Abstract
Background and Aim
Acute kidney injury (AKI) in severe acute pancreatitis (SAP) has a high mortality rate. Traditionally used serum creatinine is an insensitive biomarker for the early detection of AKI. We aimed to study the role of plasma and urinary neutrophil gelatinase‐associated lipocalin (NGAL) in predicting AKI and a severe course in patients with acute pancreatitis (AP).
Methods
Consecutive patients of AP who presented within 72 h of symptom onset and age‐ and gender‐matched healthy controls were included. Urinary and serum NGAL levels [enzyme‐linked immunosorbent assay (ELISA)] were evaluated within 24 h of and 72 h after admission and once in controls. Urine and serum NGAL levels were correlated with development of AKI, severity, and outcomes of AP.
Results
Fifty patients with AP and 30 controls were enrolled. The mean serum and urine NGAL levels in patients on day 1 were significantly higher than the serum and urine NGAL levels in controls (P < 0.001). After excluding patients with AKI on day 1 (n = 10), both serum and urinary NGAL levels on days 1 and 3 were significantly higher in patients who subsequently developed AKI (n = 11) compared to those who did not (n = 29) (P = 0.02, 0.01 and P < 0.001, 0.03). A urinary NGAL level of 221.03 ng/mL on day 1 predicted AKI with a sensitivity and specificity of 82 and 80%, respectively (AUC = 0.9). Mean serum and urinary NGAL levels on day 1 were significantly elevated in patients with SAP compared to those without SAP (P = 0.04 and <0.001).
Conclusion
NGAL levels in urine and serum can predict severity of AP and development of AKI.
Keywords: acute kidney injury, acute pancreatitis, creatinine, neutrophil gelatinase‐associated lipocalin, organ failure
Introduction
Acute kidney injury (AKI) is a common complication of severe acute pancreatitis (SAP) and is associated with a high mortality despite optimal intensive care.1 There are no reliable and sensitive markers of early AKI that can be used for prevention and early management strategies.2 Recent studies have identified many emerging markers of early AKI, including cystatin C,3 interleukin‐18 (IL‐18),4 kidney injury molecule‐1 (KIM‐1),5 and neutrophil gelatinase‐associated lipocalin (NGAL).2
NGAL is a 24 kDa glycoprotein belonging to the lipocalin superfamily of proteins and is released by activated neutrophils from areas of infection and inflammation and acts as a bacteriostatic agent.6, 7 NGAL is an established biomarker of renal injury.8, 9, 10 Urinary and serum/plasma NGAL levels are elevated within 6 h of the kidney injury, while the rise in serum creatinine levels occurs only after more than 50% of renal function is lost, which may take days.11, 12 Expression of NGAL mRNA increases more than 1000‐fold in response to kidney injury and manifests as rapid elevation of urine and blood NGAL levels, making it useful as an early biomarker.9, 13 It has been shown that elevated NGAL predicts the development of AKI before serum creatinine in conditions such as cardiac surgery‐associated AKI, after renal transplantation, contrast AKI, and AKI in the critical care setting. Studies have also demonstrated the utility of early NGAL test in predicting the prognosis of AKI.14
There are limited data showing serum and urinary NGAL as a marker of severe AP and as a predictor of AKI in AP.15, 16, 17 The aim of this prospective study was to compare urinary and serum NGAL levels in predicting the development of AKI, severity, and mortality in patients with AP.
Methods
This prospective study was conducted in the departments of Gastroenterology and General Surgery at Postgraduate Institute of Medical Education and Research, a tertiary care referral center in Northern India, between January 2011 and June 2012. The study was approved by the Institute's Ethics Committee, and the Indian Council of Medical Research guidelines for conducting a research were followed.
After informed consent, patients older than 12 years of age who were admitted with a diagnosis of AP18 and presented to us within 72 h of the onset of symptoms were enrolled in the study. The revised Atlanta classification was used to categorize the severity of AP as mild, moderately severe, or severe.19 Patients were excluded if they had a diagnosis of chronic pancreatitis (CP) (definitive features on imaging like pancreatic duct dilation, pancreas atrophy, and presence of pancreatic calcifications), chronic kidney disease,20 and significant comorbidities (including chronic obstructive airway disease, cirrhosis, bronchial asthma, and congestive heart failure). Serum creatinine, blood urea, serum sodium, and potassium levels were measured every day in all the patients who developed AKI until they became normal, after which they were repeated every third day. Serum and urine samples from consenting healthy blood donors without any comorbidities, especially kidney disease, in the past/present, without any recent history of nephrotoxic drug intake or urinary tract infection were taken for NGAL measurement.
Management of acute pancreatitis (AP)
Management of patients was according to the standard guidelines.18 Aggressive hydration with 250–500 mL per hour infusion of crystalloid solution was given to all patients in the first day. In the next few days, fluid correction was guided by the patient's blood pressure, urine output, heart rate, and hematocrit. No prophylactic antibiotics were given. All the patients were managed in a ward or intensive care setting based on their clinical situation. Patients underwent contrast‐enhanced computed tomography (CT) scan after day 5. Patients were monitored for the presence and severity of organ failure (Modified Marshall organ failure score)21 every day during the first week and periodically after that. Subsequent local complications, sepsis, and death during the same hospital admission were recorded. The course in the hospital and final outcomes were recorded.
AKI
According to the Modified Marshall organ failure score, an organ failure is defined as a score greater than or equal to 2, which in the case of AKI is defined by serum creatinine level ≥1.9 mg/dL.21 Transient organ failure was defined as organ failure present for less than 48 h, and persistent organ failure was recorded when organ failure was present for ≥48 h.22, 23, 24 The Acute Kidney Injury Network (AKIN) classification was also used to identify patients with AKI (increase in serum creatinine by ≥0.3 mg/dL or a percentage increase of greater than 50%).25 Patients with AKI by AKIN criteria on day 1 after admission were excluded from analysis.
Measurement of NGAL
Serum and urine were collected from all patients within 24 h of admission and again after 72 h. They were collected from all controls once only. The samples of serum and urine were labeled and stored frozen and evaluated for NGAL level using ELISA test kits (ELISA, BioPortoDiagnositics, Copenhagen, Denmark).
Data analysis
The data were analyzed using SPSS software (IBM corp., Armonk, NY, USA) Quantitative data were described in terms of means and standard deviations, with 95% confidence intervals for continuous variables with normal distribution, median with range for nonparametric variables, and categorical data shown as proportions. We checked the data for normal distribution using the Kolomogorov Smirnov test. Normally distributed continuous variables were compared using the student t‐test. Nonparametric data were compared using Mann–Whitney test. For more than two groups, one‐way anova was used. Cut‐off values for predicting AKI, Atlanta severity, and mortality were evaluated using the area under the receiver operating curve (AUC). The ability of this cut‐off value to predict the above variables was then evaluated. A P‐value of <0.05 was considered statistically significant, and two‐tailed P‐values were used as indicated.
Results
A total of 345 patients with AP were admitted to our facility during the study duration. Fifty patients who fulfilled the inclusion criteria and consented for the study and 30 (age‐ and gender‐matched) healthy controls were included in the study. Most patients (43, 86%) were admitted with their first attack of AP. All the patients who presented with a repeat attack had alcohol as their etiology. The severity distribution of AP was mild in 15, moderately severe in 12, and severe in 23 patients. Patients with kidney dysfunction were identified by the Modified Marshall Score AKI criteria and the same cohort of patients also fulfilled the AKIN classification criteria25 for AKI. AKI developed in 21 (42%) patients, of whom 10 (48%) had transient and 11 (52%) had persistent AKI. The median duration of persistent AKI was 8 days (range 2–14). Dialysis was required in 4 of 11 of those with persistent AKI, hemodialysis in 3, and peritoneal dialysis in 1 patient. AKI was associated with other organ failures, with 13 patients developing acute lung injury and 6 patients developing acute cardiovascular failure; 15 patients had acute lung injury (ALI) performed. All patients with cardiovascular failure had AKI. Patients with AKI had a significantly longer (6.2 ± 9.7 days) intensive care unit stay than those without AKI (0.7 ± 2.8 days, P = 0.02) (Table 1 ). Ten (20%) patients died; 4 of these patients had transient AKI, and 6 had persistent AKI.
Table 1.
Characteristics of AP patients with AKI and without AKI
| AKI (21) | No AKI (29) | P value | |
|---|---|---|---|
| Gender (Males – %) | 13 (62) | 17 (58.6) | 0.8 |
| Age | 47.1 ± 16.4 | 35 ± 15.5 | 0.01 |
| Etiology | – | – | 0.2 |
| Alcohol | 10 (47.6%) | 12 (41.4%) | NA |
| Gall stone | 6 (28.6%) | 14 (48.5%) | NA |
| Idiopathic | 2 (9.5%) | 2 (6.9%) | NA |
| Alcohol + gall stone | 3 (4.3%) | 0 | NA |
| Drug induced | 0 | 1 (3.4%) | NA |
| SAP | 16 (76.2%) | 7 (24.1%) | <0.001 |
| Hospital stay (days) | 10 (2–64) | 8 (3–31) | 0.5 |
| ICU stay (days) | 6.2 ± 9.7 | 0.7 ± 2.8 | 0.02 |
| Surgery | 3 (14.3%) | 0 | 0.06 |
| Death | 10 (47.6%) | 0 | <0.001 |
AKI, acute kidney injury; AP, acute pancreatitis; ICU, intensive care unit; SAP; severe acute pancreatitis.
NGAL levels
The mean serum and urine NGAL levels in controls were 15.10 ± 6.3 and 4.26 ± 4.5 ng/mL, respectively. The mean serum and urine NGAL levels on day 1 in patients with AP (587.6 ± 251.5 ng/mL & 252.8 ± 165.9 ng/mL) were significantly higher than the values in the controls (P < 0.001). There was no significant difference between serum NGAL levels on day 1 and day 3 (P = 0.66), but the mean urine NGAL level on day 1 was significantly higher than the level on day 3 (P = 0.01) (Table 2 ). There was moderate positive correlation between day 1 serum and urine NGAL levels (r = 0.6, P = 0.001). Similarly, there was moderate positive correlation between day 3 serum and urine NGAL levels (r = 0.5, P < 0.001). There was a strong positive correlation between day 1 and day 3 serum NGAL levels (r = 0.7, P < 0.001) and day 1 and day 3 urine NGAL levels (r = 0.7, P < 0.001). Serum and urine NGAL levels were significantly higher in patients with AKI on day 1 (n = 10) compared to those without AKI on day 1 (n = 40). Table 3 gives mean NGAL levels in urine and serum in different subgroups of patients of AKI.
Table 2.
Mean NGAL serum and urine on day 1 and day 3 in patients with AP
| Mean NGAL levels | Range (ng/mL) | Mean ± SD (ng/mL) | P value |
|---|---|---|---|
| Serum day‐1 | 175.63–1029.41 | 587.66 ± 251.5 | – |
| Serum day‐3 | 125.88–1029.41 | 573.98 ± 259.86 | 0.7 |
| Urine day‐1 | 54.99–658.23 | 252.84 ± 165.89 | – |
| Urine day‐3 | 45.29–514.70 | 202.36 ± 132.46 | 0.01 |
AP, acute pancreatitis; NGAL, neutrophil gelatinase‐associated lipocalin; SD, standard deviation.
Table 3.
Subgroup of patients with AP and their serum and urine NGAL levels
| Category of patient (n) | Serum NGAL (ng/mL) | Urine NGAL(ng/mL) |
|---|---|---|
| Transient AKI at admission (4) | 834.1 ± 170.3 | 281.9 ± 203.1 |
| Persistent AKI at admission (6) | 742.4 ± 194.3 | 406.6 ± 131.4 |
| New‐onset transient AKI after admission (6) | 654.5 ± 337.8 | 343.8 ± 123.6 |
| New‐onset persistent AKI after admission (5) | 787.4 ± 220 | 478.6 ± 167.4 |
| No AKI at admission (40) | 507.2 ± 234.4 | 215 ± 156 |
| No new‐onset AKI after admission (29) | 473.3 ± 197.8 | 158.2 ± 99.9 |
AKI, acute kidney injury; AP, acute pancreatitis; NGAL, neutrophil gelatinase‐associated lipocalin.
NGAL levels in predicting AKI
After excluding patients with AKI on day 1, the mean of both serum and urine NGAL levels on day 1 were significantly higher in patients who subsequently developed AKI compared to those who never had AKI (Table 4). After excluding patients with AKI on day 1, serum NGAL on day 1 at a cut‐off level of 790.9 ng/mL predicted AKI with a sensitivity and specificity of 64 and 96%, respectively (AUC = 0.8, P = 0.012). Similarly, urine NGAL on day 1 at a cut‐off level of 221 ng/mL predicted AKI with a sensitivity and specificity of 82 and 80%, respectively (AUC = 0.9, P < 0.001) (Fig. 1). Serum NGAL on day 1 at a cut‐off level of 790.9 ng/mL predicted persistent AKI with a sensitivity and specificity of 80 and 89%, respectively (AUC = 0.8, P = 0.019). Similarly, urine NGAL on day 1 at a cut‐off level of 395.3 ng/mL predicted persistent AKI with a sensitivity and specificity of 80 and 92%, respectively (AUC = 0.9, P = 0.003).
Table 4.
Serum and urine NGAL levels in AP patients with and without AKI (after excluding those who had AKI on day 1)
| NGAL | NGAL levels in patients with AKI (ng/mL) | NGAL level in patients without AKI (ng/mL) | P‐value |
|---|---|---|---|
| Serum day‐1 | 714.94 ± 285.04 | 473.29 ± 197.77 | 0.02 |
| Serum day‐3 | 742.61 ± 255.97 | 463.86 ± 222.33 | 0.01 |
| Urine day‐1 | 405.1 ± 154.11 | 158.25 ± 99.98 | <0.001 |
| Urine day‐3 | 286.37 ± 147.26 | 150.28 ± 93.54 | 0.03 |
AKI, acute kidney injury; AP, acute pancreatitis; NGAL, neutrophil gelatinase‐associated lipocalin.
Figure 1.
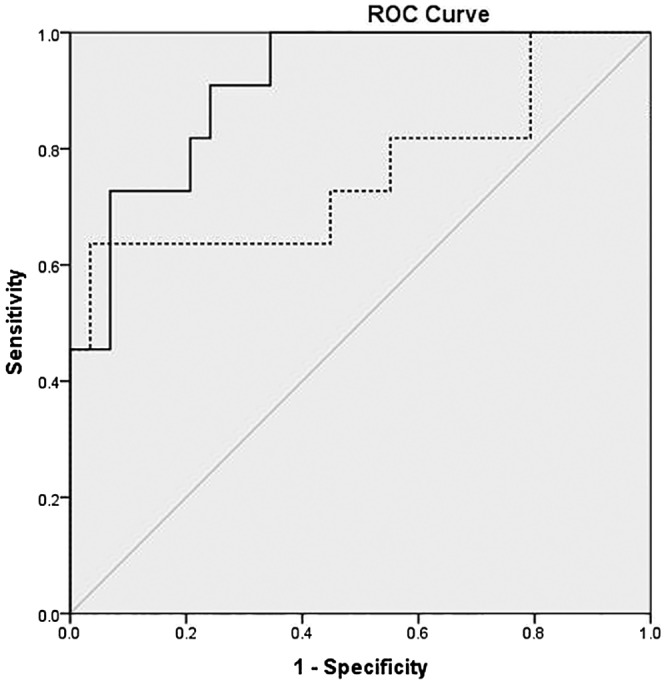
Receiver operating characteristic (ROC) curve of day 1 serum and urine neutrophil gelatinase‐associated lipocalin (NGAL) levels for prediction of acute kidney injury (AKI) (after excluding those who had AKI on day 1). ( ) Serum NGAL day 1; (
) Serum NGAL day 1; ( ) urine NGAL day 1; (
) urine NGAL day 1; ( ) reference line. AUC, area under the receiver operating curve.
) reference line. AUC, area under the receiver operating curve.
NGAL levels in predicting severity
The mean day 1 serum NGAL levels in patients with mild, moderate, and severe AP after excluding those with AKI on day 1 were 433.6 ± 213.5 ng/mL, 532 ± 181.8 ng/mL, and 669.3 ± 286.2 ng/mL, respectively (P = 0.037) (Fig. 2). Similarly, the mean day 1 urine NGAL levels in patients with mild, moderate, and severe AP were 128.3 ± 62.6 ng/mL, 189.3 ± 123.6 ng/mL, and 372.9 ± 168.3 ng/mL, respectively (P < 0.00) (Fig. 2). The mean serum and urine NGAL levels in the non‐severe group (mild and moderate, n = 27) were 477.3 ± 286.2 ng/mL and 155.4 ± 97.6 ng/mL, respectively. The serum and urine NGAL levels were significantly higher in the severe compared to the non‐severe group (P = 0.04 & P < 0.001). A cut‐off serum NGAL level on day 1 of 706.4 ng/mL had a sensitivity and specificity of 61.5 and 85.2%, respectively, to predict the development of severe AP (AUC = 0.7, P = 0.45). Similarly, the cut‐off NGAL urine level on day 1 of 195.5 ng/mL had a sensitivity and specificity of 84.6 and 87.8%, respectively, to predict severe AP (AUC = 0.87, P < 0.01). By definition, patients with AKI either have moderate or severe AP. After the exclusion of patients with AKI on day 1, all the other patients who developed AKI also had SAP either due to AKI or other organ failures. None of these patients had moderate AP despite having transient organ failure.
Figure 2.
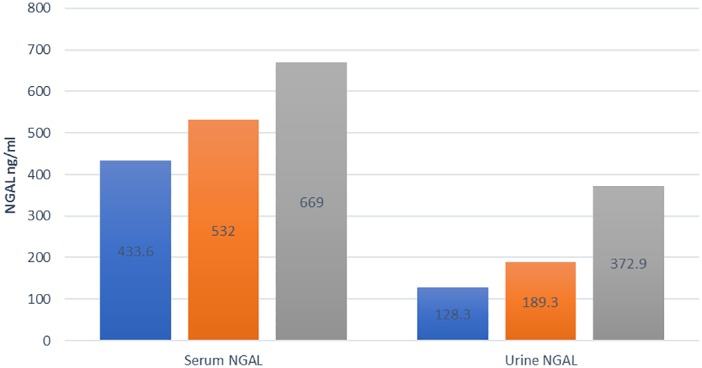
Mean serum and urine neutrophil gelatinase‐associated lipocalin (NGAL) in mild, moderate, and severe acute pancreatitis (AP). After excluding patients with acute kidney injury on day 1, n = 40. Mean ± standard deviation of serum and urine NGAL levels in patients with mild, moderate, and severe AP. anova (combined data), F = 3.61 and 14.45, P = 0.037 and < 0.001, respectively). ( ), Mild AP; (
), Mild AP; ( ), moderate AP; (
), moderate AP; ( ), severe AP.
), severe AP.
NGAL levels in predicting death
Overall, 10 (20%) patients died in the study group of patients with AP. After excluding patients with AKI on day 1, mean urine NGAL level on day 1 was significantly higher in patients who died compared to those who did not (226.8 ± 150.9 and 443.3 ± 155.9 ng/mL, P = 0.02). However, there was no significant difference between the mean serum NGAL level on day 1 between patients who died compared to those who survived (570.9 ± 245.5 and 709.9 ± 284.5 ng/mL, P = 0.2). Day 1 serum NGAL levels at a cut‐off value of 420.58 ng/mL predicted death in patients with AP with a sensitivity and specificity of 75 and 35%, respectively, with an AUC of 0.56. Day 1 urine NGAL at a cut‐off value of 378.2 ng/mL predicted death in patients with AP with a sensitivity and specificity of 83 and 80%, respectively, with an AUC of 0.838.
Discussion
We found AKI to be a common complication of AP. Most patients developed AKI within the first week of illness, with half of them having AKI at presentation. NGAL was significantly elevated in patients with severe AP and in patients who died.
We found that urinary and serum NGAL levels were elevated in patients with AKI, when compared with those without AKI. These results are similar to a recent published study.19 In addition, when we looked at the value of NGAL on day 1 in predicting subsequent development of AKI, we found that both serum and urine NGAL levels were significantly higher in patients who went on to develop AKI. At a cut‐off value of 221 ng/mL, urinary NGAL on day 1 had an AUC of 0.909 to predict the development of AKI. In a systematic review and meta‐analysis by Haase et al. on the role of urinary and or serum NGAL as a biomarker for predicting AKI in different diseases, the AUC for NGAL to predict AKI was 0.8. The AUC for serum and urinary NGAL was similar (0.7 and 0.8, respectively).14 None of the studies in the meta‐analysis included AP patients.
Serum NGAL levels were higher than urinary NGAL levels on day 1 as well as day 3 in our study. This is probably due to predominantly extrarenal source(s) of NGAL in patients with AP, which is not completely filtered by the glomeruli and is also reabsorbed by the tubules. Our study also found that urinary NGAL levels were better than serum NGAL levels in predicting the subsequent development of AKI. Koyner et al. studied the role of serum and urinary NGAL levels in patients undergoing elective cardiac surgery and found that urine NGAL was better in predicting the development of AKI postsurgery.26A recent meta‐analysis, however, concluded that serum NGAL was as good a predictor of AKI as urinary NGAL.14
There are two studies that have evaluated the utility of NGAL as a marker of severity in patients with AP. Chakraborty et al. used serum NGAL levels to differentiate mild and severe AP. In their study, NGAL levels were quantified in serum from 28 mild acute pancreatitis (MAP) and 16 SAP cases and compared with 28 CP and 30 healthy control samples. Mean serum NGAL levels were significantly higher in SAP compared to MAP, CP, and healthy controls.15 In another study, urinary NGAL levels were found to be significantly elevated in patients with severe AP and in patients who died. Elevated NGAL levels at admission and after 24 h could predict severe AP and mortality.16 In our study, we also found that urinary and serum NGAL levels could differentiate mild, moderate, and severe AP. NGAL is a glycoprotein released from areas of inflammation from activated neutrophils, so it stands to reason that NGAL levels are elevated in pancreatic inflammation. It can be argued that organ failure reflects the severity of pancreatitis, and hence, NGAL levels are expected to be elevated in its presence and would thus correlate with the severity of AP. All patients with AKI in our group, after excluding those with AKI on day 1, had SAP, which goes to show that AKI is, in fact, a manifestation of SAP rather than the cause of it.
Chakraborty et al. found that there was a steady decline in serum NGAL levels 48 h from the onset of severe AP.15 In a mouse model of AP, they observed that serum NGAL levels after 6 h of saline or taurocholate infusion into the common bile duct (to induce AP) were significantly higher compared to control mice. Serum NGAL levels in SAP were significantly higher than those with MAP at 24 h and 48 h; the levels started declining after 24 h. Although most of our patients presented to emergency after 48 h of onset of symptoms, there was a decline in serum and urine NGAL levels from day 1 to day 3 of admission, which was statistically significant only for urinary levels. In addition, most of the patients had AKI in the first week of illness. This supports the case for using day 1 NGAL levels as a marker of AKI and for prognosticating AP.
The source of NGAL rise in patients with AP can be both renal, due to injury or stress, and extrarenal. There are no prior studies looking at the precise origin of NGAL in patients with AP. Extrapolating the available evidence, NGAL appears to come from both pancreatitis and renal damage. However, the bulk of NGAL that is released into the bloodstream is probably related to either direct pancreas injury or its effects on the rest of the body including kidney. This is shown by the fact that the mean NGAL level is much higher in the AP population compared to the levels quoted in literature in patients with AKI alone. We also excluded patients who had AKI at the time of evaluation to remove this bias. Renal cause of NGAL rise could be due to ischemia and cytokine‐mediated injury to the tubules.27 We hypothesize that fluid sequestration, systemic inflammatory response syndrome, and hypovolemia in AP lead to renal hypoperfusion and hypoxia‐induced injury to the medullary tubules of the kidney. In a mouse model where lipopolysaccharide (LPS) was injected to induce renal injury over the next 3–12 h, there was elevation of NGAL mRNA expression within the tubular epithelia during the same time. There was a transient increase in tumor necrosis factor (TNF)‐alpha levels within 3 h of LPS administration, with a strong correlation between TNF‐alpha and NGAL mRNA expression. They concluded that NGAL upregulation was sensitive to LPS‐induced AKI.28 TNF‐alpha is known to be elevated early in patients with AP and is believed to mediate many of the detrimental consequences of AP, including AKI.29 The probable extrarenal source of NGAL is most likely from activated neutrophils and macrophages, which could explain the rise of NGAL levels in all patients with AP. This is further supported by evidence from a study where a significant correlation was found between urinary NGAL levels in patients with AKI and serum neutrophil myeloperoxidase levels.30 We believe that the renal damage is more a manifestation of the SAP. The presence of AKI shows that the inflammation was more severe, which led to, possibly, renal hypoperfusion that was enough to cause acute tubular necrosis (ATN).
Haase et al., in their meta‐analysis, showed the utility of NGAL as a sensitive and specific prognostic marker with respect to predicting requirements for renal replacement therapy and hospital mortality in various predisposing conditions, such as critically ill adults and children and after cardiac surgery. The cut‐off values to predict requirement for renal replacement ranged from >80 to >680 ng/mL, and the cut‐off value to predict mortality ranged from >80 to >570 ng/mL.14 Our study also found that a cut‐off value of 378.2 ng/mL urine NGAL predicted mortality with a sensitivity of 83% and specificity of 80%.
Our study was limited by the small number of patients; further studies with larger numbers of patients would establish it as a marker of AKI and severity of AP. Most patients of AP who present to our tertiary establishment are referred from smaller hospitals from surrounding areas with a delay for close to 24–48 h. We believe that using NGAL on the first day of symptom onset would predict the onset of AKI and severity better, and study designs to include this as criteria would be prudent. We could not exclude pre‐existing renal disease in patients who had elevated urea and creatinine levels at admission. However, all such patients (survivors) reverted to normal renal functions at the time of discharge or on follow‐up.
In conclusion, the present study shows that NGAL levels in urine and serum can predict the development of AKI, including the more severe ‘persistent AKI’ in patients with AP. NGAL levels also predict severity of AP. To the best of our knowledge, ours is the only study to use urine and serum NGAL for prediction of AKI in patients of AP. Development of renal injury is a critical event that significantly increases the morbidity and mortality of patients with AP, and so, its early prediction would improve chances of salvaging renal function. Hence, it makes sense to recommend NGAL estimation at admission. Urine NGAL appears to be more sensitive and specific compared to serum NGAL in predicting AKI and severity of AP as well as mortality.
Acknowledgments
The authors were supported by grants from the Postgraduate Institute of Medical Education and Research (PGIMER). The study sponsors (PGIMER, Chandigarh) approved the proposal with regard to its design and data analysis but had no role in data collection, analysis, interpretation, drafting, and overviewing of the paper. The corresponding author had full access to all the data in the study and had final responsibility for the decisions to submit for publication.
Declaration of conflict of interest: Pradeep K Siddappa certifies that none of the authors of this manuscript have any conflict of interest that could either directly or indirectly, purposefully or inadvertently affect the conduct, outcome, or reporting of any scholarly activity related to this document. Authors have nothing to disclose.
Funding support: Postgraduate Institute of Medical Education and Research
REFERENCES
- 1. LjutiĆ D, PiploviĆ‐VukoviĆ T, Raos V, Andrews P. Acute renal failure as a complication of acute pancreatitis. Ren. Fail. 1996; 18: 629–33. [DOI] [PubMed] [Google Scholar]
- 2. Devarajan P. Neutrophil gelatinase‐associated lipocalin (NGAL): a new marker of kidney disease. Scand. J. Clin. Lab. Invest. 2008; 68 (Suppl. 241): 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta‐analysis. Am. J. Kidney Dis. 2002; 40: 221–6. [DOI] [PubMed] [Google Scholar]
- 4. Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin‐18 is a marker of human acute tubular necrosis. Am. J. Kidney Dis. 2004; 43: 405–14. [DOI] [PubMed] [Google Scholar]
- 5. Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule‐1 (KIM‐1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002; 62: 237–44. [DOI] [PubMed] [Google Scholar]
- 6. Grzyb J, Latowski D, Strzałka K. Lipocalins–a family portrait. J. Plant Physiol. 2006; 163: 895–915. [DOI] [PubMed] [Google Scholar]
- 7. Friedl A, Stoesz S, Buckley P, Gould M. Neutrophil gelatinase‐associated lipocalin in normal and neoplastic human tissues. Cell type‐specific pattern of expression. Histochem. J. 1999; 31: 433–41. [DOI] [PubMed] [Google Scholar]
- 8. Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore‐mediated iron acquisition. Mol. Cell. 2002; 10: 1033–43. [DOI] [PubMed] [Google Scholar]
- 9. Schmidt‐Ott KM, Mori K, Li JY et al Dual action of neutrophil gelatinase–associated lipocalin. J. Am. Soc. Nephrol. 2007; 18: 407–13. [DOI] [PubMed] [Google Scholar]
- 10. Flo TH, Smith KD, Sato S et al Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004; 432: 917–21. [DOI] [PubMed] [Google Scholar]
- 11. Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Med. 2004; 30: 33–7. [DOI] [PubMed] [Google Scholar]
- 12. Cruz DN, Goh CY, Palazzuoli A et al Laboratory parameters of cardiac and kidney dysfunction in cardio‐renal syndromes. Heart Fail. Rev. 2011; 16: 545–51. [DOI] [PubMed] [Google Scholar]
- 13. Mori K, Lee HT, Rapoport D et al Endocytic delivery of lipocalin‐siderophore‐iron complex rescues the kidney from ischemia‐reperfusion injury. J. Clin. Investig. 2005; 115: 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase‐Fielitz A. Group NM‐aI. Accuracy of neutrophil gelatinase‐associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta‐analysis. Am. J. Kidney Dis. 2009; 54: 1012–24. [DOI] [PubMed] [Google Scholar]
- 15. Chakraborty S, Kaur S, Muddana V et al Elevated serum neutrophil gelatinase‐associated lipocalin is an early predictor of severity and outcome in acute pancreatitis. Am. J. Gastroenterol. 2010; 105: 2050–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lipinski M, Rydzewska‐Rosolowska A, Rydzewski A, Rydzewska G. Urinary Neutrophil Gelatinase–Associated Lipocalin as an Early Predictor of Disease Severity and Mortality in Acute Pancreatitis. Pancreas. 2015; 44: 448–52. [DOI] [PubMed] [Google Scholar]
- 17. Sporek M, Gala‐Błądzińska A, Dumnicka P et al Urine NGAL is useful in the clinical evaluation of renal function in the early course of acute pancreatitis. Folia Med. Cracov. 2016; 56: 13–25. [PubMed] [Google Scholar]
- 18. Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am. J. Gastroenterol. 2006; 101: 2379. [DOI] [PubMed] [Google Scholar]
- 19. Banks PA, Bollen TL, Dervenis C et al Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013; 62: 102–11. [DOI] [PubMed] [Google Scholar]
- 20. Levey AS, Coresh J, Bolton K et al K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. 2002; 39 (2 Suppl): 1–237. [PubMed] [Google Scholar]
- 21. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit. Care Med. 1995; 23: 1638–52. [DOI] [PubMed] [Google Scholar]
- 22. Johnson C, Abu‐Hilal M. Persistent organ failure during the first week as a marker of fatal outcome in acute pancreatitis. Gut. 2004; 53: 1340–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mofidi R, Duff M, Wigmore S, Madhavan K, Garden O, Parks R. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br. J. Surg. 2006; 93: 738–44. [DOI] [PubMed] [Google Scholar]
- 24. Lytras D, Manes K, Triantopoulou C et al Persistent early organ failure: defining the high‐risk group of patients with severe acute pancreatitis? Pancreas. 2008; 36: 249–54. [DOI] [PubMed] [Google Scholar]
- 25. Mehta RL, Kellum JA, Shah SV et al Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit. Care. 2007; 11: R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koyner JL, Bennett MR, Worcester EM et al Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008; 74: 1059–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mishra J, Ma Q, Prada A et al Identification of neutrophil gelatinase‐associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003; 14: 2534–43. [DOI] [PubMed] [Google Scholar]
- 28. Han M, Li Y, Liu M, Li Y, Cong B. Renal neutrophil gelatinase associated lipocalin expression in lipopolysaccharide‐induced acute kidney injury in the rat. BMC Nephrol. 2012; 13: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malleo G, Mazzon E, Siriwardena AK, Cuzzocrea S. Role of tumor necrosis factor‐α in acute pancreatitis: from biological basis to clinical evidence. Shock. 2007; 28: 130–40. [DOI] [PubMed] [Google Scholar]
- 30. Mårtensson J, Bell M, Oldner A, Xu S, Venge P, Martling C‐R. Neutrophil gelatinase‐associated lipocalin in adult septic patients with and without acute kidney injury. Intensive Care Med. 2010; 36: 1333–40. [DOI] [PubMed] [Google Scholar]


