Abstract
Purpose:
Prior clinical trials evaluating cisplatin for non–muscle-invasive bladder cancer (NMIBC) were stopped due to local and systemic toxicity. Currently, there is still a need for improved intravesical therapies, and nanoparticle-based CDDP may be efficacious without the toxicity of free cisplatin observed in the past.
Experimental Design:
Cisplatin nanoparticles (CDDP NPs) were developed using biocompatible poly(L-aspartic acid sodium salt; PAA), both with and without low and high grafting density of methoxy-polyethylene glycol (PEG). In vitro cytotoxicity studies confirmed activity of CDDP NPs and CDDP solution against a papillary bladder cancer cell line. Local toxicity was assessed by three weekly intravesical administrations of CDDP formulations. CDDP NPs and CDDP solution were evaluated for bladder absorption in murine models 1 and 4 hours after intravesical administration. In vivo efficacy was evaluated in an immunocompetent carcinogen model of NMIBC.
Results:
CDDP NPs showed decreased local toxicity, as assessed by bladder weight, compared with CDDP solution. Furthermore, >2 μg/mL of platinum was observed in mouse serum after intravesical administration of CDDP solution, whereas serum platinum was below the limit of quantification after intravesical administration of CDDP NPs. CDDP NPs provided significantly increased (P < 0.05) drug levels in murine bladders compared with CDDP solution for at least 4 hours after intravesical administration. In vivo, CDDP NPs reduced cancer cell proliferation compared with untreated controls, and was the only treatment group without evidence of invasive carcinoma.
Conclusions:
Cisplatin-loaded PAA NPs have the potential to improve intravesical treatment of NMIBC while reducing local and systemic side effects.
Introduction
Bladder cancer is the seventh most common malignancy worldwide, with a 5-year global prevalence of approximately 1.3 million people (1). Approximately 70% of patients present with non–muscle-invasive bladder cancer (NMIBC), which is managed primarily by trans-urethral (endoscopic) resection of the bladder tumor followed by intravesical instillation of anticancer therapeutics (2). For more than 35 years, intravesical Bacillus Calmette-Guerin (BCG) has been the mainstay for intravesical treatment of NMIBC. However, for the more than 50% of patients that will recur, progress, or be intolerant to BCG, there is no standard alternative intravesical therapy (3). Indeed, a major limitation in designing more effective intravesical therapies is the inherent dilution and low retention of the therapeutic agent due to urine production and bladder voiding (4, 5).
For patients with locally advanced or metastatic bladder cancer, systemic cisplatin (CDDP)-based chemotherapy is the mainstay of treatment, with superior efficacy to other chemotherapeutic agents (6). On the basis of this experience, in 1981 the European Organization for Research on the Treatment of Cancer (EORTC GU Group) initiated a prospective randomized trial comparing intravesical thiotepa, doxorubicin, and CDDP for the treatment of superficial bladder cancer (EORTC 30782). Of the 50 patients who received CDDP for more than 4 months, three (6%) stopped treatment early due to chemical cystitis, and seven (14%) had an anaphylactic reaction attributed to systemic drug absorption resulting in hypotensive shock (7). As a result, the CDDP arm of the study was suspended, which effectively ended investigations of CDDP for the treatment of NMIBC. Therefore, there is a great need to not only develop intravesical chemotherapies with prolonged bladder retention and absorption, but also to minimize local and systemic toxicity. Because CDDP is the most effective treatment for metastatic and advanced cancers, it has great promise for treating NMIBC if the toxicity can be mitigated.
Nanotechnology approaches can improve the efficacy of and/or reduce adverse effects associated with various chemotherapeutics by controlling drug release and modulating biodistribution (8). In the case of CDDP delivery, polyethylene glycol (PEG)-coated CDDP NPs have been explored for systemic treatment of various cancers in preclinical animal models and phase I/II human trials in solid tumors, and have shown promise for reducing systemic side effects associated with CDDP while maintaining efficacy (9–16). Furthermore, our previous studies demonstrated that densely PEG-coated CDDP NPs improved local CDDP delivery, efficacy, and survival compared to uncoated CDDP NPs in preclinical orthotopic models of lung carcinoma and glioblastoma (17, 18). Thus, we hypothesized that intravesical NP-mediated delivery of CDDP may reduce the local bladder toxicity observed in clinical trials while promoting intravesical drug uptake and prolonged bladder retention, and that PEG-coated NPs would be more efficacious than uncoated particles as an intravesical CDDP treatment for NMIBC.
NPs were composed of CDDP conjugated to poly(L-aspartic acidsodiumsalt) (PAA) or methoxy-poly(ethyleneglycol)-block-PAA (PEG–PAA) polymers with low or high PEG content. We show that, unlike intravesical administration of free CDDP, intravesical administration of PAA-CDDP nanoparticles (PAA-CDDP NPs) or PEG-PAA-CDDP nanoparticles (PEG-PAA-CDDP NPs) in mice did not result in detectable systemic CDDP levels. Furthermore, repeated intravesical administration of free CDDP caused extensive bladder tissue hyperplasia and increases in bladder weights, whereas intravesical administration of PAACDDP NPs and PEG-PAA-CDDP NPs did not. Unexpectedly, only PAA-CDDP NPs provided a statistically-significant increase in CDDP levels in bladder tissues at 1- and 4-hour time points, whereas PEG-containing NP formulations did not. Finally, using an immune-competent rat model of NMIBC, we demonstrate that PAA-CDDP NPs had improved antiproliferative activity compared to untreated tumors and was the only treatment group without evidence of invasive carcinoma. Taken together, our data suggest that CDDP NPs hold promise as a potentially safer and/or more effective method for intravesical CDDP treatment for patients with NMIBC who are found to be BCG unresponsive.
Materials and Methods
Materials
PAA with molecular weight (MW) of 27 kDa and linear methoxy-poly(ethylene glycol)-block-PAA (PEGlow-PAA, 5 kDa PEG block/27 kDa PAA block) were purchased from Alamanda Polymers. PEGhigh-PAA (27 kDa PAAwith 10 × 5 kDa PEG chains) was synthesized in the lab according to reported methods (see Supplementary Methods). Cis-diamminedichloroplatinum (cisplatin or CDDP, 99.9%), silver nitrate, and 100-kDaAmicon-Ultra-2mL filters were purchased from Sigma-Aldrich. Twenty- and 50-kDa dialysis cassettes were obtained from Spectrum Labs. Alexa Fluor 647-cadaverine was purchased from Thermo Fisher Scientific. PEG (NH2-PEG-OCH3; MW: 5 kDa) was purchased from Creative PEG works and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) was purchased from Invitrogen.
Nanoparticle formulation
PAA and CDDP were dissolved in nuclease-free water at a concentration of 1 and 1.5 mg/mL, respectively. PAA solution was mixed with CDDP solution in 1:1 volume ratio, and the mixture was continuously stirred and protected from light. After 3 days at room temperature, self-assembled PAA-CDDP NPs were formed. PAA-CDDP NPs were washed once with ultrapure water using 100 kDa Amicon-Ultra centrifugal filters to remove excess CDDP and PAA. For formulation of fluorescent PAA-CDDP-NPs (AF647-PAA-CDDP NPs), AF647-PAA (synthesis described in Supplementary Methods) and PAA were dissolved in water at a ratio of 2:3 to obtain a total concentration of 1 mg/mL. The remaining process for NP formation was the same as described for PAA-CDDP NPs. Details on the formulation of PEG-PAA-CDDP NPs are given in Supplementary Methods.
Nanoparticle characterization
Following purification, all NP formulations were characterized for particle size, polydispersity index (PDI), and surface charge (as measured by ζ-potential) using a Malvern Zetasizer Nano ZS90. Particle size measurements were carried out at a scattering angle of 173°. For ζ-potential, NPs were diluted with 10 mmol/L NaCl solution at pH 7. Transmission electron microscopy (TEM) images were obtained using a Hitachi H7600 microscope. Drug loading was determined using atomic absorption spectroscopy (AAS). Briefly, 10 μL of nanoparticle stock solution was diluted with 990 μL of 2.2% nitric acid, and platinum (Pt) content was measured using an AAnalyst 800 atomic absorption spectrometer (Perkin-Elmer Inc.). A 1.0 mL volume of NP stock solution was then lyophilized in preweighed tubes and subsequently weighed to determine the mass of lyophilized NPs. Drug loading was calculated and reported as wt% (wt cisplatin/wt particles).
In vitro cytotoxicity assay
In vitro cytotoxicity of CDDP, carboplatin, PAA-CDDP NPs, PEGlow-PAA-CDDP NPs, and PEGhigh-PAA-CDDP NPs was evaluated using a superficial bladder cancer cell line (RT4) as well as invasive cell lines (J82 and 5673) in triplicate. Cells were purchased from the ATCC and cultured in McCoy’s 5A (Thermo Fisher Scientific) at 37°C under 5% CO2. Cells were seeded in 96-well tissue-treated culture plates at a density of 5 × 103 cells in 100 μL of complete medium and cultured overnight. Then, the supernatant was removed, and a known concentration of CDDP, carboplatin, or CDDP NPs (100 μL) was added into the wells. After 72 hours, cell viability was measured using CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega) according to manufacturer’s protocol. Acquired data were analyzed to depict sigmoidal dose–response curve with Graph Pad Prism 5 (Graph Pad Software Inc.) using the equation log(inhibitor) versus response-variable slope. To further characterize the cytotoxicity of each drug, RT4 cells were treated with either 3 μg/mL of CDDP or 3 μg/mL of PAA-CDDP NPs in a 37°C, 5% CO2 incubator for 48 hours. Cells were subsequently incubated with Hoechst33342 (Thermo Fischer Scientific), Apoxin (Abcam), and propidium iodide (Thermo Fischer Scientific). Images were taken by EVOS FL Cell Auto Imaging System (Thermo Fischer Scientific).
Animal welfare statement
All protocols involving animals strictly adhered to U.S. NIH guidelines and were approved by the Johns Hopkins Medical Institutions Animal Care and Use Committee.
Bladder uptake and retention of fluorescent NP in mice
Female CF-1 mice (age, 8 weeks) were purchased from Harlan and acclimated in the animal facility for 4 weeks. Mice were anesthetized with an isoflurane vaporizer and nose cone system and catheterized using polyethylene tubing mounted on a 30G needle. After catheterization, the bladder was emptied of urine by aspiration and/or gentle pressure on the abdomen. A total of 100 μL of AF647-PAA-CDDP NPs (CDDP content: 0.7 mg/mL) was instilled into the bladders of mice (n = 3 per group). Mice were maintained under anesthesia for 1 hour and then allowed to wake up, during which time they resumed free access to food and water. Bladder tissues were obtained at 1 and 4 hours to evaluate uptake and retention AF647-PAA-CDDP NP. Bladder tissues were dipped in PBS, gently squeezed with forceps to remove any residual fluid, transferred to plastic cryomolds filled with optimal cutting temperature (OCT) medium (Tissue-Tek), and subsequently frozen in liquid nitrogen. Frozen bladders were cryosectioned into 6-μm sections with a cryostat (Leica CM 3050S, Leica) and mounted onto positively charged microscope slides. Slides were washed with 1 × tris-buffered saline (TBS, Mediatech), dried, stained with DAPI (ProLong Gold antifade reagent with DAPI; Invitrogen), sealed with a cover slip, and imaged using a Zeiss confocal 710 laser scanning microscope in the DAPI and Cy5 channels.
Assessment of toxicity in mice
Mice were randomly divided into different groups (n ≥ 4), anesthetized, and catheterized as described above. Then, 100 μL of CDDP solution, PAA-CDDP NP, or PEGlow-PAA-CDDP NPs at 0.7 mg/mL CDDP content was instilled into the bladder. Mice were maintained under anesthesia for 1 hour and then allowed to wake up, during which time they resumed free access to food and water. To assess systemic exposure, mice were euthanized at 1, 4, and 24 hours and plasma samples were obtained for analysis of CDDP content. To assess local toxicity of CDDP solution and CDDP NPs, mice received a total of three intravesical doses spaced 1 week apart. Bladder tissues were obtained 24 hours after the third dose, photographed, and weighed.
CDDP uptake and retention in the bladders of rats
Female Fischer 344 rats (age, 7 weeks) were purchased from Harlan and acclimated in the animal facility for 1 week. Before starting the experiment, rats were randomly divided into different groups (n ≥ 5). Rats were anesthetized with an isoflurane vaporizer and nose cone system and catheterized using a 20G angiocatheter sheath. After catheterization, the bladder was voided using aspiration and/or gentle pressure on the abdomen. Then, 300 μL of CDDP solution, PAA-CDDP NP, PEGlow-PAA-CDDP NP, or PEGhigh-PAA-CDDP NP, each with CDDP content of 0.7 mg/mL, was instilled. Rats were maintained under anesthesia for 1 hour and then allowed to wake up, during which time they resumed free access to food and water. Plasma and bladder tissues were obtained at 1 and 4 hours for analysis of CDDP content. Bladder tissues were dipped in PBS and gently squeezed with forceps to remove any residual fluid. Details for the analysis of CDDP content in plasma and tissue samples can be found in the Supplementary Methods.
N-methyl-N-nitrosourea induced rat bladder carcinogenesis model
The N-methyl- N-nitrosourea (MNU) rat model of bladder cancer was chosen because it recapitulates human NMIBC, with carcinoma in situ (CIS), non-invasive papillary carcinoma (Ta), and high-grade invasive papillary carcinoma (T1) histologies, and progresses from dysplasia to NMIBC to MIBC (19). In addition, this model has been recently validated as a model reflective of human NMIBC, particularly with regard to its immune competency and clinical and immunologic response to intravesical immunotherapy and chemotherapy (20). In this model, the presence of NMIBC progresses from week 8 to week 15, at which point all rats have NMIBC. Fischer 344 female rats (age, 7 weeks) were anesthetized with an isoflurane vaporizer and nose cone system. After complete anesthesia and preparation of the surgical area, a 20G angiocatheter (BD) was placed into the rat’s urethra and the bladder was emptied of urine. MNU (1.5 mg/kg MNU dissolved in 0.30 mL of 0.9% saline, pH 6.0) was then instilled into the bladder under 45 minutes of continued sedation to prevent spontaneous micturition and allow absorption. To explore the potential for treatment to prevent recurrence, as previously described, we initiated treatment weekly from weeks 8 to 14. Treatment groups (n = 5 per group) included CDDP and PAA-CDDP NP dosed weekly. All formulations were delivered at a concentration of 0.7 mg/mLin 300 μL. Rats were sacrificed at week 15 for tissue analysis.
Tissue staining and analysis
Bladders were paraffin embedded, sectioned, and stained with hematoxylin–eosin for classification of tumor stage according to the World Health Organization/International Society of Urological Pathology consensus. Tumor staging was performed in a blinded fashion by a board certified genitourinary pathologist. For IHC staining, high-temperature antigen retrieval (18–23 psi/126°C) was performed by immersing the slides in Trilogy (Cell Marque). Endogenous peroxidase activity was blocked for 5 minutes in using Dual Endogenous Enzyme Block (Dako S2003). Primary Antibodies used were KI 67 (Abcam; ab16667) and cleaved caspase-3 (Cell Signaling Technology; ab9661). Slides were stained with Impact DAB (Vector Labs) for 3 minutes and counterstained with hematoxylin (Richard-Allen). For each section, Ki67+ cells were counted in 10 random 400× fields. Immunofluorescence was performed on a separate set of slides which were stained for TUNEL per manufacturer instructions (ApopTag Peroxidase In Situ Apoptosis Detection Kit, Millipore) and counterstained with DAPI (Vector Laboratories). For each section, apoptotic cells were counted in five random fields.
Statistical analysis
Statistical analysis was performed using Prism 5 (GraphPad). One-way ANOVA tests with Bonferroni adjustment for multiple comparisons were conducted and results were considered statistically significant at P ≤ 0.05.
Results
Formulation and physicochemical characterization of CDDP NPs
PAA-CDDP NPs were 140 ± 5 nm in size with a narrow size distribution (PDI 0.2 ± 0.1) and negative ζ-potential (−35 ± 2.0 mV) due to the presence of carboxylate groups that did not react with cisplatin on the polymer backbone. Partitioning of PEG to the NP surface led to comparably smaller size and near-neutral ζ-potential for PEGlow-PAA-CDDP NP (49 ± 2 nm, 3.9 ± 0.1 mV) and PEGhigh-PAA-CDDP NP (45 ± 2 nm, −3.3 ± 0.5 mV; Supplementary Table S1). These observations are in accordance with published data from our group and others (17, 18, 21, 22). All types of CDDP NPs were spherical in shape (Supplementary Fig. S1), and CDDP loading ranged between 17% and 40% (Supplementary Table S1).
CDDP NP retain anticancer activity of CDDP
Drugs like carboplatin are prepared by coordination of platinum with carboxylate groups, and due to this process, they slowly release CDDP which results in considerably lower cytotoxicity to cancer cells in vitro compared to CDDP (23, 24). CDDP NP formation involves physical association between CDDP and the carboxylate groups of PAA, leading to sustained dissociation and release of CDDP in vitro (18). To assess the impact of CDDP coordination to PAA, we evaluated the anticancer activity of CDDP NP in vitro. Specifically, we tested the cytotoxicity of CDDP, carboplatin, and various CDDP NP in vitro against a superficial bladder cancer cell line (RT4), as well as two MIBC cell lines (J82, 5637). All types of CDDP NP showed IC50 values that were by two- to sixfold higher compared to CDDP, likely reflecting the effect of the slow CDDP release on cytotoxicity. However, all CDDP NP were considerably more cytotoxic (two- to 11-fold) compared to carboplatin (Supplementary Table S2). Additional in vitro cell death assays confirmed the cytotoxic effects of both CDDP and PAA-CDDP NPs (Supplementary Fig. S2).
Intravesically administered PAA-CDDP NPs are retained in the bladder, yet reduce systemic exposure and local toxicity of CDDP in mice
Healthy mice were utilized to compare bladder uptake and systemic absorption of CDDP in order to identify NP formulations with the highest likelihood of improving efficacy and reducing toxicity. We found that CDDP levels in the mouse bladders were similar 1 hour after intravesical administration of CDDP compared with PAA-CDDP NPs and PEGlow-PAA-CDDP NPs (Fig. 2A). In contrast, CDDP levels were much lower in bladder tissue 1 hour after intravesical administration of PEGhigh-PAA-CDDP NPs (Fig. 2A). At 4 hours after intravesical administration (3 hours of ambulatory time), only PAA-CDDP NP-treated mice had detectable levels of CDDP in their bladders (Fig. 2A). Moreover, the concentration of CDDP 4 hours after PAA-CDDP NP administration was >80% of the amount observed at 1 hour, suggesting that the PAA-CDDP NPs were retained in the bladder after urination. We confirmed this observation by fluorescent imaging of fluorescently labeled CDDP-PAA NPs with similar physicochemical properties to PAA-CDDP NPs (Supplementary Table S1). Confocal imaging of bladder sections 1 hour after intravesical administration of fluorescent PAA-CDDP NP showed uniform distribution and tissue accumulation that persisted for 4 hours (Fig. 1). At 24 hours, CDDP levels in the bladder were BLQ for all formulations (Fig. 2A).
Figure 2.
CDDP NPs improve retention of CDDP and reduce systemic exposure and local toxicity of CDDP in mice. A, CDDP levels in the mouse bladder 1 hour after intravesical administration of CDDP solution, PAA-CDDP NPs, or PEGlow-PAA-CDDP NPs were similar. In contrast, CDDP levels in bladder tissue were much lower 1 hour after intravesical administration of PEGhigh-PAA-CDDP NPs. Four hours after intravesical administration, CDDP was only detectable in bladder tissue in mice that received intravesical PAA-CDDP NPs. CDDP levels in tissue were BLQ for all formulations 24 hours after intravesical administration. B, Intravesical administration of CDDP solution to mice resulted in ~2 μg/mL of CDDP in plasma within 1 hour, whereas CDDP levels were BLQ after intravesical administration of CDDP-loaded NPs. C, Three weekly intravesical administrations of CDDP solution led to significant increase (P < 0.01) in bladder weight compared to mice treated with PAA-CDDP NPs, PEGlow-PAA-CDDP NPs, and untreated controls. PEGhigh-PAA-CDDP NPs were not included due to low CDDP absorption in bladder tissue. CDDP content in all the formulations used for the experiments was 0.7 mg/mL and CDDP limit of quantification was 250 ng/mL.
Figure 1.
Intravesically administered PAA-CDDP NPs are retained in the bladder for up to 4 hours. Fluorescent PAA-CDDP NPs (CDDP content: 0.7 mg/mL) were intravesically administered to mice maintained under anesthesia for 1 hour. Bladders were resected at 1 and 4 hours to evaluate uptake and retention of PAA-CDDP NPs confocal imaging of bladder sections 1 hour after intravesical administration of fluorescent PAA-CDDP NPs showed uniform distribution and tissue accumulation that was visible for up to 4 hours after administration.
We then evaluated how the uptake and retention of CDDP NP impacted local and systemic CDDP-related toxicity. We found that intravesical administration of CDDP solution in mice resulted in rapid systemic exposure at 1 hour (>2 μg/mL in plasma), whereas none of the mice dosed with CDDP NP had detectable amounts of CDDP in their plasma (Fig. 2B). At 4 and 24 hours, CDDP was below the limit of quantification (BLQ; <250 ng/mL) in plasma for all treatment groups (not shown). Furthermore, three weekly intravesical administrations of the CDDP solution led to significant increases in bladder weights due to hyperplasia compared to PAA-CDDP NP, PEGlow-PAA-CDDP NPs, and untreated controls (Fig. 2C). No differences in animal weights were observed between treatments. PEGhigh-PAA-CDDP NPs were not tested for local bladder toxicity due to the low CDDP absorption shown in Fig. 2. The increases in bladder tissue weights after CDDP treatment were also confirmed by gross observations of bladder size (Supplementary Fig. S3). Given that bladders treated with PAA-CDDP NPs continued to retain drug 4 hours after delivery with no evidence of systemic exposure, this formulation was selected for efficacy experiments in the rat MNU model of NMIBC.
PAA-CDDP NPs improve uptake and retention of CDDP in rats
Prior to initiating studies for in vivo efficacy in a rat model of NMIBC, we examined whether PAA-CDDP NPs provided improved bladder retention of CDDP compared to a CDDP solution in healthy rats. PAA-CDDP NPs provided significantly increased CDDP concentration (sixfold increase) in rat bladders at 1 hour after intravesical administration compared to CDDP solution (P < 0.05; Fig. 3). Similarly, CDDP levels were BLQ in rat bladders 4 hours after administration of CDDP solution, whereas the CDDP concentration 4 hours after PAA-CDDP NPs dosing was maintained at 45% of the concentration at 1 hour (Fig. 3). Similar to what was observed in mice (Fig. 2), CDDP levels at 1 hour were much lower for PEGhigh-PAA-CDDP NPs, and CDDP levels were BLQ in bladder tissues 4 hours after dosing with both the PEGlow- and PEGhigh-NP formulations (Fig. 3). Having observed in both mice and rats that CDDP absorption and retention in bladder tissue was reduced with PEG-containing CDDP NPs, we focused on comparing PAA-CDDP NPs to cisplatin solution for in vivo efficacy studies.
Figure 3.
PAA-CDDP NPs improve uptake and retention of CDDP in rats. PAA-CDDP NPs provided significantly increased CDDP levels in the rat bladder (P < 0.05) compared to CDDP solution 1 hour after intravesical administration. PEGylated PAA-CDDP NPs provided decreased CDDP absorption compared to PAA-CDDP NPs, although not significantly different. Four hours after administration, CDDP levels were only detectable in bladder tissue in animals receiving intravesical PAA-CDDP NPs. CDDP content in all the formulations used for the experiment was 0.7 mg/mL.
In vivo efficacy assessment
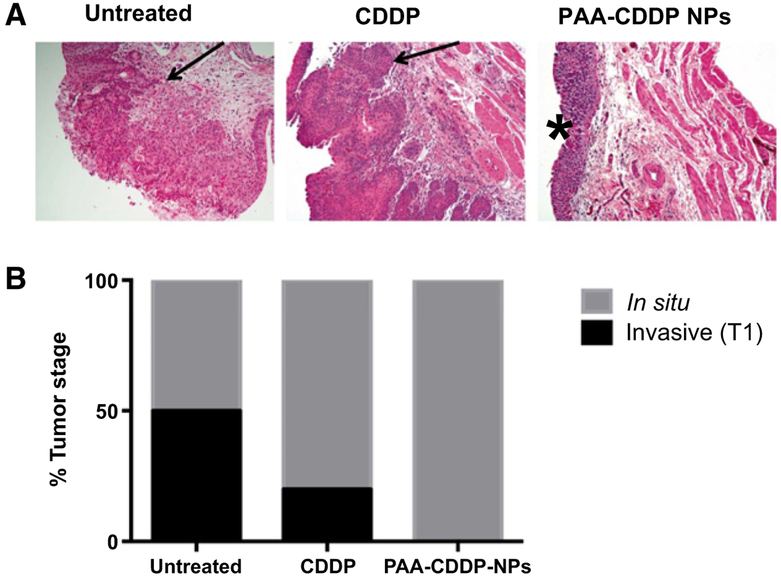
Histopathologic and IHC analysis of rat bladder tissues showed significant progression of bladder cancer in untreated controls by week 16 after MNU instillation, with evidence of CIS or high-grade invasive papillary carcinoma (T1) in all specimens, validating the MNU-induced rat bladder carcinogenesis model (Fig. 4). Gross histopathologic analysis of bladder tissues showed that rats treated with intravesical PAA-CDDP NPs had no signs ofT1 grade tumors at week 16, whereas at least 20% of rats treated with CDDP and 50% of untreated rats had T1 high-grade tumors in the bladder at week 16 (Fig. 4). Furthermore, bladder tissues from rats treated with intravesical PAA-CDDP NPs had significantly decreased Ki67+ staining, a marker of cancer cell proliferation, compared to untreated controls (P< 0.05; ref. 25). Bladder tissues from rats treated with intravesical CDDP solution had decreased Ki67+ staining, although statistically indistinguishable from the untreated control group (Fig. 5). TUNEL staining of apoptotic cells showed no statistically significant differences in apoptosis between treatment groups or untreated tumor controls, a finding that was confirmed with IHC of cleaved caspase-3 (Fig. 6 and Supplementary Fig. S4).
Figure 4.
PAA-CDDP NPs show efficacy in MNU-induced rat bladder carcinogenesis model. A, Representative histologic sections of untreated rats and rats treated with intravesical CDDP solution or PAA-CDDP NPs, and the corresponding (B) bladder cancer staging of untreated rat bladders and rat bladders treated with CDDP solution and PAA-CDDP NPs. CDDP solution and PAA-CDDP NPs both reduced progression of bladder tumors to T1 stage, although none of the rats treated with PAA-CDDP NPs progressed to T1 stage. Lamina propria invasion is indicated with an arrow, and CIS with a (*). CDDP content in all the formulations used for the experiment was 0.7 mg/mL.
Figure 5.
PAA-CDDP NPs reduce proliferation of bladder cancer in MNU-induced rat bladder carcinogenesis model. A, Representative IHC sections at 10× and 20× showing presence of Ki67+ cells (staining brown) in untreated rats and rats treated with intravesical CDDP solution or intravesical PAA-CDDP-NPs. B, Rats treated with intravesical PAA-CDDP NPs had significantly decreased (P < 0.05) numbers of Ki67+ cells compared to untreated controls, indicating decreased cell proliferation. CDDP content in all the formulations used for the experiment was 0.7 mg/mL.
Figure 6.
PAA-CDDP NPs and CDDP have similar apoptotic effects in MNU-induced rat bladder carcinogenesis model. A, Representative immunofluorescence (IF) sections stained for TUNEL (green) and counter stained with DAPI (blue) of the control rats, untreated rats, and rats treated with intravesical CDDP solution or intravesical PAA-CDDP NPs. B, No significant differences in TUNEL+ cells were identified between treatment groups. CDDP content in all the formulations used for the experiment was 0.7 mg/mL.
Discussion
Ineffective retention of treatment modalities in the bladder is a major factor that limits the success of intravesical therapies in benign and malignant disease of the bladder. Intravesical therapies are inherently limited by duration of retention in the bladder. Current clinical practice for intravesical NMIBC therapies includes catheterization of the patient to administer therapeutic agent into the bladder followed by temporarily sealing the catheter to facilitate absorption and retention of the therapeutic agent in the bladder. During this time, urine production dilutes the therapeutic agent in the bladder (4, 5). Moreover, the majority of the drug in the bladder is subsequently lost by urination following catheterization. Hence, strategies that can prolong the residence time of therapeutics in the bladder are of great interest for intravesical therapy. To date, few reports of NPs developed for intravesical therapy have evaluated bladder retention in vivo (26–29). Our studies showed that only animals (both mice and rats) treated with PAA-CDDP NPs had detectable bladder CDDP levels for at least 4 hours after intravesical administration. Although these drug levels were BLQ by 24 hours, the concentrations of CDDP at 4 hours after PAA-CDDP NPs administration were only decreased by at most twofold compared to levels at 1 hour. Using fluorescently labeled PAA-CDDP NPs with similar characteristics as PAA-CDDP NPs, we were able to confirm the observation of fluorescence in bladder tissues 4 hours after intravesical administration. It is possible that the poly-amino acid backbone, the surface charge, and the size play a key role in the extent and duration of bladder retention. However, it must be noted that due to the nature of the PAA-CDDP complex formation, the PEG-PAA-CDDP NP formulations tested here differed in size and CDDP content compared to PAA-CDDP NPs, which limits direct comparison based on surface chemistry or size alone.
The significant decrease in Ki67+ cells in tumors from rats treated with PAA-CDDP NPs compared to conventional CDDP suggests that further study is warranted (30, 31). The antiproliferative properties of cisplatin have previously been established, and adjunctive delivery and costimulatory agents are known to work synergistically to affect proliferation (32, 33). Recent work has demonstrated that DNA damage repair genes, notably ERCC2, play a central role in cisplatin sensitivity for MIBC. During the process of DNA damage and mutation, quiescent cells can be induced to proliferate, a process that cisplatin may slow. In this study, the enhanced therapeutic effect associated with NP cisplatin appears more likely to be linked to inhibition of proliferation, rather than induction of cell apoptosis, at least in this non-muscle invasive animal model of bladder cancer. The rat MNU model is an aggressive tumor model, and nearly all bladders had residual CIS (19). However, no rats treated with PAA-CDDP NPs had evidence of invasion into the lamina propria, which is notable because patients with lamina propria invasion (T1) have a worse prognosis [5-year cancer-specific survival (CSS) 88%] than patients with CIS or high grade papillary disease (5-year CSS 98%; ref. 34). In addition, T1 invasion is a significant risk factor for stage progression to MIBC, which is also associated with a worse cancer prognosis (CSS 63%). Furthermore, muscle invasion often necessitates chemoradiation or bladder extirpation and urinary reconstruction, both of which are associated with significant morbidity. As a result, preventing invasive disease is an important endpoint for any trial in NMIBC, and is a major reason why preclinical studies should be performed in clinically relevant NMIBC animal models (19, 35).
Although systemic CDDP therapy is the gold standard for the treatment of metastatic bladder cancer, clinical experience with intravesical CDDP to treat NMIBC is limited and cautionary. The high rate of anaphylaxis in randomized trials and incidences of local toxicity precluded further exploration of intravesical CDDP for the treatment of NMIBC (7). Hypersensitivity associated with intravesical administration of CDDP was likely due to translocation of CDDP into the systemic circulation. Our studies showed that more than 2 μg/mL of cisplatin can be detected in plasma within 1 hour after intravesical administration of CDDP solution to mice, supporting the hypersensitivity observed with intravesical CDDP therapy in humans. This level of serum platinum is notable when considering that platinum concentrations as low as 0.23 ng/mL have been documented years after systemic treatment and have been linked to oto- and neuro-toxicities, as well as hypogonadism, hypercholesterolemia, and hypertension (36, 37). Hypersensitivity reactions, which can occur with small quantities of circulating platinum, are present in 5% and 20% of the cases during systemic CDDP therapy (38). Patients undergoing systemic chemotherapy for advanced disease are more likely to accept the risks associated with chemotherapy hypersensitivity than patients with NMIBC. Hence, in order to safely and efficiently deliver intravesical CDDP to the bladder for local treatment of NMIBC, it is important to develop a strategy that can prevent systemic translocation of CDDP, while also minimizing local toxicity to noncancerous cells in the bladder.
Local toxicity and systemic exposure with intravesical administration of CDDP have limited the use of CDDP for NMIBC treatment. Indeed, we found that >2 μg/mL of cisplatin could be detected in plasma within 1 hour after intravesical administration of CDDP solution to mice. However, we found that CDDP levels in plasma were BLQ when CDDP was incorporated into NP administered intravesically. Furthermore, we found that three weekly intravesical administrations of CDDP led to a significant increase in bladder weight compared with sham control. In contrast, three weekly intravesical administrations of PAA-CDDP NPs and PEGlow-PAA-CDDP NP did not cause an increase in bladder weight, despite delivering similar amounts of CDDP to the bladder tissue. It is likely that the sustained release of CDDP from NP leads to the decreased systemic exposure and decreased local toxicity compared to intravesical administration of CDDP solution. Previous experience with sustained-release CDDP NP formulations has demonstrated their potential to mitigate toxicity without compromising anticancer activity. Preclinical and clinical studies have demonstrated that systemically administered CDDP-loaded NPs were associated with significantly reduced common side effects, including renal toxicity, neurotoxicity, and ototoxicity (9–16). Currently, polypeptide–cisplatin conjugate NPs are being explored in phase III clinical trials for the systemic treatment of pancreatic cancer, and the data reported to date indicate that these NPs can significantly reduce the toxicity of CDDP after systemic administration (16).
More studies are warranted to determine which factors led to decreased CDDP delivery in the bladder for the PEG-containing PAA-CDDP NP formulations. Little is known about the impact of PEG coating of NPs on their uptake and retention in the bladder following intravesical administration. Recently, Mun and colleagues showed that PEGylation of silica NPs reduced their adhesion to ex vivo porcine bladder tissue (39). They observed that PEGylated silica NPs were more rapidly washed off of tissue by artificial urine solution compared to non-PEGylated silica NPs, and that increased PEG molecular weight further decreased adhesion. The NP surface PEG density was not characterized (39). Although it is now well-known that NPs densely coated with PEG provide much more uniform distribution over mucosal epithelial surfaces, including the gastrointestinal tract (40), respiratory system (17, 41, 42), female reproductive tract (43–45), and the ocular surface (46), the bladder epithelium is not coated in a soluble mucin gel layer (47). We also previously demonstrated that a similar PEGhigh-PAA-CDDP NP formulation was more efficacious for local treatment of orthotopic murine glioblastoma than PAACDDP NP (18, 22), although enhanced efficacy correlated with increased spread of small, non-adhesive PEG-coated NPs through brain tumor tissue. However, in the absence of a mucus barrier in the bladder, small, non-adhesive PEG-PAA-CDDP NPs may be more readily expelled by urine production. Although, the differences in size and CDDP loading between the PEG-PAA-CDDP NP formulations and the PAA-CDDP NPs limit direct comparison based on surface chemistry alone.
This study has limitations that should be noted. The animal model used in these experiments was chosen because it progresses from dysplasia to NMIBC between weeks 8 and week 16, making it an ideal time to initiate treatment. Although this interval best recapitulates a chemopreventative treatment model, there are currently no reliable animal models in which a non–muscle-invasive bladder tumor can be first resected prior to treatment induction. However, the MNU model represents an outbred strain of NMIBC with an intact immune system that responds clinically to intravesical immunotherapy and chemotherapy, as opposed to an inbred strain utilizing immortal clones of genetically identical tumor strains (20). Although an inbred strain derived from a cell line provides consistent tumor size and growth, its homogeneity means that it is not clear whether any treatment effects observed will necessarily translate beyond only a small group of genetically similar tumor subtypes. In addition, there are currently no reliable xenograft models of NMIBC that progress to MIBC in a time-dependent fashion similar to human urothelial cancer. As a result, a carcinogen model was chosen to reflect the heterogeneity of human disease, to study the antineoplastic potential, and to reflect the typical progression of non-muscle to muscle invasive cancer.
In conclusion, we demonstrate that PAA-CDDP NPs may reduce CDDP-related side effects upon intravesical use by limiting both systemic exposure and local toxicity, while maintaining CDDP’s primary antitumor efficacy in vitro and in vivo. Following further analyses, we hope to initiate early phase clinical trials to evaluate the safety and efficacy of intravesical CDDP NPs in patients with NMIBC.
Supplementary Material
Translational Relevance.
Systemic platinum-based chemotherapy is the first-line standard of care treatment for patients with metastatic bladder cancer. However, prior clinical trials evaluating intravesical cisplatin for non–muscle-invasive bladder cancer (NMIBC) were halted due to systemic translocation and toxicity. Here, we describe cisplatin nanoparticles using biocompatible poly (L-aspartic acid sodium salt; PAA) that provide enhanced platinum absorption into the bladder urothelium while mitigating systemic absorption and toxicity. In murine pharmacokinetic experiments, we demonstrate than the intravesical CDDP NP provide increased penetration into the bladder wall with undetectable platinum level in the systemic circulation, an improvement over conventional CDDP. In an immuno-competent murine model of NMIBC, we show that CDDP NPs reduce cancer cell proliferation compared with untreated controls. This study represents a significant improvement for intravesical platinum drug delivery that is highly applicable for patients with NMIBC.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
M. Kates, A. Date, J. Hanes, L. Ensign, and T.J. Bivalacqua are listed as coinventors on a provisional patent on nanoparticle formulations for enhanced drug delivery to the bladder, which is owned by Johns Hopkins University. N.M. Hahn is a consultant/advisory board member for AstraZeneca, Genentech, Merck, and TARIS Biomedical. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer 2013;132:1133–45. [DOI] [PubMed] [Google Scholar]
- 2.David KA, Mallin K, Milowsky MI, Ritchey J, Carroll PR, Nanus DM. Surveillance of urothelial carcinoma: stage and grade migration, 1993–2005 and survival trends, 1993–2000. Cancer 2009;115:1435–47. [DOI] [PubMed] [Google Scholar]
- 3.Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol 2016;196:1–9. [DOI] [PubMed] [Google Scholar]
- 4.GuhaSarkar S, Banerjee R. Intravesical drug delivery: challenges, current status, opportunities and novel strategies. J Control Release 2010;148:147–59. [DOI] [PubMed] [Google Scholar]
- 5.Douglass L, Schoenberg M. The future of intravesical drug delivery for non-muscle invasive bladder cancer. Bladder Cancer 2016;2:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068–77. [DOI] [PubMed] [Google Scholar]
- 7.Denis L Anaphylactic reactions to repeated intravesical instillation with cisplatin. Lancet 1983;1:1378–9. [DOI] [PubMed] [Google Scholar]
- 8.Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST. Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv Drug Deliv Rev 2016;108:25–38 [DOI] [PubMed] [Google Scholar]
- 9.Mizumura Y, Matsumura Y, Hamaguchi T, Nishiyama N, Kataoka K, Kawaguchi T, et al. Cisplatin-incorporated polymeric micelles eliminate nephrotoxicity, while maintaining antitumor activity. Jpn J Cancer Res 2001;92:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchino H, Matsumura Y, Negishi T, Koizumi F, Hayashi T, Honda T, et al. Cisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Br J Cancer 2005;93: 678–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plummer R, Wilson RH, Calvert H, Boddy AV, Griffin M, Sludden J, et al. A phase I clinical study of cisplatin-incorporated polymeric micelles (NC-6004) in patients with solid tumours. Br J Cancer 2011;104:593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paraskar AS, Soni S, Chin KT, Chaudhuri P, Muto KW, Berkowitz J, et al. Harnessing structure-activity relationship to engineer a cisplatin nanoparticle for enhanced antitumor efficacy. Proc Natl Acad Sci U S A 2010;107: 12435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sengupta P, Basu S, Soni S, Pandey A, Roy B, Oh MS, et al. Cholesterol-tethered platinum II-based supramolecular nanoparticle increases antitumor efficacy and reduces nephrotoxicity. Proc Natl Acad Sci 2012;109:11294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oberoi HS, Nukolova NV, Kabanov AV, Bronich TK. Nanocarriers for delivery of platinum anticancer drugs. Adv Drug Deliv Rev 2013;65:1667–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumura Y The drug discovery by nanomedicine and its clinical experience. Jpn J Clin Oncol 2014;44:515–25. [DOI] [PubMed] [Google Scholar]
- 16.Nishiyama N, Matsumura Y, Kataoka K. Development of polymeric micelles for targeting intractable cancers. Cancer Sci 2016;107:867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chisholm J, Schneider C, Suk JS, Peacock CJH. Mucus-penetrating cisplatin nanoparticles for the local treatment of lung cancer. NanoDDS 2014;1:84–5. [Google Scholar]
- 18.Zhang C, Nance EA, Mastorakos P, Chisholm J, Berry S, Eberhart C, et al. Convection enhanced delivery of cisplatin-loaded brain penetrating nanoparticles cures malignant glioma in rats. J Control Release 2017. March 7 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinberg GD, Brendler CB, Ichikawa T, Squire RA, Isaacs JT. Characterization of an N-methyl-N-nitrosourea-induced autochthonous rat bladder cancer model. Cancer Res 1990;50:6668–74. [PubMed] [Google Scholar]
- 20.Kates M, Nirschl T, Sopko NA, Matsui H, Kochel CM, Reis LO, et al. Intravesical BCG induces CD4+ T-cell expansion in an immune competent model of bladder cancer. Cancer Immunol Res 2017;5:594–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishiyama N, Kataoka K. Preparation and characterization of size-controlled polymeric micelle containing cis-dichlorodiammineplatinum(II) in the core. J Control Release 2001;74:83–94. [DOI] [PubMed] [Google Scholar]
- 22.Timbie KF, Afzal U, Date A, Zhang C, Song J, Wilson Miller G, et al. MR image-guided delivery of cisplatin-loaded brain-penetrating nanoparticles to invasive glioma with focused ultrasound. J Control Release 2017. March 11 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alberts D, Dorr R. New perspectives on an old friend: optimizing carboplatin for the treatment of solid tumors. Oncologist 1998;3:15–34. [PubMed] [Google Scholar]
- 24.Powles T, Perry J, Shamash J, Liu W, Oliver T, Joel S. A comparison of the platinum analogues in bladder cancer cell lines. Urol Int 2007;79:67–72. [DOI] [PubMed] [Google Scholar]
- 25.Margulis V, Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y. Ki-67 Is an independent predictor of bladder cancer outcome in patients treated with radical cystectomy for organ-confined disease. Clin Cancer Res 2006;12: 7369–73. [DOI] [PubMed] [Google Scholar]
- 26.Martin DT, Hoimes CJ, Kaimakliotis HZ, Cheng CJ, Zhang K, Liu J, et al. Nanoparticles for urothelium penetration and delivery of the histone deacetylase inhibitor belinostat for treatment of bladder cancer. Nanomedicine 2013;9:1124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang M, Yang G, Place RF, Charisse K, Epstein-Barash H, Manoharan M, et al. Intravesical delivery of small activating RNA formulated into lipid nanoparticles inhibits orthotopic bladder tumor growth. Cancer Res 2012;72:5069–79. [DOI] [PubMed] [Google Scholar]
- 28.Mugabe C, Matsui Y, So AI, Gleave ME, Baker JHE, Minchinton AI, et al. In vivo evaluation of mucoadhesive nanoparticulate docetaxel for intravesical treatment of non-muscle-invasive bladder cancer. Clin Cancer Res 2011;17:2788–98. [DOI] [PubMed] [Google Scholar]
- 29.Mugabe C, Raven PA, Fazli L, Baker JHE, Jackson JK, Liggins RT, et al. Tissue uptake of docetaxel loaded hydrophobically derivatized hyperbranched polyglycerols and their effects on the morphology of the bladder urothelium. Biomaterials 2012;33:692–703. [DOI] [PubMed] [Google Scholar]
- 30.Van Allen EM, Mouw KW, Kim P, Iyer G, Wagle N, Al-Ahmadie H, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4:1140–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patrick O’Neill J DNA damage, DNA repair, cell proliferation, and DNA replication: how do gene mutations result? Proc Natl Acad Sci U S A 2000;97:11137–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viale M, Fontana A, Maric I, Monticone M, Angelini G, Gasbarri C. Preparation and antiproliferative activity of liposomes containing a combination of cisplatin and procainamide hydrochloride. Chem Res Toxicol 2016;29:1393–5. [DOI] [PubMed] [Google Scholar]
- 33.Ercoli A, Scambia G, De Vincenzo R, Alimonti A, Petrucci F, Fattorossi A, et al. Tamoxifen synergizes the antiproliferative effect of cisplatin in human ovarian cancer cells: enhancement of DNA platination as a possible mechanism. Cancer Lett 1996;108:7–14. [DOI] [PubMed] [Google Scholar]
- 34.Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25–41. [DOI] [PubMed] [Google Scholar]
- 35.Kamat AM, Sylvester RJ, Böhle A, Palou J, Lamm DL, Brausi M, et al. Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: recommendations from the International Bladder Cancer Group. J Clin Oncol 2016;34:1935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boer H, Proost JH, Nuver J, Bunskoek S, Gietema JQ, Geubels BM, et al. Long-term exposure to circulating platinum is associated with late effects of treatment in testicular cancer survivors. Ann Oncol 2015;26:2305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hjelle Lv, Bremnes RM, Ole Gundersen P, Sprauten M, Brydoy M, Tandstad T, et al. Impact of long-term serum platinum on neuro- and ototoxicity, cardiovascular disease, and hypogonadism in testicular cancer survivors. J Clin Oncol 32:15s, 2014. (suppl; abstr4518). [Google Scholar]
- 38.Makrilia N, Syrigou E, Kaklamanos I, Manolopoulos L, Saif MW. Hypersensitivity reactions associated with platinum antineoplastic agents: a systematic review. Met Based Drugs 2010;2010: pii: 207084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mun EA, Williams AC, Khutoryanskiy VV. Adhesion of thiolated silica nanoparticles to urinary bladder mucosa: effects of PEGylation, thiol content and particle size. Int J Pharm 2016;512: 32–8. [DOI] [PubMed] [Google Scholar]
- 40.Maisel K, Ensign L, Reddy M, Cone R, Hanes J. Effect of surface chemistry on nanoparticle interaction with gastrointestinal mucus and distribution in the gastrointestinal tract following oral and rectal administration in the mouse. J Control Release 2015;197:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suk JS, Kim AJ, Trehan K, Schneider CS, Cebotaru L, Woodward OM, et al. Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier. J Control Release 2014;178:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mastorakos P, da Silva AL, Chisholm J, Song E, Choi WK, Boyle MP, et al. Highly compacted biodegradable DNA nanoparticles capable of overcoming the mucus barrier for inhaled lung gene therapy. Proc Natl Acad Sci U S A2015;112:8720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maisel K, Reddy M, Xu Q, Chattopadhyay S, Cone R, Ensign LM, et al. Nanoparticles coated with high molecular weight PEG penetrate mucus and provide uniform vaginal and colorectal distribution in vivo. Nanomedicine 2016;11:1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang M, Yu T, Wang Y-Y, Lai SK, Zeng Q, Miao B, et al. Vaginal delivery of paclitaxel via nanoparticles with non-mucoadhesive surfaces suppresses cervical tumor growth. Adv Health Mat 2014;3:1044–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ensign LM, Tang BC, Wang Y-Y, Tse TA, Hoen T, Cone R, et al. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci Transl Med 2012;4:138ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schopf L, Enlow E, Popov A, Bourassa J, Chen H. Ocular pharmacokinetics of a novel loteprednol etabonate 0.4% ophthalmic formulation. Ophthalmol Ther 2014;3:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.N’Dow J, Jordan N, Robson CN, Neal DE, Pearson JP. The bladder does not appear to have a dynamic secreted continuous mucous gel layer. J Urol 2005;173:2025–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.