Abstract
Although sea urchins are one of the oldest and most widely used marine model systems, few species have been routinely kept in culture through multiple generations. The workhorse of the field is the purple urchin Strongylocentrotus purpuratus. However, one disadvantage of S. purpuratus is its long generation time, making it impractical as a model for generating and maintaining transgenic lines. In an effort to develop a sea urchin that is suitable for transgenerational experiments and the generation of transgenic lines, we have focused on development of updated culturing methods and genomic resources for the painted sea urchin, Lytechinus pictus. Compared to S. purpuratus, L. pictus have relatively large eggs, develop into optically clear embryos, and the smaller adults can become gravid in under a year. Fifty years ago, Hinegardner developed culturing methods for raising L. pictus through metamorphosis. Here, we provide an updated protocol for establishing and maintaining L. pictus in the laboratory, and describe a new genome resource for this urchin. In our hands, L. pictus reach the 4-armed pluteus stage at 4 days; become competent to metamorphosis at 24 days; and are gravid by 6 months. Plutei and juveniles are fed on a diet of algae and di-atoms, and adults are fed on kelp. We also make available a L. pictus transcriptome generated from developmental stages (eggs to 2-day-old plutei) to support the annotation of our genome sequencing project, and to enhance the utility of this species for molecular studies and transgenesis.
1. INTRODUCTION
The generation and use of genetically defined and modified lines of laboratory animals have been a cornerstone of modern biology. Yet, it remains the case that the vast majority of sea urchins used in the lab continue to be animals harvested from the wild. The primary reasons for this have been: (1) a lack of molecular tools for generating mutants in marine organisms and (2) the use of echinoderm species with long generation times. The “molecular tools bottleneck” has recently been relaxed, with the demonstration of successful F0 genome editing using the CRISPR/Cas9 system in, among many other organisms, the purple sea urchin Strongylocentrotus purpuratus (Cui, Lin, & Su, 2017; Lin & Su, 2016; Mellott, Thisdelle, & Burke, 2017; Oulhen, Swartz, Laird, Mascaro, & Wessel, 2017; Oulhen & Wessel, 2016; Shevidi, Uchida, Schudrowitz, Wessel, & Yajima, 2017). As such, the next bottleneck to address is the shift to species with generation times suitable for maintaining stable germline transgenic lines in the lab.
In 1969, Hinegardner published a method for raising Lytechinus pictus sea urchins from egg to egg in 4–6 months in the laboratory. He noted that their generation time is on par with that of mice and corn, and argued that “it should now be both possible and practical to use genetic techniques in the study of sea urchin development.” As with several other sea urchin species used in the lab, L. pictus also produce large amounts of gametes that are easy to fertilize externally and their robust embryos can be easily manipulated in the lab. Indeed L. pictus has already been used widely on the west coast of North America and Mexico as a model species for studies in the fields of developmental biology, cell biology, environmental sciences, and toxicology (e.g., Anitole-Misleh & Brown, 2004; Leong & Manahan, 1997; Pace & Manahan, 2006; Shilling & Manahan, 1990).
Although S. purpuratus has been the sea urchin species for which the most molecular resources have been generated, L. pictus have several key advantages. First, the generation of L. pictus is at least three times shorter than that of S. purpuratus (Leahy, 1986). Second, the L. pictus egg and embryo are larger and more transparent than those of S. purpuratus (120 μm vs 80 μm diameter), making them ideal for microscopy. Third, the synchronously growing embryos of L. pictus can be reared at room temperature and reach key stages more quickly than their S. purpuratus counterparts, which are best reared in an incubator at 15°C. Thus, less time and equipment are required to raise L. pictus embryos. Fourth, the smaller adult size of L. pictus is more suitable for lab culture as the gravid adults are full size at approximately 2.5–4cm diameter, and thus more individuals can be housed in a single tank than S. purpuratus, which grow to body sizes ~10cm diameter.
Taking advantage of these benefits of L. pictus as a new transgenic model system will require the implementation of efficient, updated culturing techniques, and the application of growing molecular/genetic resources for this species. Here we present an updated culturing procedure for raising L. pictus in the laboratory, and discuss our efforts for building genomic resources to support the generation of germline transgenics that will be of use to any lab using this sea urchin as a model.
2. CULTURING OF ADULT AND LARVAL L. PICTUS
2.1. MAINTENANCE OF ADULT ANIMALS
L. pictus are native to the southern coast of California from Point Conception to Baja California. Our broodstock at Scripps are from wild populations found at 30m depth off the coast of California near La Jolla Canyon. These animals are maintained in our flow through aquarium facility at Scripps Institution of Oceanography. Sea water for the facility is pumped through custom intake cages and a drum filter at the end of the Scripps Pier and passes through a sand filter with size #20 sand grain and 3/8” gravel bottom before entering a settling tank and being gravity-fed into the aquaria.
Glass 20-gal aquaria are used to raise juveniles, and are connected to open-flow-through sea water lines at either ambient (ranging between 14 and 16°C, averaging 15°C) or chilled (10°C) temperatures. Outflow pipes from each tank are covered with wire mesh prevent animals from entering the pipe (Fig. 1). We maintain our tanks at flow rates of 2L/min. Animals can be spawned year-round in the lab, though the optimum time is from late Spring through summer months (May–September) (Strathman, 1987). Light cycle for the animals in the facility is 12:12 light-dark. Continuous exchange of fresh sea water keeps animals well-oxygenated and flushes out waste products in this open-flow system.
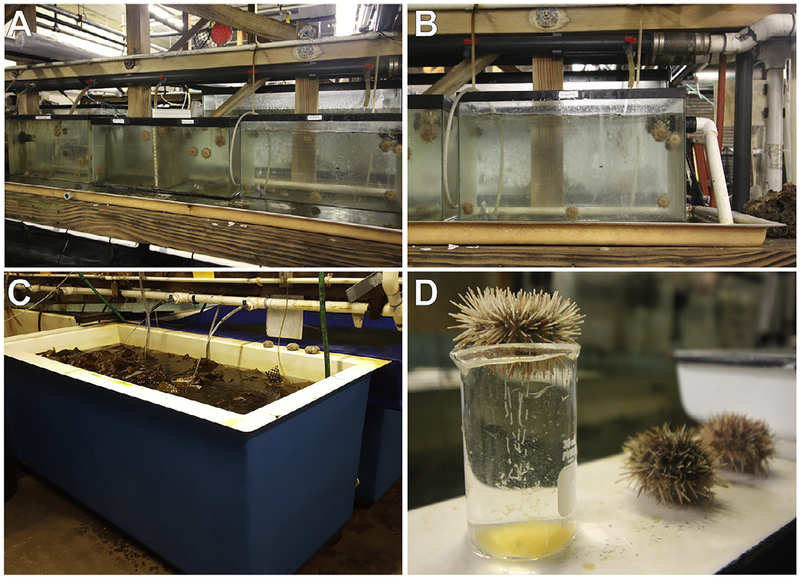
FIG. 1.
Husbandry of adult Lytechinus pictus. (A) Adult animals are kept in communal 20-gallon tanks connected to an open-flow chilled sea water line. (B) Each tank has a water line running into it, and a hole drilled in the side of the tank with PVC piping attached for outflow. The outflow pipe has a mesh covering to prevent animals from crawling into the outflow pipe. Animals are fed kelp (Macrocystis pyrifera) twice weekly. (C) Kelp is collected from the field and stored in a large open-flow tank. (D) Individual animals are selected for spawning. Females are placed aboral side down over a beaker after KCl injection and release ribbons of eggs from the gonopores. Sperm is collected from the aboral surface of the animal. Males and females should not be exposed to air for more than a few minutes. Gently streaming water over females while spawning or placing spawning males in a shallow dish of sea water minimizes stress from air exposure.
Adults are fed a diet of fresh kelp, Macrocystis, collected from the coast near La Jolla Canyon. Fresh kelp is kept in a large open-flow tank. Dried kelp that has been rehydrated in sea water is also sufficient nutrition for adult animals. Animals are fed twice weekly; five blades of kelp satisfy approximately 40 animals. Tanks are cleaned twice a week by siphoning out waste, and any unconsumed kelp is exchanged for fresh kelp.
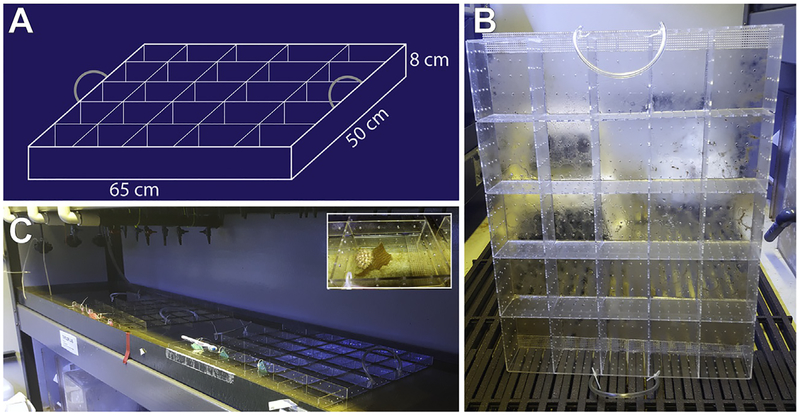
In addition to the large communal tanks, we use a plastic tray system for keeping individual animals separated (Fig. 2). The individualized tanks are constructed from custom-cut 1/8” thick plexiglass (San Diego Plastics, Inc.), with 1/8” holes drilled into the pieces so that each individual compartment has a 4 × 4 grid of holes on each “wall” and the “floor.” The holes allow water to flow through and flush out waste. Pieces in the trays interlock and are also adhered with Weld-on liquid cement (purchased from San Diego Plastics Inc., but available at most hardware stores). Each housing tray has handles made from rubber tubing attached with zip ties. Each individual compartment has dimensions of 13cm × 10cm × 8cm (L × W × H) but can be subdivided further with the addition of more interlocking pieces if additional compartments are needed. The individual trays are kept in an open-flow water table (260cm × 50cm × 8cm) with input from ambient sea water line. Trays are cleaned twice weekly and animals are fed pieces of kelp (about 5cm2) twice weekly. Animals are separated by sex (sexed after spawning) in the trays and the compartments are labeled with laminated ID tags with relevant information such as gender, ID number, date of last spawn, and any relevant manipulations (e.g., Female 2, Spawned: DD/MM/YY).
FIG. 2.
Individual housing for Lytechinus pictus. (A) Blueprints for individual housing units for animals constructed from 1/8″ plexiglass. (B) Each tray of housing units has handles made of rubber tubing attached with zip ties. (C) The trays rest inside a flooded water table that is filled by a chilled sea water line and flows out a pipe at the end of the table. To clean, trays are removed from the water and kelp waste is flushed out, then the table is drained and hosed down before re-flooding and placing the trays back. Inset shows an adult animal inside one of the compartments of the tray.
2.2. COLLECTION OF GAMETES AND FERTILIZATION
L. pictus are spawned using similar procedures to other species of urchins, although greater care needs to be taken to keep animals wet during spawning and to keep KCl level low. In our lab we use a 27-gauge needle to inject through the peristomal membrane with 0.55 M KCl. For an adult with body sizes ranging between 2 and 3.5cm, injection of 100μL (0.1 cc) is sufficient. Animals as small as 1.2cm diameter have been spawned using 50–100μL of KCl (0.05–0.1cc) Animals are gently shaken after injection and gamete release is initiated within minutes. In the event that spawning is delayed, an additional injection on the opposite side of the membrane can be administered to the animal without hindering its recovery. Each animal is shaken by hand immediately before and after KCL injection.
When spawning begins, gender identification is conducted through inspection of released gametes. Females are placed over a beaker filled with sea water, aboral side down (Fig. 1). It is suggested to rinse the animal with sea water as it spawns to prevent stress on the animal. Prolonged exposure (more than a few minutes) to air can hinder the recovery of the animal. Spawning females can be placed within a larger beaker filled with sea water such that the animal is completely submerged for the duration of the spawn. Females release large batches of gametes, generally 1–2mL in volume totaling several hundreds of thousands of eggs. Spawning is completed typically within 15–20min for body sizes between 2.5 and 3cm. Males are placed in a petri dish with sea water, and sperm is collected from the surface of the animal. Sperm should be kept concentrated (dry) for best preservation. A single male (2.5–3cm diameter) can yield between 100 and 300mg of concentrated sperm.
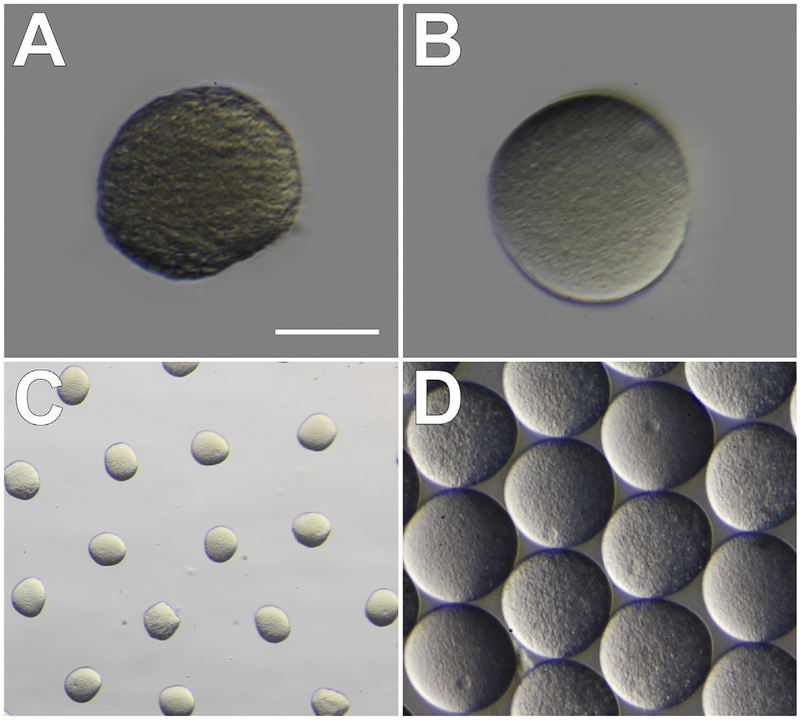
Gametes are prepared for fertilization as for other urchins. Dry-collected sperm can be stored at 4°C until just before fertilization. Eggs are washed 3–4 times with 0.22μM filtered sea water (FSW); gentle stirring with the bulb end of a plastic Pasteur pipette and allowing the eggs to settle for 10–15min before siphoning out 75% of the volume is sufficient. Eggs can be examined under the microscope for quality and cleanliness. Poorer quality eggs will appear grainy or are irregularly shaped (Fig. 3A). High quality eggs with little jelly coating remaining will have a smooth even texture and regular color throughout (Fig. 3B) and will be tightly packed in the middle of a shallow dish when swirled after dejellying (Fig. 3C and D). Immature eggs will have large germinal vesicles and are not ready for fertilization. Eggs from L. pictus are fragile and do not handle screening through Nytex mesh as well as other urchin gametes do (e.g., S. purpuratus) for removal of jelly coating. We do not recommend screening L. pictus eggs, but if necessary they can be passed through a >125μm mesh (eggs are approximately 120μm in diameter) to filter out larger debris or remove fertilization envelopes. In other species of sea urchin, it is recommended to wash the eggs in an acidic solution (4–5 drops of 0.5M citric acid into 40mL FSW, pH 4.8) to remove the jelly coating. However, we usually choose to omit acid washing for L. pictus because the eggs can be sufficiently de-jellied with FSW washes.
FIG. 3.
Quality and washing of Lytechinus pictus eggs. (A) Poor quality eggs have a wrinkled appearance at the edges and a grainy texture, in addition to uneven color throughout. (B) High quality eggs are smooth and round, with even color and texture. Scale bar in A and B = 60μm. (C) Unwashed eggs are covered in a transparent jelly-like substance and space evenly apart when resting in a dish. (D) Eggs washed in filtered sea water (FSW) are dejellied and will cluster tightly together when swirled in a dish.
To assess quality of washed eggs and of sperm, test fertilizations are performed. Eggs of L. pictus are prone to polyspermy (V. Vacquier, personal communication), thus we recommend a more dilute sperm solution (5μL of concentrated sperm into 25mL of 0.22μM FSW) for performing fertilizations than is typically recommended with other urchin species. The dilution should not be used after 15min, but rather a fresh dilution should be made. The fertilization envelope should encase the newly fertilized zygote within a minute or two (Fig. 4A and B). Fertilization success of >95% can be achieved from gametes that are well-washed and of superior quality. Sperm can be stored at 4°C for 2–3days with little decrease in quality.
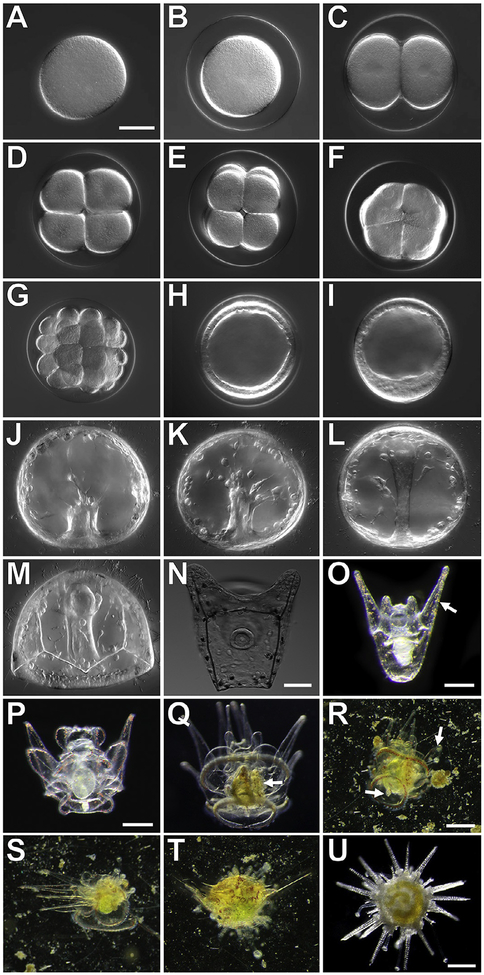
FIG. 4.
Developmental stages of the sea urchin, Lytechinus pictus. A–M and N imaged in DIC, scale bars = 50μM; O scale bar = 0.25mm; P and Q scale bar = 0.25mm; R–T scale bar = 0.25mm; U scale bar = 0.25mm. (A) Unfertilized egg; (B) Fertilized egg; (C) 2-cell embryo; (D) 4-cell embryo; (E) 8-cell embryo; (F) 16-cell embryo; (G) 32-cell embryo;(H) Early blastula stage; (I) Hatched mesenchyme blastula; (J) early gastrulation;(K) mid-gastrulation; (L) late gastrulation; (M) prism larva; (N) early 2-arm pluteus;(O) late 2-arm pluteus, red pigment cells (arrow) are visible in the ectoderm; (P) 8-arm pluteus without a rudiment; (Q) competent 8-arm pluteus with a rudiment (white arrow);(R) Early metamorphosis, a competent larvae swims to the substrate and tube feet (arrow) extend from the rudiment; (S) Mid-stage metamorphosis of a competent larvae, the animal moves the arms to the side and further attaches tube feet to the settlement surface, tissue along the arms is resorbed, revealing bare skeletal rods; (T) Late stage metamorphosis, the animal has extended the walking spines and the arms are now pushed to the top of the juvenile body as further tissue reorganization occurs; (U) Juvenile sea urchin at 1 week post-metamorphosis.
2.3. EARLY DEVELOPMENT AND LARVAL CARE
We culture L. pictus embryos and larvae between 15 and 20°C (Figs. 4 and 5; Table 1). We establish long-term cultures of embryos at densities no >1 embryo/mL FSW during early development. However, densities of up to 100 embryos/mL can be reared up to hatching. The use of antibiotics (i.e., penicillin-streptomycin) during larval culture is generally not necessary if using high quality, finely filtered sea water, in our case 0.22μM filtered with a bottle top filter (e.g., Corning, # CLS431475).
FIG. 5.
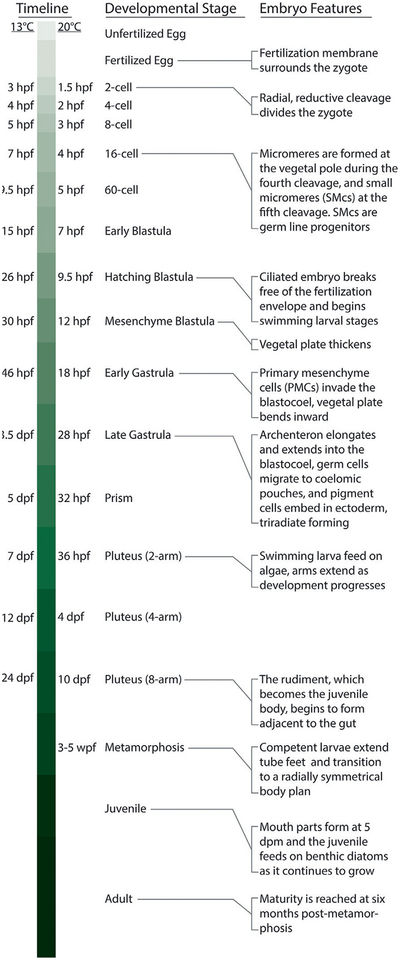
A quick guide to development in the sea urchin, Lytechinus pictus. “hpf” hours post-fertilization; “dpf” days post-fertilization; “wpf” weeks post-fertilization; “dpm” days post-metamorphosis.
Table 1.
Comparison of Developmental Time Course of L. pictus and S. purpuratus at Typical Culture Temperatures
| Developmental Stage | L. pictus | S. purpuratus |
|---|---|---|
| 2-cell | 1.5hpf | 2 hpf |
| 4-cell | 2hpf | 4 hpf |
| 16-cell | 4hpf | 7 hpf |
| Early Blastula | 7hpf | 16 hpf |
| Hatching Blastula | 9.5 hpf | 22–24 hpf |
| Gastrulation | 18 hpf | 32–44 hpf |
| Prism | 32 hpf | 72dpf |
| Pluteus (2-arm) | 36–38 hpf | 4dpf |
| Pluteus (4-arm) | 4dpf | - |
| Pluteus (8-arm) | 10dpf | - |
| Metamorphosis | 3–5 wpf | 6–12 wpf |
| Maturity | 6mpm | 24mpm |
L. pictus are cultured at room temperature (20°C), and S. purpuratus at 15°C; “hpf” hours post-fertilization, “dpf” days post-fertilization, “wpf” weeks post-fertilization, “mpm” months post-metamorphosis.
When cultured at room temperature (~20°C), embryos undergo the first cell division approximately 1.5h post-fertilization (hpf) (Fig. 4C). By 4hpf the embryo has reached the 16-cell stage (Fig. 4D–F). The early cleavages occur considerably faster than those in S. purpuratus, which undergoes the fourth cleavage after approximately 7h when grown at 15°C (Table 1). By 7hpf the L. pictus embryo is at the early blastula stage (Fig. 4H). The ciliated embryo will spin around within the fertilization envelope until hatching to begin its free-swimming stages at approximately 9.5hpf, whereas the S. purpuratus blastula does not hatch until close to 24hpf. By 12hpf a mesenchyme blastula stage is achieved (Fig. 4). The primary mesenchyme cells begin to ingress into the blastocoel initiating primary gastrulation by 18hpf. The vegetal plate invaginates and, after a brief pause, the archenteron elongates into the blastocoel. By 20–22hpf primary mesenchymal cells form a typical arrangement of ventrolateral clusters and PMCs fuse and form a syncytium within the blastocoel (Fig. 4). Between 22 and 28hpf pigment cells migrate and embed into the ectoderm, the triradiate begins to mineralize and extend to form the skeletal rods, and germ cells migrate to the coelomic pouches by late gastrulation. The gut fuses to the oral side of the animal by 32hpf when the embryo has reached the early prism stage (Fig. 4). Between 36 and 38hpf the prism has grown to an early pluteus with two short, rounded anterior lateral arms supported by the extending skeletal rods. The gut is differentiated at this time, and the larvae are diluted to a concentration of 1 larva/3mL FSW.
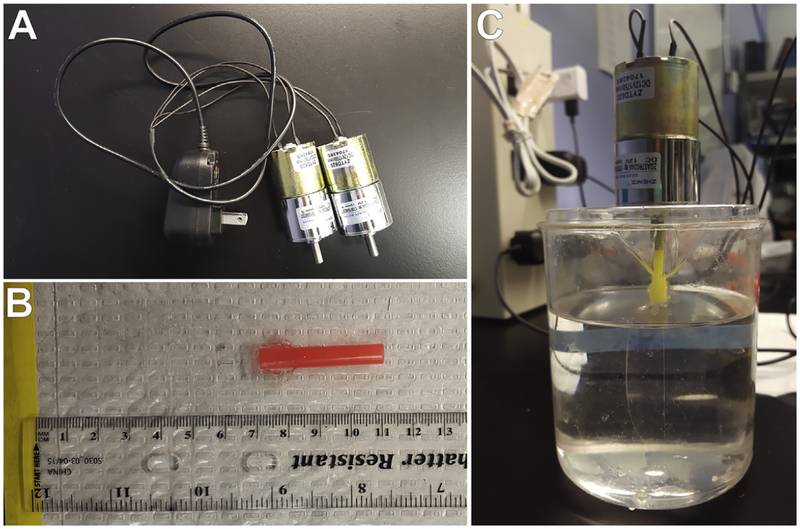
Water in the culture beakers is slowly stirred to prevent larvae from congregating at the surface of the culture and getting trapped in the surface tension. We use a 12V DC Micro Gear Box Motor (Uxcell, Part. No. a16071400ux0596) and 12V AC/DC Power Supply Adapters (Amazon, SMART-POWER: CUGLB; Fig. 6A) attached to oval-shaped paddles made from plastic sheeting to stir cultures at 30rpm (Fig. 6C). Stir paddles are constructed using thin plastic sheeting and straws (Fig. 6B), and can be adhered together with hot glue or Weld-On liquid cement, and thoroughly washed with fresh water and FSW before placing into culture to keep residual material from washing into cultures and impacting larval health. Alternatively, plastic sheeting and straws can remain attached without adhesive (in smaller vessels, e.g., 500 mL beakers) or by taping them together (for larger vessels, e.g., ~3000mL beakers).
FIG. 6.
Culture apparatus for rearing larvae of L. pictus. (A) A power supply is modified to connect with two motors, and wiring is sealed with heat-shrink tubing. (B) Stir paddles are constructed from plastic straws and plastic sheeting. The paddle is connected to the straw by slicing the straw with a razor blade and placing the plastic paddle into the cut. This can be secured using hot glue or Weld-on, but will also remain in place without adhesive. The plastic sheeting making the paddle is clear, and cut into an oval approximately 8cm long and 4cm wide. The plastic sheeting has a 1cm overlap with the plastic straw to stay secured. The straw can be cut-to-length as needed. (C) The open end of the straw is inserted onto the rotating part of the motor. The motor and paddle is connected to an outlet and held above the culture vessel (in our case a 500mL beaker) by resting it on a 13mm plastic petri dish with a hole cut in the middle.
Water in the cultures is exchanged every 2days starting at the 2-arm pluteus stage. Debris and unconsumed food are gently washed away using an overflow method. The culture is slowly poured over a 125μM mesh submerged in a dish filled with FSW. Mesh is constructed by taking 2-in. PVC piping and hot-gluing a piece of 125μM nylon mesh (Component Supply Co., Part No. U-CMN-125) around one end of the pipe (Fig. 7A). The larvae in the mesh are passed through the FSW dish in a gentle scooping motion (Fig. 7B and C) and rinsed gently with a squirt bottle filled with FSW before rinsing the larvae off the mesh and back into the culture beaker filled with fresh FSW (Fig. 7D).
FIG. 7.
Washing of larval cultures using the overflow technique. (A) Larvae are collected over a 125μm mesh, secured to PVC piping. (B) The culture is slowly poured through the mesh submerged in a shallow dish of FSW. (C) The larvae are rinsed using a gentle scooping motion, and rinsed with fresh FSW, allowing the excess water and waste to “over flow” out of the shallow dish while retaining the larvae. (D) Larvae are carefully rinsed back into a culture vessel with fresh FSW by using a squirt bottle filled with FSW to wash them from the mesh.
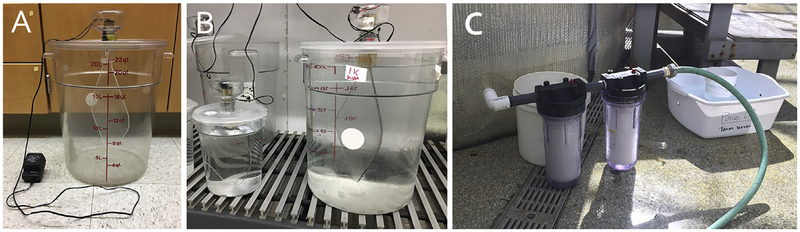
It is also possible to culture L. pictus (as well as other echinoderm larvae) at higher volumes (Fig. 8). This is particularly important if measurements of interest require a large amount of tissue or if day-to-day sampling is moderate but needs to continue throughout the entire larval stage. We use 20-L food-grade polycarbonate, plastic containers made by Cambro (catalog # RFSCW22135) in combination with a polypropylene lid (catalog # RFS12SCPP190). Both of these items are commonly available at restaurant supply stores as well as Amazon. Cultures are stirred (necessary for ensuring equal distribution of larvae and algal food) using small gearhead motors that are mounted on top of the lid. The motors are attached to a plexiglass diamond shape paddle (custom made). It is important to ensure that motors used for this purpose have a low rpm speed and high torque (necessary for driving the paddle through the culture water). We use Buehler gearhead motors (18rpm, 23.5V DC). Motor speed is further controlled using a Tatco Universal AC/DC Adapter with six different voltage settings (ranging from 3 to 12V). We generally mix the cultures at 6–8rpm. This setup can also be used for a range of smaller volumes (3 – 10L) simply by changing the paddle and container size. Water is changed every 2 days by gently siphoning the culture water onto a 50μm Nitex mesh filter (typically mounted on PVC tubing) and allowing seawater to pass through. Larvae are then resuspended in freshly filtered sea-water and appropriate algal feeding conditions are established. Seawater is filtered down to 0.2μm using a two-step filtration process (10 and 0.2μm). This allows for filtration rates up to 5gal per minute. Filter cartridge housing (catalog # 1ECP8) and filter cartridges (catalog numbers: 10 μm = 2MJG1; 0.2μm = 4NVU4) are available through Grainger.
FIG. 8.
Equipment used for culturing L. pictus in 20-L format. (A) 20-L culture vessel showing food grade container, DC gearhead motor mounted to a plexiglass paddle for mixing, and an AC/DC Adapter with variable speed control. The motor has been protected from seawater using an empty 250mL plastic container with holes drilled on the top to help vent out heat generated by motor. (B) Example of 20-L and 3-L vessels. The 3-L vessel is setup up the same as the 20-L but with a smaller paddle. (C) Seawater filtration rig. The upstream cartridge holds a 10μm filter cartridge and the downstream holds a 0.2μm filter cartridge. Background shows the Nitex mesh where larvae are filtered on to during water change.
2.4. FEEDING OF LARVAE
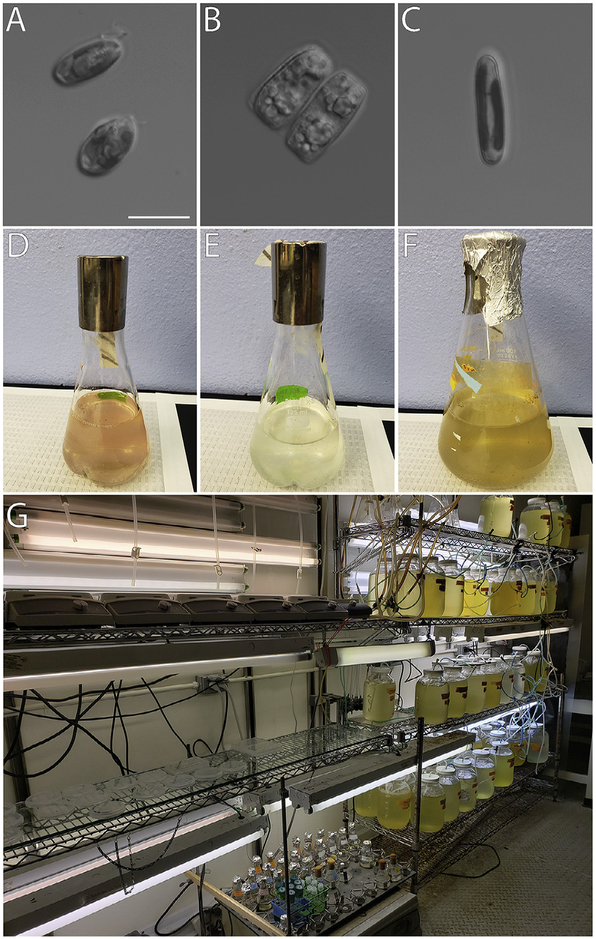
We always exchange the culture water before feeding the larvae. The plutei are given Rhodamonas sp. (Fig. 9B) daily at a concentration of 5000cells/mL culture. Stocks can be purchased from the National Center for Marine Algae and Microbiota (NCMA) at Bigelow Laboratories. The axenic Rhodamonas cultures are raised unagitated in the commercially available ProLine F/2 algae food, equivalent to Guillard’s (Guillard, 1975; Guillard & Ryther, 1962) media, at room temperature on a 12:12 light-dark cycle. Cultures are propagated by transferring 15–30mL of a dense culture into 250mL of fresh F/2 media, aseptically. Within 2 weeks the newly inoculated culture should be growing stably and can be used to inoculate subsequent cultures. Cultures will turn a brownish-reddish color when they are old and dying (typically around 3 weeks), whereas a dark pink culture indicates healthy algal growth. Aliquots of 40–45mL of algae in F/2 are spun down (500 × g for 3–5min), resuspended in 20mL 0.22μM FSW, and counted on a hemocytometer before feeding.
FIG. 9.
Algal food sources for Lytechinus pictus throughout development. (A) Rhodamonas sp. are fed to larval sea urchins. Scale bar = 10 μm. (B) Nitzschia alba is a benthic diatom and can be fed ad libidum to newly settled juveniles. (C) Navicula incerta is a benthic diatom that may serve as a temporary/alternative food source for newly settled juveniles instead of N. alba. (D)–(F) Cultures of Rhodamonas sp., N. alba, and N. incerta are grown in 250 mL flasks on a 12:12 light:dark cycle at 20°C, 25°C, and 18°C, respectively. Flasks are kept agitated at approximately 120 rpm to prevent clumping. Healthy cultures are pink (Rhodamonas), white (N. alba), and greenish-brown (N. incerta) in color. (G) Large scale culturing of these strains in temperature-controlled rooms is optimal.
2.5. COMPETENCY, METAMORPHOSIS, AND JUVENILE REARING
With care, plutei will reach the 4-arm stage between 4 and 6days post-fertilization (dpf). By 10dpf the larvae have 8 arms and the beginnings of the rudiment are visible adjacent to the gut (Fig. 4). Healthy, well-fed larvae can be induced to metamorphose once competency is achieved. We have induced metamorphosis as early as 3weeks post-fertilization (wpf), but on average we find that most larvae settle between 4 and 6wpf.
Metamorphosis of L. pictus is induced by placing competent larvae into 13mm round plastic petri dishes that have grown biofilms by being left to incubate for several weeks in the adult animal tanks. Competent larvae will hover near the bottom of the dish and metamorphose over the course of an hour. Juvenile tube feet will extend from the rudiment, and the larval arms will begin to recede leaving bare skeletal fibers protruding from the animal (Fig. 4). Spines will extend, and the metamorphosing animal will anchor itself to the bottom of the dish. Newly settled juveniles are typically about 1mm in diameter. They have 10 long spines, 5 juvenile tube feet, and will not develop mouth parts until 4 or 5days post-settlement. As the juvenile grows, more tube feet will form, and additional spines extend. Distinct red pigment cells from the larvae can be seen on the aboral side of the animal. Additional pigmentation, presumably from the gut contents, is visible through the translucent juveniles.
Upon completion of metamorphosis the juveniles are moved to fresh 13mm round plastic petri dishes, with no >10 juveniles per dish. Alternatively, if keeping individuals isolated, juveniles are moved into 6-well plates. 30% of the water is exchanged daily. A transitional diet, between the ciliated larval food (Rhodamonas; Fig. 9B) and the adult diet (kelp) is important for maintaining healthy juveniles. The standard recommendation for juvenile diets is the benthic diatom Nitzschia (Fig. 9A), and, the benthic diatoms, Navicula incerta (Fig. 9C), can serve as an alternative/supplemental early juvenile diet. Axenic Nitzschia and Navicula cultures from the National Center for Marine Algae and Microbiota (NCMA) at Bigelow Laboratories for Ocean Sciences (Strains CCMP2426 and CCMP542, respectively). Both species of diatoms are grown in F/2 media (Guillard, 1975; Guillard & Ryther, 1962). 40–45mL aliquots of the diatoms in F/2 are spun down (500 × g for 2min) and resuspended in 10mL of FSW, before provided to the urchin ad libidum. The diatoms will settle in the petri dish and form a “lawn” for the juveniles to graze on. Tracks are left behind the juveniles as they move around the dish and feed on the lawn of diatoms. Additionally, juvenile feeding can be confirmed by the presence of small scratch marks on the dish from the juvenile mouthparts. If diatom growth begins to exceed the rate of consumption, juveniles are carefully transferred with a plastic pasteur pipette to a fresh petri dish with fresh, diluted food. Growth of the juveniles is slower on the Navicula diet than on the optimum food source, Nitzschia.
At 4–6weeks post-metamorphosis, smaller macroalga can be presented to the animals. We suggest Ulva, which we collect from the field. This macroalga can also be obtained commercially (Ebay) or from an aquarium hobbyist. Ulva is fed to the juveniles for 4–5 weeks before transitioning to kelp (Macrocystis pyrifera) for nutrition. Though we have not observed this, if juveniles do not appear to be eating, it is possible that a more enriched food source is required. Scrambled egg whites blended with kelp and/or market squid are a protein-rich diet option (V. Vacquier, personal communication). If this supplementary diet is provided it should be administered in small quantities [e.g., pieces no larger than a grain of rice] and removed from the dish after 24h to avoid rotting. Urchins will not eat this supplement for extended periods of time, thus we recommend only offering it for 1–2 weeks before returning to the algal diet.
Once the juveniles have grown to sufficient size (approximately 3mm diameter) they can be moved into the individual housing boxes previously described, or into a communal tank. The tanks should be cleaned, and animals fed, twice weekly. As early as 6months post-metamorphosis the animals may achieve sexually maturity. Animals at this young age will not release large quantities of gametes (Hinegardner, 1969), but certainly sufficient amounts of egg and sperm can be obtained to propagate a line and culture out a subsequent generation.
2.6. GENOMIC RESOURCES FOR L. PICTUS
A fully sequenced and annotated L. pictus genome is necessary for generating genetically modified lines. The L. pictus genome was recently sequenced by Dovetail Genomics (Santa Cruz, CA) and its size is estimated at 743Mb. Current resources include a mix-staged developmental transcriptome to support genomic and molecular genetic studies. This resource is available on echinobase (http://bouzouki.bio.cs.cmu.edu/Echinobase/LpAbout). The developmental transcriptome represents pooled transcripts from 14 time points ranging from unfertilized eggs to 2-day-old plutei.
The sequenced library resulted in 129,856,831 raw reads. Quality of the reads was assessed using FastQC v0.11.7 (Andrews, 2010), followed by trimming of adapter sequences and filtering for quality control with trimmomatic v0.36 (Bolger, Lohse, & Usadel, 2014). De novo assembly (kmer length = 23) was performed using Trinity v2.66 (Haas et al., 2013) on an Amazon EC2 instance. Trinity assembled the reads into 188,907 genes and 294,633 transcripts. Based on all transcript contigs in the assembly, the median contig length was 403bp, the average contig length was 866.77bp, and the N50 was 1681bp. Transcriptome completeness was quantified using BUSCO v3.0.2 (Simão, Waterhouse, Ioannidis, Kriventseva, & Zdobnov, 2015) and assembly quality assessment was conducted with TransRate (Smith-Unna, Boursnell, Patro, Hibberd, & Kelly, 2016). The transcriptome of L. pictus was 98.7% complete, containing 965 of 978 near-universal single-copy BUSCO orthologs ([S:36.5%,D:62.2%], F:0.8%,M:0.5%,n:978) and had a Transrate score of 0.0473 (Table 2).
Table 2.
Assembly Statistics for the Developmental Transcriptome of Lytechinus pictus
| Sequencing | Total Reads | 129,856,831 |
|---|---|---|
| Trimming | Dropped Reads | 837,154 |
| Both Surviving | 113,415,437 | |
| Assembly | Assembler | Trinity |
| kmer size | 25 | |
| Total Trinity Transcripts | 294,633 | |
| Total Trinity Genes | 188,907 | |
| Percent GC | 38.12 | |
| All transcript contigs | N5O | 1681 |
| Max length | 40,649 | |
| Mean length | 866.77 | |
| Median length | 403 | |
| Assembled bases | 255,377,848 | |
| Assembly quality assessment | Transrate score | 0.0473 |
| Transcripts with ORF (transrate) | 40,526 | |
| BUSCO score | C:98.7%, F:0.8%, M:0.5% |
We are currently maintaining larvae from the cross used for the transcriptome. Sea urchins are highly heterogeneous and this variation will be reduced with generating highly inbred lines, which could help with designing efficient CRISPR guide RNAs. Upon completion of genome annotation, the data will be made publicly available on echinobase for the community to use.
3. DISCUSSION
With the protocols we describe, L. pictus can be kept through multiple generations in the lab, and thus should prove to be a valuable marine model system for a range of fields. One of the most important factors in choosing a species for long-term culture is how resistant it is to diseases and how readily it produces gametes in the lab. Hinegardner (1969, 1975) noted that L. pictus is remarkably resistant to disease, and that the adults could be spawned as frequently as every 1–2 months for at least a year. Hinegardner also reported that virtually no diseased animals were observed in his culture.
Another concern, especially for indirect developing species, is identifying an appropriate diet for each stage in the life cycle. Here we provide a simple protocol for raising L. pictus on flagellated marine algae (during the larval stage), biofilm-associated diatoms (during the newly metamorphosed juvenile stage), and macroalgae (during the adult stage). The effects on reproduction have been studied with more complex diets, for example, the Watts lab has experimented with complex mixtures of fats, protein, and carbohydrates in Lytechinus variegatus (Heflin et al., 2016; Taylor, Heflin, Powell, Lawrence, & Watts, 2017). With the possibility of making genetically modified lines of L. pictus, nutrition and metabolism could be studied at the molecular level in specific genetic backgrounds.
Finally, an important feature of a marine model for transgenesis is that it can easily be maintained in inland aquaria. While our protocol describes how we keep L. pictus in a free flowing system with natural sea water, this system can be adapted to recirculating systems and artificial sea water (see chapter “Procuring animals and culturing of eggs and embryos” by Adams et al., this volume, for suppliers). We maintain the adults of other marine animals in an inexpensive closed system like that described by Henry et al. (2017). The small size of L. pictus adults makes them compatible with inland aquaria. Multiple tanks can be used to keep males and females separate after they have been identified from their first spawns.
The purpose of updating culturing methods and generating transcriptomic and genomic resources for L. pictus is to enable our ultimate goal: to establish genetically altered lines that can be maintained over many generations. With robust culturing techniques and the transcriptome and genome sequence in hand, we will make transgenic lines using traditional transposon (e.g., minos) and meganuclease-based trans-genesis methods, and CRISPR/Cas9 via Non-Homologous End Joining (NHEJ) or Homology-Directed Repair (HDR). In these ways many useful lines of L. pictus can be produced; for example, lines expressing Cas9 under ubiquitous or cell-type specific promotors; inducible gene expression for over-expression or conditional gene knock-down; lines expressing nano-bodies, and cell-type specific transcriptional and translational reporters.
REFERENCES
- Andrews S (2010). FastQC: A quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- Anitole-Misleh KG, & Brown KM (2004). Developmental regulation of catecholamine levels during sea urchin embryo morphogenesis. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 137, 39–50. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, & Usadel B (2014). Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics, 30, 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Lin CY, & Su YH (2017). Recent advances in functional perturbation and genome editing techniques in studying sea urchin development. Briefings in Functional Genomics, 16, 309–318. [DOI] [PubMed] [Google Scholar]
- Guillard RR (1975). Culture of phytoplankton for feeding marine invertebrates In Culture of marine invertebrate animals (pp. 29–60). Boston, MA: Springer. [Google Scholar]
- Guillard RR, & Ryther JH (1962). Studies of marine planktonic diatoms:I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Canadian Journal of Microbiology, 8, 229–239. [DOI] [PubMed] [Google Scholar]
- Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. (2013). De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nature Protocols, 8, 1494–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heflin LE, Makowsky R, Taylor JC, Williams MB, Lawrence AL, & Watts SA (2016). Production and economic optimization of dietary protein and carbohydrate in the culture of juvenile sea urchin Lytechinus variegatus. Aquaculture, 463, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JQ, Lesoway MP, Perry KJ, Osborne CC, Shankland M, & Lyons DC (2017). Beyond the sea: Crepidula atrasolea as a spiralian model system. The International Journal of Developmental Biology, 61, 479–493. [DOI] [PubMed] [Google Scholar]
- Hinegardner RT (1969). Growth and development of the laboratory cultured sea urchin. The Biological Bulletin, 137, 465–475. [DOI] [PubMed] [Google Scholar]
- Hinegardner RT (1975). Morphology and genetics of sea urchin development. American Zoologist, 15, 679–689. [Google Scholar]
- Leahy PS (1986). Laboratory culture of Strongylocentrotus purpuratus adults, embryos, and larvae. Methods in Cell Biology, 27, 1–13. Academic Press. [DOI] [PubMed] [Google Scholar]
- Leong P, & Manahan D (1997). Metabolic importance of Na+/K+− ATPase activity during sea urchin development. The Journal of Experimental Biology, 200, 2881–2892. [DOI] [PubMed] [Google Scholar]
- Lin CY, & Su YH (2016). Genome editing in sea urchin embryos by using a CRISPR/Cas9 system. Developmental Biology, 409, 420–428. [DOI] [PubMed] [Google Scholar]
- Mellott DO, Thisdelle J, & Burke RD (2017). Notch signaling patterns neurogenic ectoderm and regulates the asymmetric division of neural progenitors in sea urchin embryos. Development, 144, 3602–3611. [DOI] [PubMed] [Google Scholar]
- Oulhen N, Swartz SZ, Laird J, Mascaro A, & Wessel GM (2017). Transient translational quiescence in primordial germ cells. Development, 144, 1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhen N, & Wessel GM (2016). Albinism as a visual, in vivo guide for CRISPR/Cas9 functionality in the sea urchin embryo. Molecular Reproduction and Development, 83, 1046–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace DA, & Manahan DT (2006). Fixed metabolic costs for highly variable rates of protein synthesis in sea urchin embryos and larvae. The Journal of Experimental Biology, 209, 158–170. [DOI] [PubMed] [Google Scholar]
- Shevidi S, Uchida A, Schudrowitz N, Wessel GM, & Yajima M (2017). Single nucleotide editing without DNA cleavage using CRISPR/Cas9-deaminase in the sea urchin embryo. Developmental Dynamics, 246, 1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilling FM, & Manahan DT (1990). Energetics of early development for the sea urchins Strongylocentrotus purpuratus and Lytechinus pictus and the crustacean Artemia sp. Marine Biology, 106, 119–127. [Google Scholar]
- Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, & Zdobnov EM (2015). BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics, 31, 3210–3212. [DOI] [PubMed] [Google Scholar]
- Smith-Unna R, Boursnell C, Patro R, Hibberd JM, & Kelly S (2016). TransRate: Reference-free quality assessment of de novo transcriptome assemblies. Genome Research, 8, 1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathman MF (1987). In Strathmann MF (Ed.), Reproduction and Development of Marine Invertebrates of the Northern Pacific Coast. Seattle, WA: The University of Washington Press. [Google Scholar]
- Taylor AM, Heflin LE, Powell ML, Lawrence AL, & Watts SA (2017). Effects of dietary carbohydrate on weight gain and gonad production in small sea urchins, Lytechinus variegatus. Aquaculture Nutrition, 23, 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]