measure paw withdrawal threshold of experimental mice using Dixon’s up-down method.19,20 The mice were placed on an elevated wire grid, and the lateral plantar surface of the hindpaw was stimulated with calibrated von Frey monofilaments (0.02–1 g). Positive responses were recognized for three lifts of five trials with 3-min intervals for each stimulus intensity.
Dynamic allodynia
The protocol of evaluating dynamic allodynia was referred to the previously published studies.21 Briefly, the operated left hindpaw was lightly stroked with paint brush (appropriately 10 hairs) in the direction from heel to toe. Scoring system was employed to determine the light touch sensitivity of mice. For each test the scores were given as follows: score 0: walking away or occasionally brief paw lifting (<1 s or less), score 1: sustained lifting (>2 s) of the stimulated paw toward the body, score 2: strong lateral lifting above the level of the body, score 3: flinching or licking of the operated paw. The test was repeated three times, with the intervals of 10 s.
Tissue preparation
The experimental mice were perfused transcardially with 0.9% normal saline followed by 4% paraformaldehyde under anesthesia with chloral hydrate (SNI at day 5). The whole spinal cord was removed and post-fixed in 4% paraformaldehyde for 2 h and subjected to 15% and 30% sucrose solution in 0.01 M phosphate-buffered saline (PBS) for dehydration, until it sunk to the bottom. Coronal lumbar spinal sections were cut into 20 µm by freezing microtome (Leica, Germany).
Immunohistochemistry
Spinal cord tissue sections (20 µm) were processed for visualization of the Iba-1 protein and Kir2.1 channel expression by immunofluorescent labeling in the dorsal horn. Sections were incubated overnight at 4°C in PBS containing 10% donkey serum, a mixture of two antibodies, rabbit monoclonal anti-Iba-1 antibody (dilution 1:500, GeneTex), and mouse polyclonal anti-Kir2.1 (dilution 1:200, Abcam). After thorough rinsing, the sections were incubated for 2 h at room temperature in a mixture of two corresponding secondary fluorescently tagged antibodies (dilution at 1:500, Alexa Fluor 488 for Iba-1 and Alexa Fluor 594 for Kir2.1, Invitrogen). Sections were covered with glass coverslips and pictured under the fluorescence microscope (Olympus, Japan). The fluorescence intensities of Iba-1 and Kir2.1 channel in spinal dorsal horn were analyzed by ImageJ software.
Statistical analysis
Data are expressed as mean ± SEM; n refer to the number of experimental mice in each group. Two-way analysis of variance (ANOVA) with Bonferroni post hoc tests were used to detect the difference among different groups of data. Statistical analysis was employed using GraphPad Prism 7 software.
Results
MEM-10 selectively prevented the induction of dynamic, but not punctate, allodynia following SNI in mice
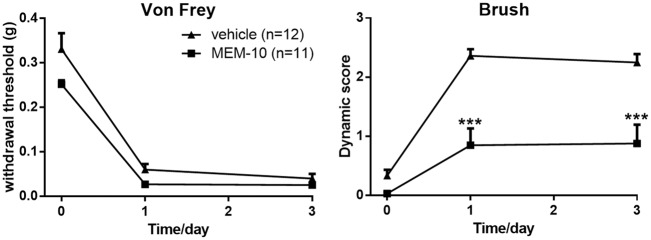
SNI could induce long-lasting ipsilateral mechanical allodynia. Consistent with our previous study,5 SNI operation induced robust punctate allodynia (sensitive to von Frey filaments) and dynamic allodynia (sensitive to paintbrush) on the ipsilateral hind paw three days after surgery (n = 12). To study the effect of MEM on punctate and dynamic allodynia, MEM was administrated intrathecally before SNI surgery. Interestingly, i.t. pretreatment with low dose of MEM (10 nmol, i.t.; MEM-10) before SNI surgery selectively prevented the induction of the dynamic allodynia (p = 0.0038, n = 11) but had no effect on the punctate allodynia (p = 0.1702; Figure 1).
Figure 1.
Selective inhibition of dynamic, but not punctate allodynia, in MEM-10 pretreated mice following SNI. (vehicle, n = 12; MEM-10, n = 11; two-way ANOVA with Bonferroni post hoc. Von Frey test, vehicle vs. MEM-10, p = 0.1702; brush test, vehicle vs. MEM-10, p = 0.0038). Graphs represent the mean response ± SEM, ***p < 0.001. MEM: memantine.
Either MK801-10 or MEM-30 prevented the induction of both punctate and dynamic allodynia
MEM was widely believed to be uncompetitive antagonist of NMDARs with a favorable pharmacokinetic profile and approved for the treatment of Alzheimer’s disease.22 To confirm whether the MEM-10’s analgesic effect toward dynamic allodynia was due to the blockade of NMDAR, MK801, another potent uncompetitive antagonist of NMDAR, and higher dose of MEM (30 nmol, i.t.; MEM-30) were employed in this study.
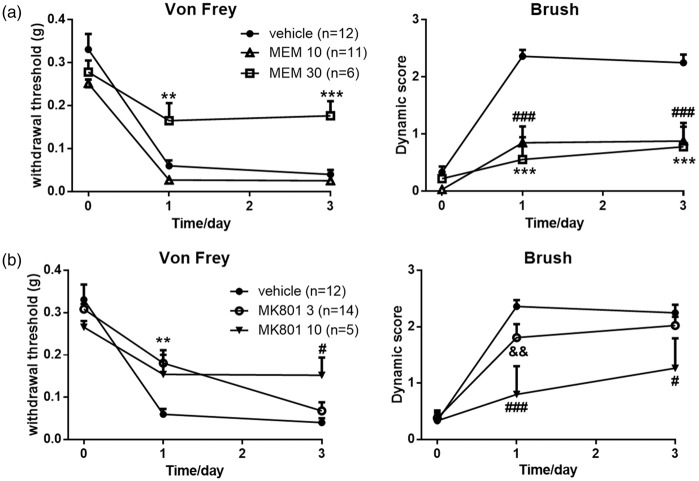
As shown in Figure 2(a), preadministration with MK801 (3 nmol, i.t., n = 14) increased the withdrawal threshold (p = 0.0088) but showed no effects on the dynamic score (p = 0.1583) following SNI. Pretreatment with MK801 (10 nmol, i.t., n = 5) prevented the induction of both punctate (p = 0.0096) and dynamic (p = 0.0057) allodynia compared with the vehicle group (n = 12) in both postoperative days 1 and 3. Similarly, MEM-30 (n = 6) prevented the induction of both punctate (p = 0.0039) and dynamic (p = 0.0001) allodynia in both postoperative days 1 and 3 following SNI (Figure 2(b)). These results indicated that the selective inhibitory effect of MEM-10 on the induction of dynamic, but not punctate, allodynia might not be correlated with the blockade of NMDARs in the spinal cord of mice.
Figure 2.
Effects of intrathecal MK801-3, MK801-10, and MEM-30 following SNI.(a) Pretreatment with MK801-3 prevented the induction of punctate allodynia at day 1 after SNI surgery and MK801-10 prevented the induction of punctate allodynia at day 3 (two-way ANOVA with Bonferroni post hoc, vehicle vs. MK801-3, p = 0.0088; vehicle vs. MK801-10, p = 0.0096). MK801-3 exerted no effects on the induction of dynamic allodynia (two-way ANOVA with Bonferroni post hoc, p = 0.1583). MK801-10 prevented the induction of dynamic allodynia (two-way ANOVA with Bonferroni post hoc, p = 0.0057). Vehicle, n = 12; MK801-3, n = 14; MK801-10, n = 5. (b) Pretreatment with MEM-30 prevented the induction of punctate allodynia (two-way ANOVA with Bonferroni post hoc, vehicle vs. MEM-30, p = 0.0039) and dynamic allodynia (two-way ANOVA with Bonferroni post hoc, vehicle vs. MEM-30, p = 0.0001). Graphs represent the mean response ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001, MEM-30 or MK801-3 vs. vehicle group. #p < 0.05; ###p < 0.001, MK801-10 versus vehicle group; &&p < 0.01, MK801-3 versus MK801-10 group. MEM: memantine.
MEM-10 prevented microglia overactivation during SNI injury in the dorsal spinal cord
Studies have implicated that the overactivation of microglia cells in response to nerve injury is important in thedevelopment of neuropathic pain.23,24 Thus, immunohistostaining was performed to investigate whether activation of microglia was inhibited by MEM-10 in spinal dorsal horn following SNI and to verify the role of microglia in the induction of dynamic allodynia.
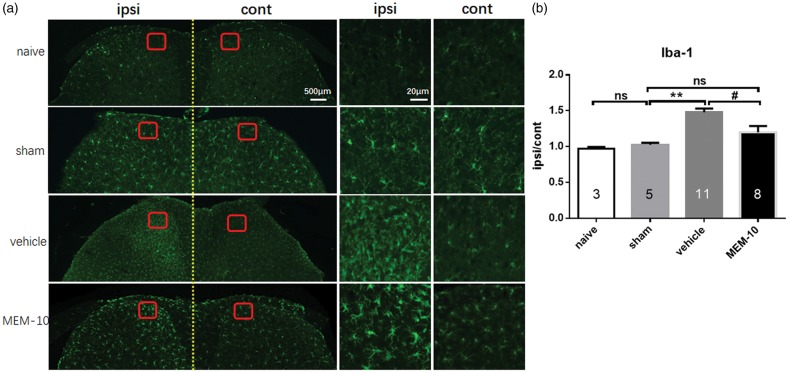
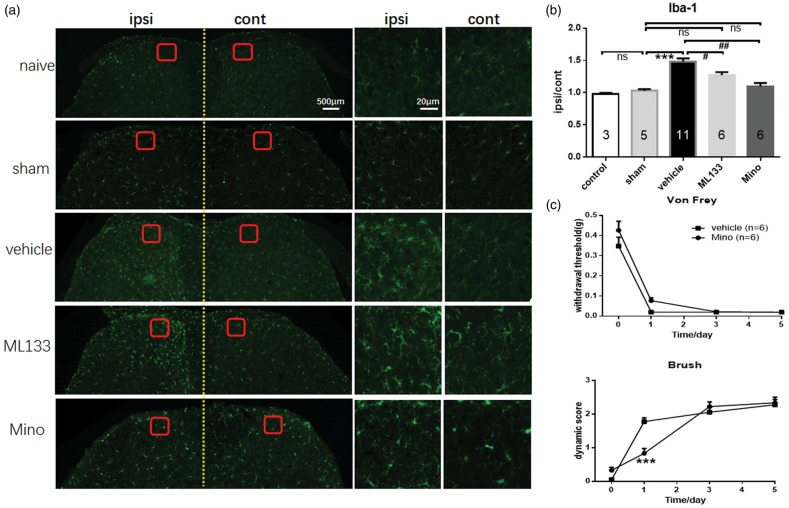
As depicted in Figure 3, sham group had no change in microglia activation (p = 0.9999, n = 5) compared with naïve group (n = 3), whereas vehicle group (n = 11) showed significant activation of microglia in the spinal dorsal horn after SNI surgery compared with sham group (p = 0.0005, n = 11). Pretreatment with MEM-10 significantly inhibited the activation of microglia induced by SNI surgery in day 5 (p = 0.0166, n = 8). The activation of microglia in the spinal cord was similar between sham and MEM-10 groups (p = 0.9704). Therefore, the inhibitory effect of MEM-10 on the activation of microglia may be correlated with the inhibitory effect displayed only in the induction of dynamic allodynia.
Figure 3.
Reduction of the microglia cells activation in the spinal cord horn of MEM-10 treatment mice after SNI. (a) Microglial immunostaining against Iba-1 in mice spinal cord dorsal horns after SNI surgery. (b) The histograms, respectively, show the quantification of ratio of immunostaining density on ipsilateral (ipsi) and control (cont) sides of dorsal spinal cord after SNI surgery (one-way ANOVA with Bonferroni post hoc, naive vs. sham, p > 0.9999; sham vs. vehicle, p = 0.0005; vehicle vs. MEM-10, p = 0.0166; sham vs. MEM-10, p = 0.9704). Naive, n = 3; sham, n = 5; vehicle, n = 11; MEM-10, n = 8. **p < 0.01, vehicle group versus sham group. #p < 0.05, MEM-10 group versus vehicle group. MEM: memantine.
MEM-10 did not affect the induction of dynamic allodynia in CFA inflammation pain model
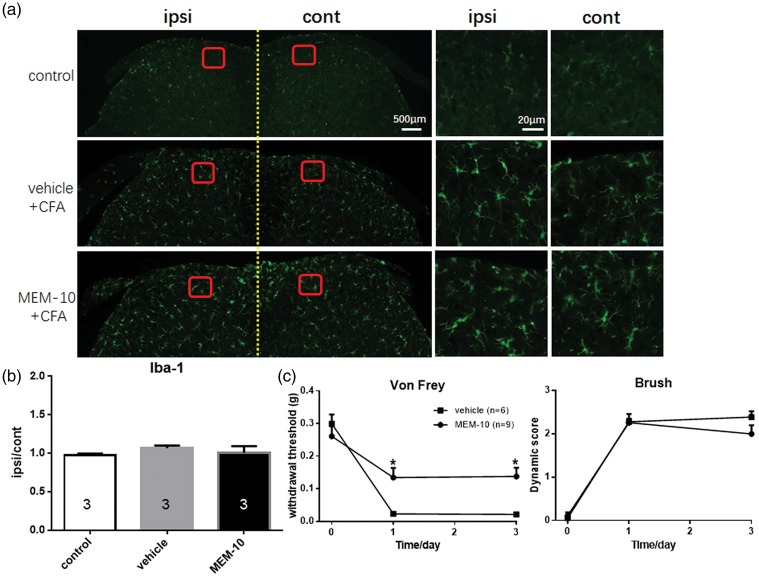
Previous study has proved that CFA treatment did not increase the proliferation of microglia in the ipsilateral side.25 Therefore, we employed CFA model as a negative control of microglia activation model to evaluate the relationship between microglia overactivation and MEM-10 inhibition of dynamic allodynia. As shown in Figure 4(a) and (b), CFA injection did not increase the proliferation of microglia in spinal dorsal horn of mice, which is in line with a previous study.25 The behavior test results proved that pretreatment with MEM-10 inhibited the induction of punctate allodynia (p = 0.0096, n = 9) while showed no significant effects on CFA-induced dynamic allodynia (p = 0.2512, n = 9, Figure 4(c)). Combined with aforementioned results, we speculated that the inhibitory effect of microglia cells in dorsal spinal cord induced by MEM-10 may be correlated with the prevention effect toward the induction of dynamic allodynia.
Figure 4.
CFA injection did not increase the activation of microglia in dorsal spinal cord of mice and MEM-10 showed no changes on the induction of dynamic allodynia following CFA injection. (a) Microglial immunostaining against Iba-1 in mice spinal cord dorsal horns after CFA injection and MEM-10 administration. (b) The histograms, respectively, show the quantification of ratio of immunostaining density on ipsilateral (ipsi) and control (cont) sides of dorsal spinal cord after CFA injection. (c) Punctate allodynia was prevented by MEM-10 injection (one-way ANOVA with Bonferroni post hoc, p = 0.0096) and dynamic allodynia was not affected by MEM-10 injection (one-way ANOVA with Bonferroni post hoc, p = 0.2512). Vehicle, n = 5; MEM-10, n = 9. Graphs represent the mean response ± SEM. *p < 0.05. MEM: memantine; CFA: complete freund′s adjuvant.
Kir 2.1 channel blocker (ML133) selectively inhibited the induction of dynamic allodynia, as well as the overactivation of microglia after SNI injury
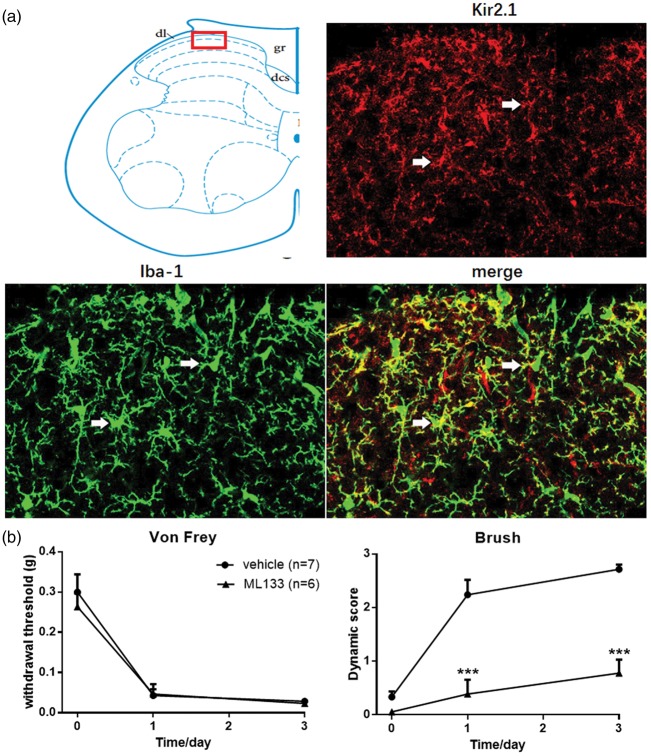
Despite of the NMDAR, Kir2.1 channel was also important target of MEM in microglia cells. MEM could suppress the Ikir2.1 amplitude of microglia cells by decreasing the slow component of mean open time and increasing the closed time of Kir2.1 channels and then depolarize the membrane potential in BV2 microglia cells.16 In the spinal cord, Kir2.1 channels are present in gray matter of dorsal and ventral horn.26 Our immunochemical staining result also proved that, similar with former published study, Kir2.1 channel distributed widely on the gray matter and located in the membrane of microglia on the spinal dorsal horn of control mice (Figure 5(a)).
Figure 5.
Loss of brush-induced dynamic allodynia in ML133 injection mice following SNI. (a) Kir 2.1 channel expressed in the membrane of microglia in dorsal spinal cord lamina I and II. Immunohistochemistry confocal scanning in ipsilateral lumbar spinal cord between Kir2.1 (red) and Iba-1 (green) showed the distribution of Kir2.1 channel in spinal cord dorsal horn. Three confocal images are zoom-in images from area in top red square. White arrows showed the co-localization of Kir2.1 channel and microglia. (b) ML133 prevented the induction of dynamic allodynia following SNI (two-way ANOVA with Bonferroni post hoc, p = 0.0002) and had no effect on the induction of punctate allodynia (two-way ANOVA with Bonferroni post hoc, p = 0.7237). Vehicle, n = 7; ML133, n = 6. Graphs represent the mean response ± SEM. ***p < 0.001.
Previous study has reported that blocking Kir2.1 channel by Ba2+ could inhibit microglia proliferation.27 To identify the precise role of Kir2.1 channel on induction of dynamic allodynia after SNI surgery, Kir channel blocker-ML133 used in this study was initially investigated for preventing the induction of mechanical allodynia. Pretreatment with ML133 (30 nmol, i.t.; n = 6) selectively inhibited the induction of dynamic allodynia (p = 0.0002) while showed no significant changes on punctate allodynia compared with vehicle group (p = 0.7237, n = 7; Figure 5(b)). The activation state of microglia in spinal cord following pretreatment with ML133 was also tested following SNI surgery. As depicted in Figure 6(a) and (b), compared with vehicle group (n = 11), pretreatment with ML133 inhibited the activation of microglia cells in spinal dorsal horn (p = 0.0370, n = 6). This result mimicked the effect of MEM-10 pretreatment on the inhibitory induction effect of dynamic allodynia and activation of microglia cells in spinal dorsal horn after SNI surgery.
Figure 6.
Loss of brush-induced dynamic allodynia in minocycline (mino) injection mice following SNI. (a) Microglial immunostaining against Iba-1 in mice spinal cord dorsal horns after SNI surgery. (b) The histograms, respectively, show the quantification of ratio of immunostaining density on ipsilateral (ipsi) and control (cont) sides of dorsal spinal cord after SNI surgery (one-way ANOVA with Bonferroni post hoc, naive vs. sham, p > 0.9999; sham vs. vehicle, p < 0.0001; vehicle vs. ML133, p = 0.0370; vehicle vs. minocycline, p = 0.0002; sham vs. mino, p > 0.9999). (c) Mino delayed the forming of dynamic allodynia following SNI (two-way ANOVA with Bonferroni post hoc, p = 0.0001) while showed no significant changes on punctate allodynia (two-way ANOVA with Bonferroni post hoc, p = 0.2280). Graphs represent the mean response ± SEM. ns: no significant changes; ***p < 0.001, vehicle group versus sham group in (b) and mino group versus vehicle group in (c); #p < 0.05, ML133 group versus vehicle group; ##p < 0.01, mino group versus vehicle group.
Minocycline inhibited the overactivation of microglia and prevented the induction of dynamic allodynia following SNI surgery
Previous study has shown that minocycline acts as an inhibitor of microglial activation.28 SNI surgery could induce significant overactivation of microglia in spinal dorsal horn (Figure 3). Whether pretreatment with minocycline (Mino) could inhibit the activation of microglia in the spinal dorsal horn and the behavior test of dynamic allodynia following SNI were evaluated in this study. As shown in Figure 6(a) and (b), the results revealed that minocycline (p = 0.0002, n = 6) significantly inhibited the activation of microglia after SNI injury. This phenomenon also matched the effects of pretreatment with MEM-10 on the inhibition effect toward activation of microglia cells in dorsal spinal cord. As shown in the Figure 6(c), pretreatment with minocycline significantly inhibited the induction of dynamic allodynia in day 1 (p = 0.0001, n = 6) but had no significant changes in day 3 after SNI surgery compared with vehicle group (n = 11). The punctate allodynia showed no significant changes in minocycline pretreatment group after SNI surgery (p = 0.2280, n = 6). This part of research revealed that microglia played crucial role in the development of dynamic allodynia, and blockage of Kir2.1 could inhibit microglia activation, which further inhibited dynamic allodynia.
Discussion
The animal pain behavior results from this study revealed that pretreatment with low dose of MEM (MEM-10) has selective analgesic property toward the induction of dynamic allodynia but not punctate allodynia. Furthermore, immunohistostaining results indicate that this selective analgesic property toward dynamic allodynia by MEM-10 is probably mediated by the inhibition of microglia overactivity induced by peripheral nerve injury of SNI through blockade of Kir2.1 channels localized in microglia in the spinal dorsal horn. This conclusion is based on the observation that (1) in in vivo SNI pain model, pretreatment with MEM-10 selectively prevented the induction of dynamic allodynia while showed no significant changes on punctate allodynia; (2) pretreatment with either MK801 or MEM-30 prevented the induction of both dynamic and punctate allodynia; (3) MEM-10 significantly inhibited the overactivation of microglia in spinal dorsal horn in SNI model; (4) microglia was not overactivated by CFA injection. MEM-10 pretreatment before CFA injection did not affect the induction of dynamic allodynia; (5) both minocycline and ML133 selectively prevented the overactivation of microglia in spinal dorsal horn as well as the induction of dynamic, but not punctate, allodynia following SNI.
Bioavailability of MEM
MEM is a novel class of Alzheimer’s disease medications acting as a moderate-affinity voltage-dependent noncompetitive antagonist at glutaminergic NMDARs.29 Except for NMDAR, Kir2.1 channel was another important target of MEM in the microglia cells. Previous study proved that MEM could inhibit the amplitude of inwardly rectifying K+ current through the Kir channels in BV2 microglia cells.16 From previous studies, MEM’s IC50 toward NMDAR was 0.54 µM (in rat wild-type NMDAR clones)30 and Kir2.1 channel was 12 µM (in clonal strain BV2 microglia cell line).16 The volume of CSF in mice was around 37 µL.31 At therapeutic concentrations, MEM effectively blocks excessive extrasynaptic NMDAR-mediated currents, while relatively sparing normal synaptic activity.7 Therefore, the inhibitory effect toward synaptic NMDARs may be much higher than 0.54 µM which was reported earlier. It is possible that MEM (i.t.) only blocks the Kir2.1 channel while showing enough effects toward synaptic NMDAR to influence mEPSC and action potential number in the spinal cord slices (Figure S1).
Antinociceptive effect toward dynamic allodynia after SNI surgery
Neuropathic pain was initiated or caused by a primary lesion or dysfunction in the nervous system.32 SNI model was established to induce neuropathic pain behaviors including punctate, dynamic, and thermal allodynia.21 Previous studies mostly paid attention to studying the analgesic effect of MEM toward punctate or thermal allodynia after nerve injury on animal.33 The precise mechanism underlying dynamic allodynia was not very clear and the effective therapy was still under exploration. In this study, we demonstrated that pretreatment with MEM-10 inhibited the induction of dynamic allodynia but not the punctate allodynia. This study is the first to show the selective analgesic role of low dose of MEM toward dynamic allodynia in the mice SNI model. At the same time, MK801 was also pretreated to verify the role of NMDAR in the induction of dynamic allodynia. The result revealed that MK801-3 only inhibited the induction of punctate allodynia, and MK801-10 prevented the induction of both punctate and dynamic allodynia, indicating that NMDAR is more likely to be involved in the formation of punctate allodynia but not dynamic allodynia. Therefore, the selective analgesic effect of low dose of MEM in dynamic allodynia may be mediated through a NMDA-independent pathway. In our study, we also found that MEM-10 could inhibit the activation of microglia. Kir2.1 channel is another target for MEM.16 Therefore, ML133 was also pretreated before SNI surgery to examine the role of Kir2.1 channel in the induction of dynamic allodynia. Both dynamic allodynia behavior and immunohistostaining results were consistent with MEM-10 preadministration in mice. What’s more, minocycline, one of the important inhibitors of microglia, prevented the induction of dynamic allodynia after SNI surgery. This part of results revealed that the selective inhibition effect of MEM-10 toward dynamic allodynia was likely mediated by blockade of Kir2.1 channel in spinal cord. Moreover, previous studies have reported that microglia were activated following nerve injury, and punctate mechanical allodynia could also be abolished via microglia silencing.34 Combined with our results together, the activation of microglia participated in the establishment of both punctate and dynamic allodynia.
Spinal mechanism of inhibition effect of MEM toward dynamic allodynia
Previous study proved that Kir2 channels composed of Kir2.1–2.3 subunits were expressed widely in neuronal and glia cells in spinal cord, contributing to sensory transduction and motor control.15 In terms of Kir2.1 channel, cell types of spinal dorsal horn were further studied to verify the precise analgesic effect of MEM-10 toward prevention of dynamic allodynia. Consistent with former studies, nerve injury would induce significant activation of microglia in spinal dorsal horn.25 Pretreatment with MEM-10 inhibited this overactivation of microglia induced by SNI injury (Figure 3). Previous studies have revealed that Kir channel blocker Ba2+ potently inhibited the proliferation of microglia.27 MEM-10 was also acted as one of Kir2.1 channel blockers, and the inhibition toward proliferation of microglia could be mediated by Kir2.1 channel. On the contrary, microglia cells were not activated by CFA injection into plantar of mice. Pretreatment with MEM-10 also did not affect the induction of dynamic allodynia after CFA injection. Kir 2.1 channel in microglia might be important target for preventing the induction of dynamic allodynia.
Except for NMDAR and Kir2.1 channel, several potassium channels are also the targets of MEM in the spinal cord, KATP, KV1.3, KCa3.1, and so forth. But the KATP channel opener treatment is an effective therapy for postoperative pain in animals, while MEM was a KATP channel blocker. MEM was believed to aggravate the pain sensation. Kv1.3 channel was weakly expressed in the spinal cord in animals. KCa3.1 did not play role in the neuropathic pain processing but in the noxious chemical stimuli in mice. Therefore, we believe that Kir2.1 channel may be one of major targets of MEM conducting the inhibition effect toward the induction of dynamic allodynia.
It is well recognized that peripheral nerve injury could lead to rapid and vigorous microglial activation. Once activated, the microglia could release excitatory amino acids, interleukin-1β,and prostaglandin E2, which all involved in the induction of central sensitization of pain sensation.10 MEM could reduce microglia-associated inflammation by decreasing the production of inflammatory factors, such as extracellular superoxide anion, intracellular reactive oxygen species, nitric oxide, and tumor necrosis factor-α.35 In this study, besides Kir2.1 channel in the spinal cord, inflammatory factors may also contribute to the analgesia effect toward dynamic allodynia which need to be further studied.
Previous studies have revealed that punctate and dynamic allodynia shared different afferent fibers and neuronal circuits in spinal cord.5,36,37 Our studies in which pretreatment with MEM-10 selectively prevented the induction of dynamic allodynia provided direct evidence toward the former theory. Previous study also reported that glycine inhibitory dysfunction induced a selectively dynamic, morphine-resistant mechanical allodynia.38,39 38However, our whole-cell patch clamp recordings’ final results showed that bath application of MEM (10 µM and 100 µM) did not change the spontaneous glycinergic inhibitory postsynaptic currents (gly-sIPSCs) in the lamina II neurons (data were not shown). Activities of glycinergic interneurons were also excluded in the mechanism underlying i.t. administration with MEM on the inhibition effect of the induction of dynamic allodynia in our study.
MEM-10 did not alleviate punctate and dynamic allodynia seven days after SNI
We also evaluated the MEM’s treatment effect after the total formation of dynamic allodynia at day 7 post-SNI surgery (Figure S3). However, the effective prevention dose of MEM on the induction of dynamic allodynia did not alleviate punctate and dynamic allodynia at all. Seven days after SNI injury, a large number of microglia cells have already been in activated stage, and the dynamic allodynia have also established. The activities of Kir2.1 channel blocked by MEM-10 or ML133 would not affect the proliferation of microglia cells even further broke the maintenance of dynamic allodynia. Our studies will further focus on the mechanism underlying the treatment doses of MEM after the formation of dynamic allodynia following nerve injury.
Functional implication
MEM-10 suppressed the function of Kir2.1 channel which further inhibited the overactivation of microglia in spinal dorsal horn, indicating a potential therapy in preventing the induction of dynamic allodynia. As one of clinical medications, the analgesic effect of MEM toward preventing induction of dynamic allodynia will provide direct evidence for exploring precise mechanism of dynamic allodynia. In clinical prevention of dynamic allodynia, MEM and spinal Kir2.1 channel blocker may have great potential for preventing the establishment of this disease before predictable injury, such as amputation in clinical treatment.
Supplemental Material
Supplemental Material for Memantine selectively prevented the induction of dynamic allodynia by blocking Kir2.1 channel and inhibiting the activation of microglia in spinal dorsal horn of mice in spared nerve injury model by Yangyang Chen, Yiqian Shi, Guoxiang Wang, Yimei Li, Longzhen Cheng and Yun Wang in Molecular Pain
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Nature Science Foundation of China (31771188, 31471027) to YW.
Supplemental material
Supplemental material is available online for this article.
Reference
- 1.Loeser JD. Herpes zoster and postherpetic neuralgia. Pain 1986; 25: 149–164. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki A, Serizawa K, Andoh T, Shiraki K, Takahata H, Kuraishi Y. Pharmacological differences between static and dynamic allodynia in mice with herpetic or postherpetic pain. J Pharmacol Sci 2008; 108: 266–273. [DOI] [PubMed] [Google Scholar]
- 3.Argoff CE, Katz N, Backonja M. Treatment of postherpetic neuralgia: a review of therapeutic options. J Pain Symptom Manage 2004; 28: 396–411. [DOI] [PubMed] [Google Scholar]
- 4.Nurmikko T, Bowsher D. Somatosensory findings in postherpetic neuralgia. J Neurol Neurosurg Psychiatry 1990; 53: 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng L, Duan B, Huang T, Zhang Y, Chen Y, Britz O, Garcia-Campmany L, Ren X, Vong L, Lowell BB, Goulding M, Wang Y, Ma Q. Identification of spinal circuits involved in touch-evoked dynamic mechanical pain. Nat Neurosci 2017; 20: 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.La JH, Chung JM. Peripheral afferents and spinal inhibitory system in dynamic and static mechanical allodynia. Pain 2017; 158: 2285–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia P, Chen HS, Zhang D, Lipton SA. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci 2010; 30: 11246–11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlton SM, Hargett GL. Treatment with the NMDA antagonist memantine attenuates nociceptive responses to mechanical stimulation in neuropathic rats. Neurosci Lett 1995; 198: 115–118. [DOI] [PubMed] [Google Scholar]
- 9.Eisenberg E, Kleiser A, Dortort A, Haim T, Yarnitsky D. The NMDA (N-methyl-D-aspartate) receptor antagonist memantine in the treatment of postherpetic neuralgia: a double-blind, placebo-controlled study. Eur J Pain 1998; 2: 321–327. [DOI] [PubMed] [Google Scholar]
- 10.Takeda K, Muramatsu M, Chikuma T, Kato T. Effect of memantine on the levels of neuropeptides and microglial cells in the brain regions of rats with neuropathic pain. J Mol Neurosci 2009; 39: 380–390. [DOI] [PubMed] [Google Scholar]
- 11.Morel V, Etienne M, Wattiez A-S, Dupuis A, Privat A-M, Chalus M, Eschalier A, Daulhac L, Pickering G. Memantine, a promising drug for the prevention of neuropathic pain in rat. Eur J Pharmacol 2013; 721: 382–390. [DOI] [PubMed] [Google Scholar]
- 12.Wilson JA, Garry EM, Anderson HA, Rosie R, Colvin LA, Mitchell R, Fleetwood-Walker SM. NMDA receptor antagonist treatment at the time of nerve injury prevents injury-induced changes in spinal NR1 and NR2B subunit expression and increases the sensitivity of residual pain behaviours to subsequently administered NMDA receptor antagonists. Pain 2005; 117: 421–432. [DOI] [PubMed] [Google Scholar]
- 13.Pickering G, Morel V. Memantine for the treatment of general neuropathic pain: a narrative review. Fundam Clin Pharmacol 2017; 32: 4–13. [DOI] [PubMed] [Google Scholar]
- 14.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 2010; 90: 291–366. [DOI] [PubMed] [Google Scholar]
- 15.Murata Y, Yasaka T, Takano M, Ishihara K. Neuronal and glial expression of inward rectifier potassium channel subunits Kir2.x in rat dorsal root ganglion and spinal cord. Neurosci Lett 2016; 617: 59–65. [DOI] [PubMed] [Google Scholar]
- 16.Tsai KL, Chang HF, Wu SN. The inhibition of inwardly rectifying K+ channels by memantine in macrophages and microglial cells. Cell Physiol Biochem 2013; 31: 938–951. [DOI] [PubMed] [Google Scholar]
- 17.Njoo C, Heinl C, Kuner R. In vivo SiRNA transfection and gene knockdown in spinal cord via rapid noninvasive lumbar intrathecal injections in mice. J Vis Exp 2014; 85: 51229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 2000; 87: 149–158. [DOI] [PubMed] [Google Scholar]
- 19.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Cai J, Wang BH, Huang L, Fan J, Wang Y. The antinociceptive effects of novel epibatidine analogs through activation of α4β2 nicotinic receptors. Sci China Life Sci 2018; 61(6): 688–695. [DOI] [PubMed]
- 21.Duan B, Cheng L, Bourane S, Britz O, Padilla C, Garcia-Campmany L, Krashes M, Knowlton W, Velasquez T, Ren X, Ross S, Lowell BB, Wang Y, Goulding M, Ma Q. Identification of spinal circuits transmitting and gating mechanical pain. Cell 2014; 159: 1417–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majlath Z, Torok N, Toldi J, Vecsei L. Memantine and kynurenic acid: current neuropharmacological aspects. Curr Neuropharmacol 2016; 14: 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao H, Alam A, Chen Q, A Eusman M, Pal A, Eguchi S, Wu L, Ma D. The role of microglia in the pathobiology of neuropathic pain development: what do we know? Br J Anaesth 2017; 118: 504–516. [DOI] [PubMed] [Google Scholar]
- 24.Tsuda M. Modulation of Pain and Itch by Spinal Glia. Neurosci Bull 2018; 34(1): 178–185. [DOI] [PMC free article] [PubMed]
- 25.Li K, Tan YH, Light AR, Fu KY. Different peripheral tissue injury induces differential phenotypic changes of spinal activated microglia. Clin Dev Immunol 2013; 2013: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pruss H, Derst C, Lommel R, Veh RW. Differential distribution of individual subunits of strongly inwardly rectifying potassium channels (Kir2 family) in rat brain. Brain Res Mol Brain Res 2005; 139: 63–79. [DOI] [PubMed] [Google Scholar]
- 27.Schlichter LC, Sakellaropoulos G, Ballyk B, Pennefather PS, Phipps DJ. Properties of K+ and Cl- channels and their involvement in proliferation of rat microglial cells. Glia 1996; 17: 225–236. [DOI] [PubMed] [Google Scholar]
- 28.Tikka TM, Koistinaho JE. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J Immunol 2001; 166: 7527–7533. [DOI] [PubMed] [Google Scholar]
- 29.Assarzadegan F, Sistanizad M. Tolerability and efficacy of memantine as add on therapy in patients with migraine. Iran J Pharm Res 2017; 16: 791–797. [PMC free article] [PubMed] [Google Scholar]
- 30.Limapichat W, Yu WY, Branigan E, Lester HA, Dougherty DA. Key binding interactions for memantine in the NMDA receptor. ACS Chem Neurosci 2013; 4: 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barten DM, Cadelina GW, Weed MR. Dosing. Collection, and quality control issues in cerebrospinal fluid research using animal models. Handb Clin Neurol 2017; 146: 47–64. [DOI] [PubMed] [Google Scholar]
- 32.Vote BJ, Newland A, Polkinghorne PJ. Humidity devices in vitreoretinal surgery. Retina 2002; 22: 616–621. [DOI] [PubMed] [Google Scholar]
- 33.Villetti G, Bergamaschi M, Bassani F, et al. Antinociceptive activity of the N-methyl-D-aspartate receptor antagonist N-(2-Indanyl)-glycinamide hydrochloride (CHF3381) in experimental models of inflammatory and neuropathic pain. J Pharmacol Exp Ther 2003; 306: 804–814. [DOI] [PubMed] [Google Scholar]
- 34.Huang Y, Li Y, Zhong X, Hu Y, Liu P, Zhao Y, Deng Z, Liu X, Liu S, Zhong Y. Src-family kinases activation in spinal microglia contributes to central sensitization and chronic pain after lumbar disc herniation. Mol Pain 2017; 13: 174480691773363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu H-M, Tzeng N-S, Qian L, Wei S-J, Hu X, Chen S-H, Rawls SM, Flood P, Hong J-S, Lu R-B. Novel neuroprotective mechanisms of memantine: increase in neurotrophic factor release from astroglia and anti-inflammation by preventing microglial activation. Neuropsychopharmacol 2009; 34: 2344–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasaki A, Inomata Y, Serizawa K, Andoh T, Kuraishi Y. Contribution of sensory C-fiber neuron injury to mechanical dynamic allodynia in a murine model of postherpetic neuralgia. Neuroreport 2013; 24: 137–141. [DOI] [PubMed] [Google Scholar]
- 37.Duan B, Cheng L, Ma Q. Spinal Circuits Transmitting Mechanical Pain and Itch. Neurosci Bull 2018; 34(1): 186–193. [DOI] [PMC free article] [PubMed]
- 38.Miraucourt LS, Moisset X, Dallel R, Voisin DL. Glycine inhibitory dysfunction induces a selectively dynamic, morphine-resistant, and neurokinin 1 receptor- independent mechanical allodynia. J Neurosci 2009; 29: 2519–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y, Chen Y, Wang Y. Kir2.1 channel regulating glycinergic transmission selectively contributes to dynamic mechanical allodynia in mice spared nerve injury model. Neurosci Bull 2019; 35(2): 301–314. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Memantine selectively prevented the induction of dynamic allodynia by blocking Kir2.1 channel and inhibiting the activation of microglia in spinal dorsal horn of mice in spared nerve injury model by Yangyang Chen, Yiqian Shi, Guoxiang Wang, Yimei Li, Longzhen Cheng and Yun Wang in Molecular Pain