Abstract
Without stringent criteria, liver transplantation for hepatocellular carcinoma (HCC) can lead to high cancer recurrence and poor prognosis in the current treatment context. Checkpoint inhibitors can lead to long survival by targeting coinhibitory pathways and promoting T-cell activity; thus, they have great potential for cancer immunotherapy. Therapeutic modulation of cosignaling pathways may shift paradigms from surgical prevention of recurrence to oncological intervention. Herein, we review the available evidence from a therapeutic perspective and focus on immune microenvironment perturbation by immunosuppressants and checkpoint inhibitors. Partial and reversible interleukin-2 signaling blockade is the mainstream strategy of immunosuppression for graft protection. Programmed cell death protein 1 (PD-1) is abundantly expressed on human liver allograft-infiltrating T-cells, which proliferate considerably after programmed death-ligand 1 (PD-L1) blockade. Clinically, checkpoint inhibitors are used in heart, liver, and kidney recipients with various cancers. Rejection can occur after checkpoint inhibitor administration through acute T-cell-mediated, antibody-mediated, or chronic allograft rejection mechanisms. Nevertheless, liver recipients may demonstrate favorable responses to treatment for HCC recurrence without rejection. Pharmacodynamically, substantial degrees of receptor occupancy can be achieved with lower doses, with favorable clinical outcomes. Manipulation of the immune microenvironment is a therapeutic niche that balances seemingly conflicting anticancer and graft protection needs. Additional translational and clinical studies emphasizing the comparative effectiveness of signaling networks within the immune microenvironment and conducting overall assessment of the immune microenvironment may aid in creating a therapeutic window and benefiting future liver recipients with HCC recurrence.
Keywords: hepatocellular carcinoma, immunotherapy, liver transplantation, microenvironment
Introduction
The management of hepatocellular carcinoma (HCC) recurrence after liver transplantation is an unmet need in therapeutics. This is because under immunosuppression, cancer develops early during the post-transplant period and has a higher chance of extrahepatic spreading, particularly if the pretransplant HCC status exceeds Milan or University of California San Francisco criteria.1–3 In this scenario, locoregional therapy, which is the first-line therapeutic choice for recurrent HCC in nontransplant patients, may be ineffective; thus, effective management strategies are urgently required.4 In liver recipients with disseminated HCC recurrence, sorafenib confers survival benefits but is associated with considerable drug toxicity.5 Most immunotherapies for organ transplantation are intended to achieve sufficient immunosuppression to prevent organ rejection or limit autoreactivity without impairing the host’s ability to protect against opportunistic infections and malignancies. Thus, patients with new or recurrent malignancies after transplantation often have a relatively low chance of undergoing another surgery; however, in these patients, the effects of other treatment approaches may be nonsignificant.6 The development of systemic therapy with sustained effectiveness is required urgently.
Cancer immunotherapy modulates the immune system to fight cancer. It includes adoptive cell transfer [chimeric antigen receptor (CAR)-T-cell engineering, T-cell receptor, and tumor-infiltrating lymphocytes], immune checkpoint inhibitors [programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1) inhibitor and CTLA-4 inhibitor], cancer vaccine, and general immunotherapy [interleukins (ILs), interferons, and colony stimulating factors]. Selective upregulation of B7-H1 and the resulting B7-H1/PD-1-mediated T-cell dysfunction in the tumor microenvironment were found to have major roles in impairing spontaneous immune responses and immunotherapy efficacy; thus, a conceptual breakthrough has occurred in understanding the limitations of immune responses to cancer.7,8 Therefore, checkpoint inhibitors have become a major treatment option for cancers, including HCC, across different anatomic sites of origin. Thus, among cancer immunotherapies, immune checkpoint inhibitors have great potential because they may provide substantially longer disease-free survival than other current target therapies, such as sorafenib.9–12 Checkpoint inhibitors currently approved as systemic treatments for HCC include nivolumab and pembrolizumab. In the phase I/II study of nivolumab, in which 30% of patients were sorafenib-experienced, the objective response rate was 20% in patients treated with 3 mg/kg nivolumab in the dose-expansion phase and 15% in the dose-escalation (0.1–10 mg/kg) phase, but the exact dose–response relationship was not clear in the latter phase.7 In the single-arm phase II trial of pembrolizumab, a 17% objective response rate was reported, including complete response in 1% and partial responses in 16% sorafenib-experienced patients.8 However, no biomarker in both studies could predict potential responders before checkpoint inhibitor therapy was initiated.
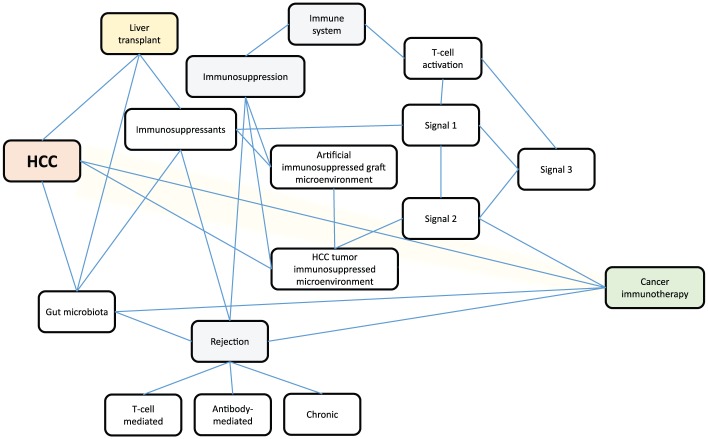
In the transplantation setting, patients with HCC are already under immunotherapy, which generates an artificially immunosuppressive microenvironment for graft protection against rejection or other immune-mediated damage.13 Although rejection is a major concern of checkpoint inhibitor therapeutics in transplant oncology, we aimed to concisely review this strategy from the transplantation perspective and elucidate approaches to modify the immune microenvironment for graft protection and tumor suppression by modulating cosignaling pathways to achieve patient survival. The schematic of the conceptual framework for this review is depicted in Figure 1.
Figure 1.
Schematic of the conceptual framework for immunotherapy in liver recipients with hepatocellular carcinoma. The three-signal model of T-cell activation: signal 1: antigen-specific (MHC/HLA-TCR/CD3) signaling; signal 2: cosignaling pathways; signal 3: IL-2-CD25/IL-2R signaling. Cosignaling pathways (including costimulatory and coinhibitory signals) are signals that accompany signal 1 to determine the final fate of T-cell activation. Optimal T-cell effector function requires costimulatory signals, and coinhibitory molecules contribute to immune suppression and exhaustion. Downstream pathways of complete T-cell activation include the IL-2-calcineurin pathway, the RAS-mitogen activated protein kinase pathway, and the IKK-NF-κB pathway.
HCC, hepatocellular carcinoma; IL, interleukin; NF-κB, nuclear factor kappa B
CD3, cluster of differentiation 3
HLA, human leukocyte antigen
IKK, I kappa B kinase
MHC, major histocompatibility complex
RAS, rat sarcoma virus
TCR, T cell receptor
Immunosuppressants create an artificially immunosuppressive microenvironment for graft protection in transplantation unless clinical tolerance develops
The major clinical immunosuppressants used for liver recipients are calcineurin inhibitors, which act dose-dependently and reversibly by partially blocking the IL-2 signaling pathway, which is critical for final T-cell activation. For graft protection in clinical transplantation, calcineurin inhibitors limit the activation of the immune system and thus antigen presentation, resembling in vivo partial T-cell anergy.13 Chimerism can be observed in liver transplant recipients.14,15 The recipient DNA in post-transplant liver biopsy specimens increased after liver transplantation as early as 1 week, peaked at approximately 30–40 weeks, and was detectable 63 weeks after transplantation.15 Moreover, most recipient-derived cells showed macrophage/Kupffer cell differentiation, and only up to 1.6% of recipient-derived cells in the liver grafts demonstrated hepatocytic differentiation.15 Although graft tolerance is the immunological holy grail in transplantation, it may not correlate with chimerism.16 The major barrier to operational tolerance is the occurrence of allograft rejection, mostly mediated by effector T-cells.17 Cosignaling pathways (detailed in Figure 1) coordinated by costimulatory and coinhibitory molecules are critical to optimal T-cell effector function.18
The PD-1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) pathways contribute to the immune tolerance of a transplanted organ,19 and the PD-1/PD-L1 pathway is critical in maintaining liver transplant tolerance in animal models.20–22 In a human study, PD-L1 was expressed by hepatocytes, cholangiocytes, and cells along the sinusoids in post-transplant liver allografts, and PD-1 was abundantly expressed on allograft-infiltrating T-cells.22 Moreover, PD-L1 blockade-enhanced the allogeneic proliferative responses of these T-cells, and the interplay between donor- and recipient-PD-1-regulated rejection activity.23 Although a cosignaling pathway is the intermediate stage in the three-signal model [signal 1 (antigen recognition, HLA-TCR/CD3), signal 2 (costimulation), and signal 3 (cytokine priming)] for T-cell activation,13 PD-L1 blockade-enhanced allogeneic proliferative responses of graft-infiltrating T-cells may lead to breakthrough rejection under the low maintenance dosage of immunosuppressants in the transplant population undergoing anti-PD therapy for cancer. In summary, major clinical immunosuppressants target signal 1, and cancer immunotherapy targets signal 2.
Liver transplantation is a curative strategy for HCC: patient selection is the primary key to preventing post-transplant recurrence
HCC can be successfully managed through liver transplantation provided that the appropriate criteria are met to predict low extrahepatic dissemination risk before transplantation.1,17 In previous studies, only 10% of patients meeting the Milan criteria showed HCC recurrence after liver transplantation, with high cure rates.2,24 Many other criteria to further expand the inclusion of transplant candidates have been developed based on regional experiences; of them, few are superior to the Milan criteria.17 HCC recurred in many liver transplantation patients who did not meet these criteria.24 Moreover, the clinical course progressed rapidly even under current treatment modalities for nontransplant HCC patients.25
The immunosuppressant load might determine cancer recurrence.26,27 Tumor-induced inflammation and reduced anticancer immune defense, expressed as a disturbed T-regulatory–CD8 lymphocyte balance, are responsible for increased recurrence after liver transplantation.28 In addition, immunosuppressant drugs may stimulate cancer cell growth, accelerating tumorigenesis.25 The strategy of minimizing immunosuppression, mainly through calcineurin inhibitors, should be explored in the expanding field of transplant oncology.29 Minimization strategies are justified by the intrinsic immunosuppressed status of cancer patients and the immunological privilege of the liver, which enables substantial reduction in the immunosuppressant load without compromising patient or graft survival.30–32 By contrast, mammalian target of rapamycin (mTOR) inhibitors interfere with carcinogenesis by inhibiting the PI3K/Akt/mTOR pathway, the key regulator of cell proliferation and angiogenesis.33,34 mTOR inhibiters are clinically applied for preventing transplant rejection (lower recommended dose, as they target signal 3) and for cancer treatment (higher recommended dose).35 The combination of either sirolimus or everolimus with reduced-dose tacrolimus is well tolerated and effective in reducing recurrence.5,36–38 However, there is inadequate evidence for this combination to recommend the optimal serum level of tacrolimus.5 Whether increased exposure to mTOR inhibitors in liver recipients already exhibiting recurrent HCC exerts net survival benefits requires further investigation.36–38 As the broadening of HCC indications for liver transplantation becomes the current trend in transplant oncology, minimized and individualized immunosuppressive strategies incorporating cosignaling pathway modulation (e.g. anti-PD therapy) are essential for managing HCC recurrence. In summary, accumulating evidence supports the contribution of immunosuppressants or costimulatory pathway modulation to T-cell activity, creating a therapeutic niche for the management of post-transplant HCC.
Post-transplant HCC recurrence: add-on immunosuppressive microenvironment
The immunosuppressive microenvironment of HCC is attributable to the abundant expression of immune checkpoint molecules, such as CTLA-4, PD-1, TIM3, lymphocyte-activating gene 3 protein, and B- and T-cell attenuator39–41; alterations in molecules and cellular pathways involved in antigen processing and presentation; and hypoxia-induced cytokine/chemokines [e.g. IL-10, transforming growth factor (TGF)-β, and arginase] and immunosuppressive molecules (e.g. PD-1 and PD-L1) from HCC and stromal cells that attract regulatory T-cells, cause defects of effector T-cells, and inhibit phagocytosis.39,42–44 Cancer stem cells are a small subset of cancer cells with high capacity for self-renewal, differentiation, and tumorigenesis.45 Given their central role in cancer initiation, metastasis, recurrence, and therapeutic resistance, liver cancer stem cells constitute a therapeutic opportunity for achieving cure and preventing the relapse of HCC.45 Studies have reported that 28–50% of HCC cells express progenitor cell markers such as CK7 and CK19, suggesting that at least a portion of HCC cells have characteristics intermediate between progenitors and differentiated mature hepatocytes.45,46 Moreover, cancer stem cells, if ever present in the microenvironment and their occurrence is rare in the nontransplantation setting,45 often express a lower level of major histocompatibility complex class I molecules than do bulk tumor cells47 and exhibit enriched PD-L1 expression through glycosylation regulation by the epithelial–mesenchymal transition/β-catenin/STT3/PD-L1 signaling axis48; this facilitates the immune escape of these cells. Responsiveness to checkpoint blockade immunotherapy is favorable when a local CD8+ T-cell-based immune response occurs in the tumor microenvironment.49 Accumulating evidence is indicating that the activation of oncogenic pathways in tumor cells can impair the induction of local antitumor immune responses.49 For instance, WNT-β-catenin signaling reduces T-cell recruitment, MYC function gain inhibits T-cell activation and infiltration, and PTEN loss reduces efficient T-cell priming.49 As an immunosuppressive microenvironment has already established for HCC cells and stromal cells,39 artificial immunosuppression achieved by partial T-cell activation suppression for liver graft protection leads to a more complex HCC tumor immunological microenvironment. Whether the selective modification of the tumor microenvironment can restore near-normal anticancer immunity while preventing graft rejection warrants further investigation. In summary, advocating immune capability to discriminate the microenvironment between HCC and liver allograft would be key to successful immunotherapy in this transplantation population.
Remote modulation of gut microbiota in liver transplant oncology
Accumulating evidence is suggesting that gut microbiota play remote roles in the liver microenvironment in transplantation, rejection, and HCC.50–53 During liver transplantation, fecal microbial communities, such as those of Actinobacillus, Escherichia, and Shigella, demonstrate a substantial decrease, whereas those of Micromonosporaceae, Desulfobacterales, Sarcina (Eubacteriaceae), and Akkermansia demonstrate a considerable increase.50 In patients with acute T-cell-mediated rejection, Bacteroides, Enterobacteriaceae, Streptococcaceae, and Bifidobacteriaceae increased, but Enterococ-caceae, Lactobacillaceae, Clostridiaceae, Rumino-coccaceae, and Peptostreptococcaceae decreased.51 Compared with healthy controls, patients with early HCC demonstrated fewer butyrate-producing bacteria but more lipopolysaccharide-producing bacteria.52 Intervention with probiotics shifts the gut microbial community abundance toward certain beneficial bacteria, including Prevotella and Oscillibacter, producers of anti-inflammatory metabolites. This subsequently reduces Th17 polarization and promotes the differentiation of anti-inflammatory Treg/Tr1 cells in the gut; this in turn alters proinflammatory cytokine levels in the extra-intestinal tumor HCC microenvironment.52 Furthermore, human studies have suggested that intestinal microbiota not only play a role in carcinogenesis but also determine the efficacy of chemotherapy and immune checkpoint inhibitors.53–56 For instance, increased microbial diversity, irrespective of species identity, was associated with improved responses to checkpoint inhibitors in humans.54,57 Furthermore, patients treated with antibiotics during the course of therapy had decreased antitumor responses.54 In summary, during decision making for therapeutic strategies in transplant oncology in the future, gut microbial modulation may ensure favorable responses to immunotherapy.
Immunotherapeutics of HCC in the transplantation setting: is rejection the bottom line?
The importance of modulating cosignaling pathways is being recognized in transplant oncology. Selective enhancement of antitumor immunity without graft rejection is the primary contemporary goal. The 4-1BB/4-1BBL blockade is an inducible costimulatory pathway and a major component of CAR-T-cell engineering, which has a central role in CAR-mediated T-cell activation and subsequent tumor clearance. This blockade has potentially lower impact on solid organ transplant outcomes than pathway blocking.58 The experimental therapeutic strategy involving the targeted delivery of PD-1-blocking single-chain variable fragments by CAR-T-cells can enhance antitumor efficacy in vivo and may provide another promising approach in the transplantation setting.59 The combination of immunosuppressants (for graft protection) with cosignaling modulation (for HCC control) can be considered a strategy in the transplantation settings. Targeting mTOR pathways in combination with PD-1 blockade may increase antitumor efficacy in cancer.60 The mechanism involves the binding of PD-1 to the downstream mTOR effectors eukaryotic initiation factor 4E and ribosomal protein S6, resulting in the promotion of their phosphorylation.60 Combining IL-2 treatment with PD-1 blockade has considerable synergistic effects in enhancing virus-specific CD8+ T-cell responses and reducing the viral load.61 Therefore, combined IL-2 therapy and PD-L1 blockade may be considered a regimen for treating chronic infections and cancer.61 However, the target of calcineurin inhibition is the IL-2 signaling pathway. Thus, the aforementioned strategy should be applied very cautiously in transplant oncology. Moreover, inhibiting calcineurin using cyclosporine A increases PD-1 ligand expression in B-cells. PD-1high B-cells are an immunosuppressive cell type specifically induced in the HCC microenvironment.62 Anti-CD20 antibody (clinically used for preventing and treating antibody-mediated rejection) can be used to transiently reduce these cells and attenuate the immunosuppressive microenvironment contributed to by PD-1high B-cells.63 Experimental models of transplantation in both mouse and nonhuman primates have revealed that CD28-mediated signal blockade impairs the generation of donor-specific antibody, the presence of which is a prerequisite for antibody-mediated rejection.64–66 Furthermore, transcriptome analyses before and during nivolumab therapy revealed increases in distinct immune cell subsets, activation of specific transcriptional networks, and more pronounced upregulation of immune checkpoint genes in melanoma patients exhibiting responses to nivolumab therapy.67 When managing multiple medications targeting different immune druggable nodes, maintaining a balance is the key to therapeutic success in transplant oncology. In summary, meticulous titration of immune composition would achieve optimal patient outcomes in this field.
Dose optimization (low dose but within the therapeutic window) of anti-PD therapy as a strategy in transplant oncology
Anti-PD therapy doses lower than the recommended dose may be a practical solution for partially meeting the transplant oncology needs. Pharmacodynamic data of 39 patients with various cancers who received anti-PD-1 therapy indicated a sustained mean occupancy of PD-1 molecules of more than 70% on circulating T-cells at least 2 months after infusion, regardless of the dose.68 Most studies on anti-PD therapeutic dose selection have investigated non-small cell lung cancer.69,70 Peripheral receptor occupancy was saturated at the nivolumab dose of ⩾0.3 mg/kg, with no apparent relationship between tumor shrinkage rate and exposure.69 Dosing of nivolumab and PD-L1 expression do not seem to lead to inferior overall survival.70 The KEYNOTE-010 study reported no difference in the efficacy of 2 and 10 mg/kg pembrolizumab; thus, the United States Food and Drug Administration approved a lower dose of 2 mg/kg, enough to achieve antitumor activity, such that further dose increases were not necessary.71 Thus, in summary, low-dose (but still within the therapeutic window) anti-PD therapy might be a feasible strategy, similar to the minimization strategy of immunosuppressants, in clinical situations where rejection is a major concern.
Real-world experiences
Checkpoint inhibitors for cancers, such as melanoma, cutaneous squamous cell cancer, non–small-cell lung cancer, HCC, and duodenal cancer,18,72–77 have been used in heart, liver, and kidney recipients. Rejection can occur through acute T-cell-mediated, antibody-mediated, or chronic allograft rejection mechanisms.72–74 CTLA-4 inhibitors, generally deemed less tolerable than PD-1 inhibitors, are associated with a significantly lower risk of allograft rejection than regimens containing a PD-1 inhibitor.75 From limited reported HCC cases, one patient showed responses to nivolumab and demonstrated 10-month survival without graft rejection.76 However, in Munker and colleagues’ review of 14 liver transplant recipients treated with immune checkpoint inhibitors, graft rejection was reported in four cases, and in three cases, rejection occurred within 3 weeks since the initiation of therapy, with lethal outcomes.76 Factors potentially affecting allograft rejection risk and treatment responses include the more integral role of the PD-1 pathway (compared with the CTLA-4 pathway) in organ acceptance, sequential implementation of different immune checkpoint inhibitor classes, time from transplantation to therapy, strength of immunosuppressive agents to prevent organ transplant rejection, and immunogenicity of the particular organ grafted.19,77 However, additional relevant studies are needed before a concrete conclusion can be drawn. Notably, in addition to other rejection, immune-mediated hepatitis can occur in the liver graft after checkpoint inhibitor therapy.78 In summary, a precision medicine approach involving cautious assessment of individualized rejection risk must be implemented before initiating immunotherapy against HCC in liver recipients.
Conclusions and future perspectives: toward overall immune assessment
Before formulating management strategies for liver recipients with HCC, the overall assessment of the immune microenvironment is essential. Donor immune cells (such as natural killer cells, natural killer T-cells, and lymphocytes) within liver graft are transplanted along with the graft into recipients,79 and numerous cellular interactions and alternate binding partners characterize and complicate T-cell costimulatory pathways at the graft site. Further detailed understanding of the kinetics, cellular distribution, binding partners, and intracellular signaling networks of cosignaling molecules in alloimmunity may aid in the rational development of immunomodulatory strategies to prolong graft survival.77 A therapeutic window for manipulation of cosignaling pathways in transplant recipients with cancer would enable the suppression of alloreactivity toward graft rejection while maintaining tumor-specific protective immune responses. Optimization of costimulation blockade-based regimens, including immunosuppressants, during and after transplantation could widely benefit liver recipients with HCC.77 Table 1 summarizes potential application of major immunotherapeutic approaches for HCC in liver transplant recipients considering specificity, advantages, and limitations.80
Table 1.
Summary of potential application of immunotherapeutic approaches for HCC in liver transplant recipients: advantages and limitations.76
| Immunotherapy for HCC | Specificity to kill HCC | Advantage | Limitation | Current clinical approval in nontransplant setting |
|---|---|---|---|---|
| Checkpoint inhibitor | No | High safety, simple administration, durable response | Lack biomarkers predicting responders | Yes |
| Adoptive cell therapy | ||||
| CAR T-cell | Yes | High efficacy | Lack HCC-associated tumor-specific antigens, risk of on-target, off-tumor toxicities | No |
| Other cells (cytokine-induced killer cells, tumor-infiltrating lymphocytes, natural killer cells) | No | Unclear | Difficulty of relevant immune cell extraction | No |
| Vaccine | ||||
| Tumor vaccine | Maybe yes | Unclear | Lack HCC-associated tumor-specific antigens | No |
| Dendritic cell vaccine | Maybe yes | Potent capacity of antigen presenting, safety | Unclear | No |
| Oncolytic virus | Yes | High efficacy | Safety | No |
CAR, chimeric antigen receptor; HCC, hepatocellular carcinoma.
Clinical and translational studies on the comparative effectiveness of immune perturbations, particularly cosignaling networks, are necessary for the rational formulation of therapeutics in transplant oncology. For instance, triple maintenance immunosuppression (calcineurin inhibitor + mycophenolate mofetil + corticosteroids) can efficiently block activation-induced upregulation of CD25 in CD8+ T-cells, but not CD4+ T-cells.81 Another proof-of-concept example is that the ICOS/B7-H2 pathway is secondary to the CD28/B7 pathway in costimulating T-cell-mediated delayed-type hypersensitivity in mice, suggesting a functional hierarchy of CD28/B7 and ICOS/B7-H2 pathways and enabling the delineation of their relative contributions to costimulate T-cell immune responses.82 Clinically, advancing age protects against acute cellular rejection.83–85 Clinically guided minimization of immunosuppression is possible and safe.86 Compared with grafts from deceased donors, lower acute T-cell-mediated rejection rates are noted after liver transplantation between biologically related living-donor–recipient pairs.87 Therefore, in long-term surviving, elderly liver recipients with HCC, the risk of rejection under anti-PD therapy may be not substantial. In principle, translational studies should establish relevant therapeutic agents and combination strategies most likely to achieve patient benefits based on solid mechanistic and clinical justifications,24 generating effective immunotherapeutic interventions with real-world benefits in clinical care.
Footnotes
Funding: This work was supported by the National Taiwan University Hospital (NTUH 108-S4162) and the Ministry of Science and Technology, Taiwan (MOST 106-2314-B-002-148-MY2).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Cheng-Maw Ho  https://orcid.org/0000-0003-1874-7615
https://orcid.org/0000-0003-1874-7615
Contributor Information
Cheng-Maw Ho, Department of Surgery, National Taiwan University Hospital and College of Medicine, 7 Chung-Shan South Road, Taipei 100, Taiwan.
Hui-Ling Chen, Hepatitis Research Center, National Taiwan University Hospital, Taipei, Taiwan.
Rey-Heng Hu, Department of Surgery, National Taiwan University Hospital and College of Medicine, Taipei College of Medicine, National Taiwan University, Taipei, Taiwan.
Po-Huang Lee, Department of Surgery, National Taiwan University Hospital and College of Medicine, Taipei College of Medicine, National Taiwan University, Taipei, Taiwan.
References
- 1. Ho CM, Lee PH, Chen CL, et al. Long-term outcomes after resection versus transplantation for hepatocellular carcinoma within UCSF criteria. Ann Surg Oncol 2012; 19: 826–833. [DOI] [PubMed] [Google Scholar]
- 2. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334: 693–699. [DOI] [PubMed] [Google Scholar]
- 3. Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33: 1394–1403. [DOI] [PubMed] [Google Scholar]
- 4. Ho CM, Lee PH, Shau WY, et al. Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: comparative effectiveness of treatment modalities. Surgery 2012; 151: 700–709. [DOI] [PubMed] [Google Scholar]
- 5. Au KP, Chok KSH. Multidisciplinary approach for post-liver transplant recurrence of hepatocellular carcinoma: a proposed management algorithm. World J Gastroenterol 2018; 24: 5081–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu M, Guo W, Zhang S. Cancer immunotherapy in patients with new or recurrent malignancies after liver transplantation. Int J Surg Oncol (NY) 2017; 2: e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8: 793–800. [DOI] [PubMed] [Google Scholar]
- 8. Kim TK, Herbst RS, Chen L. Defining and understanding adaptive resistance in cancer immunotherapy. Trends Immunol 2018; 39: 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018; 19: 940–952. [DOI] [PubMed] [Google Scholar]
- 11. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 12. Schrag D, Basch E. Oncology in transition: changes, challenges, and opportunities. JAMA 2018; 320: 2203–2204. [DOI] [PubMed] [Google Scholar]
- 13. Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med 2004; 351: 2715–2729. [DOI] [PubMed] [Google Scholar]
- 14. Alexander SI, Smith N, Hu M, et al. Chimerism and tolerance in a recipient of a deceased-donor liver transplant. N Engl J Med 2008; 358: 369–374. [DOI] [PubMed] [Google Scholar]
- 15. Ng IO, Chan KL, Shek WH, et al. High frequency of chimerism in transplanted livers. Hepatology 2003; 38: 989–998. [DOI] [PubMed] [Google Scholar]
- 16. Starzl TE, Lakkis FG. The unfinished legacy of liver transplantation: emphasis on immunology. Hepatology 2006; 43: S151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho CM, Lee PH, Cheng WT, et al. Succinct guide to liver transplantation for medical students. Ann Med Surg (Lond) 2016; 12: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ansari AW, Khan MA, Schmidt RE, et al. Harnessing the immunotherapeutic potential of T-lymphocyte co-signaling molecules in transplantation. Immunol Lett 2017; 183: 8–16. [DOI] [PubMed] [Google Scholar]
- 19. Kittai AS, Oldham H, Cetnar J, et al. Immune checkpoint inhibitors in organ transplant patients. J Immunother 2017; 40: 277–281. [DOI] [PubMed] [Google Scholar]
- 20. Morita M, Fujino M, Jiang G, et al. PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am J Transplant 2010; 10: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riella LV, Paterson AM, Sharpe AH, et al. Role of the PD-1 pathway in the immune response. Am J Transplant 2012; 12: 2575–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ono Y, Perez-Gutierrez A, Nakao T, et al. Graft-infiltrating PD-L1hi cross-dressed dendritic cells regulate antidonor T cell responses in mouse liver transplant tolerance. Hepatology 2018; 67: 1499–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi XL, Mancham S, Hansen BE, et al. Counter-regulation of rejection activity against human liver grafts by donor PD-L1 and recipient PD-1 interaction. J Hepatol 2016; 64: 1274–1282. [DOI] [PubMed] [Google Scholar]
- 24. Flynn MJ, Sayed AA, Sharma R, et al. Challenges and opportunities in the clinical development of immune checkpoint inhibitors for hepatocellular carcinoma. Hepatology 2019; 69; 2258–2270. [DOI] [PubMed] [Google Scholar]
- 25. Yokoyama I, Carr B, Saitsu H, et al. Accelerated growth rates of recurrent hepatocellular carcinoma after liver transplantation. Cancer 1991; 68: 2095–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vivarelli M, Cucchetti A, La Barba G, et al. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrence. Ann Surg 2008; 248: 857–862. [DOI] [PubMed] [Google Scholar]
- 27. Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol 2013; 59: 1193–1199. [DOI] [PubMed] [Google Scholar]
- 28. Cescon M, Bertuzzo VR, Ercolani G, et al. Liver transplantation for hepatocellular carcinoma: role of inflammatory and immunological state on recurrence and prognosis. World J Gastroenterol 2013; 19: 9174–9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lerut J, Iesari S, Foguenne M, et al. Hepatocellular cancer and recurrence after liver transplantation: what about the impact of immunosuppression? Transl Gastroenterol Hepatol 2017; 2: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodríguez-Perálvarez M, De la Mata M, Burroughs AK. Liver transplantation: immunosuppression and oncology. Curr Opin Organ Transplant 2014; 19: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olson JC, Wiesner RH. Immunomodulating therapy in liver transplantation: principles and practice. Immunotherapy 2012; 4: 793–805. [DOI] [PubMed] [Google Scholar]
- 32. Lerut JP, Pinheiro RS, Lai Q, et al. Is minimal, [almost] steroid-free immunosuppression a safe approach in adult liver transplantation? Long-term outcome of a prospective, double blind, placebo-controlled, randomized, investigator-driven study. Ann Surg 2014; 260: 886–891. [DOI] [PubMed] [Google Scholar]
- 33. Andrassy J, Graeb C, Rentsch M, et al. mTOR inhibition and its effect on cancer in transplantation. Transplantation 2005; 80(Suppl.): S171–S174. [DOI] [PubMed] [Google Scholar]
- 34. Mazzola A, Costantino A, Petta S, et al. Recurrence of hepatocellular carcinoma after liver transplantation: an update. Future Oncol 2015; 11: 2923–2936. [DOI] [PubMed] [Google Scholar]
- 35. Zheng Y, Jiang Y. mTOR inhibitors at a glance. Mol Cell Pharmacol 2015; 7: 15–20. [PMC free article] [PubMed] [Google Scholar]
- 36. Vivarelli M, Dazzi A, Zanello M, et al. Effect of different immunosuppressive schedules on recurrence-free survival after liver transplantation for hepatocellular carcinoma. Transplantation 2010; 89: 227–231. [DOI] [PubMed] [Google Scholar]
- 37. Zhou J, Wang Z, Wu ZQ, et al. Sirolimus-based immunosuppression therapy in liver transplantation for patients with hepatocellular carcinoma exceeding the Milan criteria. Transplant Proc 2008; 40: 3548–3553. [DOI] [PubMed] [Google Scholar]
- 38. Jeng LB, Lee SG, Soin AS, et al. Efficacy and safety of everolimus with reduced tacrolimus in living-donor liver transplant recipients: 12-month results of a randomized multicenter study. Am J Transplant 2018; 18: 1435–1446. [DOI] [PubMed] [Google Scholar]
- 39. Nishida N, Kudo M. Immunological microenvironment of hepatocellular carcinoma and its clinical implication. Oncology 2017; 92(Suppl. 1): 40–49. [DOI] [PubMed] [Google Scholar]
- 40. Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009; 15: 971–979. [DOI] [PubMed] [Google Scholar]
- 41. Shi F, Shi M, Zeng Z, et al. PD-1 and PD-L1 upregulation promotes CD8 (+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer 2011; 128: 887–896. [DOI] [PubMed] [Google Scholar]
- 42. Willimsky G, Schmidt K, Loddenkemper C, et al. Virus-induced hepatocellular carcinomas cause antigen-specific local tolerance. J Clin Invest 2013; 123: 1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumor-associated macrophages inhibits phagocytosis and tumor immunity. Nature 2017; 545: 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2015; 12: 681–700. [DOI] [PubMed] [Google Scholar]
- 45. Wang N, Wang S, Li MY, et al. Cancer stem cells in hepatocellular carcinoma: an overview and promising therapeutic strategies. Ther Adv Med Oncol 2018; 10: 1758835918816287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mishra L, Banker T, Murray J, et al. Liver stem cells and hepatocellular carcinoma. Hepatology 2009; 49: 318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Desai A, Yan Y, Gerson SL. Concise Reviews: cancer stem cell targeted therapies: toward clinical success. Stem Cells Transl Med. Epub ahead of print 17 October 2018. DOI: 10.1002/sctm.18-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hsu JM, Xia W, Hsu YH, et al. STT3-dependent PD-L1 accumulation on cancer stem cells promotes immune evasion. Nat Commun 2018; 9: 1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat Rev Cancer 2018; 18: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sun LY, Yang YS, Qu W, et al. Gut microbiota of liver transplantation recipients. Sci Rep 2017; 7: 3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kato K, Nagao M, Miyamoto K, et al. Longitudinal analysis of the intestinal microbiota in liver transplantation. Transplant Direct 2017; 3: e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ren Z, Li a, Jiang J, et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. Epub ahead of print 25 July 2018. DOI: 10.1136/gutjnl-2017-315084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li J, Sung CY, Lee N, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci USA 2016; 113: E1306–E1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359: 91–97. [DOI] [PubMed] [Google Scholar]
- 55. Schramm C. Bile Acids, the microbiome, immunity, and liver tumors. N Engl J Med 2018; 379: 888–890. [DOI] [PubMed] [Google Scholar]
- 56. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 2019; 19; 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018; 359: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kean LS, Turka LA, Blazar BR. Advances in targeting co-inhibitory and co-stimulatory pathways in transplantation settings: the Yin to the Yang of cancer immunotherapy. Immunol Rev 2017; 276: 192–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rafiq S, Yeku OO, Jackson HJ, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol 2018; 36: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li H, Li X, Liu S, et al. Programmed cell death-1 (PD-1) checkpoint blockade in combination with a mammalian target of rapamycin inhibitor restrains hepatocellular carcinoma growth induced by hepatoma cell-intrinsic PD-1. Hepatology 2017; 66: 1920–1933. [DOI] [PubMed] [Google Scholar]
- 61. West EE, Jin HT, Rasheed AU, et al. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest 2013; 123: 2604–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ou X, Xu S, Li YF, et al. Adaptor protein DOK3 promotes plasma cell differentiation by regulating the expression of programmed cell death 1 ligands. Proc Natl Acad Sci USA 2014; 111: 11431–11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ren Z, Peng H, Fu Y-X. PD-1 shapes B cells as evildoers in the tumor microenvironment. Cancer Discov 2016; 6: 477–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen J, Yin H, Xu J, et al. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA-4Ig. Am J Transplant 2013; 13: 2280–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ford ML, Koehn BH, Wagener ME, et al. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med 2007; 204: 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim EJ, Kwun J, Gibby AC, et al. Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am J Transplant 2014; 14: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Riaz N, Havel JJ, Makarov V, et al. Tumor and microenvironment evolution during immunotherapy with Nivolumab. Cell 2017; 171: 934–949.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28: 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Agrawal S, Feng Y, Roy A, et al. Nivolumab dose selection: challenges, opportunities, and lessons learned for cancer immunotherapy. J Immunother Cancer 2016; 4: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yoo SH, Keam B, Kim M, et al. Low-dose nivolumab can be effective in non-small cell lung cancer: alternative option for financial toxicity. ESMO Open 2018; 3: e000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 72. Alhamad T, Venkatachalam K, Linette GP, et al. Checkpoint inhibitors in kidney transplant recipients and the potential risk of rejection. Am J Transplant 2016; 16: 1332–1333. [DOI] [PubMed] [Google Scholar]
- 73. Tanaka K, Albin MJ, Yuan X, et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J Immunol 2007; 179: 5204–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Friend BD, Venick RS, McDiarmid SV, et al. Fatal orthotopic liver transplant organ rejection induced by a checkpoint inhibitor in two patients with refractory, metastatic hepatocellular carcinoma. Pediatr Blood Cancer 2017; 64: e26682. [DOI] [PubMed] [Google Scholar]
- 75. Chae YK, Galvez C, Anker JF, et al. Cancer immunotherapy in a neglected population: the current use and future of T-cell-mediated checkpoint inhibitors in organ transplant patients. Cancer Treat Rev 2018; 63: 116–121. [DOI] [PubMed] [Google Scholar]
- 76. Munker S, De Toni EN. Use of checkpoint inhibitors in liver transplant recipients. United European Gastroenterol J 2018; 6: 970–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ford ML. T cell cosignaling molecules in transplantation. Immunity 2016; 44: 1020–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jennings JJ, Mandaliya R, Nakshabandi A, et al. Hepatotoxicity induced by immune checkpoint inhibitors: a comprehensive review including current and alternative management strategies. Expert Opin Drug Metab Toxicol 2019; 15; 231–244. [DOI] [PubMed] [Google Scholar]
- 79. Freitas-Lopes MA, Mafra K, David BA, et al. Differential location and distribution of hepatic immune cells. Cells 2017; 6: E48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xie Y, Xiang Y, Sheng J, et al. Immunotherapy for hepatocellular carcinoma: current advances and future expectations. J Immunol Res 2018; 2018: 8740976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chen CC, Koenig A, Saison C, et al. CD4+ T Cell help is mandatory for naive and memory donor-specific antibody responses: impact of therapeutic immunosuppression. Front Immunol 2018; 9: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wong SC, Tan AH, Lam KP. Functional hierarchy and relative contribution of the CD28/B7 and ICOS/B7-H2 costimulatory pathways to T cell-mediated delayed-type hypersensitivity. Cell Immunol 2009; 256: 64–71. [DOI] [PubMed] [Google Scholar]
- 83. Kueht ML, Cotton RT, Galvan NT, et al. Profiling immunologic risk for acute rejection in liver transplantation: recipient age is an important risk factor. Transpl Immunol 2016; 38: 44–49. [DOI] [PubMed] [Google Scholar]
- 84. Au KP, Chan SC, Chok KS, et al. Clinical factors affecting rejection rates in liver transplantation. Hepatobiliary Pancreat Dis Int 2015; 14: 367–373. [DOI] [PubMed] [Google Scholar]
- 85. Wiesner RH, Demetris AJ, Belle SH, et al. Acute hepatic allograft rejection: incidence, risk factors, and impact on outcome. Hepatology 1998; 28: 638–645. [DOI] [PubMed] [Google Scholar]
- 86. Charlton M, Levitsky J, Aqel B, et al. International Liver Transplantation Society Consensus Statement on immunosuppression in liver transplant recipients. Transplantation 2018; 102: 727–743. [DOI] [PubMed] [Google Scholar]
- 87. Abu-Gazala S, Olthoff KM. Status of adult living donor liver transplantation in the United States: results from the adult-to-adult living donor liver transplantation cohort study. Gastroenterol Clin North Am 2018; 47: 297–311. [DOI] [PubMed] [Google Scholar]