Abstract
Functional bowel disorders, including irritable bowel syndrome (IBS), are a chronic condition that can significantly reduce patients’ quality of life. Therefore, this paper will review the roles of a low fermentable oligosaccharides, disaccharides, monosaccharides, and polypols (FODMAP) diet in treating IBS, particularly in an Asian setting. About 20% of the general population is diagnosed with IBS. However, there are limited effective medical therapies available for treating IBS. Therefore, IBS presents a major challenge to the health‐care providers. Recently, there is an increasing interest in the use of a diet low in FODMAP for the treatment of IBS. A low FODMAP diet can decrease the delivery of readily fermentable substrates to the small intestine and colon, thereby improving functional gastrointestinal symptoms.
Keywords: Asian; fermentable oligosaccharides, disaccharides, monosaccharides, and polypols; gastrointestinal; irritable bowel syndrome
Introduction
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder1 worldwide, with significant effects on quality of life (QOL). The prevalence ranges from 10 to 20% in the West,2 including some parts of Asia and Malaysia, and largely depends on diagnostic criteria used.3 The prevalence is generally higher in South America than in Southeast Asia and is higher among women than men.4, 5
Up to now, the pathogenesis of IBS is not exactly known, although postulated mechanisms included visceral hypersensitivity,6 intestinal dysmotility,7 altered gut microbiota,8 low‐grade bowel inflammation,9 altered gut–brain axis,10 and psychosocial factors.11 IBS is usually identified symptomatically, including abdominal pain, bloating, diarrhea, and constipation after exclusion of organic diseases.12 Currently, the diagnosis of IBS is based on the recently revised Rome IV criteria (Table 1).13, 14
Table 1.
Rome IV criteria for the diagnosis of IBS
| Rome IV criteria† |
|---|
Recurrent abdominal pain on average at least 1 day per week in the last 3 months associated with two or more of the following:
|
These criteria fulfilled in the last 3 months with symptom onset at least 6 months prior to diagnosis.
IBS, irritable bowel syndrome.
Methods
Management of IBS is largely symptom‐based, but in the recent years, as we understand the disease better, certain disease‐modifying interventions, including diets that contain low fermentable oligosaccharides, disaccharides, monosaccharides, and polypols (FODMAP), have been suggested.15 In this review, we discussed the role of diet in IBS, issues of existing interventions in IBS, and how the low FODMAP diet changes the management landscape of IBS particularly in Asia. An electronic literature search was conducted using Medline (OvidSP), PubMed, Cochrane CENTRAL, and Web of Science. Additional articles were further identified from references in the retrieved articles. The search was restricted to articles in English that addressed the review of our scope.
The rise of IBS in Asia may be due to changing diet across the globe
Based on a meta‐analysis, the pooled global prevalence of IBS was approximately 11.2% but can range from 1.1 to 45% depending on countries and diagnostic criteria used.16 The prevalence of IBS is generally higher in North America and Western Europe (between 3 and 32%) than in Asia (between 5 and 10%).17, 18, 19 However, in recent years, the prevalence of IBS in Asia, including Malaysia, has seen an increase to a proportion similar to the West.3 The reason for this rise in Asia is not entirely clear, but the increase is more common in the urban community, and therefore, it may reflect the rapid socioeconomic development seen in Asia.19, 20
The pathogenesis of IBS remains incompletely understood to date, but environmental factors are probably one of the most important factors. Dietary pattern has drastically changed due to accessibility across the globe, including Asia, and such changes are closely linked with socioeconomic development. More than half (63%) of patients with IBS associated their symptoms with foods, especially ones that are rich in carbohydrates and fats.21 IBS patients also reported higher frequencies of eating refined and processed foods, including canned food, processed meat, fruits, legumes, and drinking tea.22, 23
In addition, exacerbation of IBS symptoms has been shown to be associated with intolerance or allergies to certain foods.24 About a third (27%) of IBS patients were lactose intolerant, and 39% of them demonstrated improvement in symptoms after a low‐lactose diet.25 Another example is gluten or wheat, reported both in the West and in some Asian countries.20 The overlap between IBS and gluten‐related disorders like celiac disease (CD) has been identified for many years. Studies showed that about 5% of patients with IBS were also diagnosed with CD, and patients with CD may conversely present with typical IBS symptoms.26 In recent years, nonceliac gluten sensitivity (NCGS) or nonceliac wheat protein sensitivity has also been increasingly reported in patients with IBS. Studies showed that the IBS symptoms were presented in the majority of patients with NCGS, and about 30% of patients with IBS suffered from wheat sensitivity.27 Food allergy that involves IgE‐mediated immune responses was hypothesized as the mechanism that underlies food‐precipitated IBS; however, the evidence remains weak.20
Dysbiosis in the gut microbiota has been shown to cause IBS, for example, exposure of environmental microbiota had led to the development of IBS after a major flood disaster.28 On the other hand, diet is an important determinant of gut microbiota abundance and diversity, especially in early life, where it may impact health and diseases in later life,29 including IBS.30, 31
Current pharmacological strategies are not effective in treating IBS
Symptom‐directed therapy remains the main treatment option in IBS. Antispasmodics, including smooth muscle relaxants, anticholinergic agents, and calcium channel blockers, are effective for abdominal pain but have other side effects.20 Similarly, laxatives (e.g. lactulose) and prokinetics (e.g. prucalopride) are often used in constipation‐predominant IBS; however, diarrhea can be an undesirable side effect.20, 32 Psychotropic agents like antidepressants are widely prescribed in primary care in the United States, but they are poorly tolerable, with other side effects that often lead to poor compliance.33
On the other hand, as we understand the pathogenesis of IBS better, especially with regard to the role of gut microbiota, newer approaches directed to pathogenesis will be more helpful. Probiotics is an attractive approach as there is evidence to indicate its effectiveness in dysbiosis, and it is generally safe except in immunocompromised states. Exact mechanisms are not entirely known but may include altering microbiota composition, enhancing the mucosal barrier function, and regulating the gut inflammatory response.34, 35 Several clinical studies of single probiotic strains, such as Bifidobacteria or Lactobacilli, have been shown to reduce global symptoms and to improve QOL in IBS,36, 37 but a mixture of strains was also effective.35 Despite the evidence, a consensus of Asian experts casts doubts on the beneficial effects of probiotics in IBS largely because of modest treatment effect, small study sample sizes, and because not all probiotics have demonstrated benefits in IBS.38
How does FODMAP affect IBS?
Given the close association between foods and IBS, dietary modification is considered an attractive nonpharmacological intervention of IBS. Developing a regular eating habit and reducing the intake of alcohol, caffeine, spicy foods, and fat are generally recommended to patients, but such advice does not usually work.39 On the other hand, the introduction of low FODMAP diet in IBS has turned out to be a more effective strategy.40, 41
What is FODMAP?
FODMAP, an acronym, was first described by Gibson and Shepherd from Melbourne, Australia, in a 2005 article on the relationship between Western diets and Crohn's disease.42 It is a group of short‐chain carbohydrates including oligosaccharides (e.g. fructans), disaccharides (e.g. lactose), monosaccharides (e.g. fructose), and polyols (e.g. sorbitol) (Fig. 1).43, 44 FODMAP is less absorbable by the intestine due to a lack of brush border enzymes or the presence of low‐capacity epithelial transporters, such as GLUT‐2, and are highly fermentable by gut microbiota into short‐chain fatty acids (SCFAs), including acetate, propionate, and butyrate. As a result, FODMAP increases water volume and intestinal gas production and promotes intestinal motility. In addition, it can increase calcium absorption, lower serum cholesterol and triacylglycerols levels, and modify gut immune function.43, 44
Figure 1.
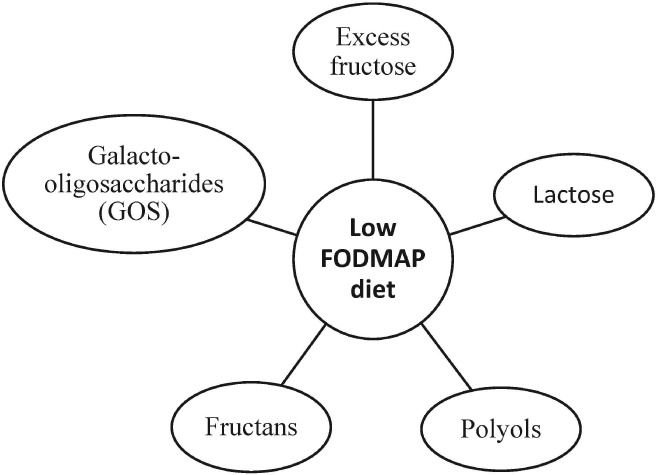
Low fermentable oligosaccharides, disaccharides, monosaccharides, and polypols (FODMAP) diet includes a reduction of the five main subgroups of carbohydrates.
How does FODMAP affect IBS symptoms?
Different molecules in FODMAP tend to have overlapping but heterogeneous effects on IBS symptoms based on their molecular size and absorption characteristics. It is generally believed that two possible mechanisms are involved. The first mechanism relates to osmotic molecules like fructose and polyols, of which the osmotic activity of these molecules in the small intestine lead to net secretion of fluids that result in luminal distension and increased water delivery to the large intestine causing diarrhea (Fig. 2).20, 41 For example, Murray et al. studied the differential effects of fructose and fructan (inulin) on luminal water and gas through magnetic resonance imaging in healthy volunteers.45 Their results showed that inulin produced more gas than fructose but had no effects on small bowel water content.45 Another study by Undseth et al. showed that small bowel water content was increased after ingestion of unabsorbed lactulose, more in the IBS than the control group. In addition, more symptoms were significantly provoked by lactulose in IBS than in healthy controls.46 A study by Ong et al. reported that gas production, as measured by the hydrogen breath test, decreased with restriction of FODMAP molecules in both IBS patients and healthy subjects.47 Moreover, in a randomized, placebo‐controlled rechallenge trial, the induction of IBS symptoms was significantly seen in patients after consuming FODMAP compared with glucose in a cumulative and dose‐dependent fashion.48
Figure 2.
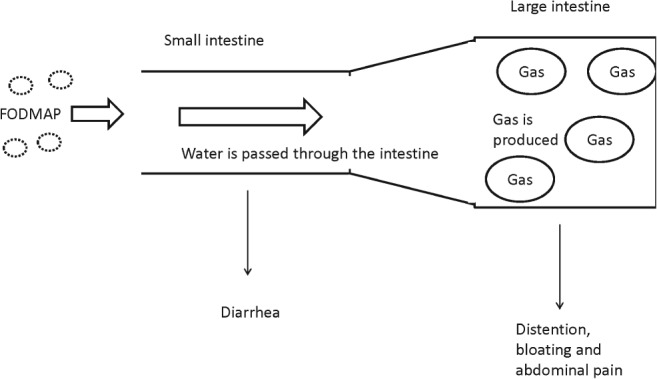
How does fermentable oligosaccharides, disaccharides, monosaccharides, and polypols (FODMAP) affect IBS symptoms?
The second mechanism relates to the rapid fermentation of FODMAP by the colonic microbiota from the delivery of unabsorbed FODMAP molecules in the small bowel. The fermented molecules led to increased gas production (hydrogen and/or methane) that causes bloating and also increased SCFAs that result in intestinal dysmotility.49 In addition, patients with IBS are known to have higher sensitivity and lower tolerance to bowel distension from fermented FODMAP molecules.50
Does the low FODMAP diet alter colonic microbiota in IBS?
Acting as substrates for bacterial fermentation, the FODMAP diet has been shown to alter gut bacterial composition/diversity and abundance.49, 51 Fructans and oligomates have ‘prebiotic’ effects, with abilities to stimulate the growth and activity of putatively beneficial colonic bacteria, such as Bifidobacteria and Lactobacilli. The study by Staudacher et al. was the first to show a significant reduction in luminal Bifidobacteria abundance after 4 weeks of low FODMAP diet in IBS.51 Furthermore, another study by Halmos et al., through a randomized controlled crossover trial, reported that IBS patients on low FODMAP versus standard diets had a significant reduction in the abundance of Clostridium coccoides and Akerkmanisa muciniphila but relative increase in abundance of Ruminococcus torques. 52 As A. muciniphila was a good bacterium, while R. torques was an unfavorable mucus‐consuming bacterium, such alterations caused by low FODMAP diet in the long run may be detrimental to health. However, the actual clinical significance remains unclear due to a lack of long‐term studies.
How do we manage IBS with low FODMAP diet?
Evidence of effectiveness of low FODMAP diet in Asian IBS
Low FODMAP diet has achieved widespread application and positive effects across Western countries, while it is relatively new and less studied in Asian regions. The British Dietetic Association recommends a low FODMAP diet as the second‐line intervention in dietary management of IBS.53 According to the latest Asian Consensus on IBS, the main recommendations for dietary modification include restriction of certain food such as dairy products, chili, and fructose, but no specific diet was recommended.54
Although several clinical studies have reported consistent symptomatic improvement of IBS with low FODMAP,41, 55, 56 high‐quality studies are sparse; most are retrospective, uncontrolled, and with a small number of participants.51 Halmos et al. conducted a randomized controlled, single‐blind crossover study where 70% of IBS subjects were shown to report lower severity of symptoms after low FODMAP diet for 21 days.57 With its robust experimental design and high degree of dietary adherence, the study can be considered high‐quality evidence supporting the role of low FODMAP diet being a first‐line therapy for IBS. In another multicenter, parallel, single‐blind study of 75 adult patients by Bohn et al. comparing the effect of low FODMAP diet with traditional dietary advice, low FODMAP diet reduced IBS symptoms compared with traditional IBS dietary advice.58 However, it may be difficult to apply in free‐living adults as such a dietary restriction has varying degrees of compliance. A meta‐analysis by Marsh et al. supported the role of low FODMAP diet in reducing severity of abdominal pain, bloating, and overall symptoms in IBS,55 and likewise, similar findings were also reported in a more recent meta‐analysis by Varju et al. with more studies and standardized outcome measurements (e.g. IBS symptom severity score) included.56 However, studies of low FODMAP diet in children with IBS are limited. Chumpitazi et al. conducted a pilot study59 followed by a randomized study in children (7 and 16 years old) with IBS,60 and their results showed that low FODMAP diet significantly reduced the frequency of abdominal pain. Moreover, the responders tended to be enriched with bacteria of greater saccharolytic capacity, such as Bacteroids and Ruminococcaceae.
How to apply low FODMAP in clinical practice
The implementation of the low FODMAP diet in clinical practice is primarily led by qualified dietitians and generally consists of three stages: FODMAP restriction, FODMAP reintroduction, and FODMAP personalization.61 The essential principle of applying a low FODMAP diet is to replace the foods with high FODMAP content with those of low FODMAP content within the same food group and do not reduce the dietary quality. Therefore, the knowledge of FODMAP content is the key to the success of a low FODMAP diet. At the beginning, dietitians educate patients on the knowledge of the FODMAP composition, provide them with some lists of suitable foods and unsuitable foods based on FODMAP composition, and help them make diet plans (Fig. 3). The strict adherence of low FODMAP diet usually lasts for 4–6 weeks and is then followed by a closely monitored reintroduction of FODMAP. Generally, dietitians may recommend less troublesome FODMAP first, such as polyol‐containing foods, and one food item is reintroduced at a time until patients are able to achieve an individual tolerance.
Figure 3.
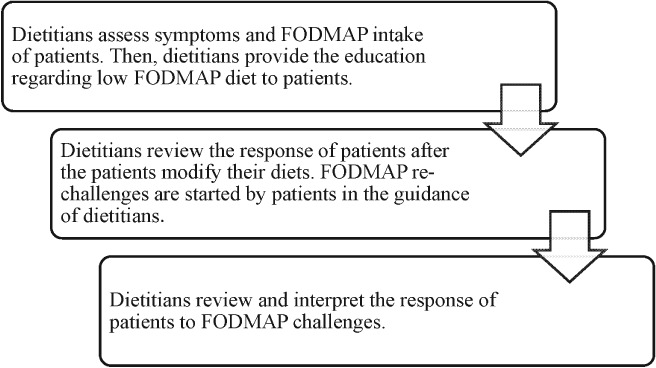
How do we apply low fermentable oligosaccharides, disaccharides, monosaccharides, and polypols (FODMAP) in clinical practice?
What are the issues of low FODMAP diet in Asian setting?
Although most of the studies concerning the effects of low FODMAP diet were performed in Western countries, it is believed that low FODMAP diet has similar efficacy in Asian settings. However, the application of the low FODMAP diet in the Asian setting might be more challenging.62 First, the dietary habits are quite different in Asian countries, which might contribute to FODMAP intake. For example, ‘kimchi’ is a traditional side dish that contains several high FODMAP ingredients such as onion and garlic, but the restriction might be very difficult as such ingredients can be found in nearly every meal in Korea. Second, the current databases of FODMAP content of foods are mostly developed in Western countries, such as The Monash University Low FODMAP Diet App, so the FODMAP contents of some foods that are unique to Asia are unavailable. Finally, the dietitians in Asia may have less knowledge and experience in delivering low FODMAP diet in practices. In addition, because most of the education resources are in English, they are not easily accessible for health professionals in some Asian countries with low English proficiency. A study in Malaysia also indicated that adherence to low FODMAP diet may also be a challenge; thus, this dietary advice requires close follow up and, potentially, patients’ motivation.63
Limitations of low FODMAP diet
There are some limitations of low FODMAP diets, especially in the longer term. First, which exact foods to avoid and replace, how much, and how long to intervene with low FODMAP diet remain unclear. While tests are carried out to compile the contents of specific FODMAP in foods, they are expensive, of long duration, and are mostly focused on Western rather than Asian foods. In addition, the content of FODMAP may vary in different conditions and degrees of maturation (e.g. storage and cooking).64
Second, nutritional inadequacy with a low FODMAP diet has been a concern among doctors and patients. FODMAP exists in many staple foods, including wheat, dairy products, and several types of vegetables and fruits, and this means limited replacement choices for patients who chose to restrict them. In a study comparing the effects of fermentable carbohydrate restriction versus control diet,51 calcium intake was significantly reduced, probably caused by a lower intake of dairy products, while other micronutrients did not show any significant reduction. In addition, a reduced fiber intake in low FODMAP diet may worsen constipation in IBS.65 However, the restriction of FODMAP is not for a lifetime as it is normally just within the first 4–6 weeks during the initial advice.
Finally, patients on dietary restriction may lose the benefits provided by FODMAP. For example, the SCFAs, byproducts of fermented carbohydrates, have several health benefits. SCFAs have been documented to be an important energy source for colonocytes and have effects in inhibiting inflammation, enhancing barrier function and faster cell proliferation, and even lowering the chance of colon cancer.66, 67 In addition, the prebiotic effects provided by fructans and galacto‐oligosaccharide may be affected. However, it is important to remember that other foods also produce SCFA; thus, this might not be a major issue.
Conclusion
A larger number of research studies have considered the epidemiology, pathogenesis, and management of IBS. IBS is believed to be a multifactorial disease, of which the complex pathogenesis has not been understood completely. Many studies have highlighted the potential association between diet, the gut microbiome, and the pathogenesis of IBS, with emerging evidence suggesting the important role of diet modification in the management of IBS. FODMAP is a group of fermentable and poorly absorbed carbohydrates, the consumption of which might exacerbate IBS symptoms by increasing the water content and gas production in the luminal. The low FODMAP diet is therefore considered a potential approach in improving the IBS symptoms. Many clinical studies have supported the efficacy of low FODMAP diet on improving the severity of IBS symptoms, while more high‐quality evidence with larger sample sizes and scientific methodology is still needed to establish a definite conclusion. On the other hand, there are studies concerned that the utilization of low FODMAP diet may lead to negative influences, such as the lack of specific nutrients and dysbiosis of intestinal microbiota. Therefore, studies considering the short‐term effect and safety of low FODMAP diet in Asian settings are needed in the future. Moreover, evidence shows that there might be certain subtypes of IBS patients, such as those enriched in the bacteria with greater saccharolytic capacity, that could respond better to the low FODMAP diet. Therefore, future studies should be conducted to determine the subtype and predict the effect of low FODMAP diet in different subtypes of IBS patients.
Declaration of conflict of interest: None.
References
- 1. Thompson WG, Heaton KW, Smyth GT, Smyth C. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut. 2000; 46: 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006; 130: 1480–91. [DOI] [PubMed] [Google Scholar]
- 3. Lee YY, Waid A, Tan HJ, Chua AS, Whitehead WE. Rome III survey of irritable bowel syndrome among ethnic malays. World J. Gastroenterol. 2012; 18: 6475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soares RL. Irritable bowel syndrome: a clinical review. World J. Gastroenterol. 2014; 20: 12144–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Staudacher HM, Irving PM, Lomer MC, Whelan K. Mechanisms and efficacy of dietary fodmap restriction in ibs. Nat. Rev. Gastroenterol. Hepatol. 2014; 11: 256–66. [DOI] [PubMed] [Google Scholar]
- 6. Accarino AM, Azpiroz F, Malagelada JR. Selective dysfunction of mechanosensitive intestinal afferents in irritable bowel syndrome. Gastroenterology. 1995; 108: 636–43. [DOI] [PubMed] [Google Scholar]
- 7. Kellow JE, Eckersley GM, Jones M. Enteric and central contributions to intestinal dysmotility in irritable bowel syndrome. Dig. Dis. Sci. 1992; 37: 168–74. [DOI] [PubMed] [Google Scholar]
- 8. Lin HC. Small intestinal bacterial overgrowth: a framework for understanding irritable bowel syndrome. J. Am. Med. Assoc. 2004; 292: 852–8. [DOI] [PubMed] [Google Scholar]
- 9. Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut. 2002; 51 (Suppl. 1): i41–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tougas G. The autonomic nervous system in functional bowel disorders. Gut. 2000; 47: iv78–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drossman DA. Personality and psychological factors in the irritable bowel syndrome. Gastroenterol. Clin. Biol. 1990; 14: 49c–53c. [PubMed] [Google Scholar]
- 12. Fass R, Longstreth GF, Pimentel M et al Evidence‐ and consensus‐based practice guidelines for the diagnosis of irritable bowel syndrome. Arch. Intern. Med. 2001; 161: 2081–8. [DOI] [PubMed] [Google Scholar]
- 13. Schmulson MJ, Drossman DA. What is new in rome IV. J. Neurogastroenterol. Motil. 2017; 23: 151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whitehead WE, Palsson OS, Simrén M. Irritable bowel syndrome: what do the new rome iv diagnostic guidelines mean for patient management? Expert Rev. Gastroenterol. Hepatol. 2017; 11: 281–3. [DOI] [PubMed] [Google Scholar]
- 15. Chey WD. Food: the main course to wellness and illness in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2016; 111: 366–71. [DOI] [PubMed] [Google Scholar]
- 16. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta‐analysis. Clin. Gastroenterol. Hepatol. 2012; 10: 712.e714–21.e714. [DOI] [PubMed] [Google Scholar]
- 17. Kang JY. Systematic review: the influence of geography and ethnicity in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2005; 21: 663–76. [DOI] [PubMed] [Google Scholar]
- 18. Choung RS, Locke GR 3rd. Epidemiology of ibs. Gastroenterol. Clin. North Am. 2011; 40: 1–10. [DOI] [PubMed] [Google Scholar]
- 19. Chang FY, Lu CL. Irritable bowel syndrome in the 21st century: perspectives from asia or south‐east asia. J. Gastroenterol. Hepatol. 2007; 22: 4–12. [DOI] [PubMed] [Google Scholar]
- 20. Gwee KA, Ghoshal UC, Chen M. Irritable bowel syndrome in asia: pathogenesis, natural history, epidemiology, and management. J. Gastroenterol. Hepatol. 2018; 33: 99–110. [DOI] [PubMed] [Google Scholar]
- 21. Simren M, Mansson A, Langkilde AM et al Food‐related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001; 63: 108–15. [DOI] [PubMed] [Google Scholar]
- 22. Guo YB, Zhuang KM, Kuang L, Zhan Q, Wang XF, Liu SD. Association between diet and lifestyle habits and irritable bowel syndrome: a case‐control study. Gut Liver. 2015; 9: 649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chirila I, Petrariu FD, Ciortescu I, Mihai C, Drug VL. Diet and irritable bowel syndrome. J. Gastrointestin. Liver Dis. 2012; 21: 357–62. [PubMed] [Google Scholar]
- 24. Bokic T, Storr M, Schicho R. Potential causes and present pharmacotherapy of irritable bowel syndrome: an overview. Pharmacology. 2015; 96: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parker TJ, Woolner JT, Prevost AT, Tuffnell Q, Shorthouse M, Hunter JO. Irritable bowel syndrome: is the search for lactose intolerance justified? Eur. J. Gastroenterol. Hepatol. 2001; 13: 219–25. [DOI] [PubMed] [Google Scholar]
- 26. Wahnschaffe U, Ullrich R, Riecken EO, Schulzke JD. Celiac disease‐like abnormalities in a subgroup of patients with irritable bowel syndrome. Gastroenterology. 2001; 121: 1329–38. [DOI] [PubMed] [Google Scholar]
- 27. De Giorgio R, Volta U, Gibson PR. Sensitivity to wheat, gluten and fodmaps in ibs: facts or fiction? Gut. 2016; 65: 169–78. [DOI] [PubMed] [Google Scholar]
- 28. Yusof N, Hamid N, Ma ZF et al Exposure to environmental microbiota explains persistent abdominal pain and irritable bowel syndrome after a major flood. Gut Pathog. 2017; 9: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee YY, Hassan SA, Ismail IH et al Gut microbiota in early life and its influence on health and disease: a position paper by the malaysian working group on gastrointestinal health. J. Paediatr. Child Health. 2017; 53: 1152–8. [DOI] [PubMed] [Google Scholar]
- 30. Simren M, Barbara G, Flint HJ et al Intestinal microbiota in functional bowel disorders: a rome foundation report. Gut. 2013; 62: 159–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kassinen A, Krogius‐Kurikka L, Makivuokko H et al The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007; 133: 24–33. [DOI] [PubMed] [Google Scholar]
- 32. Jiang C, Xu Q, Wen X, Sun H. Current developments in pharmacological therapeutics for chronic constipation. Acta Pharm. Sin. B. 2015; 5: 300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ladabaum U, Boyd E, Zhao WK et al Diagnosis, comorbidities and management of irritable bowel syndrome in patients in a large health maintenance organization. Clin. Gastroenterol. Hepatol. 2012; 10: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee BJ, Bak Y‐T. Irritable bowel syndrome, gut microbiota and probiotics. J. Neurogastroenterol. Motil. 2011; 17: 252–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Korpela R, Niittynen L. Probiotics and irritable bowel syndrome. Microb. Ecol. Health Dis. 2012; 23 10.3402/mehd.v23i0.18573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whorwell PJ, Altringer L, Morel J et al Efficacy of an encapsulated probiotic bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am. J. Gastroenterol. 2006; 101: 1581–90. [DOI] [PubMed] [Google Scholar]
- 37. O'Mahony L, McCarthy J, Kelly P et al Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005; 128: 541–51. [DOI] [PubMed] [Google Scholar]
- 38. Gwee KA, Lee WW, Ling KL et al Consensus and contentious statements on the use of probiotics in clinical practice: a south east asian gastro‐neuro motility association working team report. J. Gastroenterol. Hepatol. 2018; 33: 1707–16. [DOI] [PubMed] [Google Scholar]
- 39. Cozma‐Petrut A, Loghin F, Miere D, Dumitrascu DL. Diet in irritable bowel syndrome: what to recommend, not what to forbid to patients! World J. Gastroenterol. 2017; 23: 3771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barrett JS. How to institute the low‐fodmap diet. J. Gastroenterol. Hepatol. 2017; 32: 8–10. [DOI] [PubMed] [Google Scholar]
- 41. Nanayakkara WS, Skidmore PM, O'Brien L, Wilkinson TJ, Gearry RB. Efficacy of the low fodmap diet for treating irritable bowel syndrome: the evidence to date. Clin. Exp. Gastroenterol. 2016; 9: 131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gibson PR, Shepherd SJ. Personal view: food for thought – western lifestyle and susceptibility to Crohn's disease. The fodmap hypothesis. Aliment. Pharmacol. Ther. 2005; 21: 1399–409. [DOI] [PubMed] [Google Scholar]
- 43. Borghini R, Donato G, Alvaro D, Picarelli A. New insights in ibs‐like disorders: pandora's box has been opened; a review. Gastroenterol. Hepatol. Bed Bench. 2017; 10: 79–89. [PMC free article] [PubMed] [Google Scholar]
- 44. Catassi G, Lionetti E, Gatti S, Catassi C. The low fodmap diet: many question marks for a catchy acronym. Nutrients. 2017; 9: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Murray K, Wilkinson‐Smith V, Hoad C et al Differential effects of fodmaps (fermentable oligo‐, di‐, mono‐saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am. J. Gastroenterol. 2014; 109: 110–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Undseth R, Berstad A, Klow NE, Arnljot K, Moi KS, Valeur J. Abnormal accumulation of intestinal fluid following ingestion of an unabsorbable carbohydrate in patients with irritable bowel syndrome: an MRI study. Neurogastroenterol. Motil. 2014; 26: 1686–93. [DOI] [PubMed] [Google Scholar]
- 47. Ong DK, Mitchell SB, Barrett JS et al Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2010; 25: 1366–73. [DOI] [PubMed] [Google Scholar]
- 48. Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo‐controlled evidence. Clin. Gastroenterol. Hepatol. 2008; 6: 765–71. [DOI] [PubMed] [Google Scholar]
- 49. Tuck CJ, Muir JG, Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols: role in irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol. 2014; 8: 819–34. [DOI] [PubMed] [Google Scholar]
- 50. Harder H, Serra J, Azpiroz F, Malagelada J‐R. Intestinal gas distribution determines abdominal symptoms. Gastroenterology. 2001; 120: A72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Staudacher HM, Lomer MC, Anderson JL et al Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 2012; 142: 1510–18. [DOI] [PubMed] [Google Scholar]
- 52. Halmos E, Christophersen C, Bird A. The low fodmap diet alters the composition of the colonic microbiota compared to a typical australian intake in patients with irritable bowel syndrome: a randomised controlled cross‐over trial. J. Gastroenterol. Hepatol. 2013; 28: 122.23034166 [Google Scholar]
- 53. McKenzie YA, Bowyer RK, Leach H et al British Dietetic Association systematic review and evidence‐based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J. Hum. Nutr. Diet. 2016; 29: 549–75. [DOI] [PubMed] [Google Scholar]
- 54. Gwee KA, Bak YT, Ghoshal UC et al Asian consensus on irritable bowel syndrome. J. Gastroenterol. Hepatol. 2010; 25: 1189–205. [DOI] [PubMed] [Google Scholar]
- 55. Marsh A, Eslick EM, Eslick GD. Does a diet low in fodmaps reduce symptoms associated with functional gastrointestinal disorders? a comprehensive systematic review and meta‐analysis. Eur. J. Nutr. 2016; 55: 897–906. [DOI] [PubMed] [Google Scholar]
- 56. Varju P, Farkas N, Hegyi P et al Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (fodmap) diet improves symptoms in adults suffering from irritable bowel syndrome (ibs) compared to standard ibs diet: a meta‐analysis of clinical studies. PLoS One. 2017; 12: e0182942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in fodmaps reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014; 146: 67.e65–75.e65. [DOI] [PubMed] [Google Scholar]
- 58. Bohn L, Storsrud S, Liljebo T et al Diet low in fodmaps reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015; 149: 1399.e1392–407.e1392. [DOI] [PubMed] [Google Scholar]
- 59. Chumpitazi BP, Hollister EB, Oezguen N et al Gut microbiota influences low fermentable substrate diet efficacy in children with irritable bowel syndrome. Gut Microbes. 2014; 5: 165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chumpitazi BP, Cope JL, Hollister EB et al Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low fodmap diet in children with the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015; 42: 418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Whelan K, Martin LD, Staudacher HM, Lomer MCE. The low fodmap diet in the management of irritable bowel syndrome: an evidence‐based review of fodmap restriction, reintroduction and personalisation in clinical practice. J. Hum. Nutr. Diet. 2018; 31: 239–55. [DOI] [PubMed] [Google Scholar]
- 62. Iacovou M, Tan V, Muir JG, Gibson PR. The low fodmap diet and its application in east and southeast asia. J. Neurogastroenterol. Motil. 2015; 21: 459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wong Z, Mok C‐Z, Majid HA, Mahadeva S. Early experience with a low fodmap diet in asian patients with irritable bowel syndrome. JGH Open. 2018; 2: 178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Biesiekierski JR, Rosella O, Rose R et al Quantification of fructans, galacto‐oligosacharides and other short‐chain carbohydrates in processed grains and cereals. J. Hum. Nutr. Diet. 2011; 24: 154–76. [DOI] [PubMed] [Google Scholar]
- 65. Molina‐Infante J, Serra J, Fernandez‐Bañares F, Mearin F. The low‐fodmap diet for irritable bowel syndrome: lights and shadows. Gastroenterol. Hepatol. 2016; 39: 55–65. [DOI] [PubMed] [Google Scholar]
- 66. Blachier F, Beaumont M, Andriamihaja M et al Changes in the luminal environment of the colonic epithelial cells and physiopathological consequences. Am. J. Pathol. 2017; 187: 476–86. [DOI] [PubMed] [Google Scholar]
- 67. Andoh A, Tsujikawa T, Fujiyama Y. Role of dietary fiber and short‐chain fatty acids in the colon. Curr. Pharm. Des. 2003; 9: 347–58. [DOI] [PubMed] [Google Scholar]


