Short abstract
Background and aim
Diabetic neuropathic pain is a refractory and disabling complication of diabetes mellitus. The pathogenesis of the diabetic neuropathic pain is still unclear, and treatment is insufficient. The aim of this study is to investigate the roles of glucose-6-phosphate dehydrogenase (G6PD) and toll-like receptor 4 (TLR4) in neuropathic pain in rats with diabetes.
Methods
Type 1 diabetes model was induced by intraperitoneal injection of streptozotocin (STZ, 75 mg/kg) in adult female Sprague-Dawley rats. Paw withdrawal threshold and paw withdrawal latency of rats were measured by von Frey filaments and thermal radiation, respectively. The expressions of G6PD and TLR4 in L4-L6 dorsal root ganglions (DRGs) were measured by western blotting and quantitative real-time polymerase chain reaction analysis. Fluorescent immunohistochemistry was employed to detect expressions of G6PD and TLR4 and co-location of G6PD with TLR4.
Results
The mRNA and protein expression levels of G6PD in DRGs were significantly decreased in diabetic rats when compared with age-matched control rats. Upregulation of G6PD by intrathecal injection of G6PD overexpression adenovirus markedly attenuated hindpaw pain hypersensitivity of diabetic rats. The mRNA and protein expression levels of TLR4 in DRGs of diabetic rats were significantly increased when compared with control rats. Intrathecal injection of TLR4-selective inhibitor CLI-095 attenuated diabetic pain in dose- and time-dependent manners. Furthermore, G6PD and TLR4 were co-localized in DRG neurons. Intrathecal injection of G6PD overexpression adenovirus greatly reduced TLR4 expression, while intrathecal injection of CLI-095 had no significant effect on G6PD expression in diabetic rats.
Conclusions
Our results suggest that decrease in G6PD expression was involved in diabetic peripheral neuropathic pain, which was most likely through upregulation of TLR4 expression in the DRGs of rats.
Keywords: Diabetes, neuropathic pain, dorsal root ganglion, glucose-6-phosphate dehydrogenase, toll-like receptor 4
Introduction
Diabetic peripheral neuropathic pain is one of the most common chronic complications of diabetes mellitus (DM).1,2 It mainly manifests as spontaneous hyperalgesia caused by mild irritation and allodynia caused by innocuous stimulation such as light touch.3 However, painkillers, the main treatment, referring to other neuropathic pain, are ineffective and insufficient to many diabetic pain patients.4 Therefore, it is believed that diabetic neuropathic pain has its own special pathogenesis.
Glucose-6-phosphate dehydrogenase (G6PD) is the first rate-limiting enzyme and the important reductase of the pentose phosphorylation pathway.5 A growing body of evidence has proved that chronic high glucose decreased the expression and activity of G6PD in different tissues, including the nervous system.6,7 G6PD deficiency induced oxidative stress and various chronic complications, such as diabetic nephropathy and retinopathy.7–9 However, whether G6PD is downregulated in primary sensory neurons of dorsal root ganglion (DRG) of rats with diabetic peripheral neuropathic pain has not been reported.
Toll-like receptors (TLRs) are the innate immune receptors, including TLR1-13. TLR4 is the first human TLR-associated protein widely expressed in various systems of the body. It has been reported that oxidative stress can upregulate the expression of TLR2/TLR4 and activate the NF-κB signaling pathway in human macrophages, thereby stimulating the release of downstream inflammatory factors.10 In addition, many studies reported that TLR4 signaling pathway play an important role in visceral pain, inflammatory pain, bone cancer pain, and neuropathic pain.11–16 Whether TLR4 is involved in diabetic neuropathic pain remains largely unclear.
In this study, we aimed to investigate the roles of G6PD and TLR4 in diabetic neuropathic pain model induced by STZ injection. The results showed that G6PD expression was decreased while TLR4 expression was upregulated in L4-L6 DRGs of diabetic rats. Overexpression of G6PD markedly attenuated hindpaw pain hypersensitivity and negatively regulated TLR4 expression in DRGs of diabetic rats. Furthermore, inhibition of TLR4 also attenuated hindpaw pain hypersensitivity but did not alter G6PD expression. Our findings suggest that G6PD downregulation contributes to diabetic neuropathic pain through upregulation of TLR4 expression. This signaling pathway may represent a potential strategy for the treatment of neuropathic pain in patients with diabetes.
Materials and methods
Generation of STZ-induced diabetes
The protocols of all animal experiments were approved by the institutional animal care and use committee of Soochow University. All methods were conformed to the guidelines of International Association for the Study of Pain (IASP). During the period of the experiment, female Sprague-Dawley (SD) rats (weighing 180–200 g) were housed three per standard cage in a temperature-controlled (25 ± 1°C) and 12 h/12 h light–dark cycle room. All rats were allowed free access to tap water and standard laboratory chow, ad libitum.
As described in previous studies,17,18 DM model was induced by a single intraperitoneal injection of streptozotocin (STZ, 75 mg/kg, Sigma Chemicals, St. Louis, MO, USA), which was dissolved freshly in citrate buffer (pH 4.3–4.4). The control (CON) rats were injected citrate buffer only in an equivalent volume. Three days later, fasting blood glucose concentration obtained from the tail vein of diabetic rats was measured. Only these rats with high blood glucose concentration (higher than 15.0 mmol/L or 270 mg/dL) were further used in the present study.
Measurement of hindpaw withdrawal threshold and latency
Before behavioral experiments, all rats were adapted to the test environment for three days. The pain threshold was measured before and after an injection of STZ, G6PD overexpression adenovirus (Ad-G6PD) and negative control adenovirus (Ad-NC), or CLI-095, et. al. Paw withdrawal threshold (PWT) in response to von Frey filament stimulation and thermal paw withdrawal latency (PWL) in response to radiant heat applied to the hindpaw plantar surface were performed, as described previously.18–22 All behavioral experiments were under double-blind conditions.
Rotarod test
Rotarod test was performed as described previously.23,24 Briefly, rats were trained by a given speed (from 5 r/min to 15 r/min) on the Rotarod for one day or three consecutive days before the experiments, until most rats could step voluntarily on the rod for 5 min. The time that every rat stayed on the rod at the given speed of 15 r/min was recorded before and after the administration of Ad-G6PD, Ad-NC, or CLI-095.
Drug administration
For behavioral experiments, Ad-G6PD or Ad-NC (Shanghai Genepharma Co., Ltd) was delivered by intrathecal injection. Briefly, three days after STZ injection, Ad-G6PD or Ad-NC (5 × 109 TU/mL, 10 μL) was injected at the L5-L6 interspinous space of SD rats with microsyringe. And the adenovirus was injected again at the last day of second week. The PWT and PWL were measured at pre, one, two, three, and four weeks. CLI-095 (InvivoGen, USA) was resolved with dimethyl sulfoxide (DMSO) and administrated by intrathecal injection. The PWT and PWL were recorded before and after drug application.
Western blotting analysis
Expressions of G6PD and TLR4 in L4-L6 DRGs were determined by western blotting analysis, as previously described in detail.18,23,25 The antibodies in the present study included mouse anti-TLR4 (1:200, Santa Cruz Biotechnology, Inc.), mouse anti-G6PD (1:200, Santa Cruz Biotechnology, Inc.), rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:1000, Goodhere Biotechnology Co., Ltd.), anti-mouse horseradish peroxidase-conjugated secondary antibody (1:2000; Jackson ImmunoResearch Laboratories, Inc.), and anti-rabbit peroxidase-conjugated secondary antibody (1:2000; Jackson ImmunoResearch Laboratories, Inc.).
Real-time polymerase chain reaction
Total RNA was extracted from L4-L6 DRGs with TRIzol (Ambion). The cDNA was synthesized from total RNA using the EasyScript One-Step gDNA Removal and cDNA Synthesis SuperMix kit (TransGen Biotech, Beijing, China) under the instructions of the supplier, as described previously.17,18 The mRNA expressions of G6PD and TLRs were detected by real-time polymerase chain reaction (RT-PCR). The sequences of the primers for G6PD, TLR4, and GAPDH are shown in Table 1.
Table 1.
The sequences of the primers used in the present study.
| Primers | Sequence (5′→3′) |
|---|---|
| G6PD-F | GCGTATCTTCACACCACTGC |
| G6PD-R | AGCCCACTCTCTTCATCAGC |
| TLR4-F | TGGTGGCTGTGGAGACAAAA |
| TLR4-R | AGGCTTGGGCTTGAATGGAG |
| GAPDH-F | TGGAGTCTACTGGCGTCTT |
| GAPDH-R | TGTCATATTTCTCGTGGTTCA |
G6PD: glucose-6-phosphate dehydrogenase; TLR4: toll-like receptor 4; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
Immunofluorescence study
Immunofluorescence technology was performed as described previously.17,18 Rabbit anti-TLR4 (1:50, Biovision), rabbit anti-G6PD (1:50, Santa Cruz Biotechnology, Inc.), mouse anti-G6PD (1:50, Santa Cruz Biotechnology, Inc.), calcitonin gene-related peptide (CGRP) (1:200, Abcam), isolectin B4 (IB4+) (1:200, Sigma), NeuN (1:50, Millipore Sigma), and glutamine synthetase (GS) (1:200, Abcam) antibodies were used in the present study.
Data analysis
All data were expressed as the mean ± SEM. Statistical analysis was conducted with Software GraphPad Prism 7.0. Significance was determined with a two-sample t-test, Dunn post hoc test following Friedman analysis of variance (ANOVA), one-way ANOVA followed by Bonferroni post hoc test, or two-way ANOVA followed by Tukey post hoc test, Dunn post hoc test, or Sidak post hoc test, as appropriate. When a p-value was less than 0.05, results were considered statistically significant.
Results
Hindpaw pain hypersensitivity was induced by STZ injection in rats
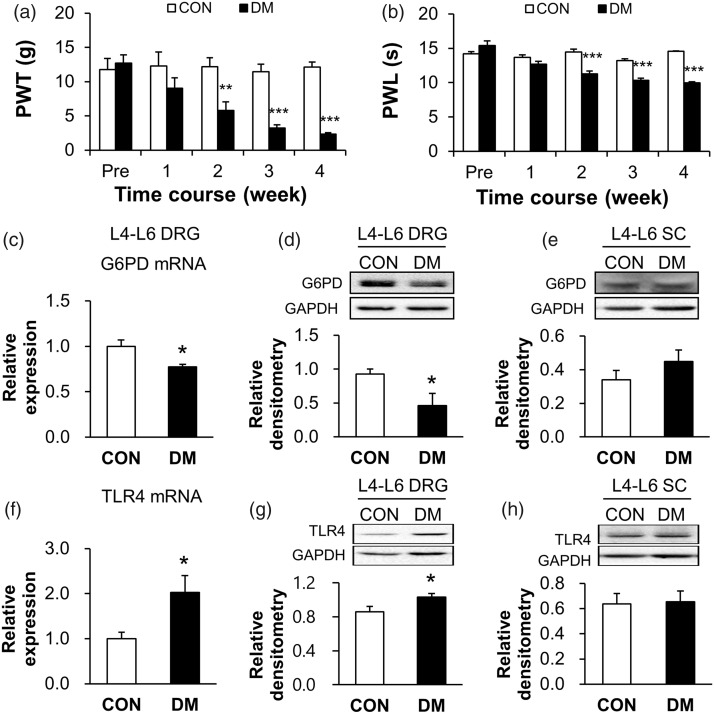
Consistent with previous studies,18–20 the hindpaw pain hypersensitivity were induced by a single injection of STZ in rats, manifested as mechanical allodynia (Figure 1(a), **p < 0.01, ***p < 0.001, compared with CON group, two-way ANOVA) and thermal hyperalgesia (Figure 1(b), ***p < 0.001, compared with CON group, two-way ANOVA). The values of PWT for CON rats (n = 6) at pre, 1, 2, 3, and 4 weeks were 11.78 ± 1.63, 12.27 ± 2.05, 12.19 ± 1.30, 11.47 ± 1.08, and 12.14 ± 0.72 g, respectively. The values of PWT for DM rats (n = 7) at pre, 1, 2, 3, and 4 weeks were 12.70 ± 1.21, 9.01 ± 1.52, 5.80 ± 1.22, 3.24 ± 0.43, and 2.31 ± 0.21 g, respectively. The values of PWL for CON rats (n = 6) at pre, 1, 2, 3, and 4 weeks were 14.23 ± 0.31, 13.70 ± 0.34, 14.46 ± 0.45, 13.23 ± 0.27, and 14.57 ± 0.06 s, respectively. The values of PWL for DM rats (n = 7) at pre, 1, 2, 3, and 4 weeks were 15.38 ± 0.68, 12.70 ± 0.42, 11.28 ± 0.43, 10.36 ± 0.31, and 9.99 ± 0.13 s, respectively.
Figure 1.
G6PD expression was significantly decreased, while TLR4 was markedly upregulated in L4-L6 DRGs of diabetic rats. (a, b) DM rat showed decreased mechanical PWT (a) and thermal PWL (b) compared with CON rats (n = 6 rats for CON and n = 7 rats for DM group, **p < 0.01, ***p < 0.001 vs. CON, two-way ANOVA). (c, d) The protein expression (c, n = 6 rats for CON and n = 7 rats for DM group) and mRNA level (d, n = 5 rats for each group) of G6PD in L4-L6 DRGs were significantly lower in DM rats than in age-matched CON rats (*p < 0.05 vs. CON, two-sample t-test). (e) Expression of G6PD was not altered in L4-L6 spinal cord of DM rats (n = 4 rats for each group, p > 0.05 vs. CON, two-sample t-test). (f, g) The mRNA level (F, n = 5 rats for each group) and protein expression (G, n = 9 rats for CON and n = 8 rats for DM group) of TLR4 in L4-L6 DRGs of DM rats were significantly increased when compared with the age-matched CON rats (*p < 0.05 vs. CON, two-sample t-test). (h) Expression of TLR4 was not altered in L4-L6 spinal cord of DM rats (n = 7 rats for CON and n = 6 rats for DM group, p > 0.05 vs. CON, two-sample t-test).
CON: control; DM: diabetes mellitus; PWT: paw withdrawal threshold; PWL: paw withdrawal latency; DRG: dorsal root ganglion; G6PD: glucose-6-phosphate dehydrogenase; TLR4: toll-like receptor 4; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
G6PD expression in L4-L6 DRGs was decreased in DM rats
To determine whether the expression of G6PD is decreased in L4-L6 DRGs after STZ injection, western blotting and RT-PCR assays were performed. The last day of the fourth week after STZ injection, proteins were isolated from both sides of L4-L6 DRGs of CON and DM rats. The expression of G6PD was significantly decreased in DM rats, when compared with age-matched CON rats (Figure 1(c), *p < 0.05, two-sample t-test). The relative densitometry of G6PD was 0.93 ± 0.07 (n = 6) in CON rats and 0.46 ± 0.18 (n = 7) in DM rats. G6PD expression at the mRNA level was also remarkably decreased after STZ injection (Figure 1(d), *p < 0.05, compared with CON group, two-sample t-test). The relative mRNA level of G6PD was 1.00 ± 0.07 (n = 5) in CON rats and 0.77 ± 0.03 (n = 5) in DM rats. In contrast, the expression of G6PD was not significantly altered in L4-L6 spinal cord after STZ injection (Figure 1(e), p > 0.05, compared with CON group, two-sample t-test). The relative densitometry of CON and DM rats was 0.34 ± 0.06 (n = 4) and 0.45 ± 0.07 (n = 4), respectively. Therefore, the expression of G6PD was downregulated at both transcriptional and translational levels in STZ-induced DM rats.
TLR4 expression in L4-L6 DRGs was increased in DM rats
To test whether TLR4 is involved in diabetic neuropathic pain, RT-PCR and western blotting analysis were performed. The TLR4 expression at mRNA level was remarkably increased when compared with age-matched CON rats (Figure 1(f), *p < 0.05, compared with CON group, two-sample t-test). The relative mRNA level was 2.03 ± 0.37 (n = 5) in DM rats and 1.00 ± 0.14 (n = 5) in CON rats. We next examined the protein expression of TLR4 in L4-L6 DRGs of DM rats by western blotting assays. The relative densitometry of TLR4 was 1.03 ± 0.04 (n = 8) in DM rats and 0.86 ± 0.06 (n = 9) in CON rats. The statistical analysis showed that TLR4 expression was significantly increased in DM rats (Figure 1(g), *p < 0.05, compared with CON group, two-sample t-test). In contrast, TLR4 expression was not significantly altered in L4-L6 spinal cord after STZ injection (Figure 1(h), respectively, p > 0.05, compared with CON group, two-sample t-test). The relative densitometry of TLR4 was 0.66 ± 0.09 (n = 6) in DM rats and 0.64 ± 0.08 (n = 7) in CON rats.
G6PD and TLR4 were co-localized in DRG neurons
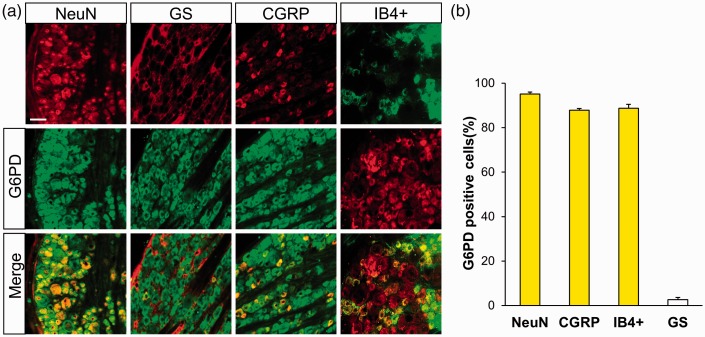
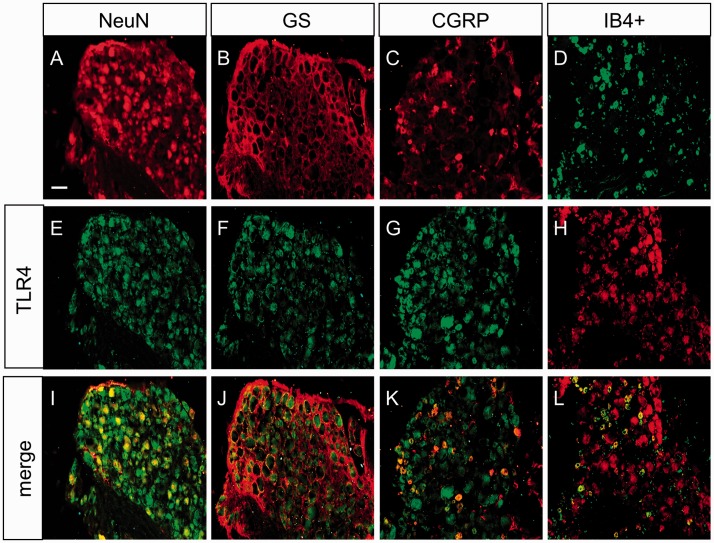
Next, the localization of G6PD and TLR4 in CON rats was detected by immunofluorescence analysis. We co-labeled G6PD with NeuN (the marker of neurons) or GS (the marker of satellite glial cells) to determine the localization of G6PD in L4-L6 DRG (Figure 2(a)). The results showed that G6PD was mainly co-expressed with NeuN, not with GS. Moreover, G6PD was expressed in CGRP and IB4+-positive neurons in DRG. The quantification analysis showed that the G6PD-positive cells of NeuN, CGRP, IB4+, and GS was 95.12%, 87.80%, 88.74%, and 2.66%, respectively (Figure 2(b), n = 3 rats). Similarly, as shown in Figure 3, TLR4 was also co-expressed exclusively with NeuN-labeled cells (Figure 3(i)) but not detected in GS-labeled cells (Figure 3(j)). TLR4 was also distributed in CGRP (Figure 3(k)) and IB4 (Figure 3(l))-positive DRG neurons.
Figure 2.
G6PD was mainly expressed in DRG neurons. (a) G6PD-positive cells (middle) were co-labeled with NeuN-positive dyed in red (top left one) but not with GS-positive cells shown in red (top left second). Double labeling of G6PD and NeuN (bottom left one) and merge of GS-positive staining and G6PD labeling (bottom left second). G6PD was mainly co-expressed in CGRP-positive cells (top right second) dyed in red and IB4-positive DRG neurons which were dyed in green (top right one). Merge of G6PD with CGRP-positive cells (bottom right second) and IB4 (bottom right one) was shown. Scale bar = 50 µm. (b) Quantified analysis showed G6PD was mainly located in small and medium sensory neurons labeled with CGRP and IB4+ but a few with GS.
GS: glutamine synthetase; CGRP: calcitonin gene-related peptide; IB4+: isolectin B4; G6PD: glucose-6-phosphate dehydrogenase.
Figure 3.
TLR4 was mainly expressed in DRG neurons. TLR4-positive cells (e–h) were co-labeled with NeuN-positive (a) dyed in red but not GS-positive cells (b) shown in red. Double labeling of TLR4 and NeuN (i) and merge of GS-positive staining and TLR4 labeling (j). TLR4 was mainly co-expressed in CGRP-positive cells (c) dyed in red and IB4-positive DRG neurons which were dyed in green (d). Merge of TLR4 with CGRP-positive cells (k) and IB4 (l) was shown. Scale bar = 50 µm.
GS: glutamine synthetase; CGRP: calcitonin gene-related peptide; IB4+: isolectin B4; TLR4: toll-like receptor 4.
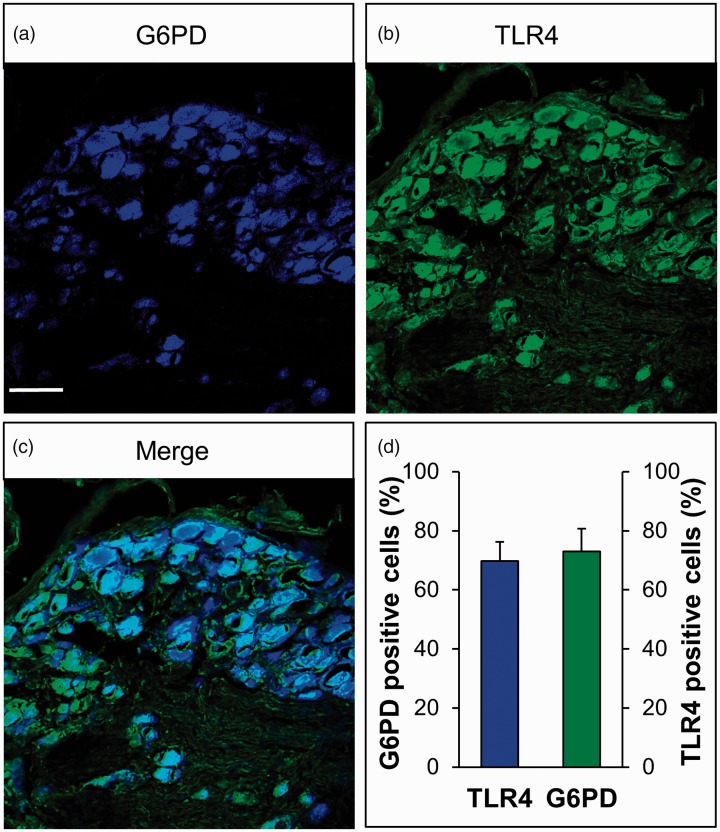
To further determine the localization of G6PD and TLR4 in the neurons, immunostaining was carried out in CON rats. Figure 4 shows G6PD-positive cells (blue, Figure 4(a)) and TLR4-positive cells (green, Figure 4(b)); G6PD was co-localized with TLR4 in the DRG (cyan, Figure 4(c)). The quantification analysis showed that the majority of G6PD was co-expressed with TLR4 in the same DRG neurons (Figure 4(d), n = 3 rats). The percentage of G6PD-positive cells in all TLR4-positive cells was 69.84%, and the percentage of TLR4-positive cells in G6PD-positive cells was 73.04%.
Figure 4.
G6PD and TLR4 were co-expressed in DRG neurons. (a) G6PD-positive cells are shown in blue. (b) TLR4-positive cells are shown in green. (c) Merge of double labeling of G6PD and TLR4. Scale bar = 50 µm. (d) Quantified analysis showed the majority of G6PD was co-expressed with TLR4-positive DRG neurons, and the majority of TLR4 was also co-expressed with G6PD-positive DRG neurons.
G6PD: glucose-6-phosphate dehydrogenase; TLR4: toll-like receptor 4.
G6PD overexpression attenuated pain hypersensitivity and reduced TLR4 expression in DM rats
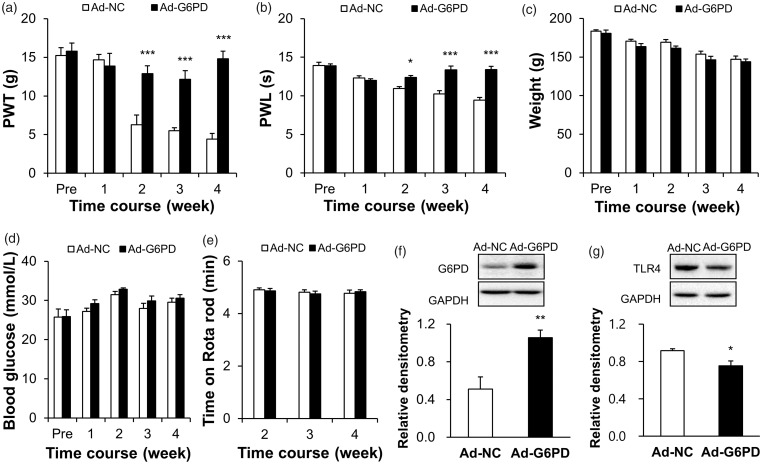
To further determine whether G6PD is involved in pain hypersensitivity of STZ-induced diabetic rats, we observed the effect of overexpression G6PD on PWT and PWL. First, Ad-G6PD was administered three days after STZ injection. At the last day of the second week, the adenovirus was injected again. Four weeks after the first injection of Ad-G6PD, we verified the effect of G6PD overexpression in L4-L6 DRGs of DM rats. The results showed that the expression of G6PD was significantly increased (Figure 5(f), **p < 0.01, compared with Ad-NC group, two-sample t-test). The relative densitometry of G6PD was 0.51 ± 0.13 (n = 6) in Ad-NC rats and 1.06 ± 0.08 (n = 5) in Ad-G6PD rats. Second, we detected the PWT and PWL of DM rats treated with adenovirus. As shown in Figure 5(a) and 5(b), intrathecal injection of Ad-G6PD markedly reversed the mechanical PWT and thermal PWL (Figure 5(a), ***p < 0.001, compared with Ad-NC group, two-way ANOVA; Figure 5(b), *p < 0.05, ***p < 0.001, compared with Ad-NC group, two-way ANOVA). The PWTs of DM rats treated with Ad-G6PD (n = 9) were 15.80 ± 1.05, 13.85 ± 1.65, 12.90 ± 1.00, 12.14 ± 1.15, and 14.82 ± 0.98 g at pre, 1, 2, 3, and 4 weeks, respectively. The PWTs of DM rats treated with Ad-NC (n = 8) were 15.24 ± 1.00, 14.68 ± 0.71, 6.29 ± 1.23, 5.50 ± 0.41, and 4.43 ± 0.72 g at pre, 1, 2, 3, and 4 weeks, respectively. The PWLs of DM rats treated with Ad-G6PD (n = 9) were 13.88 ± 0.25, 12.01 ± 0.22, 12.38 ± 0.24, 13.35 ± 0.50, and 13.40 ± 0.41 s at pre, 1, 2, 3, and 4 weeks, respectively. The PWLs of DM rats treated with Ad-NC (n = 8) were 13.92 ± 0.41, 12.33 ± 0.26, 10.93 ± 0.26, 10.24 ± 0.42, and 9.45 ± 0.32 s at pre, 1, 2, 3, and 4 weeks, respectively.
Figure 5.
Overexpression of G6PD attenuated hindpaw pain hypersensitivity and reduced the TLR4 expression of diabetic rats. (a, b) Ad-G6PD significantly reversed PWT (a) and PWL (b) of DM rats (n = 8 rats for Ad-NC and n = 9 rats for Ad-G6PD, respectively, *p < 0.05, ***p < 0.001 vs. Ad-NC, two-way ANOVA). (c, d) Ad-G6PD did not alter the body weight (c) and blood glucose (d) of DM rats (n = 8 rats for Ad-NC and n = 9 rats for Ad-G6PD, p > 0.05 vs. Ad-NC, two-way ANOVA). (e) Ad-G6PD did not alter the motor function of DM rats (n = 8 rats for Ad-NC and n = 9 rats for Ad-G6PD, p > 0.05 vs. Ad-NC, two-way ANOVA). (f) G6PD expression was obviously upregulated in DM rats by intrathecal injection of Ad-G6PD (n = 6 rats for Ad-NC and n = 5 rats for Ad-G6PD, **p < 0.01 vs. Ad-NC, two-sample t-test). (g) The TLR4 expression in L4-L6 DRGs from DM rats in which injection of Ad-G6PDs showed a significant decrease when compared with the age-matched DM rats which delivered with negative control adenovirus (n = 4 rats for each group, *p < 0.05 vs. Ad-NC, two-sample t-test).
Ad-NC: negative control adenovirus; Ad-G6PD: G6PD overexpression adenovirus; G6PD: glucose-6-phosphate dehydrogenase; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; PWT: paw withdrawal threshold; PWL: paw withdrawal latency; TLR4: toll-like receptor 4.
In addition, we also detected the body weight, blood glucose, and motion on Rotarod of DM rats, and they were not altered after Ad-G6PD injection (Figure 5(c), p > 0.05, compared with Ad-NC group, two-way ANOVA; Figure 5(d), p > 0.05, compared with Ad-NC group, two-way ANOVA; Figure 5(e), p > 0.05, compared with Ad-NC group, two-way ANOVA). The weights of DM rats treated with Ad-G6PD (n = 9) were 180.89 ± 4.05, 163.67 ± 3.63, 161.33 ± 2.98, 146.44 ± 4.56, and 143.89 ± 3.66 g at pre, 1, 2, 3, and 4 weeks, respectively. Their blood glucose (n = 9) was 25.88 ± 1.71, 29.21 ± 0.97, 32.87 ± 0.33, 29.9 ± 1.23, and 30.59 ± 0.91 mmol/L at pre, 1, 2, 3, and 4 weeks, respectively. And their time on the Rotarod (n = 9) was 4.87 ± 0.09, 4.76 ± 0.10, and 4.84 ± 0.07 min at 2, 3, and 4 weeks, respectively. The weights of DM rats treated with Ad-NC (n = 8) were 183.75 ± 1.52, 170.75 ± 2.27, 169.25 ± 3.45, 153.63 ± 4.08, and 147 ± 4.47 g at pre, 1, 2, 3, and 4 weeks, respectively. The blood glucose (n = 8) was 25.75 ± 2.04, 27.2 ± 0.85, 31.5 ± 0.83, 27.96 ± 1.29, and 29.56 ± 1.03 mmol/L at pre, 1, 2, 3, and 4 weeks, respectively. The time on the Rotarod (n = 8) was 4.91 ± 0.07, 4.81 ± 0.09, and 4.78 ± 0.12 min at 2, 3, and 4 weeks, respectively.
We next determined whether G6PD downregulation contributed to the upregulation of TLR4 expression. Four weeks after the first injection of Ad-G6PD, the L4-L6 DRGs were dissected out for western blotting assays. Intrathecal injection of Ad-G6PD significantly reduced the expression of TLR4 (Figure 5(g), *p < 0.05, compared with Ad-NC group, two-sample t-test). The relative densitometry of TLR4 was 0.92 ± 0.02 (n = 4) in Ad-NC rats and 0.75 ± 0.05 (n = 4) in Ad-G6PD rats. This result suggested that G6PD downregulation led to an increase in TLR4 expression, which might contribute to neuropathic pain in diabetic rats.
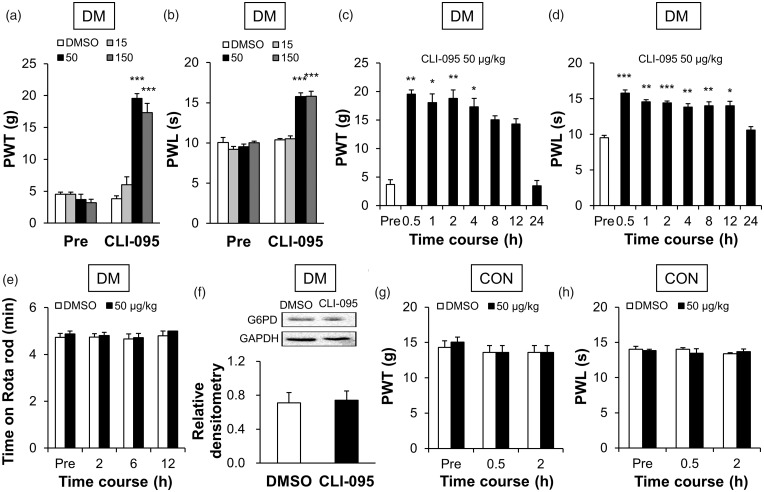
TLR4-selective inhibitor CLI-095 suppressed pain hypersensitivity in DM rats
To further confirm the role of TLR4 in diabetic pain hypersensitivity, we next determined whether treatment with TLR4-selective inhibitor CLI-095 attenuated mechanical allodynia and thermal hyperalgesia. As shown in Figure 6, after intrathecal injection of CLI-095, the PWT was reversed in dose-dependent (Figure 6(a), ***p < 0.001, compared with DMSO group, two-way ANOVA) and time-dependent manners (Figure 6(c), *p < 0.05, **p < 0.01, compared with pre, Friedman ANOVA followed by Dunn post hoc test) in DM rats. The PWTs of DM rats before the administration (pre) were 4.52 ± 0.36 g (DMSO, n = 6), 4.52 ± 0.36 g (15 μg/kg, n = 6), 3.68 ± 0.86 g (50 μg/kg, n = 6), and 3.20 ± 0.55 g (150 μg/kg, n = 6). After the treatment of CLI-095, the PWTs were 3.81 ± 0.48 g (DMSO, n = 6), 6.02 ± 1.23 g (15 μg/kg, n = 6), 19.54 ± 0.76 g (50 μg/kg, n = 6), and 17.31 ± 1.49 g (150 μg/kg, n = 6). The PWTs of DM rats treated with 50 μg/kg CLI-095 (n = 6) at pre, 0.5, 1, 2, 4, 8, 12, and 24 h were 3.68 ± 0.86, 19.54 ± 0.76, 18.06 ± 1.52, 18.82 ± 1.48, 17.31 ± 1.49, 15.04 ± 0.72, 14.31 ± 0.91, and 3.49 ± 0.90 g, respectively. The maximal inhibitory effects on PWT for CLI-095 were observed at a dose of 50 μg/kg, and the CLI-095-induced analgesic effect lasted for ∼4 h. A similar effect of CLI-095 was found in PWL (Figure 6(b), ***p < 0.001, compared with DMSO group, two-way ANOVA; Figure 6(d), *p < 0.05, **p < 0.001, ***p < 0.001, compared with pre, one-way ANOVA). The PWLs of DM rats before the administration (pre) were 10.07 ± 0.61 s (DMSO, n = 6), 9.21 ± 0.34 s (15 μg/kg, n = 6), 9.54 ± 0.33 s (50 μg/kg, n = 6), and 10.03 ± 0.18 s (150 μg/kg, n = 6). After the treatment of CLI-095, the PWLs were 10.37 ± 0.19 s (DMSO, n = 6), 10.52 ± 0.34 s (15 μg/kg, n = 6), 15.79 ± 0.45 s (50 μg/kg, n = 6), and 15.82 ± 0.61 s (150 μg/kg, n = 6). And the PWLs of DM rats treated with 50 μg/kg CLI-095 (n = 6) at pre, 0.5, 1, 2, 4, 8, 12, and 24 h were 9.54 ± 0.33, 15.79 ± 0.45, 14.56 ± 0.31, 14.42 ± 0.27, 13.83 ± 0.49, 14.00 ± 0.57, 13.99 ± 0.66, and 10.60 ± 0.47 s, respectively. The maximal inhibitory effects for CLI-095 were observed at a dose of 50 μg/kg, and the CLI-095-induced analgesic effect lasted for ∼12 h.
Figure 6.
CLI-095 suppressed hindpaw hypersensitivity but did not alter G6PD expression in DRGs of diabetic rats. (a, b) Intrathecal injection of CLI-095 reversed the PWT (a) and PWL (b) of DM rats in a dose-dependent manner (n = 6 rats for each group, ***p < 0.001 vs. DMSO, two-way ANOVA). The maximal inhibitory effects for CLI-095 were observed at a dose of 50 μg/kg. (c, d) A single injection of 50 μg/kg induced analgesic effect in PWT (c), which lasted for ∼4 h (n = 6 rats, *p < 0.05, **p < 0.01 vs. pre, Friedman ANOVA). Injection of 50 μg/kg also induced analgesic effect in PWL (d), which lasted for ∼12 h (n = 6 rats, *p < 0.05, **p < 0.01, ***p < 0.001 vs. pre, one-way ANOVA). (e) CLI-095 at 50 μg/kg did not affect motion on Rotarod of DM rats (n = 6 rats for DMSO and n = 7 rats for CLI-095 group, p > 0.05 vs. DMSO, two-way ANOVA). (f) G6PD expression was not significantly altered after intrathecal injection of CLI-095 (n = 4 rats for each group, p > 0.05 vs. DMSO, two-sample t-test). (g, h) CLI-095 at a dose of 50 μg/kg did not influence the PWT (g) and PWL (h) of healthy CON rats (n = 6 rats for each group, p > 0.05 vs. DMSO, two-way ANOVA).
DM: diabetes mellitus; CON: control; DMSO: dimethyl sulfoxide; PWT: paw withdrawal threshold; PWL: paw withdrawal latency; G6PD: glucose-6-phosphate dehydrogenase; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
In addition, the time on Rotarod of DM rats treated with CLI-095 (n = 7) was 4.87 ± 0.13, 4.80 ± 0.13, 4.72 ± 0.18, and 5.00 ± 0.00 min before (pre), and 2, 6, and 12 h after injection, respectively. The time of DM rats treated with DMSO (n = 6) were 4.73 ± 0.17, 4.74 ± 0.15, 4.67 ± 0.21, and 4.80 ± 0.20 min before (pre), and 2, 6, and 12 h after injection, respectively. Statistical analysis showed that CLI-095 at 50 μg/kg did not affect motion on the Rotarod of DM rats (Figure 6(e), p > 0.05 compared with DMSO group, two-way ANOVA). Moreover, intrathecal injection of CLI-095 had no significant effect on the expression of G6PD (Figure 6(e), p > 0.05, compared with DMSO group, two-sample t-test). The relative densitometry of G6PD was 0.71 ± 0.12 (n = 4) in DMSO rats and 0.74 ± 0.11 (n = 4) in CLI-095 rats. These results suggested that upregulation of TLR4 induced hindpaw pain hypersensitivity of diabetic rats but did not influence G6PD expression.
To investigate the possible side-effect, we observed the effect of CLI-095 on CON rats. CLI-095 at the dose of 50 μg/kg did not affect the PWT and PWL in healthy CON rats (Figure 6(g and h), p > 0.05, compared with DMSO, two-way ANOVA). The PWTs of CON rats before the administration (pre) were 14.31 ± 0.91 g (DMSO, n = 6) and 15.04 ± 0.72 g (CLI-095, n = 6). After treatment with CLI-095 for 0.5 h and 2 h, the PWTs were 13.59 ± 0.97 and 13.59 ± 0.97 g (DMSO, n = 6), and 13.59 ± 0.97 and 13.59 ± 0.97 g (CLI-095, n = 6), respectively. The PWLs before administration were 14.02 ± 0.42 s (DMSO, n = 6) and 13.83 ± 0.20 s (CLI-095, n = 6). After treatment with CLI-095 for 0.5 h and 2 h, the PWLs were 14.02 ± 0.26 and 13.39 ± 0.15 s (DMSO, n = 6), and 13.48 ± 0.63 and 13.71 ± 0.36 s (CLI-095, n = 6), respectively.
Discussion
According to the latest epidemiological survey from China, the prevalence of diabetes is 10.9% in China,26 and following lots of refractory complications, which has become a public health problem that threatens human health.27–29 Diabetic neuropathic pain refers to symptoms and/or signs associated with peripheral neurological dysfunction in diabetic patients, except for other reasons, manifested mainly as symmetric multiple sensory neuropathy, characterized by burning or needle- or knife-like abnormal pain in feet, lower legs, and upper limbs. These complications seriously affect patients’ quality of life. As the disease progresses, the prevalence rate also increases. Diabetic neuropathic pain has become a clinically common and refractory chronic pain.2,30 Constantly exploring the pathogenesis of diabetic neuropathic pain is a major task and challenge for physicians and scientists.
It was reported that high glucose could downregulate G6PD expression and lead to oxidative stress, which is one of the most important mechanisms underlying diabetic chronic complications.6,31 And inhibition of G6PD produced oxidative stress, which contributes to diabetic nephropathy.7,8 G6PD deficiency accelerates the microvascular complications of diabetes, which could increase prevalence of proliferative retinopathy.9 Increased reactive oxygen species by the activation of NADPH oxidase contributes to the development of diabetic neuropathic pain.32 In the present study, we provided the evidence to prove for the first time that G6PD in DRGs was involved in the process of diabetic neuropathic pain. We showed that the mRNA and protein expression levels of G6PD in L4-L6 DRGs were significantly lower in diabetic rats than in age-matched CON rats. Most importantly, upregulation of G6PD in diabetic rats by intrathecal injection of Ad-G6PD markedly attenuated hindpaw pain hypersensitivity. To exclude the nonspecific effect, we showed that G6PD overexpression did not influence the motion function of these rats. However, why and how G6PD was downregulated in DM rats remains unclear. Some researchers reported in other pathology models and pointed out that the causes of G6PD deficiency or downregulation mainly included four factors: DNA methylation,33 histone deacetylation,5,34 alteration in miRNA expression,35,36 and DNA damage.37 So, the upstream mechanism of G6PD deficiency or downregulation in diabetic neuropathic pain needs to be further investigated.
The most important thing is to study how G6PD downregulation contributes to diabetic pain. In the present study, we showed that the mRNA and protein expression levels of TLR4 were remarkably increased in DRGs of diabetic rats. We also detected the mRNA levels of other subtypes of TLRs, including the cell membrane expressing proteins such as TLR1 and endosome expressing proteins such as TLR7. The expression of these two subtypes of TLR was not altered between control and diabetic rats (Supplementary figure 1). Recent studies have reported that TLRs were involved in neuropathic pain. Activation of TLRs can lead to release of proinflammatory mediators and cytokines, thus contributing to the generation and maintenance of neuropathic pain.38–41 Oxidative stress can upregulate the expression of TLR2/TLR4 and activate the NF-κB signaling pathway in human macrophages.10 In addition, our previous study demonstrated that NF-κB, the main downstream target of TLRs, is involved in diabetic neuropathic pain.18,20 In the present study, we showed that intrathecal injection of TLR4-selective inhibitor CLI-095 significantly reverses the PWT and PWL of diabetic rats. These data further confirm our previous conclusion that TLR4 was involved in the development of diabetic peripheral neuropathic pain hypersensitivity of rats.
It was widely considered that both myelinated and unmyelinated afferents were implicated in mediating diabetic neuropathic pain, and small nerve fibers, such as C-fibers, were the most significant and earliest affected ones.42,43 Immunofluorescence results showed that G6PD and TLR4 were both co-expressed with NeuN-labeled cells but not detected in GS-labeled cells, and furthermore, they both distributed in CGRP- and IB4-positive DRG neurons. These data suggested G6PD and TLR4 work in the same DRG neurons and may play important roles in diabetic neuropathic pain. Interestingly, we provided evidences to support that G6PD interacts with TLR4 in DRGs of DM rats. Immunofluorescence results showed that G6PD was co-expressed with TLR4 in the L4-L6 DRG neurons of rats. Most importantly, we showed that intrathecal injection of Ad-G6PD downregulated TLR4 expression, while injection of CLI-095 did not affect G6PD expression. These data support that G6PD is an upstream factor to regulate the expression of TLR4 in DRG neurons. However, what is the specific regulatory mechanism between G6PD and TLR4 remains largely unknown. As mentioned earlier, previous studies reported that G6PD deficiency led to oxidative stress, thus regulating the expression and function of TLRs. It has been well documented that oxidative stress is associated with epigenetic regulation.44–46 Since TLR4 gene does not have CpG islands according to methprimer, alteration in methylation and demethylation of the gene is excluded. It is, therefore, reasonable to hypothesize that G6PD might cause histone modification of tlr4 gene. It is also possible that there is a decrease in expression of some miRNAs, which are the upstream regulators of TLR4 expression via oxidative stress in the process of diabetic neuropathic pain. Of course, this demands further investigations.
In conclusion, our results suggest that G6PD and TLR4 in DRGs were involved in diabetic peripheral pain hypersensitivity. The decreased G6PD might contribute to diabetic neuropathic pain by upregulating TLR4 expression. This study might provide a potential strategy for clinical treatment of diabetic neuropathic pain.
Supplemental Material
Supplemental Material for Downregulation of glucose-6-phosphate dehydrogenase contributes to diabetic neuropathic pain through upregulation of toll-like receptor 4 in rats by Qian Sun, Bing-Yu Zhang, Ping-An Zhang, Ji Hu, Hong-Hong Zhang and Guang-Yin Xu in Molecular Pain
Author Contributions
QS and B-YZ performed experiments, analyzed data, and prepared figures and manuscript. P-AZ and JH prepared figures and manuscript. H-HZ analyzed data, prepared figures, and edited the manuscript. G-YX designed experiments, supervised the experiments, and finalized the manuscript. All the authors have read and approved the paper.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
This work was performed in accordance with the recommendations of the IASP. The protocol was approved by the Institutional Animal Care and Use Committee of Soochow University, P. EEEeeeR. China.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present work was supported by grants from the National Natural Science Foundation of China (81471137, 31730040, 81471041) and Natural Science Foundation of Jiangsu (BK20181172) and the Jiangsu Youth Medical Talents Project (QNRC2016874) and from the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Teixeira JM, dos Santos GG, Neves AF, Athie MCP, Bonet IJM, Nishijima CM, Farias FH, Figueiredo JG, Hernandez-Olmos V, Alshaibani S, Tambeli CH, Müller CE, Parada CA. Diabetes-induced neuropathic mechanical hyperalgesia depends on P2X4 receptor activation in dorsal root ganglia. Neuroscience 2019; 398: 158–170. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal N, Helmstadter J, Rojas DR, Bali KK, Gangadharan V, Kuner R. Evoked hypoalgesia is accompanied by tonic pain and immune cell infiltration in the dorsal root ganglia at late stages of diabetic neuropathy in mice. Mol Pain 2018; 14: 174480691881797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman EL, Nave KA, Jensen TS, Bennett D. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 2017; 93: 1296–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ang L, Cowdin N, Mizokami-Stout K, Pop-Busui R. Update on the management of diabetic neuropathy. Diabetes Spectr 2018; 31: 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang YP, Zhou LS, Zhao YZ, Wang SW, Chen LL, Liu LX. Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J 2014; 33: 1304–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z, Liew CW, Handy DE, Zhang Y, Leopold JA, Hu J, Guo L, Kulkarni RN, Loscalzo J, Stanton RC. High glucose inhibits glucose-6-phosphate dehydrogenase, leading to increased oxidative stress and beta-cell apoptosis. FASEB J 2010; 24: 1497–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Y, Osborne BW, Stanton RC. Diabetes causes inhibition of glucose-6-phosphate dehydrogenase via activation of PKA, which contributes to oxidative stress in rat kidney cortex. Am J Physiol Renal Physiol 2005; 289: F1040–F1047. [DOI] [PubMed] [Google Scholar]
- 8.Pessoa BS, Peixoto EB, Papadimitriou A, Lopes de Faria JM, Lopes de Faria JB. Spironolactone improves nephropathy by enhancing glucose-6-phosphate dehydrogenase activity and reducing oxidative stress in diabetic hypertensive rat. J Renin Angiotensin Aldosterone Syst 2012; 13: 56–66. [DOI] [PubMed] [Google Scholar]
- 9.Cappai G, Songini M, Doria A, Cavallerano JD, Lorenzi M. Increased prevalence of proliferative retinopathy in patients with type 1 diabetes who are deficient in glucose-6-phosphate dehydrogenase. Diabetologia 2011; 54: 1539–1542. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Igwe OJ. Exogenous oxidants activate nuclear factor kappa B through toll-like receptor 4 stimulation to maintain inflammatory phenotype in macrophage. Biochem Pharmacol 2018; 147: 104–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao L, Tanga FY, Deleo JA. The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain. Neuroscience 2009; 158: 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan B, Tang WH, Lu LJ, Zhou Y, Zhu HY, Zhou YL, Zhang HH, Hu CY, Xu GY. TLR4 upregulates CBS expression through NF-kappaB activation in a rat model of irritable bowel syndrome with chronic visceral hypersensitivity. WJG 2015; 21: 8615–8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Liu W, Wang X, Wan Z, Liu Y, Leng Y. Dexmedetomidine relieves acute inflammatory visceral pain in rats through the ERK pathway, toll-like receptor signaling, and TRPV1 channel. J Mol Neurosci 2018; 66: 279–290. [DOI] [PubMed] [Google Scholar]
- 14.Kong X, Wei J, Wang D, Zhu X, Zhou Y, Wang S, Xu G-Y, Jiang G-Q. Upregulation of spinal voltage-dependent anion channel 1 contributes to bone cancer pain hypersensitivity in rats. Neurosci Bull 2017; 33: 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing F, Zhang W, Wen J, Bai L, Gu H, Li Z, Zhang J, Tao YX, Xu JT. TLR4/NF-kappaB signaling activation in plantar tissue and dorsal root ganglion involves in the development of postoperative pain. Mol Pain 2018; 14: 1744806918807050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su M, Ran Y, He Z, Zhang M, Hu G, Tang W, Zhao D, Yu S. Inhibition of toll-like receptor 4 alleviates hyperalgesia induced by acute dural inflammation in experimental migraine. Mol Pain 2018; 14: 1744806918754612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H-H, Hu J, Zhou Y-L, Hu S, Wang Y-M, Chen W, Xiao Y, Huang L-Y M, Jiang X, Xu G-Y. Promoted interaction of nuclear factor-kappaB with demethylated cystathionine-beta-synthetase gene contributes to gastric hypersensitivity in diabetic rats. J Neurosci 2013; 33: 9028–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H-H, Hu J, Zhou Y-L, Qin X, Song Z-Y, Yang P-P, Hu S, Jiang X, Xu G-Y. Promoted interaction of nuclear factor-kappaB with demethylated purinergic P2X3 receptor gene contributes to neuropathic pain in rats with diabetes. Diabetes 2015; 64: 4272–4284. [DOI] [PubMed] [Google Scholar]
- 19.Xu GY, Li G, Liu N, Huang LY. Mechanisms underlying purinergic P2X3 receptor-mediated mechanical allodynia induced in diabetic rats. Mol Pain 2011; 7: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi L, Zhang HH, Xiao Y, Hu J, Xu GY. Electroacupuncture suppresses mechanical allodynia and nuclear factor kappa B signaling in streptozotocin-induced diabetic rats. CNS Neurosci Ther 2013; 19: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu T, Li L, Liu H, Li H, Liu Z, Li Z. KCNQ2/3/5 channels in dorsal root ganglion neurons can be therapeutic targets of neuropathic pain in diabetic rats. Mol Pain 2018; 14: 1744806918793229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang ZC, Li LH, Bian C, Yang L, Lv N, Zhang YQ. Involvement of NF-kappaB and the CX3CR1 signaling network in mechanical allodynia induced by tetanic sciatic stimulation. Neurosci Bull 2018; 34: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, Yang P-P, Song Z-Y, Feng Y, Hu D-M, Hu J, Xu G-Y, Zhang H-H. Alpha-lipoic acid suppresses neuronal excitability and attenuates colonic hypersensitivity to colorectal distention in diabetic rats. J Pain Res 2017; 10: 1645–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozas G, Labandeira García JL. Drug-free evaluation of rat models of parkinsonism and nigral grafts using a new automated rotarod test. Brain Res 1997; 749: 188–199. [DOI] [PubMed] [Google Scholar]
- 25.Hu J, Qin X, Song ZY, Yang PP, Feng Y, Sun Q, Xu GY, Zhang HH. Alpha-lipoic acid suppresses P2X receptor activities and visceral hypersensitivity to colorectal distention in diabetic rats. Sci Rep 2017; 7: 3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, Zhou M, Tang X, Hu Y, Wang L. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017; 317: 2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Xu W, Zhu Y, Deng H, Tan Y, Zeng L. Decreased beta-cell function is associated with cardiovascular autonomic neuropathy in Chinese patients newly diagnosed with type 2 diabetes. Neurosci Bull 2019; 35: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrei Cristian B, Amorin Remus P. Diabetic neuropathy prevalence and its associated risk factors in two representative groups of type 1 and type 2 diabetes mellitus patients from Bihor County. Maedica 2018; 13: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aye-Mon A, Hori K, Kozakai Y, Nakagawa T, Hiraga S, Nakamura T, Shiraishi Y, Okuda H, Ozaki N. CCR2 upregulation in DRG neurons plays a crucial role in gastric hyperalgesia associated with diabetic gastropathy. Mol Pain 2018; 14: 1744806917751322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiyama H, Yano Y, So K, Imai S, Nagayasu K, Shirakawa H, Nakagawa T, Kaneko S. TRPA1 sensitization during diabetic vascular impairment contributes to cold hypersensitivity in a mouse model of painful diabetic peripheral neuropathy. Mol Pain 2018; 14: 1744806918789812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang HC, Cheng ML, Hua YS, Wu YH, Lin HR, Liu HY, Ho HY, Chiu DTY. Glucose 6-phosphate dehydrogenase knockdown enhances IL-8 expression in HepG2 cells via oxidative stress and NF-kappaB signaling pathway. J Inflamm (Lond) 2015; 12: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olukman M Onal A Celenk FG Uyanikgil Y Cavusoglu T Duzenli N, andÜlker S.. Treatment with NADPH oxidase inhibitor apocynin alleviates diabetic neuropathic pain in rats. Neural Regen Res 2018; 13: 1657–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ingrosso D, Cimmino A, D'Angelo S, Alfinito F, Zappia V, Galletti P. Protein methylation as a marker of aspartate damage in glucose-6-phosphate dehydrogenase-deficient erythrocytes: role of oxidative stress. Eur J Biochem 2002; 269: 2032–2039. [DOI] [PubMed] [Google Scholar]
- 34.Makarona K, Caputo VS, Costa JR, Liu B, O'Connor D, Iskander D, Roper D, Robertson L, Bhatnagar N, Terpos E, Georgiou E, Papaioannou M, Layton D M, Luzzatto L, Roberts I, Karadimitris A. Transcriptional and epigenetic basis for restoration of G6PD enzymatic activity in human G6PD-deficient cells. Blood 2014; 124: 134–141. [DOI] [PubMed] [Google Scholar]
- 35.Hu T, Chang Y-F, Xiao Z, Mao R, Tong J, Chen B, Liu G-C, Hong Y, Chen H-L, Kong S-Y, Huang Y-M, Xiyang Y-B, Jin H. miR-1 inhibits progression of high-risk papillomavirus-associated human cervical cancer by targeting G6PD. Oncotarget 2016; 277: 86103–86116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He C, Yang J, Ding J, Li S, Wu H, Xiong Y, Zhou F, Jiang Y, Teng L, Yang J. Downregulation of glucose-6-phosphate dehydrogenase by microRNA-1 inhibits the growth of pituitary tumor cells. Oncol Rep 2018; 40: 3533–3542. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Lee JH, Paull TT, Gehrke S, D'Alessandro A, Dou Q. Mitochondrial redox sensing by the kinase ATM maintains cellular antioxidant capacity. Sci Signal 2018; 11: pii: eaaq0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji D, Zhou Y, Li S, Li D, Chen H, Xiong Y, Zhang Y, Xu H. Anti-nociceptive effect of dexmedetomidine in a rat model of monoarthritis via suppression of the TLR4/NF-kappaB p65 pathway. Exp Ther Med 2017; 14: 4910–4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin G, Jin X, Zhou S. Sparstolonin B selectively suppresses toll-like receptor-2 and -4 to alleviate neuropathic pain. Mol Med Rep 2018; 17: 1247–1252. [DOI] [PubMed] [Google Scholar]
- 40.Thakur KK, Saini J, Mahajan K, Singh D, Jayswal DP, Mishra S, Bishayee A, Sethi G, Kunnumakkara AB. Therapeutic implications of toll-like receptors in peripheral neuropathic pain. Pharmacol Res 2017; 115: 224–232. [DOI] [PubMed] [Google Scholar]
- 41.Liu XJ, Liu T, Chen G, Wang B, Yu XL, Yin C, Ji RR. TLR signaling adaptor protein MyD88 in primary sensory neurons contributes to persistent inflammatory and neuropathic pain and neuroinflammation. Sci Rep 2016; 6: 28188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Levine JD. Hyper-responsivity in a subset of C-fiber nociceptors in a model of painful diabetic neuropathy in the rat. Neuroscience 2001; 102: 185–192. [DOI] [PubMed] [Google Scholar]
- 43.Khan GM, Chen SR, Pan HL. Role of primary afferent nerves in allodynia caused by diabetic neuropathy in rats. Neuroscience 2002; 114: 291–299. [DOI] [PubMed] [Google Scholar]
- 44.Feng B, Ruiz MA, Chakrabarti S. Oxidative-stress-induced epigenetic changes in chronic diabetic complications. Can J Physiol Pharmacol 2013; 91: 213–220. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Li C, Sun G. Histone acetylation and its modifiers in the pathogenesis of diabetic nephropathy. J Diabetes Res 2016; 2016: 4065382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hui Y, Yin Y. MicroRNA-145 attenuates high glucose-induced oxidative stress and inflammation in retinal endothelial cells through regulating TLR4/NF-kappaB signaling. Life Sci 2018; 207: 212–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Downregulation of glucose-6-phosphate dehydrogenase contributes to diabetic neuropathic pain through upregulation of toll-like receptor 4 in rats by Qian Sun, Bing-Yu Zhang, Ping-An Zhang, Ji Hu, Hong-Hong Zhang and Guang-Yin Xu in Molecular Pain