Abstract
Aims
In clinical practice, patients of ulcerative colitis showing divergence between the histological findings and endoscopic findings are often encountered. Therefore, we compared histological findings with endoscopic findings, indicating the severity of the inflammation of ulcerative colitis.
Methods and Results
The study group comprised 191 patients (527 biopsy specimens) with ulcerative colitis who underwent lower gastrointestinal endoscopy with biopsy in our hospital from July 2015 to June 2016. Endoscopic findings of the mucosa at the biopsy site were classified into seven levels according to the severity of inflammation: noninflamed mucosa, red signs, loss of visible vascular patterns, granular mucosa, friable mucosa, spontaneous bleeding, and erosions/ulcers (E/U). All biopsy samples were examined for the presence or absence of five histological findings (basal plasmacytosis [BP], neutrophil infiltration, cryptitis, crypt abscess [CAb], and E/U), and the results were contrasted with endoscopic findings. The 191 patients comprised 123 (64.4%) males and 68 (35.6%) females, with a median age of 47 years (range, 8–82). Among the 527 specimens, the detection rates of BP, CAb, and E/U in mucosa with endoscopic E/U were 58.5, 27.4, and 18.3%. The detection rate of BP in mucosa with red signs was 22.4%; in mucosa, with loss of visible vascular patterns, it was 16.9%; in granular mucosa, it was 35.7%, and in mucosa with E/U, it was 58.5%. BP was frequently seen in severely inflamed mucosa associated with E/U on endoscopic examination.
Conclusion
BP was considered an important finding, suggesting the presence of active and severe inflammation.
Keywords: basal plasmacytosis, degree of inflammation, endoscopic findings, histological findings, ulcerative colitis
Background and Aim
Ulcerative colitis is a representative inflammatory bowel disease. The incidence of ulcerative colitis has been increasing throughout the world, and the disease is often encountered by clinicians as well as pathologists. Ulcerative colitis is characterized by a chronic clinical course, during which many patients have recurrent exacerbations of symptoms. Therefore, surveillance of lower gastrointestinal endoscopy should be routinely performed to evaluate inflammation and detect dysplasia and cancer.1
Histological findings of ulcerative colitis are not specific and include diffuse inflammatory cell infiltration, cryptitis (C), crypt abscess (CAb), structural abnormalities of crypts, erosion, and goblet cell depletion and loss. Screening for dysplasia and cancer is thus particularly important. The grade of inflammation is histologically classified according to the classifications proposed by Matts2 or Geboes.3 In particular, the Geboes classification includes histological findings during remission and is considered easy to use clinically, and basal plasmacytosis (BP) is considered an early and highly specific finding in patients with inflammatory bowel disease.4, 5, 6, 7, 8, 9, 10
The endoscopic findings of ulcerative colitis can be evaluated according to the Matts classification, Mayo endoscopic subscore,11 Ulcerative Colitis Endoscopic Index of Severity,12 Baron index,13 and Rachmilewitz Endoscopic Index.14 Although the most important variables differ depending on the method used for classification, the grade of inflammation is evaluated on the basis of the presence or absence of factors such as red signs, loss of visible vascular patterns, granular mucosa, friable mucosa, spontaneous bleeding, and erosions/ulcers (E/U).
In clinical practice, however, patients showing divergence between the aforementioned histological findings and endoscopic findings are often encountered. We therefore studied the correlations between histological findings and endoscopic findings as indicators of the grade of inflammation associated with ulcerative colitis to identify important histological findings.
Methods
Among patients who had been given a diagnosis of ulcerative colitis and were receiving medical treatment, we studied 190 patients who underwent lower gastrointestinal endoscopy, including biopsy in our hospital, from July 2015 to June 2016. The biopsy specimens obtained were fixed in 10% neutral buffered formalin and then embedded in paraffin. The paraffin‐embedded specimens were thinly sliced into 4‐μm‐thick sections and stained with hematoxylin and eosin. Among the 537 biopsy specimens obtained, 10 specimens were excluded: 9 were severely crushed and could not be evaluated histologically, and 1 included dysplasia. The remaining 527 specimens were studied. Endoscopic findings of the inflamed mucosa at the biopsy site were classified into seven levels: noninflamed mucosa, redness, loss of visible vascular patterns, granular mucosa, friable mucosa, spontaneous bleeding, and E/U (Fig. 1). Because, generally, the grade of inflammation is evaluated on the basis of the presence or absence of factors such as the above findings in some criteria,2, 11, 12, 13, 14 we also evaluated them in this study. All biopsy samples and all areas of each sample were examined to evaluate the presence or absence of five histological findings of ulcerative colitis (BP, neutrophil infiltration [NI], C, CAb, and E/U) (Fig. 2), and the results were contrasted with endoscopic findings. BP was evaluated according to the criteria proposed by Tanaka et al.4 as follows: ‘the presence of 3 or more plasma cells per width of one crypt between the crypt base and the muscularis mucosa’. NI was defined as neutrophil infiltration into lamina propria. C was defined as NI of crypt epithelium. CAb was defined as a collection of neutrophils in a crypt. E/U was defined as a mucosal defect with NI or precipitation of fibrin. Because these histological findings are common and objective in famous criteria,2, 3 they were adopted.
Figure 1.
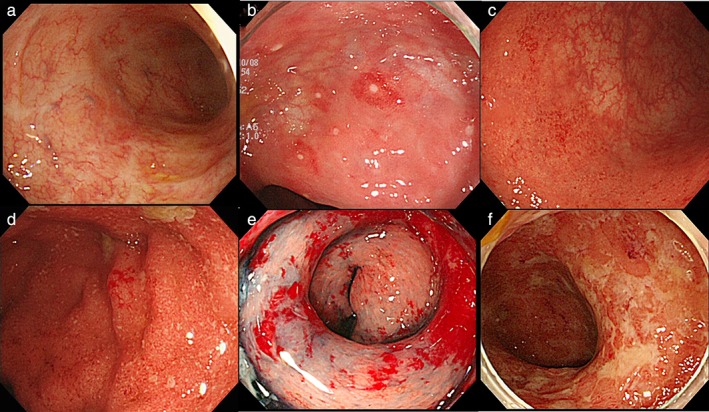
Representative endoscopic findings shown in the order of the severity of inflammation on endoscopy. a. Noninflamed mucosa in remission, with scar formation after ulcer healing. b. A small red sign was found in the noninflamed mucosa, and a small erosion can be seen in the small red sign. c. In the distal portion of the picture (near side), visible vascular patterns disappeared. In the proximal portion (far side), visible vascular patterns remained, suggesting noninflamed mucosa. d. An edematous, granular, rough mucosa. In the center of the picture, microbleeding can be observed, a finding of friable mucosa that bled on contact with the endoscope tip. e. Diffuse exudative spontaneous bleeding. f. Multiple map‐like ulcers arose in diffusely inflamed mucosa.
Figure 2.
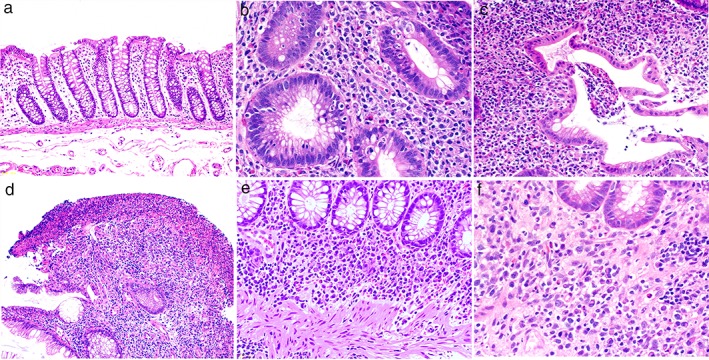
Representative histological findings shown in order of the severity of inflammation. a. Normal intestinal mucosa in healthy subjects. b. Neutrophil infiltration (NI) and cryptitis. c. Crypt abscess. d. Erosion. Loss of the surface layer of the mucosa, fibrin precipitation, and NI. e, f. Basal plasmacytosis. Three or more plasma cells per the width of one crypt can be seen between the crypt base and the muscularis mucosa.
As this retrospective study used the biopsy specimens obtained from the patients of ulcerative colitis in routine medical treatment, informed consent was not obtained from the individual patients. We disclosed the information about our protocol and provided patients the opportunity to refuse participation in our study. Our study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Jikei University School of Medicine for Biomedical Research (approval number, 28–100 [8343]).
Results
The study group comprised 191 patients with ulcerative colitis, consisting of 123 (64.4%) males and 68 (35.6%) females, with a median age of 47 years (range, 8–82). A total of 537 biopsy samples were obtained. The maximum number of samples taken per patient was 9 (Table 1). Among the 537 biopsy specimens obtained, 10 specimens were excluded: 9 (1.7%) were severely crushed and could not be evaluated histologically, and 1 (0.2%) included dysplasia. The remaining 527 specimens (98.1%) obtained from 191 patients were studied.
Table 1.
Patients and specimens in this study
| No. of total specimens (no. of patients) | 538 (191) |
| Excluded specimens | |
| Severely crushed | 9 |
| Dysplasia | 1 |
| No. of studied specimens (no. of patients) | 527 (191) |
| Gender | |
| Male | 123 (64.0%) |
| Female | 68 (36.0%) |
| Median age (yr) (range) | 47 (8–82) |
The severity of inflammation at the biopsy site was endoscopically classified into seven levels. From noninflamed mucosa, 211 specimens (40.0%) were taken. From lesions associated with E/U, 164 specimens (31.1%) were taken. Nearly all of the specimens from endoscopic E/U were obtained from small erosions in part of the inactive inflamed mucosa using targeted biopsy. The number of biopsy specimens taken from friable mucosa or mucosa with spontaneous bleeding was only two (0.4%) and zero (0%), respectively (Table 2).
Table 2.
The presence of histological findings (%) according to the severity of inflammation on endoscopy
| Classification of inflammation on endoscopy | No. of specimens (%) | Presence of histological findings (%) | ||||
|---|---|---|---|---|---|---|
| BP | NI | C | CAb | E/U | ||
| Noninflamed mucosa | 211 (40.0) | 9 (4.3) | 39 (18.5) | 28 (13.3) | 5 (2.4) | 0 (0) |
| Redness | 49 (9.3) | 11 (22.4) | 37 (75.5) | 27 (55.1) | 5 (10.2) | 1 (2.0) |
| Loss of visible vascular patterns | 59 (11.2) | 10 (16.9) | 48 (81.4) | 44 (74.6) | 11 (18.6) | 3 (5.1) |
| Granular mucosa | 42 (7.7) | 15 (35.7) | 38 (90.5) | 36 (85.7) | 11 (26.2) | 0 (0) |
| Friable mucosa | 2 (0.4) | 0 (0) | 2 (100) | 1 (50.0) | 0 (0) | 0 (0) |
| Spontaneous bleeding | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| E/U | 164 (31.1) | 96 (58.5) | 156 (95.1) | 127 (77.4) | 45 (27.4) | 30 (18.3) |
| Total | 527 | 141 (26.8) | 320 (60.7) | 263 (49.9) | 77 (14.6) | 34 (6.5) |
BP, basal plasmacytosis; C, cryptitis; CAb, crypt abscess; E/U, erosions/ulcers; NI, neutrophil infiltration.
The frequencies of histological findings are shown according to the endoscopic classification of the grade of inflammation in Table 2. The frequencies of NI and C in noninflamed mucosa were only 18.5 and 13.3% and, in mucosa with red signs, were 75.5 and 55.1%, respectively. Both differences were significant on analysis using a chi‐square test (P < 0.01). Even small lesions on endoscopic examination were frequently associated with distinct histological evidence of inflammation. The detection rates of E/U and CAb on histological examination were frequent in severely inflamed mucosa, such as endoscopic E/U; however, the rates were only 18.3 and 27.4%, respectively.
BP could be evaluated in the 407 (77.2%) specimens containing muscularis mucosa. We treated the specimens not containing muscularis mucosa as BP negative because we wanted to analyze the frequency of BP, including the influence of specimen collection. On the other hand, the detection rate of BP in mucosa with red signs was 22.4%; in mucosa, with loss of visible vascular patterns, 16.9%; in granular mucosa, 35.7%; and in mucosa with E/U, 58.5%. The frequency of BP in mucosa with endoscopic E/U was significantly higher than in mucosa with loss of visible vascular patterns on analysis with a chi‐square test (P < 0.01). BP was frequently seen in severely inflamed mucosa on endoscopic examination. Furthermore, of the 141 specimens with BP, the detection rate of NI was 97.9%, C was 83.7%, CAb was 36.2%, and E/U was 13.5% (Table 3). BP was frequently seen in active and early‐stage inflamed mucosa.
Table 3.
The presence of histological findings (%) in 141 specimens with basal plasmacytosis
| Presence of histological findings (%) | |
|---|---|
| N | 138 (97.9) |
| C | 118 (83.7) |
| CAb | 51 (36.2) |
| E/U | 19 (13.5) |
BP, basal plasmacytosis; C, cryptitis; CAb, crypt abscess; E/U, erosions/ulcers; NI, neutrophil infiltration.
Discussion
BP refers to plasma cell infiltration in the deep layer of the mucosa. In a study by Tanaka et al.,4 BP was defined as ‘the presence of 3 or more plasma cells per width of 1 crypt between the crypt base and the muscularis mucosa’. In our study, this definition was used. It is noteworthy that, in our study, BP was seen in 58.5% of specimens of severely inflamed mucosa associated with E/U on endoscopy. The frequency was significantly higher than in mucosa with a loss of visible vascular patterns. In granular mucosa on endoscopy, BP was seen in 35.7% of specimens. It is suggested that granular mucosa might contain very small erosions on endoscopy. Jinno et al.15 reported that mild plasma cell infiltration is present at the ulcer base in steroid‐resistant ulcerative colitis and pointed out the association between severely inflamed mucosa and BP. Then, Bessissow et al.16 reported that the presence of BP in noninflamed mucosa is a useful index of flare‐ups. In our study, BP was found in 9 (4.3%) of 211 specimens of noninflamed mucosa; however, all of the specimens were from patients who had severely inflamed mucosa in other areas. BP was associated with NI at a high frequency; however, advanced inflammation such as CAb and E was infrequent. These results suggest that BP might be a histological finding, suggesting the potential for inflammatory activity in noninflamed mucosa and the early‐onset feature of severely active inflammation associated with ulcerative colitis. As mentioned above, BP is considered an important finding in patients of ulcerative colitis with active inflammation. One problem is that BP cannot be evaluated in biopsy specimens that do not include the muscularis mucosa. Therefore, it is important that endoscopists biopsy specimens containing muscularis mucosa.
On the other hand, histological evidence of CAb or E/U is considered to reflect severe inflammation. However, these findings have a low frequency and are not considered to be very specific for ulcerative colitis.17
In our study, many patients with ulcerative colitis were in remission. The reason is that effective medical treatment reduces the disease activity in many patients, and screening for dysplasia and cancer should be performed during remission. Many of the specimens in our study were obtained by random biopsy of the noninflamed mucosa or by targeted biopsy of small red regions or small erosions. Very few biopsy specimens were obtained from friable mucosa or mucosa with spontaneous bleeding because the endoscopists were concerned about the risk of worsening the bleeding by biopsy.
Distinct histological evidence of inflammation, such as NI, was found even in small lesions on endoscopy. This is consistent with the findings of Lemmens et al.,18 who reported that that the presence of mild inflammation on endoscopic examination can be associated with severe inflammation on histological examination. This is attributed to the fact that endoscopy can generally evaluate lesions in a given region and therefore might underestimate the severity of mucosal inflammation in small regions of redness or small erosions against a backdrop of noninflamed mucosa. In addition, biopsy specimens may include microlesions that cannot be visualized on endoscopy.
Conclusions
Histological findings of ulcerative colitis tend to be more severe than endoscopic findings. In particular, BP was frequently seen in severely inflamed mucosa associated with E/U on endoscopic examination. BP was considered an important finding, suggesting the presence of active and severe inflammation. For evaluation of BP, it is very important to collect sufficient biopsy specimens containing muscularis mucosa.
Acknowledgments
The author acknowledges all doctors of the department of gastroenterology and hepatology and the department of pathology, The Jikei University School of Medicine, for their examination, diagnosis, and treatment of the patients.
Declaration of conflict of interest: The authors declare that they have no conflict of interest.
References
- 1. Leong RW, Ooi M, Corte C et al Full‐spectrum endoscopy improves surveillance for dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2017; 152: 1337–1447. [DOI] [PubMed] [Google Scholar]
- 2. Matts SG. The value of rectal biopsy in the diagnosis of ulcerative colitis. Q. J. Med. 1961; 30: 393–407. [PubMed] [Google Scholar]
- 3. Geboes K, Riddell RH, Ost A, Jensfelt B, Persson T, Löfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000; 47: 404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tanaka M, Saito H, Fukuda S, Sasaki Y, Munakata A, Kudo H. Simple mucosal biopsy criteria differentiating among Crohn disease, ulcerative colitis, and other forms of colitis: measurement of validity. Scand. J. Gastroenterol. 2000; 35: 281–6. [DOI] [PubMed] [Google Scholar]
- 5. Tanaka M, Kusumi T, Oshitani N et al Validity of simple mucosal biopsy criteria combined with endoscopy predicting patients with ulcerative colitis ultimately requiring surgery: a multicenter study. Scand. J. Gastroenterol. 2003; 38: 594–8. [DOI] [PubMed] [Google Scholar]
- 6. Lessells AM, Beck JS, Burnett RA et al Observer variability in the histopathological reporting of abnormal rectal biopsy specimens. J. Clin. Pathol. 1994; 47: 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Villanacci V, Antonelli E, Reboldi G, Salemme M, Casella G, Bassotti G. Endoscopic biopsy samples of naïve “colitides” patients: role of basal plasmacytosis. J. Crohns Colitis. 2014; 8: 1438–43. 10.1016/j.crohns.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 8. Canavese G, Villanacci V, Antonelli E et al Eosinophilia ‐ associated basal plasmacytosis: an early and sensitive histologic feature of inflammatory bowel disease. APMIS. 2017; 125: 179–83. [DOI] [PubMed] [Google Scholar]
- 9. Schumacher G, Sandstedt B, Kollberg B. A prospective study of first attacks of inflammatory bowel disease and infectious colitis. Clinical findings and early diagnosis. Scand. J. Gastroenterol. 1994; 29: 265–74. [DOI] [PubMed] [Google Scholar]
- 10. Yantiss RK, Odze RD. Diagnostic difficulties in inflammatory bowel disease pathology. Histopathology. 2006; 48: 116–32. [DOI] [PubMed] [Google Scholar]
- 11. D' Haens G, Sandborn WJ, Feagan BG et al A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007; 132: 763–86. [DOI] [PubMed] [Google Scholar]
- 12. Travis SP, Schnell D, Krzeski P et al Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. 2013; 145: 987–95. [DOI] [PubMed] [Google Scholar]
- 13. Baron JH, Connell AM, Lennard‐Jones JE. Variation between observers in describing mucosal appearances in proctocolitis. Br. Med. J. 1964; 1: 89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rachmilewitz D. Coated mesalazine (5‐aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989; 298: 82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jinno Y, Ohtani H, Nakamura S et al Infiltration of CD19+ plasma cells with frequent labeling of Ki‐67 in corticosteroid‐resistant active ulcerative colitis. Virchows Arch. 2006; 448: 412–21. [DOI] [PubMed] [Google Scholar]
- 16. Bessissow T, Lemmens B, Ferrante M et al Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am. J. Gastroenterol. 2012; 107: 1684–92. [DOI] [PubMed] [Google Scholar]
- 17. Cerilli LA, Greenson JK. The differential diagnosis of colitis in endoscopic biopsy specimens: a review article. Arch. Pathol. Lab. Med. 2012; 136: 854–64. [DOI] [PubMed] [Google Scholar]
- 18. Lemmens B, Arijs I, Van Assche G et al Correlation between the endoscopic and histologic score in assessing the activity of ulcerative colitis. Inflamm. Bowel Dis. 2013; 19: 1194–201. [DOI] [PubMed] [Google Scholar]


