Abstract
New and improved vaccines are needed against challenging diseases such as malaria, tuberculosis, Ebola, influenza, AIDS, and cancer. The majority of existing vaccine adjuvants lack the ability to significantly stimulate the cellular immune response, which is required to prevent the aforementioned diseases. This study designed a novel particulate based pathogen-mimicking vaccine delivery system (PMVDS) to target antigen-presenting-cells (APCs) such as dendritic cells. The uniqueness of PMVDS is that the polymer used to prepare the delivery system, Inulin Acetate (InAc), activates the innate immune system. InAc was synthesized from the plant polysaccharide, inulin. PMVDS provided improved and persistent antigen delivery to APCs as an efficient vaccine delivery system, and simultaneously, activated Toll-Like Receptor-4 (TLR-4) on APCs to release chemokine’s/cytokines as an immune-adjuvant. Through this dual mechanism, PMVDS robustly stimulated both the humoral (> 32 times of IgG1 levels vs alum) and the cell-mediated immune responses against the encapsulated antigen (ovalbumin) in mice. More importantly, PMVDS stimulated both cytotoxic T cells and natural killer cells of cell-mediated immunity to provide tumor (B16-ova-Melanoma) protection in around 40% of vaccinated mice and significantly delayed tumor progression in rest of the mice. PMVDS is a unique bio-active vaccine delivery technology with broader applications for vaccines against cancer and several intracellular pathogens, where both humoral and cellular immune responses are desired.
Keywords: Pathogen mimicking vaccine delivery system; Immune-active polymer; Toll-like receptor-4 (TLR-4); Vaccine adjuvant; Inulin acetate, Cellular immunity
1. Introduction
Vaccines are considered one of the most important scientific interventions for the prevention and treatment of numerous infectious diseases [1,2]. Despite extensive research efforts, effective vaccines are yet to be available against several challenging diseases such as AIDS, cancer, Ebola, influenza, malaria, and tuberculosis [1,2]. For immunological clearance of several of these intracellular pathogens and abnormal cancer cells, strong cell-mediated immunity is required in addition to humoral immune response. The lack of their ability to sufficiently stimulate both humoral and cellular immune responses at safe doses is one of the major reasons for the failure of existing vaccine technologies against the aforementioned diseases [3]. Therefore, there is an urgent medical need to discover new vaccine adjuvants or technologies which can address the above pitfalls before major pandemic or epidemic disease outbreaks.
For decades, alum has been serving as a vaccine adjuvant in several marketed vaccines. Alum lacks the ability to significantly stimulate cell-mediated immunity; therefore, it could not provide the desired protection in the aforementioned diseases [4–6]. Recent advancements in immunology, chemistry, material sciences, and drug delivery provide new opportunities for the discovery of the next generation vaccine adjuvants [3–5]. These strategies are mainly based on targeting the innate immune system and its specific signaling pathways [4,6–10]. The innate immune system recognizes pathogens as a foreign, insoluble, and particulate material containing multiple antigens [8,9,11]. Most importantly, innate immune cells such as antigen presenting cells (APCs) identify pathogens through their interaction with highly conserved molecules from pathogens other than antigens [11]. These are called pathogen-associated molecular patterns (PAMPs) or danger signals. APCs interact with PAMPs through their unique pattern recognition receptors (PRRs) such as toll-like receptors (TLRs) and nucleotide binding and oligomerization domain (NOD)-like receptors (NLRs) [11–14]. In addition to assisting in the recognition of pathogens, PRRs also play an integral role in modulating the adaptive immune response after infection or vaccination. Therefore, PRRs become a target for the discovery of next-generation vaccine adjuvants [5,12–17]. Of several TLRs discovered so far (10 in humans and 13 in mice), TLR-4 is the most studied TLR for modulating immune function [17]. Thus, in a quest for novel bioactive polymers to function as advanced vaccine adjuvants, we have screened several polymers that mimic PAMPs in stimulating APCs. We have discovered that inulin, a natural plant fiber, could stimulate APCs. However, this occurs at very high concentrations [18].
In the current study, we have rationally designed a novel “pathogen mimicking vaccine delivery system” (PMVDS) by encapsulating an antigen into a particulate delivery system that stimulated innate immune signaling for strong cellular and humoral immune activation. PMVDS was prepared using inulin acetate (InAc) as a bio-active polymer. The InAc-PMVDS particles not only delivered encapsulated antigen efficiently to dendritic cells but also stimulated them to release cytokines through the activation of TLR-4 on their surface. The engineered- PMVDS will mimic several characteristics of pathogens for an immune system such as being foreign and particulate in nature, having a potential to carry multiple antigens, and most importantly, providing contextual danger signals as PAMPS through their interaction with TLR-4 on immune cells. The multi-functionality of PMVDS as both a delivery system and an immune adjuvant resulted in robust humoral and cellular immune response in mice. As a proof-of-concept study using a melanoma mouse model, vaccination with PMVDS technology protected 40% of vaccinated mice from tumor development.
2. Materials and methods
2.1. Chemicals and reagents
Inulin from dahlia tubers, ovalbumin (ova), aluminum hydroxide gel (Alum, 2%), and all other chemicals were purchased from Fisher Scientific (Pittsburg, PA, USA). Lipopolysaccharide (LPS), Zymosan and poly(lactic-co-glycolic acid) (PLGA; Resomer 503) were purchased from Sigma-Aldrich (St Louis, MO, USA). A lipopolysaccharide from the photosynthetic bacterium Rhodobacter sphaeroides (LPS-RS) and Monophosphoryl Lipid A (MPLA) were purchased from Invivogen, (San Diego, CA, USA). Goat anti-mouse IgG/IgG1/IgG2a-HRP conjugates were purchased from Southern Biotech (Birmingham, AL, USA). All other chemicals, buffers, reagents and analytical or HPLC grade organic solvents were purchased from Fisher Scientific (Pittsburgh, PA, USA).
2.2. Cell lines and cell culture
Homo sapiens HEK-TLR-4YFP-MD-2 cell line (NR-9315) and mouse dendritic cells (DC2.4) were cultured in DMEM-high glucose medium (Thermofisher Scientific, USA) supplemented with antibiotics (penicillin/streptomycin) and 10% fetal bovine serum (FBS). A final concentration of 50 μM of β-mercaptoethanol was maintained in the medium for culturing DC2.4 cells.
2.3. Synthesis of inulin acetate (InAc)
Inulin acetate was synthesized by acetylating hydroxyl groups of inulin using acetic anhydride. In brief, acetic anhydride was added to a solution of inulin in dimethyl formamide (DMF) with a dropper. The reaction was carried out at 40 °C under nitrogen gas with sodium acetate (0.1% w/v) as a catalyst [15,19]. After 24 h, InAc was precipitated by adding an excess of sterile cold water, collected via filtration and washed multiple (3–5) times with cold sterile water to remove unreacted inulin and acetic acid [15,19]. The precipitate was allowed to dry overnight in an oven at 37 °C. InAc forms into a dense, free flowing white powder. The formation of InAc was confirmed by Fourier transform infrared (FTIR) and proton NMR spectroscopy.
2.4. Characterization of inulin acetate
2.4.1. Nuclear magnetic resonance (NMR) spectroscopy
Approximately 30–50 mg of polymers were dissolved in DMSO-d6 and transferred to an NMR tube. 1H NMR spectrum of the polymer solutions was recorded using a Bruker 400 MHz NMR spectrometer [15,20].
2.4.2. Fourier transform infrared (FTIR) Spectroscopy
The formation of InAc from inulin was confirmed by the FTIR spectrum of the two polymers (Nicolet 380 ATR-FTIR spectrophotometer, Thermo Electron Corp., Madison, WI). The spectrum was recorded between 4000 cm −1 and 400 cm −1 at a resolution of 4 cm −1 and 50 scans. The average of 50 scans of data is represented [15,20,21].
2.5. Preparation of inulin acetate (InAc) and PLGA particles
InAc particles encapsulated with an antigen, ovalbumin (PMVDS) were prepared by two different methods; single emulsion (o/w/) or double (w/o/w) emulsion solvent evaporation techniques. Both methods were compared for antigen loading (μg of antigen per mg of particles) and control on the burst release (% of encapsulated antigen released within first 30 min of incubation). The antigen solution was dissolved in 200 μl of 10 mM phosphate buffer (PB), pH 7.4 containing 0.25% of Pluronic F-68 as a surfactant. Simultaneously, 100 mg of InAc was dissolved in 5 ml of dichloromethane (DCM). In single emulsion method, both polymer and protein solutions were added simultaneously as small droplets into 30 ml of sterile water containing 0.5% polyvinyl alcohol (PVA) while stirring. The resultant o/w emulsion was stirred for 10 h to evaporate DCM. In the double emulsion method, the aqueous protein solution was emulsified with the polymer-DCM mixture, resulting in the formation of primary (w/o) emulsion. This primary emulsion was then added dropwise into another aqueous phase (30 ml sterile water containing 0.5% w/v PVA) with continuous stirring to form double (w/o/w) emulsion for approximately 10 h. In both cases, the precipitated particles were collected via centrifugation at 50,000g for 30 min at 4 °C. The collected particles were washed twice and resuspended in 100 mM citrate buffer pH 7.4, and subsequently lyophilized using mannitol as a cryoprotectant (VirTis, Gardiner, NY). The blank InAc particles were prepared in the same manner as ova loaded InAc particles except that PB was used during primary emulsion instead of ova solution.
PLGA particles were also prepared by the double emulsion-solvent evaporation method as described above with InAc particles [15,18,22,23]. The antigen solution in PB was emulsified in DCM containing PLGA polymer using Pluronic F-68 as a surfactant. The resulted w/o emulsion was dropwise added to 30 ml of water containing 0.5% PVA as a stabilizer to form a secondary emulsion (w/o/w). The double emulsion was stirred for 10 h at room temperature to evaporate the DCM. The precipitated PLGA particles containing the antigen were collected by centrifugation and processed as mentioned above with InAc particles.
2.6. Characterization of particles: size, shape, and surface charge
2.6.1. Dynamic light scattering (DLS) [15,18]
For determination of particle properties such as size and charge (ς-potential) of InAc particles, DLS technique was employed. Initially, InAc particles were dispersed in a filter sterilized citrate buffer (10 mM, pH 7.4), and then further diluted using filter sterilized deionized water before recording particle size and ς-potential using Malvern Zeta-Sizer, Malvern Ltd., MA, USA.
2.6.2. Scanning electron microscopy (SEM)
Scanning electron microscope (SEM, Model S-3400N, Hitachi, Japan) was used to investigate the shape and the morphology of InAc particles. For the preparation of samples, lyophilized powder free from cryoprotectant was mounted on the metal holder using conductive double-sided tape. The particles were sputter coated with a 10-nm gold layer before analysis. The micrographs were captured at an accelerating voltage of 5–25 kV, with a working distance of 5–15 mm and spot size of three. The diameter of the particles was measured by the ImageJ software after collecting images. The data is represented as an average diameter of at least 100 particles [15,18,20].
2.6.3. Transmission electron microscopy (TEM)
The morphology of the InAc particles was studied using TEM (Technai G2 Spirit, OR, USA). Specimen for TEM was prepared by placing a drop of the InAc particle on a copper grid coated with carbon. The sample was completely dried under vacuum and the images were captured using the microscope at an operating voltage of 60 kV [21].
2.7. Determination of ova loading
A measured amount of PMVDS particles was added to acetone and incubated at 37 °C for 4 h. The polymer InAc dissolved in acetone. However, the precipitated ovalbumin was pelleted by centrifuging at 10,000 g for 30 min. The pellet was dissolved in 1% sodium dodecyl sulfate (SDS) solution. The ova content in the SDS solution was measured via micro-bicinchoninic acid (BCA) protein assay. The standard curve was prepared using blank InAc particles spiked with known amount of ova. The ova loading (n = 3–4) is reported as μg of ova present per mg of InAc particles (w/w).
2.8. Determination of endotoxin levels
Endotoxins usually cause inflammation through the activation TLRs on immune cells. Therefore, the presence of significant levels of endotoxins in the formulation could interfere both in-vitro and in-vivo assessment of the technology as a vaccine adjuvant. Therefore, to rule out such possible interference, the endotoxin levels in the final formulation were determined by using commercial Kit (ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit) from GenScript (Piscataway, NJ) by following the manufacturer’s instructions. The endotoxin levels in all formulations used in this study were within the limits for parenteral injections as described in the United States of Pharmacopeia (5 EU/dose/kg of body weight).
2.9. In-vitro antigen (ova) release studies
Ova loaded InAc particles (10 mg) were dispersed in 1 ml of 10 mM PB (pH 7.4) and incubated at 37 °C with 100 rpm of agitation. At predetermined time intervals, an aliquot the dispersion was withdrawn centrifuged at 20,000g to collect the soluble supernatant. The supernatant was passed through 0.2 μm filter before measuring the released ova in the supernatant by using BCA protein assay.
2.10. Toll-like receptor-4 (TLR-4) activation
The concentration dependent TLR-4 activation by InAc particles was investigated using DC2.4 and HEKTLR-4YFPMD2 cells. DC2.4 cells represent naive dendritic cells from mouse origin [15,18,24]. HEKTLR- 4yfpMD2 cells are genetically modified human kidney epithelial cells that exclusively express TLR-4 on their surface and are capable of downstream signaling upon the activation of TLR-4 [25,26]. DC2.4 or HEKTLR-4yfpMD2 cells were incubated with different concentrations of blank InAc particles (10, 100 and 250 μg/ml) or PLGA particles (250 μg/ml) for 48 h. A well-established TLR-4 agonist-based vaccine adjuvant MPLA was used as a positive control. The activation of the cells after treatment was assessed by measuring the concentration of a chemokine IL-8 in the culture supernatant using Human IL-8 ELISA Ready-SET-Go kit (e-Bioscience, San Diego, CA).
To confirm the role of TLR-4 activation in the stimulation of dendritic cells, DC2.4 cells were incubated with an established TLR-4 specific antagonist LPS-RS (120 ng/ml) for 1 h before treating with InAc particles (250 μg/ml) or MPLA (2 μg/ml) for 48 h. After treatment, the concentration of IL-8 in the supernatant was measured as described above. In both experiments, the concentration of IL-8 released in the medium in untreated cells was considered as a background to cytokine levels.
2.11. Activation of swine peripheral blood mononuclear cells (PBMCs)
2.11.1. Treatment of PBMCs and cDNA synthesis
The peripheral blood was collected from three months old swine in heparinized vacationer tubes (BD Vacutainer, Franklin Lakes, NJ). The PBMCs were isolated from the whole blood using 65% percoll gradient centrifugation and cultured as described previously [15,27]. The PBMCs were treated with 200 μg/ml of InAc or PLGA microparticles for 24 h. Subsequently, the RNA from the treated PBMCs was isolated using the RNeasy mini kit (Qiagen Valencia, CA) and converted to cDNA using First-Strand cDNA Synthesis kit (Thermo Scientific, Waltham, MA).
2.11.2. Measurement of (TNF-α) gene expression through RT-PCR
The relative gene expression levels of TNF-α and ribosomal protein large subunit in treated PBMCs were measured through RT-PCR using SYBR green dye (Applied Biosystems, Foster City, CA) with the following primers: TNF-α forward primer-GGGGTCCTTGGGTTTGGATT, TNF-α reverse primer-TTGGAACCCAAGCTTCCCTG, RPL4 forward primer-GGCGTAAAGCTGCTACCCTC and RPL4 reverse primer-GGATCTCTGGGCTTTTCAAGATT. The conditions for RT-PCR were; initial heat for 95 °C for 10 min, followed by 45 cycles of 95 °C for 15 s and 60 °C for 40 s. All PCRs were performed in triplicate [15].
2.12. In-vitro antigen delivery and antigen persistence assay on Naïve dendritic cells (DC2.4)
The ability of PMVDS to deliver encapsulated antigen to dendritic cells was assessed using an in-vitro system with DC2.4 cells. The antigen (ova) was labeled with FITC to facilitate quantification of the antigen delivery. The cells were incubated with 25 μg of FITC-ova as a solution in 50 mM phosphate buffered saline, pH 7.4 (PBS), encapsulated inside InAc particles (PMVDS) or PLGA particles as a delivery system. Control cells were treated with PBS without FITC-ova. After 1 h of incubation at 37 °C and 5% CO2, the cells were washed thrice with PBS, trypsinized, and fixed in 4% w/v paraformaldehyde for 5 min. The proportion of cells receiving FITC-ova and the amount of FITC-ova received per cell was quantified using flow cytometer (BD Biosciences FACS LSR Fortessa and analyzed with FlowJo™). Similarly, to study the persistence of antigen inside the dendritic cells, DC 2.4 cells were cultured on glass coverslips and treated as mentioned above. After 1 h of treatment, the cells were washed thrice to remove FITC-ova or particles, which are not associated with the cells. Subsequently, the cells were incubated in a medium devoid of the FITC-ova. At different time points, (1 h, 12 h, and 24 h) the cells were washed, fixed with 4% w/v paraformaldehyde, and the antigen levels inside the cells were observed under a fluorescence microscope [15,18].
2.13. Immunization studies
All animal experiments were performed in accordance with guidelines and regulations of the National Institutes of Health and Institutional Animal Care and Use Committee (IACUC) of South Dakota State University. Male BALB/C mice (n = 4–5 per group, 6–8 weeks old) purchased from Charles River laboratories (Wilmington, MA) were immunized through intradermal route (i.d.) using 27G needle at 10–15° angle with the following injections: i) ova (100 μg per mouse); ii) physical mixture of ova (100 μg per mouse) with blank InAc particles (~2.0mg per mouse); iii) 100 μg ova with 250 μg alum (from 2% aluminum hydroxide); and iv) ova (100 μg per mouse) encapsulated in InAc particles (~ 2.0 mg). Immunizations were performed twice as primary and booster injections 21 days apart.
Sera were collected at week 1 and week 3, after both the primary and booster immunizations. The titers for total IgG, IgG1, and IgG2a in the sera were determined by indirect ELISA [16]. The titer is defined as the reciprocal of the highest dilution of a serum sample from immunized mice which yields an optical density (OD) value more than two standard deviations above the average OD generated from non- immunized sera samples. The titers were plotted as Log10 scale.
To study the effect of antigen dose on immune response, the mice (n = 4–5) were immunized through subcutaneous route at three dose levels of ova (1, 10 or 100 μg/mice) delivered either in saline or in PMVDS. The mice were immunized twice with the same formulation as a primary and booster dose with 21 days apart. The sera were collected at week 3 after the primary and booster immunizations. The titers for total-IgG, IgG1, and IgG2a in the sera were determined by indirect ELISA and represented as described above.
2.14. Splenocyte proliferation assay: ex-vivo
The generation of memory T cells by antigen-loaded InAc particles was determined using T cell proliferation assay, which measures the recall response of T cells when re-challenged with the antigen (ova) in ex-vivo conditions. Single cell suspension of the splenocytes was prepared from the spleens of the immunized mice in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% penicillin/streptomycin, 1 mM sodium pyruvate and 50 μM 2-mercaptoethanol (complete-RPMI medium). The RBCs in the cell suspension were lysed using 0.1 M NH4Cl. The viability of the splenocytes was assessed by trypan blue exclusion using Cellometer (Nexcelom Bioscience, Lawrence, MA). The splenocytes (1 × 106 cells/well) were seeded into a 96 well plate and incubated with 200 pl of complete RPMI medium containing ova (100 μg/ml) or concanavalin A (Con A) (2.5 μg/ml) as a challenge. After 72 h of incubation, the supernatant was collected for the analysis of secreted cytokines. Subsequently, the cells were incubated with 50 pl of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) solution (0.5 mg/ml) for 4 h. At the end, 150 pl of dimethyl sulfoxide (DMSO) was added and the plates were further incubated at 37 °C for 10 min. The absorbance of the wells at 540 nm wavelength was recorded using Spectra Max UV-Vis spectrophotometer. The stimulation index (SI) was defined as a ratio of absorbance values of Con A or ova treated cells to untreated cells containing the only medium.
2.15. Cytokine analysis
Cytokines in the supernatants of the splenocytes culture were measured using sandwich ELISA. The assay was performed using Mouse Th1/Th2 ELISA Ready-SET-Go kit (e-Bioscience, San Diego, CA). Cytokine concentration was determined by comparison with the standard curves generated from murine recombinant cytokines (IL-2, IL-4, IL-10, and IFN-γ) after linear regression analysis [18].
2.16. Ex-vivo cytotoxic T cell (CTL) activation assay
To investigate the ex-vivo cytotoxicity of cultured splenocytes from immunized mice (effector cells), the splenocytes were incubated at different ratios with fixed number of target cells. The target cells include B16-F10 cells (mouse melanoma cells), B16-ova cells (genetically modified B16-F10 cells expressing ova on the surface), and YAC-1 cells (target for Natural Killer cells). After 6 h of incubation, lactate dehydrogenase (LDH) release into culture supernatant was measured using LDH assay kit as an indication of cell lysis [28]. The base levels of LDH activity in the supernatants of the splenocytes after incubation without target cells were subtracted from the other values as blank reading.
2.17. In-vivo tumor prevention
Six to eight-week-old, male C57BL/6J mice (8 mice per group) were selected for in-vivo tumor prevention studies, as these mice are syngeneic with tumor cells used in the study. Six-week-old mice were immunized, on day 1 and day 21 as primary and booster doses respectively, with the following intradermal injections: PBS, ova (100 μg) with alum (250 μg) as an adjuvant or ova (100 μg) encapsulated InAc-PMVDS in saline. Fourteen days after the booster dose, mice were challenged subcutaneously with 1 × 105 B16-ova melanoma cells. Tumor length and width were measured on every alternate day as an indication of tumor growth. Tumor volume was calculated by using the following formula: 0.5 × length × width2[29]. Animals were euthanized when the tumor size exceeded 1.8 cm in any one direction and considered as mortality.
2.18. Statistics
All the experiments were performed in triplicate unless specified, and the results are expressed as mean ± SD. Student’s t-test (Instat, Graph Pad software, CA), one-way or two-way ANOVA as required followed by Bonferroni’s or Dunnett’s post-hoc multiple comparison test was used to compare the variance between groups.
3. Results and discussion
3.1. Characterization of inulin acetate (InAc) polymer and pathogen mimicking InAc particles
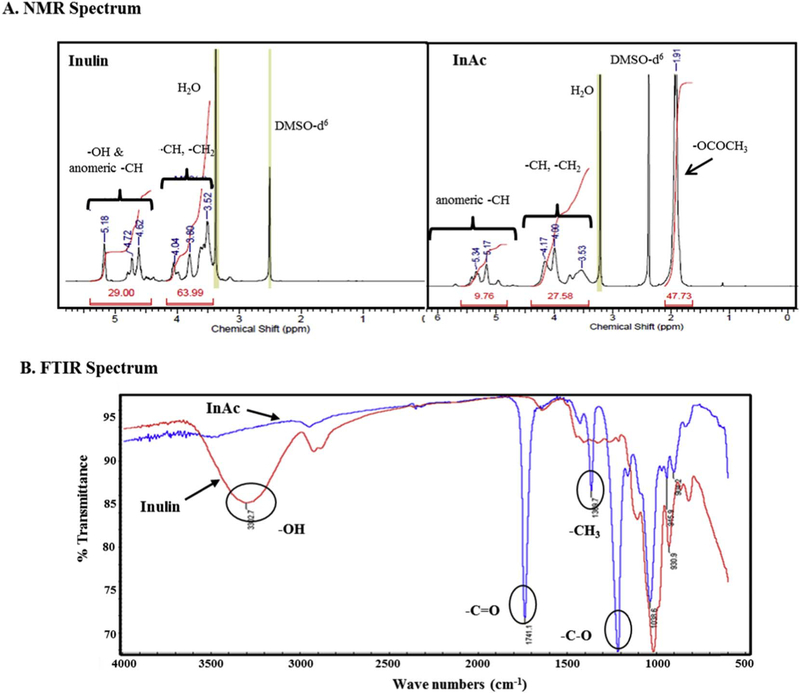
Inulin is a polysaccharide that contains multiple fructose units linked with glucose units at the terminal. InAc was prepared by acet- ylating the hydroxyl functional groups of the inulin by using acetic anhydride [15,19]. The acetylation of inulin was confirmed by 1H NMR spectroscopy and Fourier transform infrared (FTIR) spectroscopy (Fig. 1A and B). NMR spectroscopic analysis of polymers showed peaks in the region of 3.35–4.20 ppm for inulin. These peaks were shifted towards high delta (4.10–4.45 ppm and 5.30–5.66 ppm) for InAc due to de-shielding on –CH generated by the acetylation of hydroxyl groups (Fig. 1A). New proton peaks representing acetyl group have appeared at 2.06–2.13 ppm for InAc. FTIR spectroscopic analysis of InAc further confirmed the presence of the acetyl group with the appearance of corresponding peaks at ~ 1743 cm−1 (C=O), ~1224 cm−1 (C–O) and at ~1369cm−1 (–CH3) (Fig. 1B). A broad peak corresponding to several hydroxyl groups of inulin at ~3326 cm −1 was absent in InAc, which suggests that there was almost complete acetylation of hydroxyl groups. The consistency of synthesized InAc from batch to batch was assessed by the absence of –OH peak in FTIR analysis and the shift in the peaks in proton NMR analysis [15,30].
Fig. 1.
Physicochemical characterization of polymers inulin and inulin acetate. (A) 1H NMR spectrum of inulin and inulin acetate in DMSO-d6 indicating additional peaks representing acetyl group in inulin acetate. (B) FTIR spectrum of inulin and inulin acetate demonstrating complete disappearance of -OH peak of inulin (~3326 cm −1) and appearance of peaks for –C=O (~ 1743 cm−1), –C—O (~ 1224 cm−1) and for –CH3 groups (~ 1369 cm−1) suggesting complete acetylation of inulin.
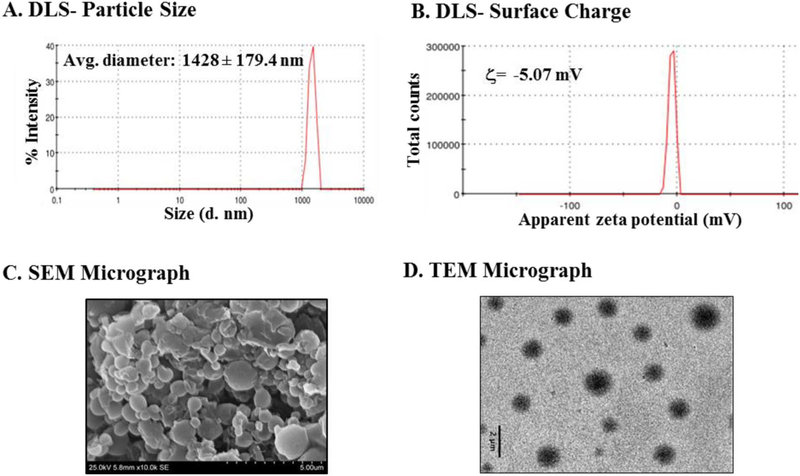
Inulin acetate particles were prepared either by encapsulating ovalbumin as an antigen (PMVDS) or without any antigen (blank particles). Both InAc as a polymer and particles prepared with InAc were water insoluble [15,18]. The characteristics of InAc particles were investigated by multiple techniques; a wet technique such as dynamic light scattering (DLS) (Fig. 2A–B), and dry techniques such as transmission electron microscopic (TEM) and scanning electron microscopy (SEM). DLS technique was used mainly to support the size measurements obtained from dry techniques. InAc particles (PMVDS) were spherical in shape with an average diameter of 1.044 ± 0.35 μm and 1.255 ± 0.47 μm determined by SEM and TEM micrographic analysis respectively (Fig. 2C–D). However, the size was slightly larger when determined by DLS technique (Fig. 2A). This may be due to the presence of a hydration layer around the particles during analysis using a wet DLS technique.
Fig. 2.
Physicochemical characterization of and inulin acetate based particulate vaccine delivery system (PMVDS). (A) Particle size distribution measured using DLS as percent intensity. (B) Surface charge (ς-potential) distribution. (C) Scanning electron micrograph showing inulin acetate microparticles with spherical morphology. (D) TEM image of inulin acetate microparticles.
3.2. The release of antigen from PMVDS is sustained over a prolonged period
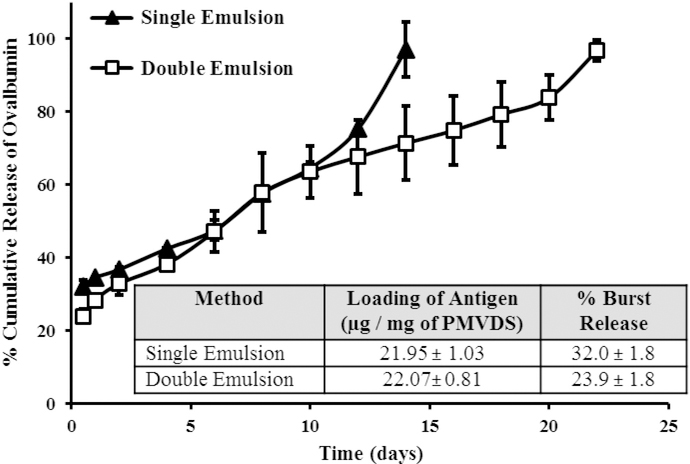
Protein encapsulation in hydrophobic particles is challenging [31,32]. To improve the antigen loading and also to tailor the release kinetics of the antigen, two different methods were used to prepare InAc particles; single emulsion and double emulsion methods. Both the methods provided comparable loading of the antigen (Fig. 3, insert), however, they differed in antigen release pattern. Kinetics of antigen release was studied in-vitro using 50 mM phosphate buffered saline, pH 7.4 (PBS) as a vehicle. Although increasing the antigen to polymer ratio enhanced the antigen loading, it also increased the burst release (% of the encapsulated antigen released within 30 min of incubation). Double emulsion-solvent evaporation method with an antigen to polymer ratio of 1 to 10 provided the required release kinetics with < 25% burst release and the rest of the antigen (> 70%) was released slowly over a period of 20 days (Fig. 3).
Fig. 3.
Antigen loading and in-vitro release kinetics of ovalbumin (ova) from InAc-PMVDS. PMVDS were prepared by either single emulsion or double emulsion methods. The insert compares the effect of the method of preparation on the ova loading and burst release. Release studies were performed in 10mM phosphate buffer, pH7.4. At different time intervals, the soluble ova concentration in the supernatant was measured by using micro-BCA assay. Data represent mean ± standard deviation (n = 3).
The immune response could be improved by controlling the release pattern of the antigen from particulate delivery systems. While the immediate release (burst release) of antigen is important for priming the immune system, the sustained release provides persistent exposure of antigen to APCs to maintain high levels of memory immune response [33–36]. In the present study, the preparation of PMVDS was optimized to achieve controlled antigen release, with immediate and sustained releasing patterns (Fig. 3). The burst release of antigen was restrained to < 25% of the total encapsulated antigen. The InAc particles were prepared with a hydrophobic polymer and a water-soluble stabilizer (PVA).
3.3. PMVDS delivers antigen efficiently and persistently to dendritic cells
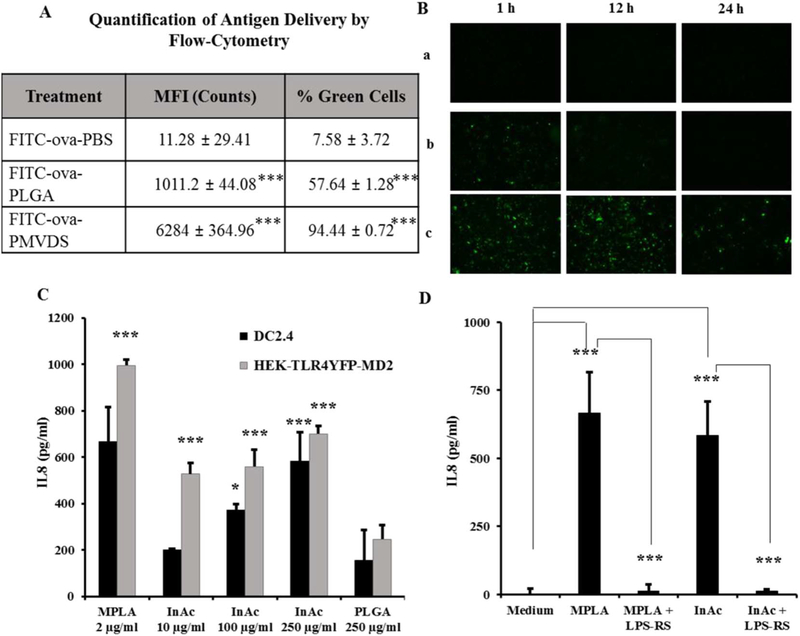
Efficient delivery of an antigen to professional APCs such as dendritic cells plays a significant role in the success of a vaccine technology. The ability of InAc-PMVDS to deliver an antigen (ovalbumin) to naive dendritic cells (DC2.4) was compared with an established PLGA based vaccine delivery system. Both InAc particles and PLGA particles used in this experiment are similar in several physicochemical characteristics such as hydrophobicity, size, surface charge, and antigen loading (Table S1). InAc-PMVDS was highly efficient in delivering antigen (FITC-ova) to dendritic cells with respect to both the number of cells that received measurable amounts of antigen and the quantity of antigen delivered per cell (Fig. 4A). Around 98% of cells received antigen when delivered through InAc-PMVDS, which is significantly more compared to other groups where the antigen was delivered through PLGA particles (~62%) or PBS (~11%) (Fig. 4A). In addition, InAc-PMVDS on average delivered 6.2 times and 557 times more antigen to each cell compared to PLGA particles or PBS respectively as measured by mean fluorescence intensity (MFI) units per cell through a flow cytometer. As expected, both particulate delivery systems (InAc and PLGA) were more efficiently engulfed by highly phagocytic naïve dendritic cells [37–39]. However, it is interesting to observe that even within particulate delivery systems, InAc particles delivered more amount of antigen per cell than PLGA particles (Fig. 4A). The above results were qualitatively confirmed by fluorescence microscopy (Fig. 4B). Not only did the InAc particles provide high amounts of antigen to dendritic cells, but also the antigen persisted for > 24 h inside dendritic cells (Fig. 4B), which could not be achieved when antigen was delivered in solution or through PLGA particles. This observed phenomenon may be due to higher intracellular levels of the antigen delivered through InAc-PMVDS (Fig. 4A), differential degradation pattern of PMVDS particles inside dendritic cells, or both. As a functional consequence, antigen persistence in dendritic cells facilitates continuous priming of T-cells, which often play a significant role in generating strong systemic and local immune memory [34–36].
Fig. 4.
PMVDS targets dendritic cells for antigen delivery (A-B) and activation (C-D). (A-B) Mouse dendritic cells (DC2.4) were Incubated with the antigen, FITC-ova delivered through PBS (a), PLGA particles (b), or InAc-PMVDS (c) as a vehicle. (A) The amount of antigen delivered in 1 h was quantified by flow cytometer by measuring the mean fluorescence intensity (MFI) of each cell. Data represent mean ± standard deviation (n = 3) and *** represents that the values are significantly different when compared to FITC-ova-PBS group (at p < 0.001) using one-way ANOVA with Bonferroni’s multiple comparison test. (B) The persistence of the antigen delivered in 1 h to dendritic cells was followed at different time periods using fluorescence imaging (1 h, 12 h, and 24 h). (C-D) InAc particles activate dendritic cells through TLR-4. (C). Dendritic cells and HEK cells expressing only TLR-4 were incubated with a known TLR-4 agonist MPLA (2 μg/ml), InAc particles at different concentration (10, 100 and 250 μg/ml), and PLGA particles (250 μg/ml). Data represents mean ± standard deviation (n = 4–5). * and *** indicates that values are significant at p ≤ 0.05 and p ≤ 0.001 respectively, as compared to PLGA treated cells using one-way ANOVA followed by Dunnett’s post-hoc multiple comparison test. (D) Simultaneously, DC2.4 cells were incubated with an established TLR-4 antagonist, LPS-RS (120 ng/ml) for 1 h before adding InAc particles (250 μg/ml) or MPLA (2 μg/ml). After 48 h of immune stimulation, cell supernatants were collected and analyzed for the levels of IL-8 by ELISA as a readout for activation. InAc particles along with MPLA stimulated DC2.4 cells. However, LPS-RS inhibited InAc induced immune-stimulation of DC2.4 cells. Data represents mean ± standard deviation (n = 4–5). *** indicates that values are significant, at (p ≤ 0.001) using one-way ANOVA, followed by post-hoc multiple comparison tests. Dunnett’s multiple comparison test was used for MPLA and InAc group to compare with the medium treated cells. Bonferroni’s multiple comparison test was used for MPLA + LPS-RS and InAc + LPS-RS groups to compare with MPLA and InAc groups, respectively.
3.4. Inulin acetate (InAc) particles activate dendritic cells
From the above experiments (Fig. 4A–B), it was clear that naïve dendritic cells recognize InAc particles better than PLGA particles of similar physical properties. Naïve dendritic cells constantly sample the surrounding environment for pathogens and recognize them through their surface PRRs such as TLRs or NLRs [11,17,40–42]. Several polysaccharides from microbes such as lipopolysaccharides (LPS), mannans, glycoinositolphospholipids, viral envelope proteins, and also modified polysaccharides are typically recognized by TLR-4 receptors on APCs [42]. Inulin is a plant polysaccharide, which has been previously identified to stimulate immune cells, however, at very high concentrations [15]. Since InAc is a modified form of inulin, we hypothesize that InAc particles could be recognized and engulfed by dendritic cells through TLR-4 receptors [43]. The hypothesis was tested by investigating the ability of InAc to activate TLR-4 receptors.
InAc based vaccine delivery system stimulated dendritic cells in a concentration-dependent manner to release a potent chemokine interleukin-8 (IL-8), similar to a well-known TLR-4 agonist monophosphoryl lipid-A (MPLA) (Fig. 4C). This activity is lost when the cells were preincubated with a TLR-4 specific antagonist (LPS-RS), which suggests that InAc stimulates dendritic cells through TLR-4 receptors (Fig. 4D). The result that InAc is a TLR-4 agonist was confirmed by using HEK-TLR-4yfp-MD2 cells. The endogenous functional TLR-4 protein exists as a heterodimer with the MD2 protein [44]. The HEK-TLR-4YFP-MD2 cells are genetically altered human kidney epithelial (HEK) cells that exclusively express both the TLR-4 and MD2 proteins on their surface (HEK-TLR-4yfp-MD2 cells). They do not actively express any other TLRs. Importantly, the expressed TLR-4 receptor is functional and signals to release IL-8 only upon the activation through ligand binding [26]. An important observation that the InAc particles activated HEK-TLR-4yfp-MD2 cells to release IL8 into the medium suggests that the InAc is a polymer based semi-synthetic TLR-4 agonist (Fig. 4C) [25]. The above results on dendritic cells with the TLR-4 specific antagonist and on genetically modified TLR-4 cells confirm that InAc is a polymer based TLR-4 agonist. Therefore, it is possible that particles prepared with a TLR-4 ligand InAc were better recognized and phagocytized (Fig. 4A–B) by dendritic cells through TLR-4-InAc interaction compared to non-immune-active particles. TLR-4 agonistic activity of InAC particles is less potent than bacterial derived MPL-A. Indeed, such a weaker activity is an advantage in preparing a vaccine delivery system (PMVDS) with TLR-4 agonist as a sole building material. If a vaccine delivery system is prepared with a potent TLR-4 agonist such as MPL-A as a building material, it would cause severe inflammation and systemic toxicity at the amount needed to prepare particles with sufficient antigen encapsulated.
As a TLR-4 agonist, the ability of InAc-PMVDS to activate swine PBMCs to release an inflammatory cytokine TNF-α was assessed using real-time PCR. PMVDS particles significantly enhanced the expression of the TNF-α gene in swine PBMCs as compared to PBMCs treated with RPMI medium (~11 fold) or PLGA particles (~1.9 fold) (Fig. S1). This is consistent with our previously reported results where InAc also activated primary immune cells to enhance the expression of another inflammatory cytokine IL-6 gene [15].
Taken together, the data from the above in-vitro experiments (Fig. 4 and S1) suggests that InAc-PMVDS is multifunctional; an efficient particulate vaccine delivery system similar to PLGA particles [39] as well as a novel immune-potentiating vaccine adjuvant such as MPLA with the TLR-4 agonistic property [42].
3.5. PMVDS generates strong antibody response in mice against an encapsulated antigen
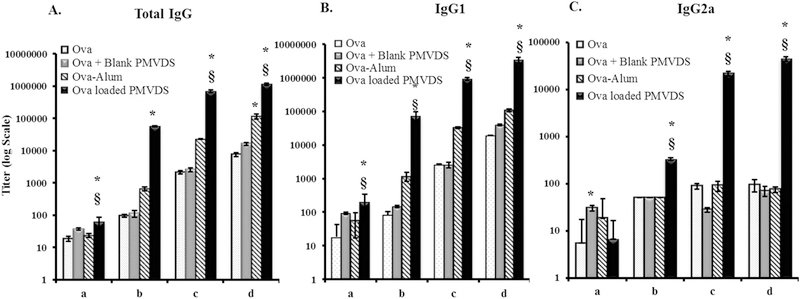
Once established in-vitro as a potential vaccine delivery system, it is important to test the potential technology in-vivo. Antigen-specific antibody response is an important aspect of successful vaccination. Generation of relative IgG2a and IgG1 antibodies indicate Th1 and Th2 polarization, respectively. The ability of multi-functional PMVDS to generate a strong antibody response against encapsulated antigen was investigated in mice. The antigen ovalbumin (100 μg) was delivered to each mouse as a solution in saline, in a bound form with alum (250 μg) as an adjuvant, encapsulated inside InAc particles (~ 2 mg) as PMVDS or as a physical mixture with blank InAc particles (~ 2 mg). Antigen delivered through PMVDS generated strong antibody titers that indicated both Th1 (IgG2a) and Th2 (IgG1) type of adaptive immune responses (Fig. 5). The magnitude of antibody response by PMVDS was robust (p < 0.001) compared to ova without adjuvant (Total IgG, 150 times; IgG1, 180 times; IgG2a, 465 times) or alum bound ova (Total IgG, 5.5 times; IgG1, 32 times; IgG2a, 592 times). The ratio of IgG2a to IgG1 titer three weeks after booster dose injection was 1:77 for PMVDS and 1:1452 for alum as an adjuvant. This suggests that PMVDS stimulated signals related to both humoral and cellular immune response. There were no significant IgE levels found in the serum. Dose sparring study (at 1, 10, 100 μg/mice) indicates that even at low doses of weak antigen like ova, PMVDS robustly stimulated the secretion of several antibodies into the serum (IgG, IgG1, and IgG2a) (Fig. S3). At 10 times less dose (10 μg/mice), PMVDS produced around 15 times more IgG1 titers than ova without adjuvant (100 μg) (Fig. S3). Such dose sparring effect will significantly reduce the antigen shortage during the pandemic attack of infections.
Fig. 5.
Ova-specific antibody titers in immunized mice serum. Mice (n = 4–5 per group) were injected intradermally with ova (100 μg) alone or along with alum (250 μg) or InAc microparticles (~2.0 mg), or loaded inside InAc-PMVDS (~2.0 mg) on days 1 and 21 as primary and booster doses. Sera were collected at 1 and 3 weeks after each immunization for analysis of IgG titers using indirect ELISA. The Y-axis is plotted as Log10 scale. Groups ‘a’ and ‘b’ represent one week and three weeks’ time point after primary immunization, respectively. Groups ‘c’ and ‘d’ represent one week and three weeks’ time points after booster injections, respectively. Data represents mean ± standard deviation (n = 4–5). * and § indicates that the results are statistically significant at p < 0.05 as compared to ova group and ova-alum group, respectively using one way-ANOVA followed by Bonferroni’s multiple comparison test.
The uniqueness of the PMVDS technology is that it is both a vaccine adjuvant and a delivery system. Purely as an adjuvant, injecting blank InAc particles along with soluble ova also enhanced the immune response to ovalbumin by two-fold (IgG1, 3 weeks after booster). However, the humoral response was tremendously boosted when ova was encapsulated inside InAc particles as PMVDS (~ 32 fold) (Fig. 5). The higher efficiency of PMVDS could be attributed to the additional benefits that it offers as a delivery system. One of such advantages is the co-delivery of antigen and adjuvant to the same APCs, which has been shown to be crucial in shaping the adaptive immune response [45]. Indeed, the efficiency of presenting antigens on MHCs by dendritic cells is dependent on the presence of TLR agonists and antigens together within phagosomes. Even the type of adaptive immune response generated by adjuvant could also be altered by changing the formulation; the context of antigen presentation. For example, muramyl dipeptide in saline produces a humoral response but induces a potent cellular response when delivered with lipophilic carrier systems [46,47]. This advantage of co-delivery was absent when blank InAc particles were injected with the antigen. In addition, the soluble antigen is expected to be eliminated very quickly from the site of injection leaving insoluble InAc particles at the site. Other advantages of PMVDS may include antigen persistence both at the injection site as a depot or inside APCs (Fig. 5B). All these advantages as a delivery system may have contributed to the differences in the magnitude and the type of immune stimulation by these two groups.
3.5.1. PMVDS stimulates cell-mediated immunity in addition to humoral immunity
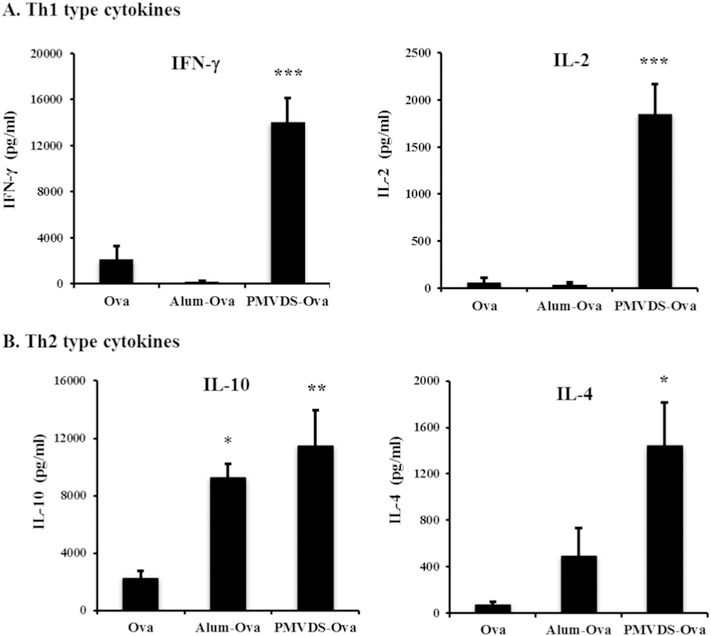
PMVDS stimulated the release of significantly higher levels of IgG2a antibodies in comparison to antigen without adjuvant or alum as an adjuvant (Fig. 5C), which is an indication of possible signals for the stimulation of cellular immune response. The IFN-γ is one of the main cytokines that represents cell-mediated immunity, which is also an important factor that controls the antibody isotype switching to IgG2a [48,49] To further evaluate whether ova loaded PMVDS induced preferential humoral or cell-mediated immune responses, the presence of secreted Th1 (IL-2 and IFN-γ) (Fig. 6A) and Th2 (IL-4 and IL-10) (Fig. 6B) cytokines in the splenocytes culture supernatants was investigated. Three weeks after the booster immunization with ova, splenocytes isolated from the spleens of immunized mice were re-stimulated with ova in ex-vivo conditions. Splenocytes from mice immunized with alum only secreted Th2 type cytokines. However, consistent with the antibody response, significantly high levels of both Th1 and Th2 cytokines were secreted by the splenocytes isolated from mice immunized with PMVDS-ova (Fig. 6). Due to their unique activation of TLR-4 on APCs, PMVDS may have stimulated strong cytokine responses representing both humoral and cell-mediated immunity types.
Fig. 6.
Quantification of Th1 type (IFN-γ and IL-2) and Th2 type (IL-10 and IL-4) cytokines from the splenocyte culture supernatants. Splenocytes were prepared from mice immunized with ova alone, ova with alum (Alum-ova), or ova loaded InAc-PMVDS (PMVDS-Ova) and cultured in the presence of ova (100 μg/ml). After 72h, the concentration of various cytokines in the culture supernatants was measured using sandwich ELISA. *, ** and *** indicates that the values are significant at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001, respectively, as compared to the splenocytes from the ova immunized mice using one-way ANOVA followed by Bonferroni’s multiple comparison test. Data represents mean ± standard deviation (n = 4–5).
Several studies have shown that PAMPs activate APCs and stimulate the expansion of antigen-specific memory T cells [46,50–52]. Splenocytes isolated from the mice immunized with InAc based PMVDS also showed higher proliferation response to the antigen challenge in comparison to splenocytes isolated from mice immunized with ova alone or ova with alum as an adjuvant (Fig. S2, Supporting information). These results suggest that PMVDS also generated strong memory cell response against encapsulated antigen.
The precursor polymer inulin could not activate TLR4 as much as InAc [15]. Inulin is also water soluble, unlike InAc. Interestingly, when the antigen was delivered through soluble inulin microparticles (sIMs) failed to generate a significant cellular immune response, unlike PMVDS [18]. This differences in the immune stimulation pattern could be attributed to the similarity with the PAMPs both with respect to the activation of PRR signaling (TLR-4) and hydrophobic or amphiphilic nature of several known PAMPs.
3.6. Antigen delivery through PMVDS activates cytotoxic T cells (CTLs) and natural killer cells
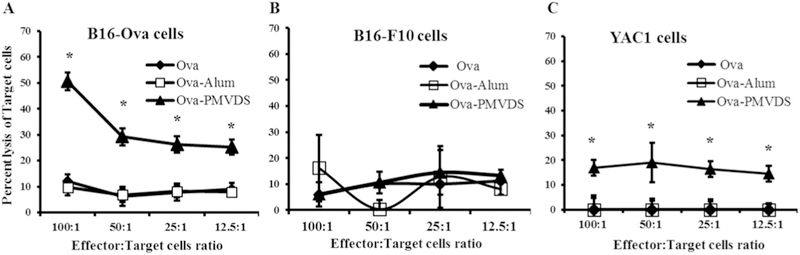
The hallmark of cellular immune response is the activation of CTLs against the antigen-expressing cells. CTLs are one of the most important components of the cellular immune system along with Natural Killer (NK) cells, which have the capability to kill the pathogen infected cells [53]. To confirm the presence of ova-specific CTLs in the mice immunized with ova and their potential to kill ova expressing cells, an ex-vivo cytotoxicity assay was performed. The splenocytes from the mice immunized with ova, which may also contain CTLs, were used as effector cells to kill target cells (B16-ova, B16-F10, and YAC-1) when incubated together. The B16-ova and B16-F10 tumor cells were used to represent antigen (ova) expressing and non-expressing target cells, respectively. B16-ova are same as B16-F10 mouse melanoma cells but genetically modified to express ova. The splenocytes from the mice immunized with PMVDS killed a significantly higher number of the target cell (B16-ova) (Fig. 7A) as compared to splenocytes from mice immunized with ova in saline or ova with alum as an adjuvant. Importantly, the same set of splenocytes from PMVDS immunized mice could not recognize and kill B16-F10 cells (Fig. 7B), which do not express ova. These results suggest that antigen delivered through InAc-PMVDS activated antigen-specific CTLs.
Fig. 7.
Ex-vivo assessment of cellular Immunity from splenocytes of Immunized mice. Splenocytes (effector cells) of Immunized mice were cultured at different ratios with fixed number of target cells; A) B16-ova B), B16-F10, and C) YAC-1 cells. After 6h of incubation, lactate dehydrogenase (LDH) release was measured using LDH assay kit as an indication of cell lysis. Statistical analysis was performed using student’s t-test. * indicates that the results are statistically significant (p < 0.05) as compared to ova immunized mice. Data represents mean ± standard deviation (n = 4–5).
NK cells are another important component of cellular immune response, but they are not antigen-specific, unlike CTLs. NK cells along with CTLs play a significant role in cancer immunity [54,55]. We evaluated the presence of NK cells in the splenocytes from immunized mice by incubating them with YAC-1 cells. NK cells are known to recognize and kill YAC-1 cells [54]. The results indicate significantly more YAC-1 cells were killed with the splenocytes from mice immunized with PMVDS in comparison to mice vaccinated with ova without adjuvant or ova with alum as an adjuvant (Fig. 7C). Alum as an adjuvant did not activate any antigen-specific CTLs or NK cells (Fig. 7). These results suggest that antigen delivered through InAc based PMVDS could stimulate strong cellular immune response to kill cells that express the encapsulated antigen.
3.7. PMVDS stimulates significant cellular immune response to prevent antigen expressing tumor growth in mice
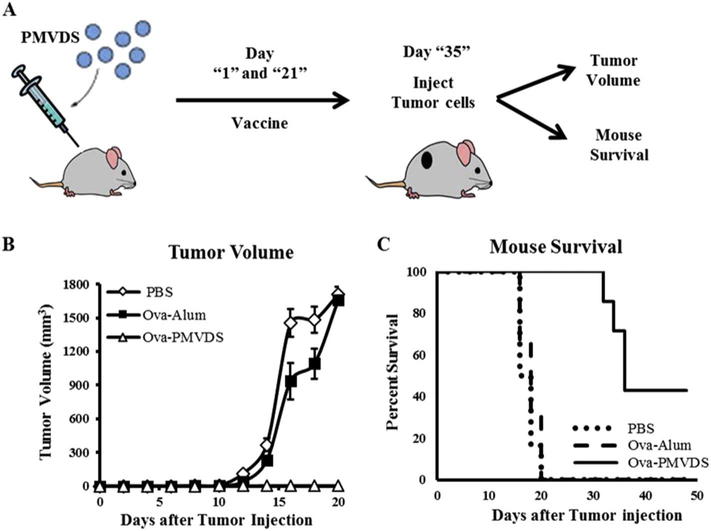
Finally, the concept that InAc based PMVDS are efficient enough in stimulating significant cellular immune response to prevent the proliferation of antigen expressing cells in-vivo conditions was tested by using a mouse melanoma tumor model [56,57]. The mice were immunized two times on day 1 and day 21 with various formulations with ova as an antigen. Fourteen days after the second dose, mice were challenged subcutaneously with 1 × 105 B16-ova melanoma cells that generate solid tumors (Fig. 8A). Immunization with alum as an adjuvant did not protect the mice from tumor development, although, it produced strong antibody response (Fig. 5). However, PMVDS based vaccine formulation showed a significant reduction in tumor volume and importantly, prevented tumor formation in around 40% of immunized mice over 48 days of study (Fig. 8B–C). However, in the rest of PMVDS immunized mice, the tumor progression is significantly delayed compared to alum-adjuvanted immunizations. This proof-of-concept study along with ex-vivo data (Fig. 7) strongly demonstrate that PMVDS is an efficient delivery system for vaccines to activate antigen-specific CTLs and NK cells to kill antigen-expressing cancer cells and prevent/delay tumor progression in animal models (Fig. 9).
Fig. 8.
Effect of PMVDS vaccination on the prevention of tumor progression. (A) Mice were administered on days 1 and 21 with PBS, ova with alum (250 μg) and ova encapsulated InAc-PMVDS. Fourteen days after the second dose, mice were challenged with a subcutaneous injection of 1 × 105 B16-ova cells. (B) Tumor volume was measured at different time intervals using the formula 0.5 × length × (width)2. (C) Percent survival of the mice is reported using Kaplan-Meier method. Data represents mean ± standard deviation (n = 8).
Fig. 9.
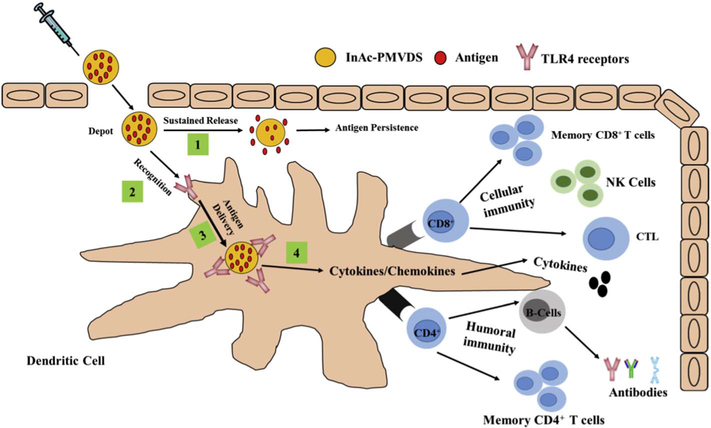
Schematic illustration of the mode of action of InAc based multi-functional pathogen mimicking vaccine delivery system (InAc-PMVDS). InAc-PMVDS is a particulate vaccine delivery system prepared with hydrophobic immune-active polymer inulin acetate (Figs. 1&2). Once immunized, being hydrophobic in nature, InAc particles form a depot at the injection site to sustain the release of antigen (Fig. 3) [1]. In addition, InAc particles are better recognized by dendritic cells through their interaction with TLR-4 on dendritic cells as they mimic PAMPS [2]. Through this recognition, InAc-PMVDS provides enhanced and sustained antigen delivery to dendritic cells (Fig. 4A–B) [3]. As a novel TLR-4 agonist, InAc-PMVDS stimulates the APCs to release pro-inflammatory cytokines and/or chemokine’s (Fig. 4C–D) [4] to initiate a cascade of signaling for a strong immune response. This unique multi-functionality of PMVDS as a delivery system and adjuvant [1–4] manifests into robust humoral and cellular immune stimulation (Figs. 5–7) along with strong memory response (Fig. S2) in mice. As a proof-of-concept study using a melanoma mouse model, vaccination with PMVDS technology protected 100% of mice from tumor development (Fig. 8).
In addition to potentiating the immune response as a next generation adjuvant, PMVDS could also function as a vaccine delivery system to protect the antigen from environmental degradation during storage. Inulin is also known as a cryoprotectant/stabilizer for protein formulations during freeze-drying [58]. Importantly, the polymer is economical and the production procedure is also simple enough to scale up. Affordability of the technology is very critical to translate lab discoveries into field/clinical applications. Being a particulate delivery system, PMVDS offers several future opportunities such as tunable physical properties, a prospect for encapsulation and controlled delivery of multiple antigens, and simultaneous incorporation of other immune-stimulatory factors or PAMPs. There is a further opportunity for surface modification InAc particles to target M-cells for an improved oral vaccine technology [59].
The paucity of adjuvants that activate clinically relevant cellular immune response has become a serious bottleneck in developing effective vaccines against the most challenging diseases, especially for intracellular pathogens. Although this is a proof-of-concept study with novel vaccine platform technology, these results are highly promising in support of a further investigation of PMVDS as a potent vaccine delivery technology not only for cancer but also for other infectious diseases where the cellular immune response is desired. More studies with disease-specific antigens and cancer antigens are underway.
4. Conclusions
In this study, a novel pathogen mimicking vaccine delivery system was designed by targeting specific signaling pathways of the innate immune system. The uniqueness of the technology is that the material/polymer used to prepare the delivery system itself is immune-active, unlike previously reported polymer based particulate vaccines. PMVDS is more potent than commercially available alum, and more importantly, it activates both humoral and cellular immune responses. This platform technology, reported for the first time in this manuscript, will have broader applications in designing the next generation of vaccines against both extracellular and intracellular pathogens and cancer where strong cell-mediated immunity is required.
Supplementary Material
Acknowledgements
We thank the Department of Pharmaceutical Sciences, SDSU (startup funds), and the Translational Cancer Research Center (TCRC, 3SA161-HT) for funding the study. We acknowledge BEI Resources, NIAID, NIH, and Dr. K. L. Rock (University of Massachusetts Medical Center, Worcester, MA) for providing the HEKTLR-4YFPMD2 and DC2.4, respectively. We acknowledge Dr. Preety Sahdev and Dr. Chandradhar Dwivedi for their assistance in mouse experiments. We thank Drs. Aravind Baride and Bruce Gray from the University of South Dakota and Mrs. Maudi Killian Marisela from the Sanford Research, for their assistance in TEM and flow-cytometry experiments, respectively. This project used the Sanford Research Flow Cytometry Core Facility that is supported in part by a COBRE grant from the National Institutes of Health (P20 GM103548).
Footnotes
Competing financial interests
Patent applications on this technology are pending. The technology is currently licensed to Medgene Labs (Brookings, SD, USA) for commercialization from South Dakota State University. Currently, Dr. Hemachand Tummala is a technical consultant for Medgene Labs.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jconrel.2017.07.003.
References
- [1].Moyer TJ, Zmolek AC, Irvine DJ, Beyond antigens and adjuvants: formulating future vaccines, J. Clin. Investig 126 (2016) 799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Demento SL, Siefert AL, Bandyopadhyay A, Sharp FA, Fahmy TM, Pathogen-associated molecular patterns on biomaterials: a paradigm for engineering new vaccines, Trends Biotechnol. 29 (2011) 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Amorij J-P, Kersten GF, Saluja V, Tonnis WF, Hinrichs WL, Slütter B, Bal SM, Bouwstra JA, Huckriede A, Jiskoot W, Towards tailored vaccine delivery: needs, challenges and perspectives, J. Control. Release 161 (2012) 363–376. [DOI] [PubMed] [Google Scholar]
- [4].Brito LA, O’Hagan DT, Designing and building the next generation of improved vaccine adjuvants, J. Control. Release 190 (2014) 563–579. [DOI] [PubMed] [Google Scholar]
- [5].O’Hagan DT, Fox CB, New generation adjuvants-from empiricism to rational design, Vaccine 33 (2015) B14–B20. [DOI] [PubMed] [Google Scholar]
- [6].Töpfer E, Boraschi D, Italiani P, Innate immune memory: the latest frontier of adjuvanticity, J Immunol Res 2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reed SG, Orr MT, Fox CB, Key roles of adjuvants in modern vaccines, Nat. Med 19 (2013) 1597–1608. [DOI] [PubMed] [Google Scholar]
- [8].Gallucci S, Lolkema M, Matzinger P, Natural adjuvants: endogenous activators of dendritic cells, Nat. Med 5 (1999) 1249–1255. [DOI] [PubMed] [Google Scholar]
- [9].Nau GJ, Schlesinger A, Richmond JF, Young RA, Cumulative toll-like receptor activation in human macrophages treated with whole bacteria, J. Immunol 170 (2003) 5203–5209. [DOI] [PubMed] [Google Scholar]
- [10].Takeuchi O, Akira S, Pattern recognition receptors and inflammation, Cell 140 (2010) 805–820. [DOI] [PubMed] [Google Scholar]
- [11].Kumar H, Kawai T, Akira S, Pathogen recognition by the innate immune system, Int. Rev. Immunol 30 (2011) 16–34. [DOI] [PubMed] [Google Scholar]
- [12].Bergmann-Leitner ES, Leitner WW, Adjuvants in the driver’s seat: how magnitude, type, fine specificity and longevity of immune responses are driven by distinct classes of immune potentiators, Vaccine 2 (2014) 252–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].De Gregorio E, The path forward, Vaccine 33 (2015) B60–B63. [DOI] [PubMed] [Google Scholar]
- [14].Maisonneuve C, Bertholet S, Philpott DJ, De Gregorio E, Unleashing the potential ofNOD-and toll-like agonists as vaccine adjuvants, Proc. Natl. Acad. Sci 111 (2014) 12294–12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kumar S, Kesharwani S, Kuppast B, Rajput M, Bakkari MA, Discovery of inulin acetate as a novel immune-active polymer and vaccine adjuvant: synthesis, material characterization, and biological evaluation as a toll-like receptor-4 agonist, J. Mater. Chem. B 4 (2016) 7950–7960. [DOI] [PubMed] [Google Scholar]
- [16].Reed SG, Hsu FC, Carter D, Orr MT, The science of vaccine adjuvants: advances in TLR4 ligand adjuvants, Curr. Opin. Immunol 41 (2016) 85–90. [DOI] [PubMed] [Google Scholar]
- [17].Dowling JK, Mansell A, Toll-like receptors: the swiss army knife of immunity and vaccine development, Clin. Transl. Immunology 5 (2016) e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kumar S, Tummala H, Development of soluble inulin microparticles as a potent and safe vaccine adjuvant and delivery system, Mol. Pharm 10 (2013) 1845–1853. [DOI] [PubMed] [Google Scholar]
- [19].Poulain N, Dez I, Perrio C, Lasne M-C, Prud’homme M-P, Nakache E, Microspheres based on inulin for the controlled release of serine protease inhibitors: preparation, characterization and in vitro release, J. Control. Release 92 (2003) 27–38. [DOI] [PubMed] [Google Scholar]
- [20].Kumar S, Kesharwani SS, Mathur H, Tyagi M, Bhat GJ, Tummala H, Molecular complexation of curcumin with pH sensitive cationic copolymer enhances the aqueous solubility, stability and bioavailability of curcumin, Eur. J. Pharm. Sci 82 (2016) 86–96. [DOI] [PubMed] [Google Scholar]
- [21].Muley P, Kumar S, El Kourati F, Kesharwani SS, Tummala H, Hydrophobically modified inulin as an amphiphilic carbohydrate polymer for micellar delivery of paclitaxel for intravenous route, Int. J. Pharm 500 (2016) 32–41. [DOI] [PubMed] [Google Scholar]
- [22].Cui C, Schwendeman SP, Surface entrapment of polylysine in biodegradable poly (DL-lactide-co-glycolide) microparticles, Macromolecules 34 (2001) 8426–8433. [Google Scholar]
- [23].Kang J, Schwendeman SP, Determination of diffusion coefficient of a small hydrophobic probe in poly (lactide-co-glycolide) microparticles by laser scanning confocal microscopy, Macromolecules 36 (2003) 1324–1330. [Google Scholar]
- [24].Shen Z, Reznikoff G, Dranoff G, Rock KL, Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules, J. Immunol 158 (1997) 2723–2730. [PubMed] [Google Scholar]
- [25].Morefield GL, Hawkins LD, Ishizaka ST, Kissner TL, Ulrich RG, Synthetic tolllike receptor 4 agonist enhances vaccine efficacy in an experimental model of toxic shock syndrome, Clin. Vaccine Immunol 14 (2007) 1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].He W, Qu T, Yu Q, Wang Z, Lv H, Zhang J, Zhao X, Wang P, LPS induces IL-8 expression through TLR4, MyD88, NF-kappaB and MAPK pathways in human dental pulp stem cells, Int. Endod. J 46 (2013) 128–136. [DOI] [PubMed] [Google Scholar]
- [27].Rajput MK, Darweesh MF, Park K, Braun LJ, Mwangi W, Young AJ, Chase CC, The effect of bovine viral diarrhea virus (BVDV) strains on bovine monocyte-derived dendritic cells (Mo-DC) phenotype and capacity to produce BVDV, Virol. J 11 (2014) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chaitra M, Shaila M, Nayak R, Characterization of T-cell immunogenicity of two PE/PPE proteins of mycobacterium tuberculosis, J. Med. Microbiol 57 (2008) 1079–1086. [DOI] [PubMed] [Google Scholar]
- [29].Zhang Z, Tongchusak S, Mizukami Y, Kang YJ, Ioji T, Touma M, Reinhold B, Keskin DB, Reinherz EL, Sasada T, Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery, Biomaterials 32 (2011) 3666–3678. [DOI] [PubMed] [Google Scholar]
- [30].Mine S, Izawa H, Kaneko Y, Kadokawa J.-i., Acetylation of a-chitin in ionic liquids, Carbohydr. Res 344 (2009) 2263–2265. [DOI] [PubMed] [Google Scholar]
- [31].Acharya AP, Lewis JS, Keselowsky BG, Combinatorial co-encapsulation of hydrophobic molecules in poly (lactide-co-glycolide) microparticles, Biomaterials 34 (2013) 3422–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yeo Y, Park K, Control of encapsulation efficiency and initial burst in polymeric microparticle systems, Arch. Pharm. Res 27 (2004) 1–12. [DOI] [PubMed] [Google Scholar]
- [33].Kamath AT, Mastelic B, Christensen D, Rochat AF, Agger EM, Pinschewer DD, Andersen P, Lambert PH, Siegrist CA, Synchronization of dendritic cell activation and antigen exposure is required for the induction of Th1/Th17 responses, J. Immunol 188 (2012) 4828–4837. [DOI] [PubMed] [Google Scholar]
- [34].Jusforgues-Saklani H, Uhl M, Blachère N, Lemaître F, Lantz O, Bousso P, Braun D, Moon JJ, Albert ML, Antigen persistence is required for dendritic cell licensing and CD8 + T cell cross-priming, J. Immunol 181 (2008) 3067–3076. [DOI] [PubMed] [Google Scholar]
- [35].Kim TS, Hufford MM, Sun J, Fu Y-X, Braciale TJ, Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection, J. Exp. Med 207 (2010) 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xu D, Walker CM, Continuous CD8 + T-cell priming by dendritic cell cross-presentation of persistent antigen following adeno-associated virus-mediated gene delivery, J. Virol 85 (2011) 12083–12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Petersen LK, Ramer-Tait AE, Broderick SR, Kong C-S, Ulery BD, Rajan K, Wannemuehler MJ, Narasimhan B, Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants, Biomaterials 32 (2011) 6815–6822. [DOI] [PubMed] [Google Scholar]
- [38].Sahdev P, Ochyl LJ, Moon JJ, Biomaterials for nanoparticle vaccine delivery systems, Pharm. Res 31 (2014) 2563–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yue H, Ma G, Polymeric micro/nanoparticles: particle design and potential vaccine delivery applications, Vaccine 33 (2015) 5927–5936. [DOI] [PubMed] [Google Scholar]
- [40].Baranov MV, ter Beest M, Reinieren-Beeren I, Cambi A, Figdor CG, van den Bogaart G, Podosomes of dendritic cells facilitate antigen sampling, J. Cell Sci 127 (2014) 1052–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sukhithasri V, Nisha N, Biswas L, Kumar VA, Biswas R, Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions, Microbiol. Res 168 (2013) 396–406. [DOI] [PubMed] [Google Scholar]
- [42].Uematsu S, Akira S, Toll-like Receptors (TLRs) and Their Ligands, Toll-like Receptors (TLRs) and Innate Immunity, Springer, 2008, pp. 1–20. [DOI] [PubMed] [Google Scholar]
- [43].Redlich S, Ribes S, Schütze S, Eiffert H, Nau R, Toll-like receptor stimulation increases phagocytosis of Cryptococcus neoformans by microglial cells, J. Neuroinflammation 10 (2013) 1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Park BS, Song DH, Kim HM, Choi B-S, Lee H, Lee J-O, The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex, Nature 458 (2009) 1191–1195. [DOI] [PubMed] [Google Scholar]
- [45].Blander JM, Medzhitov R, Toll-dependent selection of microbial antigens for presentation by dendritic cells, Nature 440 (2006) 808–812. [DOI] [PubMed] [Google Scholar]
- [46].Carelli C, Audibert F, Chedid L, Persistent enhancement of cell-mediated and antibody immune responses after administration of muramyl dipeptide derivatives with antigen in metabolizable oil, Infect. Immun 33 (1981) 312–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Parant MA, Audibert F, Chedid L, Level M, Lefrancier P, Choay J, Lederer E, Immunostimulant activities of a lipophilic muramyl dipeptide derivative and of desmuramyl peptidolipid analogs, Infect. Immun 27 (1980) 826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Finkelman F, Katona IM, Mosmann TR, Coffman RL, IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses, J. Immunol 140 (1988) 1022–1027. [PubMed] [Google Scholar]
- [49].Maloy KJ, Odermatt B, Hengartner H, Zinkernagel RM, Interferon y-producing y8 T cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection, Proc. Natl. Acad. Sci 95 (1998) 1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Liu Q, Jia J, Yang T, Fan Q, Wang L, Ma G, Pathogen-mimicking polymeric nanoparticles based on dopamine polymerization as vaccines adjuvants induce robust humoral and cellular immune responses, Small 12 (2016) 1744–1757. [DOI] [PubMed] [Google Scholar]
- [51].Chinnery F, King CA, Elliott T, Bateman AR, James E, Viral antigen mediated NKp46 activation of NK cells results in tumor rejection via NK-DC crosstalk, Oncoimmunology 1 (2012) 874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Smith CM, Wilson NS, Waithman J, Villadangos JA, Carbone FR, Heath WR, Belz GT, Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity, Nat. Immunol 5 (2004) 1143–1148. [DOI] [PubMed] [Google Scholar]
- [53].Zamai L, Ponti C, Mirandola P, Gobbi G, Papa S, Galeotti L, Cocco L, Vitale M, NK cells and cancer, J. Immunol 178 (2007) 4011–4016. [DOI] [PubMed] [Google Scholar]
- [54].Wright S, Bonavida B, Studies on the mechanism of natural killer cell-mediated cytotoxicity. IV. Interferon-induced inhibition of NK target cell susceptibility to lysis is due to a defect in their ability to stimulate release of natural killer cytotoxic factors (NKCF), J. Immunol 130 (1983) 2965–2968. [PubMed] [Google Scholar]
- [55].Luna JI, Grossenbacher SK, Murphy WJ, Canter RJ, Targeting cancer stem cells with natural killer cell immunotherapy, Expert. Opin. Biol. Ther (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].De Titta A, Ballester M, Julier Z, Nembrini C, Jeanbart L, van der Vlies AJ, Swartz MA, Hubbell JA, Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose, Proc. Natl. Acad. Sci 110 (2013) 19902–19907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nakamura T, Miyabe H, Hyodo M, Sato Y, Hayakawa Y, Harashima H, Liposomes loaded with a STING pathway ligand, cyclic di-GMP, enhance cancer immunotherapy against metastatic melanoma, J. Control. Release 216 (2015) 149–157. [DOI] [PubMed] [Google Scholar]
- [58].de Jonge J, Amorij J-P, Hinrichs WL, Wilschut J, Huckriede A, Frijlink HW, Inulin sugar glasses preserve the structural integrity and biological activity of influenza virosomes during freeze-drying and storage, Eur. J. Pharm. Sci 32 (2007) 33–44. [DOI] [PubMed] [Google Scholar]
- [59].Jiang T, Singh B, Li H-S, Kim Y-K, Kang S-K, Nah J-W, Choi Y-J, Cho C-S, Targeted oral delivery of BmpB vaccine using porous PLGA microparticles coated with M cell homing peptide-coupled chitosan, Biomaterials 35 (2014) 2365–2373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.