Abstract
Years of tremendous study have dawned a new era for the treatment of cystic fibrosis (CF). For years CF care was rooted in the management of organ dysfunction resulting from the mal-effects of absent anion transport through the CF transmembrane regulator (CFTR) protein. CFTR, an adenosine triphosphate binding anion channel, has multiple functions, but primarily regulates the movement of chloride anions, thiocyanate and bicarbonate across luminal cell membranes. Additional roles include effects on other electrolyte channels such as the epithelial sodium channel (ENaC) and on pulmonary innate immunity.
Inappropriate luminal anion movement leads to elevated sweat chloride concentrations, dehydrated airway surface liquid, overall viscous mucous production, and inspissated bile and pancreatic secretions. As a result, patients develop the well-known CF symptoms and disease-defining complications such as chronic cough, oily stools, recurrent pulmonary infections, bronchiectasis, chronic sinusitis and malnutrition.
Traditionally, CF has been symptomatically managed, but over the past 6 years those with CF have been offered a new mode of therapy; CFTR protein modulation. These medications affect the basic defect in CF: abnormal CFTR function. Ivacaftor, approved for use in the United States in 2012, is the first medication in CF history to improve CFTR function at the molecular level. Its study and approval were followed by two additional CFTR modulators, lumacaftor/ivacaftor and tezacaftor/ivacaftor.
To effectively use currently available CF therapies, clinicians should be familiar with the side effects of the drugs and their impacts on patient outcomes. As many new modulators are on the horizon, this information will equip providers to discuss the benefits and shortcomings of modulator therapy especially in the context of limited healthcare resources.
Keywords: amplifier, corrector, cystic fibrosis, cystic fibrosis transmembrane conductance regulator, ivacaftor, lumacaftor, tezacaftor, potentiator
Historical background and clinical recognition
Cystic fibrosis (CF) symptoms have been described in children for hundreds of years.1 Early medical literature bemoans afflicted infants as living in a ‘cursed’ state because a ‘child who tastes salty [when kissed]… soon must die’.2 Today, however, those with CF can anticipate a median predicted survival of 44 years.3
Despite medieval recognition of a link between an infant’s salty skin and infant mortality, true understanding of CF did not begin until the late 19th and early 20th centuries. While now widely recognized primarily as a pulmonary disease, CF manifestations were initially described in the gastrointestinal (GI) tract; meconium peritonitis in 1838 by Carl von Rokitansky and meconium ileus in 1905 by Karl Landsteiner. Many children with intestinal obstruction, oily stools, and malnutrition were felt to have a form of celiac disease. In 1930 however, Margaret Harper, an Australian pediatrician at the Royal Alexandra Hospital for Children, distinguished CF intestinal disease from celiac disease.
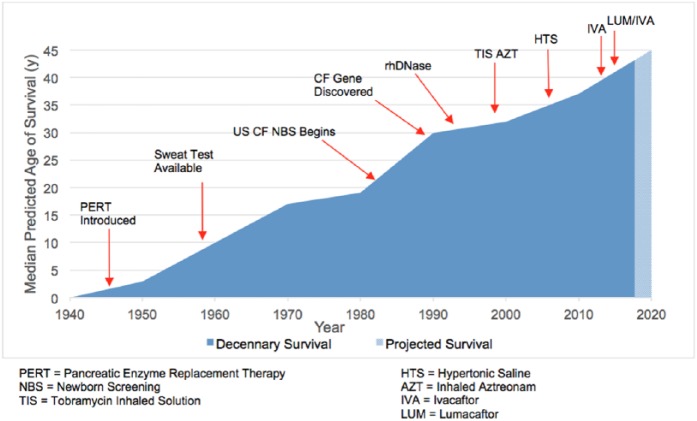
Despite these early reports, exploration of a newly described disease did not take foot until 1938 when Dr Dorothy H. Andersen, Chief of Pathology at the Babies Hospital of Columbia Presbyterian Medical Center described ‘cystic fibrosis of the pancreas’.4 As a result, work during the 1940s revealed that CF was a multiorgan disease. The 1948 heat wave in New York city, USA, led pediatricians Kessler and Anderson to report that patients with CF were more prone to the ill effects of sweating during heat prostration.5 Dr Paul di Sant’Agnese, founder of the United States (US) Cystic Fibrosis Foundation, performed several studies in the early 1950s demonstrating the altered electrolyte concentration in CF sweat.6,7 These data resulted in Gibson and Cooke’s 1959 description of pilocarpine iontophoresis.8 Their method of sweat collection replaced placing babies in heated blankets and bags (which led to death in some cases) and supplanted duodenal intubation for measurement of duodenal trypsin concentration. In 1979, measurement of immunoreactive trypsinogen on a blood spot made universal screening for CF feasible, and in 1982 Colorado was the first US state to place CF on its newborn screening panel.9 A new frontier of research opened later that decade when, in 1989, the CF gene was discovered by Canada’s Dr Lap-Chee Tsui.10 This unprecedented genomic understanding combined with organ-specific targeted therapies, has led to an exponential increase in CF survival (Figure 1).
Figure 1.
Change in cystic fibrosis survival as related to therapies and scientific advancement.9–12
CFTR protein dysfunction
CF occurs in persons with two pathologic CFTR genes located in trans; that is, one mutation on each allele. Currently more than 2000 genetic variations are described, but only about 300 are known to cause clinical disease.12 Mutations often affect both CFTR synthesis and function, but each is classified by the primary mechanism leading to protein malfunction.13 Mutations affecting synthesis and processing (Class I-II) result in more severe disease because no or nominal numbers of proteins reach the cell surface.14 Abnormal proteins maintaining some residual function (RF), that is, conduct some anions, are processed by the endoplasmic reticulum (ER) and trafficked to the luminal surface. Individuals with these mutations often experience less severe symptoms as the abnormal proteins maintain some open probability or transport some anions (classes III–IV respectively).14
Representing approximately 10% of mutations,15 class I (often delineated with an ‘X’ such as G542X) refers to defects in biosynthesis such as frameshift mutations, insertions and nonsense mutations. Also, in this class are canonical splice mutations and chromosomal deletions.13 Many of these ultimately result in premature termination codons (PTCs).16 The most common mutation, F508del, belongs principally to class II. It is present in almost 90% of the CF population and 46% of people with CF are homozygous for this mutation.17 These abnormalities result in an improperly folded CFTR that is poorly processed or insufficiently trafficked to the cell surface. The few that are trafficked function poorly and are quickly turned over.18
Class III and IV abnormalities yield proteins that, although present in the membrane, transport inadequate numbers of anions. As there is some appreciable movement of anions (i.e. there is some RF of the protein), those with these mutations tend to have less severe disease than those with class I and II mutations.3 Class III mutations, known as gating mutations, do not open with sufficient frequency. Class IV, termed conductance mutations, fail to conduct adequate quantities of chloride ions. Splice mutations (the insertion or deletion of nucleotides in regions of DNA coding for protein cuts), belong to class V. Finally, class VI refers to abnormalities resulting in protein instability once inserted into the epithelial surface.
CFTR mutations affect distinctive stages of protein synthesis and function. Thus, to rescue CFTR, modulator therapy targets a specific source of the protein’s malfunction. As some proteins result in multiple defects, more than one modulator is required to render sufficient protein activity. For example, ivacaftor, subclassified as a CFTR protein potentiator, augments chloride secretion of membranal CFTR.19 Augmentation results in clinical improvement for persons with specific gating and conductance mutations (classes III and IV).20–23 Consequently, for those who are F508del homozygous, ivacaftor monotherapy is clinically ineffective24 as F508del produces a CFTR protein that is not adequately expressed on the luminal surface. If present on the luminal surface, however, its conductance is responsive to potentiator therapy.19 As a protein corrector increases the concentration of F508del in the membrane by improving ER processing and subsequent protein trafficking, the combination of a corrector and potentiator results in clinical benefit for those F508del homozygous and most recently those with F508del/RF mutations.25–27 Currently in development is a third modulator class, termed ‘amplifier’, that increases the steady-state levels of the CFTR. It is mutation-agnostic and independently compliments the activity of both correctors and potentiators.28
Consequently, as historical therapies could only affect complications of disease, current therapies target the underlying cause of the disease. Thus, as 97.7% of those in the US CF Registry have been genotyped, a person’s genetic mutations are used to direct therapeutic changes.3,29
CFTR modulators: safety, effectiveness and use
Safety, effectiveness and use
Presently, three CFTR modulators are approved on the international market: ivacaftor, lumacaftor/ivacaftor and most recently tezacaftor/ivacaftor. Although the effect of modulators on long-term survival can only be estimated,30,31 short-term improvements such a decrease in sweat chloride, fewer pulmonary exacerbations (PExs), increases in percentage of predicted forced expiratory volume in 1 second (ppFEV1) and increases in body mass index (BMI) have been well documented in several phase III clinical trials.20,25–27
Although ivacaftor was initially approved only for people with CF and a G551D mutation,32 clinical trial data and cell models of CF33 supported the US Food and Drug Administration (FDA)’s indication expansion to include 38 mutations for people >1 year of age. Lumacaftor-ivacaftor was approved in the US for use in people with CF homozygous for the F508del mutation in 2015.34 Chest tightness and its multiple drug interactions (such as rendering hormonal contraceptives ineffective and significantly interfering with rifampin), further limit its use.35,36 Tezacaftor/ivacaftor is a new combination modulator (corrector and potentiator) that expands the population for which modulator therapy is available; those heterozygous for F508del and a RF mutation, and it is a second option for those homozygous for F508del. The two large phase III clinical trials, EVOLVE25 and EXPAND,26 were designed to expressly demonstrate the effectiveness of the new corrector tezacaftor and known potentiator ivacaftor in these different CF populations.
EVOLVE was a 24-week, randomized, double-blind, placebo-controlled, parallel-group trial studying tezacaftor/ivacaftor in F508del homozygotes. Persons 12 and older with stable CF disease and a ppFEV1 between 40% and 90% at screening, were randomized 1:1 to receive either tezacaftor 100 mg daily with ivacaftor 150 mg twice daily or placebo twice daily. The primary endpoint was the absolute change in ppFEV1 from baseline though week 24. Secondary endpoints over the same timeframe included the relative change in ppFEV1, total number of PExs, the absolute change in BMI, and absolute symptom change as measured using the respiratory domain score of the Cystic Fibrosis Questionnaire-Revised (CFQ-R), a validated CF-specific quality of life measure for which increased scores represent improved patient-reported quality of life; the established minimal clinically important difference for the respiratory domain score is 4 points.31
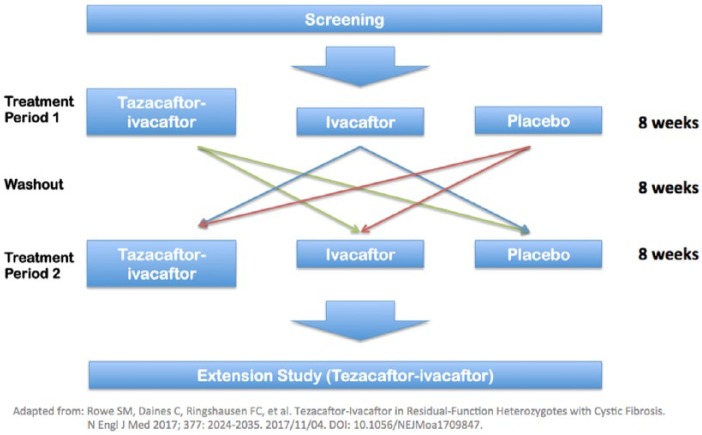
EXPAND was a randomized, multicenter, placebo-controlled, crossover trial describing the effect of tezacaftor/ivacaftor in 248 patients heterozygous for F508del and a RF mutation. The inclusion criteria were identical to EVOLVE, except that in addition to being heterozygous for a RF mutation, candidates also needed to have a sweat chloride of greater than or equal to 60 mmol/l or, if lower, evidence of chronic sinopulmonary disease. The primary endpoint was the absolute change in ppFEV1 and secondary endpoints included CFQ-R respiratory domain scores, relative change in ppFEV1, and absolute change in sweat chloride. The investigators chose a crossover design (Figure 2) because there are an insufficient number of patients with RF mutations to conduct a sufficiently large parallel-group trial (as fewer than 400 CF mutations have been fully characterized11 and the majority of those mutations are incredibly rare).3
Figure 2.
EXPAND study design26.
Both studies demonstrated a significant improvement in their primary (absolute change in ppFEV1) endpoints. In EVOLVE (F508del homozygote population) the ppFEV1 increased by 4% . In EXPAND (F508del heterozygote population) the ppFEV1 improved by 6.8%. An important secondary endpoint was the CFQ-R respiratory domain score. In EVOLVE, although nominally significant, there was a 5.1 point improvement in the CFQ-R respiratory domain score, and in EXPAND, the CFQ-R respiratory domain score increased by 11.1 points when compared with placebo. Additionally, in EXPAND, use of tezacaftor-ivacaftor was associated with a statistically significant 2.1% improvement in ppFEV1 when compared with ivacaftor alone. This finding is important as it demonstrates the increased effectiveness of dual corrector/potentiator therapy over an already available monotherapy (ivacaftor) for many in the heterozygous F508del/RF population.
Secondary endpoints, including the effect of tezacaftor/ivacaftor on the rate of pulmonary exacerbations were generally encouraging in both studies. EXPAND demonstrated a 10% reduction in risk for PExs. Although a supportive endpoint, persons in EVOLVE experienced a 35% reduction in infective PExs. Thus, as more frequent PExs have been associated with an increased rate of ppFEV1 decline, a sustained effect of tezacaftor/ivacaftor could decrease this complication.37 On a functional level, when compared with placebo, the absolute change in sweat chloride concentration from baseline was -10.1 mmol/l (EVOLVE) and -9.5 mmol/l (EXPAND). Surprisingly, although patients in both studies gained weight over the 6-month study period, there was no statistically different improvement in BMI in the treatment arms.
Use
The US FDA, delineates the genetic mutations for which each CFTR protein modulator may be prescribed, despite some persons being clinically found to respond to the approved therapies.38 For an individual person, the results of EXPAND and EVOLVE should be evaluated in the context of prior studies involving CFTR protein modulators, noting medication side effects (SEs), patient co-morbidities, differences in measured outcomes and US FDA approval criteria (See Tables 1 and 2 for a brief description of important studies and side effects referenced in the following text). For example, although immediate effects of modulator therapy include improvements in ppFEV1 and rates of PExs, markers of cellular efficacy such as changes in sweat chloride suggest improved baseline physiology. The clinical consequences of the latter finding are not yet clear as individual sweat chloride responses do not predict outcomes related to ppFEV1 or PExs.39 Perhaps sustained exposure to a more improved clinical milieu could retard organ dysfunction.
Table 1.
Brief description of referenced CFTR protein modulator studies.
| Study | Description |
|---|---|
| STRIVE20 | A randomized, double-blind, placebo-controlled trial studying ivacaftor in patients ≥12 years with at least one G551D-CFTR mutation |
| EVOLVE25 | A phase III randomized, double-blind, placebo-controlled, multicenter, parallel trial evaluating the effects of tezacaftor-ivacaftor in patients ≥ 12 years homozygous for F508del |
| EXPAND26 | A phase III randomized, double-blind, placebo-controlled, crossover trial examining the efficacy and safety of ivacaftor monotherapy or in combination with tezacaftor in those ≥ 12 years heterozygous for F508del and an RF mutation |
| Phase II LUMACAFTOR-IVACAFTOR40 | A phase II randomized control trial in patients ≥ 18 years studying the effects of lumacaftor combined with ivacaftor |
| TRAFFIC/TRANSPORT34 | Two phase III, randomized, double-blind, placebo-controlled studies studying the effects of lumacaftor combined with ivacaftor in patients ≥12 years of age F508del homozygous |
| LUMACAFTOR-IVACAFTOR Open-Label 6–11y/o41 | An open-label phase III trial, evaluating the safety, tolerability, pharmacodynamics, and efficacy of lumacaftor/ivacaftor in patients 6–11 years homozygous for F508del |
| LUMACAFTOR-IVACAFTOR Randomized 6–11y/o42 | A phase III, randomized, double-blind, placebo-controlled, multicenter study evaluating the efficacy and safety of lumacaftor/ivacaftor combination in F508del homozygotes 6–11 years of age |
| PROSPECT43 | Two-part multicenter prospective, longitudinal study of CFTR-dependent disease profiling in CF to explore biomarkers, clinical, and physiological characteristics across various degrees of CF severity |
| LUMACAFTOR-IVACAFTOR 24 Week Open Label44 | A safety, tolerability, and efficacy study of lumacaftor/ivacaftor 400 mg/200 mg Q12 hours in patients ≥ 12 years homozygous for F508del with ppFEV1 < 40% |
CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane regulator; ppFEV1, percentage of predicted forced expiratory volume in 1 second; RF, residual function.
Table 2.
Summary of modulator effects on clinically important outcome measures#.
| Study (drug used) | Mutations |
Week 24 Δ sweat chloride
(mmol/l) [CI] |
Week 24 absl Δ ppFEV1
(%) [CI] |
Week 24 Δ BMI
(kg/m 2 ) [CI] |
Week 24
PEx rate reduction (%) [CI] |
|---|---|---|---|---|---|
| STRIVE20 | G551D | ‒47.9 [-51.3 to -44.5] |
10.6 [8.6–12.6] |
2.8 (kg) (BMI NM) [1.8–3.7] |
60 [36–78] |
| EXPAND26 | F508del/RF | ‒9.5@
[-11.7 to -7.3] |
6.8@
[5.7–7.8] |
NS | NS |
| Phase 2 Lumacaftor/Ivacaftor40 | F508del homozygotes | ‒11.1*
[-18.5 to -3.7] |
NS* | NM | NM |
| TRAFFIC/ TRANSPORT34 |
F508del homozygotes | NM See Phase II lum/iva |
2.8≠
[1.8–3.8] |
0.24 [0.11–0.37] |
39 [24–51] |
| Lumacaftor/Ivacaftor-Open Label 6–11y/o 41 | F508del homozygotes | ‒24.8+
[-29.1 to -20.5] |
NS | 0.64+
[0.46–0.83] |
NM |
| Lumacaftor/Ivacaftor Phase III Randomized 6–11y/o42 |
F508del homozygotes | ‒20.8++
[-23.4 to -18.2] |
2.4 [0.4–4.4] |
NS | NM |
| PROSPECT43 | Variable | ‒17 [NR] |
1.6 [NR] |
0.6 [NR] |
NM |
| Lumacaftor/Ivacaftor 24 Week Open-Label 44 | F508del homozygotes | ‒20.2 [-24.3 to -16.1] |
NS | NS | NM |
| EVOLVE25 | F508del homozygotes | ‒10.1 [-11.4 to-8.8] |
4.0 [3.1–4.8] |
NS | 35 [12–52] |
Outcomes at week 24 versus placebo unless otherwise noted.
Day 56, lumacaftor 400 mg every 12 hours.
For patients who received approved lumacaftor-ivacaftor dose.
change from the baseline to the average of the week 4 and week 8 measurement compared with placebo.
change from baseline to week 24.
change at day 15 and week 4.
BMI, body mass index; CI, confidence interval; CF, cystic fibrosis; iva, ivacaftor; lum, lumacaftor; NM, not measured; NR, not reported; NS, not statistically significant; ppFEV1, percentage of predicted forced expiratory volume in 1 second; PEx, pulmonary exacerbation.
Tezacaftor-ivacaftor is available in the US (and approved for use in Canada) for those 12 years and older who are either F508del homozygotes or are heterozygous for F508del and one of several RF mutations (US approval only; Table 3).45 There are two advantages that favor use of tezacaftor-ivacaftor versus lumacaftor-ivacaftor: Tezacaftor-ivacaftor is associated with an improved SE profile and has fewer medication interactions than lumacaftor-ivacaftor. For example: In the EXPAND and EVOLVE, no patients discontinued the study due to respiratory SEs. Also, no increase in dyspnea was noted with tezacaftor-ivacaftor initiation as had been with lumacaftor-ivacaftor.25–27 Importantly, females can reliably use hormonal contraception with tezacaftor-ivacaftor.45 However, as with lumacaftor-ivacaftor, use of rifampin and other strong CYP3A inducers is not recommended and the dose of azole antifungals should be reduced when co-administered tezacaftor-ivacaftor.45 Important for pregnancy counseling, although animal data are not highly concerning (Table 4) and there are scattered case reports of successful human pregnancy following use of ivacaftor alone or ivacaftor-lumacaftor during the pregnancy,45,33,35–42 the effects of these modulators on human fetal development and lactation are largely unknown. The older adult population presents a different set of prescribing concerns as comorbid conditions require treatment with medications not historically encountered in CF care. Serum digoxin concentrations, for example, are variably affected by combination therapy and may need to be followed more closely.45 Finally, combination therapy also alters serum levels of several commonly used immunosuppressive medications such as cyclosporine, everolimus, sirolimus, and tacrolimus.45
Table 3.
CFTR mutations beyond F508del homozygous approved for tezacaftor/ivacaftor use.*
|
Symdeko indicated mutations
• F508del/F508de1 or • At least one responsive mutation from the following list: | |||
|---|---|---|---|
| 711+3A→ G | 2789-5G → A | 3272-26A → G | 3846+10kbC →T |
| A455E | A1067T | D110E | D110H |
| D579G | D1152H | D1270N | E56K |
| E193K | E831X | F1052V | F1074L |
| K1060T | L206W | P67L | R74W |
| R117C | R347H | R352Q | R1070W |
| S945L | S977F | ||
These CFTR mutations have been shown to yield a clinical FEV1 response or in vitro data demonstrating an increase in chloride transport to at least 10% of untreated normal over baseline in response to tezacaftor/ivacaftor.45
CFTR, cystic fibrosis transmembrane regulator; FEV1, forced expiratory volume in 1 second.
Table 4.
Drug use in specific populations.
| Organ | Ivacaftor33,35 | Lumacaftor-ivacaftor35 | Tezacaftor-ivacaftor45 |
|---|---|---|---|
| Renal insufficiency | |||
| Mild | No dose adjustment | No dose adjustment | No dose adjustment |
| Moderate | No dose adjustment | No dose adjustment | No dose adjustment |
| GFR < 30 ml/min or ESRD | Caution with use | Caution with use | Caution with use |
| Hepatic insufficiency | |||
| CP class A | No dose adjustment | No dose adjustment | No dose adjustment |
| CP class B | Dose adjustment recommended | Dose adjustment recommended | Dose adjustment recommended |
| CP class C | Not studieda | Not studieda | Not studieda |
| Fertility | No significant effect in animals at nontoxic dosec | No significant effect in animals at toxic dose35
(lumacaftor alone) |
No significant effect in animals at toxic dose45
(tezacaftor alone) |
| Pregnancy/ teratogenicity |
No significant effect in animals at nontoxic dosee | No significant effect at toxic dosef
(lumacaftor alone, not tested in combination) |
Varied effects at different dosingg
(tezacaftor alone, not tested in combination) |
| Miscarriage | Unknown | Unknown | Unknown |
| Lactation (humans) | Present 46, h | Present 46, h | Unknownh |
CP, Child–Pugh; ESRD, end-stage renal disease; GFR, glomerular filtration rate; LFBW, low fetal birth weight; MRHD, maximum recommended human dose.
Use with caution and monitor liver function closely.
Rats: none at three33, four45, or five-times35 (females) and six45,34, eight-times35 (males) the MRHD receiving 100 mg/kg/day.
Rats: none at three33, four45, or five-times35 (females) and six45,33, eight-times35 (males) the MRHD receiving 100 mg/kg/day. Reduced fertility noted at 645, 735 and 833 (females) and 945 or 15-times33,37 (males) the MRHD receiving 200 mg/kg/day.
Rats: none at eight-times (females) and three-times (male) the MRHD.
Rats: none at three-times33 the MRHD receiving 100 mg/kg/day. LFBW noted at five-times (toxic dose)33 the MRHD receiving 200 mg/kg/day.
(1) Rats: none at three-times the MRHD receiving 100 mg/kg/day based on embryo-fetal development, but LFBW at two-times the MRHD receiving 50 mg/kg/day based on pre and postnatal development.
(2) Rabbits: based on embryo-fetal development, none at 0.2-times the MRHD receiving 25 mg/kg/day, but LFBW at 0.4-times the MRHD receiving 50 mg/kg/day.
(3) Noncongenital lens opacities/cataracts have been reported in pediatric patients. Rats: Noncongenital cataracts noted in rats from postnatal days 7 to 35, cataracts were observed at all dose levels.
Rats and Rabbits: during organogenesis, no teratogenicity or adverse effects on fetal development at doses up to approximately eight (rats) and five (rabbits) times the exposure at the MRHD. Rats: from organogenesis to lactation, no developmental adverse events at eight-times the MHRD.37
Rats and Rabbits: during organogenesis, no teratogenicity or adverse developmental effects at three-times (MRHD) in rats and 0.2 times the MRHD in rabbits. Please see package insert for more details at higher doses.45
Present in milk of lactating rats. Due to species-specific lactation physiology animal data may not reflect human findings.
As previously mentioned, ivacaftor provides substantial clinical efficacy in adults and children with RF mutations; typically gating and conductance abnormalities (Table 1 STRIVE).20–22 It is now approved for use in those with CF 1 year of age or older with multiple gating and conductance altering mutations as delineated in the package insert.33 It has not been associated with significant pulmonary symptoms,20 but like each modulator, may cause hepatotoxicity.33 Animal studies revealed development of noncongenital cataracts in the young, and thus children require serial ophthalmologic evaluation.33 It also has several drug–drug interactions. Its dose needs to be reduced when co-administered with moderate and strong CYP3A inhibitors (such as azole antifungals)33 and its concentration is significantly reduced in the presence of rifamycins. Whether used alone or in combination with ivacaftor or tezacaftor, it also may affect serum dioxin levels and commonly used immunosuppressants.33 Uniquely, as the modulator with the longest commercial experience, long-term safety and efficacy have been better established; no new significant safety concerns have arisen and its use continues to be associated with fewer PExs, decreased need for transplantation and lower risk of death.47
Lumacaftor/ivacaftor, the first approved combination modulator, may be prescribed for those who are F508del homozygous and 2 years or older. While it provides improvement in clinically important endpoints such as ppFEV1 and PExs, it remains a challenging drug to use in some patients. Initiation is sometimes associated with significant chest tightness, wheezing and increased pulmonary events in those with advanced lung disease.27,35,48 As a strong CYP3A inducer, lumacaftor has the highest number of drug–drug interaction of all the modulators49; hormonal contraception is unreliable, serum concentrations of many selective serotonin reuptake inhibitors are reduced, and a rifampin-based nontuberculous mycobacteria treatment regimen is not recommended.35 Importantly, it may decrease the serum concentration of corticosteroids which has direct implications when concomitantly treating allergic bronchopulmonary aspergillosis or an acute asthma exacerbation.35
An interesting finding is the change in sweat chloride seen with lumacaftor-ivacaftor compared with the changes seen with other CFTR modulators. Notably, patients with the G551D mutation had a robust decrease in sweat chloride of 48.1 mmol/l, but such marked improvement was not matched in dual combination modulator trials including in those with other RF mutations26 (Table 2, EXPAND). The lumacaftor/ivacaftor phase II study documented improvement in sweat chloride of approximately 11 mmol/l (changes in sweat were not measured in the lumacaftor/ivacaftor phase III studies).40,36 Data from lumacaftor/ivacaftor phase IV studies demonstrate that the average improvement in sweat chloride in children ⩾ 6 years is approximately 20 mmol/l. Recently, Graeber and colleagues described changes in sweat chloride, nasal potential difference (NPD) and intestinal current measurement (ICMs) in 53 patients homozygous for F508del.50 Their work demonstrated improvement in NPD and ICMs to 10–20% of wildtype function. Most intriguing is that the extent of CFTR rescue found was comparable to that present in pancreatic sufficient F508del/RF heterozygotes. This finding suggests that lumacaftor/ivacaftor combination therapy may provide sufficient CFTR rescue to improve pancreatic exocrine dysfunction. Data from the study of lumacaftor-ivacaftor in children ages 2–5 years who are homozygous for F508del demonstrated improvements in fecal elastase (a measure of pancreatic function) of −262.1 ng/ml [standard deviation (SD) 343.1] in patients <14 kg and an improvement of −71.1 ng/ml (SD 120.5) in children >14 kg following 24 weeks of lumacaftor-ivacaftor therapy (ClinicalTrials.gov identifier: NCT02797132). These findings suggest that early combination therapy may have a bearing beyond ppFEV1 outcomes; for example: the potential to decrease overall management burden by supporting ppFEV1 through improved nutrition. How the differences in improvement in sweat chloride between lumacaftor-ivacaftor and tezacaftor-ivacaftor will impact clinical outcomes over longer periods of study is unclear, but is being evaluated (ClinicalTrials.gov identifier: NCT03445793).
Given the similarities and differences between the two drugs, there are some patients for whom the rationale for a change in therapy from lumacaftor-ivacaftor to tezacaftor-ivacaftor is clear. For example, a patient who had chest tightness and ppFEV1 decline in spite of a step-up approach to therapy would be an excellent candidate for tezacaftor-ivacaftor. However, for other patients who have been stable or had markedly improved lung function or other outcomes on lumacaftor-ivacaftor, the decision to change therapy remains unclear. Other additional questions also remain: When should a modulator be prescribed outside of the indication label? (e.g. in the case of a rare RF mutation). Might the greater improvement in sweat chloride noted with lumacaftor-ivacaftor provide better pulmonary protection and prolong endocrine pancreatic function such that the SE profile of lumacaftor-ivacaftor is more acceptable? How should CFTR modulator use be managed when a patient requires an antifungal or nontuberculous mycobacaterial (NTM) therapy? These challenges will continue to require thoughtful, informed discussions between clinician and patient.
Cost–utility analysis
The lifetime cost of CF care (considering equipment, dietary needs, and maintenance therapies, alone) is expensive and rises as new therapies become available. For example, in 1997, the total lifetime cost in Germany was €396,000/patient and rose to €858,604/patient in 2007.51 In 2006, the US spent €39,278/patient (US$48,098) of which 40% was delegated to medication costs.51 In this decade, that percentage will rise significantly as the annual patient cost of ivacaftor, lumacaftor/ivacaftor, or tezacaftor/ivacaftor is approximately US$260,000–300,000.52,53
As a result, payment for CFTR modulators has spurred substantial debate as these therapies are amongst some of the most highly priced medications on the US market.52 Viewpoints range between two extremes: a utilitarian approach in which expenditures benefit the greatest portion of a population and a ‘right-to-health’ approach in which all members of a population are provided with a defined minimum quality of life.54 To syncretize these two vantagepoints some suggest one evaluate drug cost in terms of a weighted quality of life adjusted life year (QUALY). Cost-effectiveness is thus evaluated in terms of direct and indirect costs with a greater value assigned to health gain but respecting a maximum monetary expenditure.54 This year, the Institute for Clinical and Economic Review (ICER; an independent, nonprofit research institute) evaluated the addition of CFTR protein modulators to standard CF care.55 They reported that despite improvement in health outcomes the current price of modulators needed at least a 40% price reduction to be cost-effective. Of note, lumacaftor-ivacaftor and tezacaftor-ivacaftor have not been available for a sufficient amount of time to assess their effect on QUALY, so their analysis is premature. Furthermore, the application of their analysis type to therapies for very rare diseases has been questioned.56 Nevertheless, the findings highlight the high cost of personalized medicine which has led to more limited use of CFTR modulators in countries with universal health care coverage,57–59 and thus may impact the immediate use of tezacaftor-ivacaftor outside the US.
Future prospects
The effects of currently available modulator therapy are exciting because of their potential short and long-term impact on both quality and longevity of life, but many questions remain: What might the sustained effects of currently available modulators be? How can current protein targeted therapy be expanded to include more genetic variants and age groups? In which populations might additional classes of modulators provide benefit? Could greater clinical and functional affect be obtained with multiple correctors in combination with potentiators?
These pressing questions are being addressed in multiple studies several of which use tezacaftor/ivacaftor as the backbone on which new hypotheses or new modulators are tested. For example: Vertex Pharmaceuticals, has launched two phase III trials evaluating the efficacy in patients 12 years and older of the combination tezacaftor/ivacaftor with one of two additional correctors: VX-659 or VX-445 (ClinicalTrials.gov identifier: NCT03447249 and NCT03525444 respectively). Additionally, pharmacokinetic studies of VX-659 and VX-445 with tezacaftor/ivacaftor are ongoing in patients ages 6 to 11 years (ClinicalTrials.gov identifier: NCT03633526 and NCT03691779 respectively). In December 2018, the University of Alabama in November 2018 plans to launch a phase I study looking at tezacaftor/lumacaftor in a novel population; those with the PTC W1282X (ClinicalTrials.gov identifier: NCT03624101).
Proteostasis Therapeutics INC is conducting a phase I multicenter, randomized, placebo-controlled, study evaluating safety, tolerability, and pharmacokinetics of triple therapy with lumacaftor-ivacaftor and PTI-801 (a third-generation corrector) in healthy volunteers and persons with CF (ClinicalTrials.gov identifier: NCT03140527). Additionally, in a phase II evaluation, this company is studying the effects of once-daily triple therapy using PTI-428 (a novel amplifier), PTI-808 (CFTR potentiator) and PTI-801 (corrector; ClinicalTrials.gov identifier: NCT03500263).60 Other companies conducting early phase studies of CFTR modulators through the CF pipeline include Galapagos, Novartis and Flatley.61
In addition to CFTR modulators, investigators are exploring use of CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats associated with Cas9 nuclease technology). This novel approach to the treatment of CF uses a protein-RNA complex that identifies defective CFTR DNA. The defective DNA is removed and replaced with a normal sequence. In 2016, the Cystic Fibrosis Foundation Therapeutics established a collaboration with Editas Medicine to develop a CRISPR-Cas9 therapy that would identify and correct the most common CFTR mutations as well as those mutations not approved for current protein modulator therapy.62
The effects of CFTR modulators on short-term (and in the case of ivacaftor, longer-term) outcome measures present significant challenges regarding design of future clinical trials. First, it may not be ethical or feasible to ask volunteers to discontinue an effective medication to substantiate potential effectiveness of another.63 Second, as current molecular therapy has resulted in robust short-term typical outcome measures, future trials will need other measures of functional improvement and creative designs to demonstrate efficacy.
Conclusion
Over the last century CF has been transformed from a fundamentally unrecognized and misunderstood disease to one that is approaching a cure. Virtually each decade since 1930 has passed with a novel intervention extending the life expectancy of those afflicted with this disorder. By addressing the multiorgan consequences of compromised chloride anion transport with first generation potentiators and correctors, the CF median predicted life expectancy has increased from school-age to the mid-40s. In the near future, early introduction of next generation CFTR protein modulators may, for the first time, offer the CF community a future in which CF is no longer the most common lethal autosomal recessive disease in Caucasian individuals, but a chronic disease with a normal life expectancy.
Acknowledgments
Assistance with table and figure designs was provided by both authors as well as Evelyn Zillmer and Steven E. Lommatzsch.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author declares that there is no conflict of interest.
References
- 1. Peckham D, Littlewood JM. A history of cystic fibrosis. www.cfmedicine.com (2011, accessed 18 June 2018). [Google Scholar]
- 2. Quinton PM. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev 1999; 79: S3–S22. [DOI] [PubMed] [Google Scholar]
- 3. Cystic Fibrosis Foundation. Patient registry 2016 annual data report. Bethesda, MD: Cystic Fibrosis Foundation, 2017. [Google Scholar]
- 4. Andersen DH. Cystic Fibrosis of the pancreas and its relation to celiac disease. Am J Dis Child 1938; 56: 344–399. [Google Scholar]
- 5. Kessler WR, Andersen DH. Heat prostration in fibrocystic disease of the pancreas and other conditions. Pediatrics 1951; 8: 648. [PubMed] [Google Scholar]
- 6. Darling RC, di Sant’Agnese PA, Perera GA, et al. Electrolyte abnormalities of the sweat in fibrocystic disease of the pancreas. Am J Med Sci 1953; 225: 67–70. [PubMed] [Google Scholar]
- 7. Di Sant’agnese PA, Darling RC, Perera GA, et al. Abnormal electrolyte composition of the sweat in cystic fibrosis: clinical significance and relationship to the disease. Pediatrics 1953; 12: 549–563. [PubMed] [Google Scholar]
- 8. Gibson LE, Cooke RE. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics 1959; 23: 545–549. [PubMed] [Google Scholar]
- 9. Grosse S. Newborn screening for cystic fibrosis; Evaluation of benefits and risks and recommendations for state newborn screening programs. MMWR Recomm Rep 2015; 53(RR13): 1–36. [PubMed] [Google Scholar]
- 10. Kerem B, Rommens JM, Buchanan JA, et al. Identification of the cystic fibrosis gene: genetic analysis. Science 1989; 245: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 11. cftr2.org. Accessed December 2018. [Google Scholar]
- 12. Clancy JP, Jain M. Personalized medicine in cystic fibrosis: dawning of a new era. Am J Respir Crit Care Med 2012; 186: 593–597. [DOI] [PubMed] [Google Scholar]
- 13. Boeck MP, De Boeck K. A new era in the treatment of cystic fibrosis: correction of the underlying CFTR defect. Lancet Respir Med 2013; 1: 158–163. [DOI] [PubMed] [Google Scholar]
- 14. Cystic Fibrosis Foundation Patient Registry. 2016 Annual data report. Bethesda, MD: Cystic Fibrosis Foundation, 2016. [Google Scholar]
- 15. Peltz SW, Morsy M, Welch EM, et al. Ataluren as an agent for therapeutic nonsense suppression. Annu Rev Med 2013; 64: 407–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grasemann H. CFTR modulator therapy for cystic fibrosis. N Engl J Med 2017; 377: 2085–2088. [DOI] [PubMed] [Google Scholar]
- 17. Cystic Fibrosis Trust. Antibiotic treatment for cystic fibrosis. 3rd ed. Report of the UK Cystic Fibrosis Trust Antibiotic Working Group, 2009. [Google Scholar]
- 18. Dalemans W, Barbry P, Champigny G, et al. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature 1991; 354: 526–528. [DOI] [PubMed] [Google Scholar]
- 19. Van Goor F, Hadida S, Grootenhuis PDJ, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA 2009; 106: 18825–18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011; 365: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davies JC, Wainwright CE, Canny GJ, et al. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med 2013; 187: 1219–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Boeck K, Munck A, Walker S, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros 2014; 13: 674–680. [DOI] [PubMed] [Google Scholar]
- 23. Moss RB, Flume PA, Elborn JS, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an Arg117His-CFTR mutation: a double-blind, randomised controlled trial. Lancet Respir Med 2015; 3: 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flume PA, Liou TG, Borowitz DS, et al. Ivacaftor in subjects with cystic fibrosis who are homozygous for the F508del-CFTR mutation. Chest 2012; 142: 718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taylor-Cousar JL, Munck A, McKone EF, et al. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med 2017; 377: 2013–2023. [DOI] [PubMed] [Google Scholar]
- 26. Rowe SM, Daines C, Ringshausen FC, et al. Tezacaftor-ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med 2017; 377: 2024–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 2015; 373: 220–231. [DOI] [PubMed] [Google Scholar]
- 28. Drew L, Green O, Villella A, et al. CFTR amplifiers: a new class of CFTR modulator that complements the substrate limitations of other CF therapeutic modalities. C60 All About Cystic Fibrosis 2016; 193: A5574-A. [Google Scholar]
- 29. Farrell PM, White TB, Ren CL, et al. Diagnosis of cystic fibrosis: consensus guidelines from the cystic fibrosis foundation. J Pediatr 2017; 181S: S4–S15.e1. [DOI] [PubMed] [Google Scholar]
- 30. Sawicki GS, McKone EF, Pasta DJ, et al. Sustained benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med 2015; 192: 836–842. [DOI] [PubMed] [Google Scholar]
- 31. Konstan MW, McKone EF, Moss RB, et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med 2017; 5: 107–118. [DOI] [PubMed] [Google Scholar]
- 32. Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med 2010; 363: 1991–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. KALYDECOTM. Kalydeco (ivacaftor) [package insert]. Cambridge, MA: Vertex Pharmaceutical Inc, 2012. [Google Scholar]
- 34. Quittner AL, Modi AC, Wainwright C, et al. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009; 135(6): 1610–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Orkambi (lumacaftor/ivacaftor) [package insert]. Boston, MA: Vertex Pharmaceuticals Inc, 2016. [Google Scholar]
- 36. Wainwright CE, Elborn JS, Ramsey BW, et al. Lumacaftor-Ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 2015. [DOI] [PubMed] [Google Scholar]
- 37. Waters V, Stanojevic S, Atenafu EG, et al. Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J 2012; 40: 61. [DOI] [PubMed] [Google Scholar]
- 38. Bratcher PE, Hunt KC, Pickard K, et al. Positive clinical response to ivacaftor treatment in an individual with the CFTR genotype F508del/V456A. J Cyst Fibros 2018; 18: e9–e10. [DOI] [PubMed] [Google Scholar]
- 39. Durmowicz AG, Witzmann KA, Rosebraugh CJ, et al. Change in sweat chloride as a clinical end point in cystic fibrosis clinical trials: the ivacaftor experience. Chest 2013; 143: 14–18. [DOI] [PubMed] [Google Scholar]
- 40. Boyle MP, Bell SC, Konstan MW, et al. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir Med 2014; 2: 527–538. [DOI] [PubMed] [Google Scholar]
- 41. Milla CE, Ratjen F, Marigowda G, et al. Lumacaftor/Ivacaftor in Patients Aged 6-11 Years with Cystic Fibrosis and Homozygous for F508del-CFTR. Am J Respir Crit Care Med 2017; 195: 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ratjen F, Hug C, Marigowda G, et al. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6-11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. Lancet Respir Med 2017; 5: 557–567. [DOI] [PubMed] [Google Scholar]
- 43. Sagel SD, Khan U, Heltshe SL, et al. Study design and clinical characteristics of subjects enrolled in the CFFT prospect study. Pediatr Pulmonol 2016; 51: S45. [Google Scholar]
- 44. Taylor-Cousar JL, Jain M, Barto TL, Haddad T, et al. Lumacaftor/ivacaftor (LUM/IVA) in patients (pts) with cystic fibrosis (CF) and advanced lung disease homozygous for F508del-CFTR: a 24-week open-label study. J Cyst Fibros 2018; 17: 228–235. [DOI] [PubMed] [Google Scholar]
- 45. Symdeko (tezacaftor/ivacaftor) [package insert]. Boston, MA: Vertex Pharmaceuticals Inc, 2018. [Google Scholar]
- 46. Trimble A, McKinzie C, Terrell M, et al. Measured fetal and neonatal exposure to lumacaftor and ivacaftor during pregnancy and while breastfeeding. J Cyst Fibros 2018; 17: 779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bessonova L, Volkova N, Higgins M, et al. Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor. Thorax 2018; 73: 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Taylor-Cousar JL, Jain M, Barto TL, et al. Lumacaftor/ivacaftor in patients with cystic fibrosis and advanced lung disease homozygous for F508del-CFTR. J Cyst Fibros 2018; 17: 228–235. [DOI] [PubMed] [Google Scholar]
- 49. Symdeko (tezacaftor/ivacaftor and ivacaftor) Initiation Guide. Boston, MA: Vertex Pharmaceuticals Incorporated, 2018. [Google Scholar]
- 50. Graeber SY, Dopfer C, Naehrlich L, et al. Effects of lumacaftor-ivacaftor therapy on cystic fibrosis transmembrane conductance regulator function in Phe508del homozygous patients with cystic fibrosis. Am J Respir Crit Care Med 2018; 197: 1433–1442. [DOI] [PubMed] [Google Scholar]
- 51. Angelis A, Tordrup D, Kanavos P. Socio-economic burden of rare diseases: a systematic review of cost of illness evidence. Health Policy 2015; 119: 964–979. [DOI] [PubMed] [Google Scholar]
- 52. Ferkol T, Quinton P. Precision medicine: at what price? Am J Respir Crit Care Med 2015; 192: 658–659. [DOI] [PubMed] [Google Scholar]
- 53. Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: Origins and prospects for reform. JAMA 2016; 316: 858–871. [DOI] [PubMed] [Google Scholar]
- 54. Hughes DA, Tunnage B, Yeo ST. Drugs for exceptionally rare diseases: do they deserve special status for funding? QJM 2005; 98: 829–836. [DOI] [PubMed] [Google Scholar]
- 55. Balk E, Trikalinos T, Mickle K, et al. Modulator treatments for cystic fibrosis: effectiveness and value. Final Evidence Report and Meeting Summary, May 2018. Institute for Clinical and Economic Review, Public Meeting. [Google Scholar]
- 56. Schlander M, Garattini S, Holm S, et al. Incremental cost per quality-adjusted life year gained? The need for alternative methods to evaluate medical interventions for ultra-rare disorders. J Comp Eff Res 2014; 3: 399–422. [DOI] [PubMed] [Google Scholar]
- 57. Elborn S, Elston C, Haworth CS. Letter from CFPMG to Simon Stevens NHS England. In: Stevens S, Executive C, England NHS. (eds) Cystic Fibrosis Trust, 2018. [Google Scholar]
- 58. The availability of Orkambi® (containing lumacaftor and ivacaftor) through the Pharmaceutical Benefits Scheme for patients with cystic fibrosis. Australian Government Department of Health, 2018. [Google Scholar]
- 59. Cohen-Cymberknoh M, Shoseyov D, Breuer O, et al. Treatment of cystic fibrosis in low-income countries. Lancet Respir Med 2016; 4: 91–92. [DOI] [PubMed] [Google Scholar]
- 60. Proteostasis announces positive data from ongoing phase 1 study of PTI-801 in cystic fibrosis patients on background Orkambi® therapy. Proteostasis Therapeutics, Inc., 2018. http://ir.proteostasis.com/news-releases/newsrelease-details/proteostasis-announces-positive-dataongoing-phase-1-study-pti
- 61. Cystic Fibrosis Foundation. Drug development pipeline. Bethesda, MD: Cystic Fibrosis Foundation, 2018. [Google Scholar]
- 62. Lancastre J. CRISPR/Cas9 approach for cystic fibrosis treatment. Cystic Fibrosis News Today January 16, 2017. https://cysticfibrosisnewstoday.com/crisprcas9-approach-forcystic-fibrosis/
- 63. VanDevanter DR, Mayer-Hamblett N, Boyle M. Feasibility of placebo-controlled trial designs for new CFTR modulator evaluation. J Cyst Fibros 2017; 16: 496–498. [DOI] [PubMed] [Google Scholar]