Abstract
Pulmonary inflammation in chronic obstructive pulmonary disease (COPD) is characterized by both innate and adaptive immune responses; however, their specific roles in the pathogenesis of COPD are unclear. Therefore, we investigated the roles of T and B lymphocytes and group 2 innate lymphoid cells (ILC2s) in airway inflammation and remodelling, and lung function in an experimental model of COPD using mice that specifically lack these cells (Rag1−/− and Rorafl/flIl7r Cre [ILC2‐deficient] mice). Wild‐type (WT) C57BL/6 mice, Rag1 −/−, and Rorafl/flIl7r Cre mice were exposed to cigarette smoke (CS; 12 cigarettes twice a day, 5 days a week) for up to 12 weeks, and airway inflammation, airway remodelling (collagen deposition and alveolar enlargement), and lung function were assessed. WT, Rag1 −/−, and ILC2‐deficient mice exposed to CS had similar levels of airway inflammation and impaired lung function. CS exposure increased small airway collagen deposition in WT mice. Rag1 −/− normal air‐ and CS‐exposed mice had significantly increased collagen deposition compared to similarly exposed WT mice, which was associated with increases in IL‐33, IL‐13, and ILC2 numbers. CS‐exposed Rorafl/flIl7r Cre mice were protected from emphysema, but had increased IL‐33/IL‐13 expression and collagen deposition compared to WT CS‐exposed mice. T/B lymphocytes and ILC2s play roles in airway collagen deposition/fibrosis, but not inflammation, in experimental COPD.
Keywords: COPD, emphysema, ILC2s, inflammation, remodelling, T cells
1. INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death globally and is characterized by progressive airway inflammation, emphysema, and reduced lung function.1, 2, 3, 4 Chronic pulmonary inflammation in COPD is underpinned by innate immune responses and primarily involves macrophages (alveolar and interstital), neutrophils, dendritic cells, natural killer cells, and innate lymphoid cells (ILCs).5 –7 This is associated with concomitant increases in the number of adaptive immune cells, primarily infiltrating CD4+ and CD8+ T and B lymphocytes.5, 6
Although limited in number in the lung, ILCs are crucial in bridging innate and adaptive immune responses and in maintaining lung homeostasis.8, 9 They are characterized into three subsets (ILC1, ILC2, ILC3) and are tissue resident lymphocytes that may display considerable plasticity depending on the stimulus.10, 11 In COPD, ILC2s phenotypically shift into ILC1 and ILC3 subsets upon stimulation with IL‐1β, IL‐4, and IL‐1210 and the increased ratio of ILC1:ILC2 directly correlates with a decline in lung function and more severe disease.11 In mouse models of COPD, cigarette smoke (CS) exposure has similar suppressive effects on ILC2s.12 Although the endogenous stimulus that switches ILC subsets and their role in exacerbations of COPD has been studied to some degree,11, 12 the role of ILC2s in the pathogenesis of COPD is poorly understood, particularly their role in airway remodelling and lung function decline.
Along with innate cells, there is an increase in adaptive immune cells, predominatory CD4+ and CD8+ T lymphocytes and B lymphocytes in both the large and peripheral airways in patients with COPD.13, 14, 15, 16 In mice exposed to CS, CD8‐deficient (−/−), but not CD4−/− mice, are protected from CS‐induced hallmark features of COPD, specifically macrophage accumulation, MMP2 and MMP9 activity, and emphysema,17, 18 indicating a key role of CD8+ lymphocytes in disease pathogenesis. In addition, B cells, in particular B cell rich lymphoid follicles, are increased in the severe stages of COPD and are most strongly linked with the emphysematous changes in the lung (reviewed in Ref. 19). Autoantibodies are also evident in the blood and lung samples from COPD patients.20, 21, 22 B cell‐activating factor (BAFF) is significantly increased around immune cells and within lymphoid follicles in COPD patients, and blocking BAFF in a CS‐induced mouse model of COPD reduces inflammation and alveolar destruction.23 Interestingly, mice lacking mature T and B lymphocytes (Rag2−/−), had no change in inflammation and emphysema compared to wild‐type (WT) controls.24 However, these mice developed COPD‐like disease when CD3+ T‐cells from CS‐exposed WT mice were adoptively transferred,24 demonstrating a key role of pathogenic T cells in the development of COPD. Crucially, the roles of T and B cell deficiency on airway remodelling (collagen deposition) and lung function in experimental COPD have not been assessed.
In this study, we further elucidated the roles of T/B lymphocytes and ILC2s in CS‐induced experimental COPD. We first used an established model of experimental COPD25, 26, 27, 28, 29, 30, 31, 32 and WT mice to define subsets of innate lymphoid cells, and ILC2s in Rag1 −/−,33 and then assessed CS‐induced changes in ILC2‐deficient mice (Rorafl/flIl7r Cre). WT, Rag1 −/−, and ILC2‐deficient mice had similar levels of airway inflammation following CS exposure. However, Rag1−/− mice developed spontaneous increases in collagen deposition in the presence or absence of CS, which was associated with increased IL‐33, IL‐13, and ILC2 numbers. ILC2‐deficient mice were protected from CS‐induced emphysema, but had increased collagen deposition, and IL‐33 and IL‐13 expression. Collectively, these data show roles for T and B lymphocytes and ILC2s in CS‐induced airway remodelling and emphysema, but not inflammation, in experimental COPD.
2. METHODS
2.1. Mice
All procedures were approved by the University of Newcastle Animal Care and Ethics Committee.
2.2. Cigarette smoke exposure
WT C57BL/6, Rag1 −/−,33 Rorafl/+Il7r Cre (heterozygous controls), and Rorafl/flIl7r Cre (ILC2‐deficient) mice34 were exposed to the smoke of twelve 3R4F reference cigarettes (University of Kentucky, Lexington, KY) twice per day, 5 times a week for up to 12 weeks, as previously described.25, 26, 27, 28, 29, 30, 31, 32
2.3. Airway inflammation, airway remodelling, and emphysema
Airway inflammation and remodelling as well as emphysema were measured, as previously described.25, 26, 27, 28, 29, 30, 31, 32 Bronchoalveolar lavage fluid (BALF) was obtained by 2 × 0.5 ml PBS washes of the left lobe only via a cannula inserted into the trachea. Airway inflammation was measured by differential enumeration of inflammatory cells with May‐Grunswald staining, as previously described.25, 26, 28 The left lobe was then perfused via the heart and inflated with, and drop‐fixed in formalin, prior to paraffin embedding and sectioning (4 μm thick). Masson's trichrome staining was used to measure collagen deposition and H&E staining for assessment of emphysema‐like alveolar enlargement, as previously described.25, 26
2.4. Qpcr
Lungs were homogenized and total RNA was isolated using TRIzol Reagent (Invitrogen, Life Technologies, Australia). Random‐primed reverse transcriptions were performed using BioScript reverse transcriptase in one times first‐strand buffer according to the manufacturer's instructions (Bioline Pty. Ltd., NSW, Australia). Real‐time qPCR assays were performed with SYBR Green Supermix (KAPA Biosystems, Inc., MA) and a Mastercycler ep realplex2 system (Eppendorf South Pacific Pty. Ltd., NSW, Australia). IL‐33 and IL‐13 gene expression was normalized to the housekeeping gene hypoxanthine guanine phosphoribosyltransferase (Hprt). Hprt: forward 5′‐AGGCCAGACTTTGTTGGATTTGAA‐3′; Hprt: reverse 5′‐CAACTTGCGCTCATCTTAGGCTTT‐3′; Il 33: forward 5′‐CCTCCCTGAGTACATACAATGACC‐3′; Il 33: reverse: 5′‐GTAGTAGCACCTGGTCTTGCTCTT‐3′; Il 13: forward: 5′‐TGCTTGCCTTGGTGGTCT‐3′; and Il 13: reverse: 5′‐GGGGAGTCTGGTCTTGTGTG‐3′.
2.5. ELISA
IL‐33 concentrations in whole lung homogenates were determined using a duoset ELISA kit (R&D Systems, InVitro Technologies, Noble Park, NSW, Australia) according to the manufacturer's instructions.35
2.6. Flow cytometry
Whole lung single cell suspensions were prepared, as previously described,35, 36 and incubated with Fc block (10 ng/ml, BD Biosciences, North Ryde, NSW, Australia; in 2% fetal calf serum in PBS). Cell surface antigens were labelled for the identification of inflammatory cells (ILC1s, ILC2s, ILC3s; Tables 1 and 2). Cells were analyzed on a BD FACSAriaIII and resulting data analyzed using FlowJo software.
Table 1.
Flow cytometry panel for ILC2s
| Antigen | Flurochrome | Company | Clone |
|---|---|---|---|
| CD45 | PerCP‐Cy5.5 | Biolegend | 30‐F11 |
| Lineage | AF700 | Biolegend | |
| CD90.2 | APC‐Cy7 | Biolegend | 30‐H12 |
| IL‐7Rα | BV605 | Biolegend | A7R34 |
| CD25 | APC | Biolegend | PC61 |
| CD2 | FITC | Biolegend | RM2‐5 |
| Rorγt | PE | Biolegend | Q31‐378 |
| GATA3 | PE‐Cy7 | BD | L50‐823 |
| T‐bet | BV421 | BD | 04‐46 |
Table 2.
Flow cytometry panel for ILCs
| Antigen | Fluorochrome | Company | Clone |
|---|---|---|---|
| ILC1, 2, 3 with transcription factors | |||
| Lineage | AF700 | Biolegend | |
| CD45 | PerCP‐Cy5.5 | Biolegend | 30‐F11 |
| CD90.2 | APC‐Cy7 | Biolegend | 30‐H12 |
| IL‐7Rα | BV605 | Biolegend | A7R34 |
| Rorγt | PE | Biolegend | Q31‐378 |
| GATA3 | PE‐Cy7 | BD | L50‐823 |
| T‐bet | BV421 | BD | 04‐46 |
| CD4 | FITC | Biolegend | GK1.5 |
ILC1: Lin‐CD90.2+CD45+IL‐7Rα+T‐bet+; ILC2: Lin‐CD90.2+CD45+IL‐7Rα+GATA3+T‐bet‐Rorγt‐CD4−; ILC3: Lin‐CD90.2+CD45+IL‐7Rα+Rorγt+CD4+/−
2.7. Lung function
Mice were anaesthetized with ketamine/xylazine prior to lung function analysis using forced oscillation techniques and restrained plethysmography with methacholine (MCh) provocation, as previously described.25, 28, 37, 38, 39, 40, 41
2.8. RT2 extracellular matrix (ECM) PCR array
RT2 ECM and adhesion molecule array (Qiagen, Chadstone Centre, VIC, Australia) was performed according to the manufacturer's instructions.
2.9. Statistical analyses
Data are expressed as mean ± sem. Nonnormally distributed data were analyzed using nonparametric equivalents. Comparisons between two groups were made using a two‐tailed Mann‐Whitney test. Multiple comparisons were made using one‐way ANOVA with Tukey's posttest, or the Kruskal‐Wallis test with multiple comparisons for nonparametric analyses. P < 0.05 was considered statistically significant.
3. RESULTS AND DISCUSSION
3.1. Airway remodelling and resistance are increased in association with elevated baseline IL‐33, IL‐13, and ILC2 numbers, but pulmonary inflammation and emphysema‐like alveolar enlargement are unaltered in Rag1−/− mice compared to WT mice with experimental COPD
Mice were exposed to CS for 8 weeks to induce experimental COPD, as we have previously shown.25, 26, 27, 28, 29, 30, 31, 32 CS exposure impaired weight gain (data not shown) and increased total leukocyte numbers (comprised primarily of neutrophils and lymphocytes) in the airways in WT mice, as previously described (Fig. 1A‐D).25, 29, 31 Similar observations were made with Rag1−/− mice. The only exception was that CS‐exposed Rag1−/− mice had reduced numbers of lymphocytes in the BALF compared to CS‐exposed WT mice (Fig. 1D). This is consistent with previous reports in Rag2−/− mice due to a lack of B and T cells.24 The differences between Rag1 and Rag2 relate to their binding to the V(D)J recombination. Depleting either Rag1 or Rag2 has the same effect, which is defective expression of pre‐TCR and pre‐BCR, meaning that Rag1−/− or Rag2−/− mice cannot generate mature T and B cells.42 The remaining lymphocytes in CS‐exposed Rag1−/− mice could potentially be ILCs; however, further flow cytometric analysis of these cells is required. We next assessed the effects of CS exposure on pulmonary remodelling and emphysema‐like alveolar enlargement, and lung function in Rag1−/− mice. CS exposure increased collagen deposition around the small airways in WT mice (Fig. 1E). Collagen deposition was increased around the small airways in CS‐exposed Rag1−/− compared to WT controls. Rag1−/− mice exposed to normal air had spontaneous collagen deposition around some airways, which was intermediate between levels in CS‐exposed WT and Rag1−/− groups (Fig. 1E). CS exposure resulted in similar levels of alveolar enlargement in WT and Rag1−/− mice (Fig. 1F). Airway resistance was increased in Rag1−/− CS‐exposed mice compared to Rag1−/− air‐exposed mice (0.17 ± 0.09 cf. 0.09 ± 0.02 cmH2O.s/mL); however, there was no significant difference compared to WT air and CS‐exposed mice (data not shown). The disconnect between increased collagen deposition and lung function needs further in‐depth study.
Figure 1.
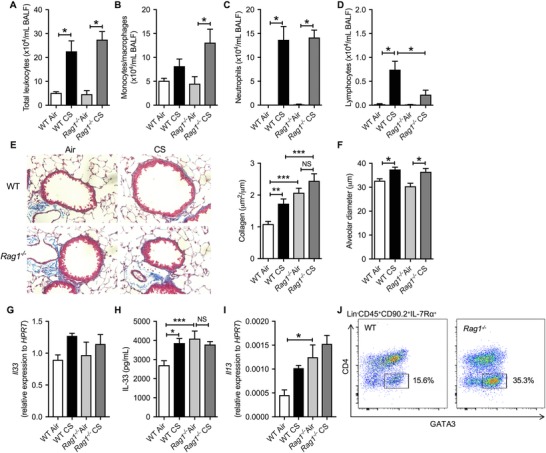
Airway remodelling is increased in association with elevated baseline IL‐33, IL‐13, and ILC2s, but inflammation and emphysema‐like alveolar enlargement are largely unaltered in CS‐exposed Rag1−/− compared to WT mice. Mice were lavaged and (A) total BAL cells, (B) monocytes/macrophages, (C) neutrophils, and (D) lymphocytes were enumerated. (E) Representative images and quantification of collagen, and (F) quantification of alveolar diameter in normal air‐ or CS‐exposed WT and Rag1−/− mice. Whole lung (G) Il33 mRNA expression, (H) IL‐33 protein levels and (I) Il13 mRNA expression. (J) Flow cytometric analysis of ILC2s in normal air‐exposed WT versus Rag1−/− mice. n = 4–6 per group. Data are expressed as mean ± sem. *P < 0.05, **P < 0.01, ***P < 0.001
We next assessed factors and cell types that are known to play roles in airway remodelling, namely, IL‐33, IL‐13, and ILC2s.43, 44 In WT mice, CS exposure increased lung IL 33 mRNA and protein, and IL‐13 mRNA expression compared to normal air‐exposed controls (Fig. 1G‐I). Conversely, there were no increases observed in CS‐exposed Rag1−/− mice compared to their normal air‐exposed controls; however, this was largely due to increased baseline levels of IL‐33 and IL‐13 in the Rag1−/− control mice (Fig. 1H, I). Flow cytometric analysis revealed increases in Lin−CD45+CD90.2+IL‐7Rα+GATA3+ ILC2s in Rag1−/− compared to WT mice at baseline (i.e., without any challenge; Fig. 1J). It is established that IL‐33 can induce the proliferation of ILC2s that leads to increased IL‐13 production and profibrogenic cytokine production resulting in collagen deposition.43 Our observations that IL‐33 protein, IL‐13 mRNA, and ILC2 numbers were all increased in normal air‐exposed Rag1−/− mice suggest that the deficiency in T/B lymphocytes increases innate immune responses involving ILC2s. In CS‐exposed Rag1−/− mice, there were no further increases in collagen deposition, IL‐33 or IL‐13 compared to their normal air‐exposed controls. Nevertheless, CS‐exposed Rag1−/− mice had increased collagen deposition and trends toward increased IL‐13 mRNA levels, but not alveolar enlargement, compared to CS‐exposed WT mice. Our results are consistent with other studies that used Rag2−/− mice, which showed that they are not protected from CS‐induced alveolar enlargement.17, 18 There is little evidence that CD8+‐deficient mice may be protected,24 suggesting a yet to be identified mechanism for the lack of protection in T and B cell deficient mice. In addition, MMP8 mRNA was further increased in Rag1−/− CS‐exposed mice compared to WT CS, and Tgfbi and Timp1 transcript levels were increased in normal air and further increased in CS‐exposed Rag1−/− mice (Supplementary Fig. 1A). MMP8, TGF‐β‐induced, and Timp1 have all been shown to contribute to other fibrotic diseases45, 46, 47; however, their specific role(s) in COPD pathogenesis are unclear. Taken together, these data show that T/B lymphocyte deficiency increases IL‐33/IL‐13/ILC2 responses and MMP8, Tgfbi, and Timp1, which may be associated with increased collagen deposition/fibrosis, but not emphysema‐like changes in experimental COPD.
3.2. Increased lung ILC1/ILC3 and reduced ILC2 numbers occur in WT mice with experimental COPD
Given that the absence of mature T and B lymphocytes in Rag1−/− mice did not protect against cardinal features of COPD, we profiled changes in ILCs following 8 weeks of CS exposure in WT mice. Similar to previously published human severe COPD patient data,10 we found that CS exposure did not alter total lung ILC numbers (Lin−CD90.2+CD45+IL‐7Rα+) in WT mice but increased the proportion of ILC1s and ILC3s and decreasing that of ILC2s (Supplementary Fig. 2A‐E). This is similar to human studies,11, 12 where the total numbers of ILCs in the lung were unaltered by CS exposure. T‐bet+ ILCs (ILC1s) were increased 6‐fold and Rorγt+ ILCs (ILC3s) increased >3‐fold following CS exposure; however, there was no change in IFN‐γ or IL‐17 protein levels in lung tissues (by ELISA, data not shown). Thus, the development of CS‐induced experimental COPD is associated with a phenotypic switch from ILC2s to ILC1s and ILC3s. It is known that CS exposure impairs lung Th2 responses, which is due to reduced ILC responsiveness to IL‐33, and compensatory increases in natural killer cell responses to IL‐33.12
3.3. Pulmonary inflammation is largely unaltered, airway neutrophil numbers are reduced, airway collagen, IL‐33 and IL‐13 expression are increased, and emphysema‐like alveolar enlargement is inhibited in CS‐exposed ILC2‐deficient mice
We then assessed the roles of ILC2s in experimental COPD using mice deficient in ILC2s (Rorafl/flIl7rCre). Normal air‐exposed ILC2‐deficient mice gained significant body weight over 12 weeks compared to their heterozygous and WT controls (data not shown). This is consistent with a previous report that dysfunctional ILC2s can lead to impaired beige adipocyte function and increase weight gain.48
Notably, when exposed to CS ILC2‐deficient mice had equivalent levels of impaired weight gain as CS‐exposed WT controls (data not shown). CS increased total leukocyte numbers in the airways, which was similar in WT and ILC2‐deficient mice (Fig. 2A). These cells were predominately macrophages (Fig. 2B) and neutrophils (Fig. 2C), with fewer neutrophils observed in CS‐exposed ILC2‐deficient compared to WT mice. Lymphocyte numbers were increased in CS‐exposed ILC2‐deficient mice (Fig. 2D). Similar weight and leukocyte numbers trajectories were observed in normal air or CS‐exposed Rorafl/+Il7rCre heterozygous mice compared to WT controls (data not shown). Although the exact mechanisms driving the decrease in neutrophils are yet to be elucidated it is known that IL‐33/ILC2 activation can regulate neutrophil IL‐5 positivity following acute lung injury.49 Thus, mice lacking ILC2s may have impaired regulation and activation of neutrophils. Our study is the first to show that ILC2s play a role in CS‐induced neutrophilic airway inflammation.
Figure 2.
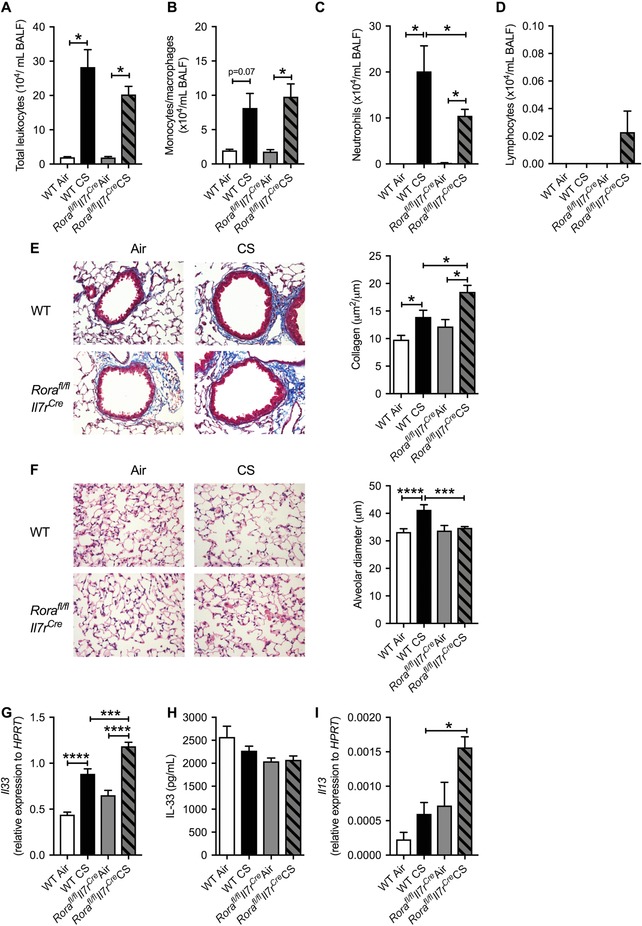
Pulmonary inflammation is largely unaltered, airway neutrophil numbers are reduced, airway remodelling is increased in association with Il33 and Il13 mRNA expression, and emphysema‐like alveolar enlargement is inhibited in ILC2‐deficient mice exposed to CS. Mice were lavaged and (A) total BAL cells, (B) monocytes/macrophages, (C) neutrophils, and (D) lymphocytes were enumerated. Representative images and quantification of (E) collagen and (F) alveolar diameter in normal air‐ or CS‐exposed WT and Rorafl/flIl7rCre (ILC2‐deficient) mice. Whole lung (G) Il33 mRNA expression, (H) IL‐33 protein levels, and (I) Il13 mRNA expression. n = 4–8 per group. Data are expressed as mean ± sem. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
We next assessed pulmonary remodelling in ILC2‐deficient mice. Again, there was increased collagen deposition around the small airways following CS exposure in WT mice (Fig. 2E). CS exposure also increased collagen levels around the small airways in ILC2‐deficient mice, with the level of deposition higher than that observed in CS‐exposed WT mice. CS exposure also induced alveolar enlargement in WT mice whereas ILC2‐deficient mice were completely protected against this major pathologic feature of COPD (Fig. 2F). These results are similar to elastase‐induced emphysematous lung pathology, whereby the absence of ILC‐intrinsic arginase‐1 prevented disease development.8 However, our results are in contrast to a study demonstrating that Nippostrongylus brasiliensis challenge in mice lacking IL‐9, which is crucial for ILC2 survival, increased emphysema compared to WT mice.50 There was no change in airway resistance at baseline (data not shown).
We next assessed the levels of lung IL‐33 and IL‐13.43, 44 CS exposure increased lung Il 33 expression in WT mice, which was further increased in CS‐exposed ILC2‐deficient mice (Fig. 2G‐I); however, there were no differences in lung IL‐33 protein levels. Il13 mRNA expression was significantly increased in CS‐exposed ILC2‐deficient mice compared to CS‐ and normal air‐exposed WT controls. These data suggest that CS‐induced increases in IL‐33, IL‐13, and collagen deposition are independent of ILC2s. Previous studies in asthmatics have shown that IL‐33 can increase IL‐13 through mast cell activation,51 and we previously demonstrated key roles for two mast cell tryptases in the pathogenesis of COPD.25, 28 In addition, MMP8, Tgfbi, and Timp1 levels were increased in CS‐ compared to normal air‐exposed WT and ILC2‐deficient mice. However, there were no additional increases in CS‐exposed ILC2‐deficient compared to WT mice (Supplementary Fig. 1B). Together, our data suggest that in the absence of ILC2s, IL‐33 can induce IL‐13 responses and downstream pro‐fibrotic pathways to increase collagen deposition and emphysema, which may in part be regulated by MMP8, TGFbi, and Timp1. In light of previous reports, these effects may also involve mast cell responses.
3.4. Body weight increase in ILC2‐deficient mice associates with airway hyperresponsiveness
Normal air‐exposed ILC2‐deficient (Rorafl/flIl7rCre) mice gained more weight than WT mice (Fig. 3A). We next assessed whether normal air‐exposed ILC2‐deficient mice had altered lung function in response to airway provocation. ILC2‐deficient mice had airway hyperresponsiveness in response to MCh at 80 mg/ml or higher (Fig. 3B‐C), and increased resistance measured by area under the curve (Fig. 3D) compared to WT controls. These data demonstrate that impaired baseline lung function in normal air‐exposed ILC2‐deficient mice is also associated with increased airway hyperresponsiveness, which may be attributed to increased weight gain. Whereas the roles of ILC2s in other respiratory diseases such as asthma are well established, the impact of depleting ILC2s on lung function parameters has not been assessed. Increased mass loading on the chest wall in obese humans can alter the balance of forces within the respiratory system that result in increased airway resistance and decreased total lung capacity as we found in ILC2‐deficient mice.52 Thus, our data also highlight a potential role for ILC2s in maintaining lung homeostasis.9
Figure 3.
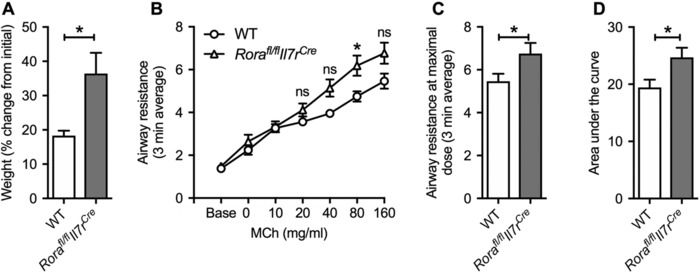
Increased weight and airway hyperresponsiveness occurs in ILC2‐deficient exposed to normal air. (A) Weights of normal air‐exposed WT and Rorafl/flIL7rCre mice. (B) Airway resistance measured in response to increasing doses of methacholine (MCh). (C) Airway resistance at the maximal 160 mg/ml dose of MCh. (D) Area under the curve analysis of airway resistance. n = 4–23 per group. Data are expressed as mean ± sem. **P < 0.01
This study demonstrates important roles for T/B lymphocytes and ILC2s in airway remodelling in COPD. It is already well known that there is a disproportionate increase in collagen and ECM deposition in COPD patients compared to asthmatics, and that this increase contributes to fixed airflow obstruction.53 Our data increases our understanding of the roles of T/B lymphocytes and ILC2s in the pathogenesis of experimental COPD, and highlights the potential for innate and adaptive immune cross‐talk in COPD.
DISCLOSURES
The authors declare no conflicts of interest.
Supporting information
Supplementary Fig. 1: Top 5 differentially expressed genes identified from RT2 ECM PCR array in WT, Rag1−/− and Rorafl/flIL7RCre mice exposed to CS. Relative abundance of the top 5 genes compared to the geometric mean of Actb, B2m, Gapdh, for normal air‐ and CS‐exposed A) Rag1−/− mice and B) Rorafl/flIL7RCre mice compared to WT controls. cDNA was pooled from 6 samples in each group.
Supplementary Fig. 2: T‐bet+ ILC1s and Rorγt+ ILC3s are increased and GATA3+ ILC2s are decreased in WT mice exposed to CS. Flow cytometric analysis of single cell suspensions from whole lungs of normal air‐ or CS‐exposed WT mice. (A) Flow cytometry gating of ILC1s, ILC2s and ILC3s. Quantification of (B) total ILCs, (C) ILC1s, (D) ILC2s, and (E) ILC3s. n = 5‐6 per group. Data are expressed as mean ± sem. * P < 0.05, ** P < 0.01. Panels: ILC1: Lin−CD90.2+CD45+IL‐7Rα+T‐bet+; ILC2: Lin−CD90.2+CD45+IL‐7Rα+GATA3+T‐bet−Rorγt−CD4−; ILC3: Lin−CD90.2+CD45+IL‐7Rα+Rorγt+CD4+/−.
ACKNOWLEDGMENTS
C.D. is supported by a National Health and Medical Research Council of Australia (NHMRC) Early Career Postdoctoral Fellowship. M.R.S. was supported by an NHMRC Early Career Postdoctoral Fellowship and is now supported by Australian Research Council Discovery Early Career Research Award. R.Y.K. is supported by a Lung Foundation Australia/Boehringer Ingelheim Chronic Obstructive Pulmonary Disease Research Fellowship. A.N.J.M. was supported by grants from the MRC UK (U105178805) and Wellcome Trust (100963/Z/13/Z). P.M.H. is supported by an NHMRC Principal Research Fellowship from the National Health and Medical Research Council of Australia, a grant from the Australian Research Council, and a Brawn Fellowship from the Faculty of Health and Medicine, The University of Newcastle. The authors thank Dr. Hans‐Reimer Rodewald for the IL7Ra‐Cre mice and Kristy Wheeldon, Nathalie Kiaos, Emma Broadfield, and Tegan Hunter for technical assistance.
AUTHORSHIP
The work presented was performed in collaboration with all authors. C.D., M.R.S., and P.M.H. designed and supervised the studies and wrote the manuscript. C.D. and M.R.S. performed all experiments and analyzed the data. B.M.J.R. and J.L.B. performed the AHR study driven by A.N.J.M. The authors R.Y.K., B.J., T.J.H., P.M.N., K.B., G.J.M.C., J.C.H. assisted with experiments. P.S.F. provided the Rag1−/ − mice and A.N.J.M. provided ILC2‐deficient mice. All authors participated in the interpretation of data, and preparation and editing of the manuscript for intellectual content. All authors have read and approved the final manuscript. C. D. and M. R. S. contributed equally to this work.
Donovan C, Starkey MR, Kim RY, et al. Roles for T/B lymphocytes and ILC2s in experimental chronic obstructive pulmonary disease. J Leukoc Biol. 2019;105:143–150. 10.1002/JLB.3AB0518-178R
REFERENCES
- 1. Global Initiative for Chronic Obstructive Lung Disease . (2017). POCKET GUIDE TO COPD DIAGNOSIS, MANAGEMENT, AND PREVENTION A Guide for Health Care Professionals 2017 REPORT. [online] Available at: http://goldcopd.org/wp-content/uploads/2016/12/wms-GOLD-2017-Pocket-Guide.pdf [Accessed 2nd August 2018]. [Google Scholar]
- 2. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. [DOI] [PubMed] [Google Scholar]
- 3. Budden KF, Gellatly SL, Wood DL, et al. Emerging pathogenic links between microbiota and the gut‐lung axis. Nat Rev Microbiol. 2017;15:55–63. [DOI] [PubMed] [Google Scholar]
- 4. Jones B, Donovan C, Liu G, et al. Animal models of COPD: what do they tell us?. Respirology. 2017;22:21–32. [DOI] [PubMed] [Google Scholar]
- 5. Curtis JL, Freeman CM, Hogg JC. The immunopathogenesis of chronic obstructive pulmonary disease: insights from recent research. Proc Am Thorac Soc. 2007;4:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pouwels SD, Heijink IH, ten Hacken NH, et al. DAMPs activating innate and adaptive immune responses in COPD. Mucosal Immunol. 2014;7:215–226. [DOI] [PubMed] [Google Scholar]
- 7. Haw TJ, Starkey MR, Pavlidis S, et al. Toll‐like receptor 2 and 4 have opposing roles in the pathogenesis of cigarette smoke‐induced chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2017. ajplung 00154 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monticelli LA, Buck MD, Flamar AL, et al. Arginase 1 is an innate lymphoid‐cell‐intrinsic metabolic checkpoint controlling type 2 inflammation. Nat Immunol. 2016;17:656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monticelli LA, Sonnenberg GF, Abt MC, et al. Innate lymphoid cells promote lung‐tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bal SM, Bernink JH, Nagasawa M, et al. IL‐1beta, IL‐4 and IL‐12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol. 2016;17:636–645. [DOI] [PubMed] [Google Scholar]
- 11. Silver JS, Kearley J, Copenhaver AM, et al. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol. 2016;17:626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kearley J, Silver JS, Sanden C, et al. Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin‐33‐dependent response to infection. Immunity. 2015;42:566–579. [DOI] [PubMed] [Google Scholar]
- 13. Zhu X, Gadgil AS, Givelber R, et al. Peripheral T cell functions correlate with the severity of chronic obstructive pulmonary disease. J Immunol. 2009;182:3270–3277. [DOI] [PubMed] [Google Scholar]
- 14. Saetta M, Di Stefano A, Turato G, et al. CD8+ T‐lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:822–826. [DOI] [PubMed] [Google Scholar]
- 15. Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:2445–2454. [DOI] [PubMed] [Google Scholar]
- 16. Gosman MM, Willemse BW, Jansen DF, et al, Groningen, Leiden Universities Corticosteroids in Obstructive Lung Disease Study, G. Increased number of B‐cells in bronchial biopsies in COPD. Eur Respir J. 2006;27:60–64. [DOI] [PubMed] [Google Scholar]
- 17. Borchers MT, Wesselkamper SC, Harris NL, et al. CD8+ T cells contribute to macrophage accumulation and airspace enlargement following repeated irritant exposure. Exp Mol Pathol. 2007;83:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, Shapiro SD. CD8+ T cells are required for inflammation and destruction in cigarette smoke‐induced emphysema in mice. J Immunol. 2007;178:8090–8096. [DOI] [PubMed] [Google Scholar]
- 19. Polverino F, Seys LJ, Bracke KR, Owen CA. B cells in chronic obstructive pulmonary disease: moving to center stage. Am J Physiol Lung Cell Mol Physiol. 2016;311:L687‐L695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee SH, Goswami S, Grudo A, et al. Antielastin autoimmunity in tobacco smoking‐induced emphysema. Nat Med. 2007;13:567–569. [DOI] [PubMed] [Google Scholar]
- 21. Nunez B, Sauleda J, Anto JM, et al. Anti‐tissue antibodies are related to lung function in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:1025–1031. [DOI] [PubMed] [Google Scholar]
- 22. Polverino F, Baraldo S, Bazzan E, et al. A novel insight into adaptive immunity in chronic obstructive pulmonary disease: B cell activating factor belonging to the tumor necrosis factor family. Am J Respir Crit Care Med. 2010;182:1011–1019. [DOI] [PubMed] [Google Scholar]
- 23. Seys LJ, Verhamme FM, Schinwald A, et al. Role of B cell‐activating factor in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:706–718. [DOI] [PubMed] [Google Scholar]
- 24. Motz GT, Eppert BL, Wesselkamper SC, Flury JL, Borchers MT. Chronic cigarette smoke exposure generates pathogenic T cells capable of driving COPD‐like disease in Rag2‐/‐ mice. Am J Respir Crit Care Med. 2010;181:1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beckett EL, Stevens RL, Jarnicki AG, et al. A new short‐term mouse model of chronic obstructive pulmonary disease identifies a role for mast cell tryptase in pathogenesis. J Allergy Clin Immunol. 2013;131:752–762.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu G, Cooley MA, Jarnicki AG, et al. Fibulin‐1 regulates the pathogenesis of tissue remodeling in respiratory diseases. JCI Insight. 2016;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hsu AC, Starkey MR, Hanish I, et al. Targeting PI3K‐p110alpha suppresses influenza virus infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:1012–1023. [DOI] [PubMed] [Google Scholar]
- 28. Hansbro PM, Hamilton MJ, Fricker M, et al. Importance of mast cell Prss31/transmembrane tryptase/tryptase‐gamma in lung function and experimental chronic obstructive pulmonary disease and colitis. J Biol Chem. 2014;289:18214–18227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haw TJ, Starkey MR, Nair PM, et al. A pathogenic role for tumor necrosis factor‐related apoptosis‐inducing ligand in chronic obstructive pulmonary disease. Mucosal Immunol. 2016;9:859–872. [DOI] [PubMed] [Google Scholar]
- 30. Jarnicki AG, Schilter H, Liu G, et al. The inhibitor of semicarbazide‐sensitive amine oxidase, PXS‐4728A, ameliorates key features of chronic obstructive pulmonary disease in a mouse model. Br J Pharmacol. 2016;173:3161–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hsu AC, Dua K, Starkey MR, et al. MicroRNA‐125a and ‐b inhibit A20 and MAVS to promote inflammation and impair antiviral response in COPD. JCI Insight. 2017;2:e90443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tay HL, Kaiko GE, Plank M, et al. Antagonism of miR‐328 increases the antimicrobial function of macrophages and neutrophils and rapid clearance of non‐typeable Haemophilus influenzae (NTHi) from infected lung. PLoS Pathog. 2015;11:e1004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maltby S, Lochrin AJ, Bartlett B, et al. Osteoblasts are rapidly ablated by virus‐induced systemic inflammation following lymphocytic choriomeningitis virus or pneumonia virus of mice infection in mice. J Immunol. 2018;2:632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oliphant CJ, Hwang YY, Walker JA, et al. MHCII‐mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thorburn AN, Foster PS, Gibson PG, Hansbro PM. Components of Streptococcus pneumoniae suppress allergic airways disease and NKT cells by inducing regulatory T cells. J Immunol. 2012;188:4611–4620. [DOI] [PubMed] [Google Scholar]
- 36. Beckett EL, Phipps S, Starkey MR, et al. TLR2, but not TLR4, is required for effective host defence against Chlamydia respiratory tract infection in early life. PLoS One. 2012;7:e39460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Essilfie AT, Horvat JC, Kim RY, et al. Macrolide therapy suppresses key features of experimental steroid‐sensitive and steroid‐insensitive asthma. Thorax. 2015;70:458–467. [DOI] [PubMed] [Google Scholar]
- 38. Horvat JC, Beagley KW, Wade MA, et al. Neonatal chlamydial infection induces mixed T‐cell responses that drive allergic airway disease. Am J Respir Crit Care Med. 2007;176:556–564. [DOI] [PubMed] [Google Scholar]
- 39. Kim RY, Horvat JC, Pinkerton JW, et al. MicroRNA‐21 drives severe, steroid‐insensitive experimental asthma by amplifying phosphoinositide 3‐kinase‐mediated suppression of histone deacetylase 2. J Allergy Clin Immunol. 2017;139:519–532. [DOI] [PubMed] [Google Scholar]
- 40. Starkey MR, Essilfie AT, Horvat JC, et al. Constitutive production of IL‐13 promotes early‐life Chlamydia respiratory infection and allergic airway disease. Mucosal Immunol. 2013;6:569–579. [DOI] [PubMed] [Google Scholar]
- 41. Barlow JL, Flynn RJ, Ballantyne SJ, McKenzie AN. Reciprocal expression of IL‐25 and IL‐17A is important for allergic airways hyperreactivity. Clin Exp Allergy. 2011;41:1447–1455. [DOI] [PubMed] [Google Scholar]
- 42. Akamatsu Y, Oettinger MA. Distinct roles of RAG1 and RAG2 in binding the V(D)J recombination signal sequences. Mol Cell Biol. 1998;18:4670–4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li D, Guabiraba R, Besnard AG, et al. IL‐33 promotes ST2‐dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J Allergy Clin Immunol. 2014;134:1422–1432.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vannella KM, Ramalingam TR, Borthwick LA, et al. Combinatorial targeting of TSLP, IL‐25, and IL‐33 in type 2 cytokine‐driven inflammation and fibrosis. Sci Transl Med. 2016;8:337ra65. [DOI] [PubMed] [Google Scholar]
- 45. Manoury B, Caulet‐Maugendre S, Guénon I, Lagente V, Boichot E. Timp‐1 is a key factor of fibrogenic response to bleomycin in mouse lung. Int J Immunopathol Pharmacol. 2006;19:471–487. [DOI] [PubMed] [Google Scholar]
- 46. García‐Prieto E, González‐López A, Cabrera S, et al. Resistance to bleomycin‐induced lung fibrosis in MMP‐8 deficient mice is mediated by interleukin‐10. PLoS One. 2010;5:e13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schwanekamp JA, Lorts A, Sargent MA, et al. TGFBI functions similar to periostin but is uniquely dispensable during cardiac injury. PLoS One. 2017;12:e0181945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brestoff JR, Kim BS, Saenz SA, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu J, Guardado J, Hoffman R, et al. IL33‐mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: a reverse translation study from a human cohort to a mouse trauma model. PLoS Med. 2017;14:e1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Turner JE, Morrison PJ, Wilhelm C, et al. IL‐9‐mediated survival of type 2 innate lymphoid cells promotes damage control in helminth‐induced lung inflammation. J Exp Med. 2013;210:2951–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kaur D, Gomez E, Doe C, et al. IL‐33 drives airway hyper‐responsiveness through IL‐13‐mediated mast cell: airway smooth muscle crosstalk. Allergy. 2015;70:556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zerah F, Harf A, Perlemuter L, Lorino H, Lorino AM, Atlan G. Effects of obesity on respiratory resistance. Chest. 1993;103:1470–1476. [DOI] [PubMed] [Google Scholar]
- 53. Jones RL, Noble PB, Elliot JG, James AL. Airway remodelling in COPD: It's not asthma! Respirology. 2016;21:1347–1356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1: Top 5 differentially expressed genes identified from RT2 ECM PCR array in WT, Rag1−/− and Rorafl/flIL7RCre mice exposed to CS. Relative abundance of the top 5 genes compared to the geometric mean of Actb, B2m, Gapdh, for normal air‐ and CS‐exposed A) Rag1−/− mice and B) Rorafl/flIL7RCre mice compared to WT controls. cDNA was pooled from 6 samples in each group.
Supplementary Fig. 2: T‐bet+ ILC1s and Rorγt+ ILC3s are increased and GATA3+ ILC2s are decreased in WT mice exposed to CS. Flow cytometric analysis of single cell suspensions from whole lungs of normal air‐ or CS‐exposed WT mice. (A) Flow cytometry gating of ILC1s, ILC2s and ILC3s. Quantification of (B) total ILCs, (C) ILC1s, (D) ILC2s, and (E) ILC3s. n = 5‐6 per group. Data are expressed as mean ± sem. * P < 0.05, ** P < 0.01. Panels: ILC1: Lin−CD90.2+CD45+IL‐7Rα+T‐bet+; ILC2: Lin−CD90.2+CD45+IL‐7Rα+GATA3+T‐bet−Rorγt−CD4−; ILC3: Lin−CD90.2+CD45+IL‐7Rα+Rorγt+CD4+/−.


