Abstract
Purpose of review:
To summarize the current studies, systematic reviews, and clinical practice guidelines pertaining to the screening, diagnosis, and treatment of osteoporosis in transgender persons.
Recent findings:
Gender affirming hormone therapy has been shown to maintain or promote acquisition of bone density as measured by dual-energy x-ray absorptiometry in transgender men and women. No differences in fracture rates have been seen in trans women or men in short, prospective trials. Trans children and adolescents on gonadotropin-releasing hormone may be at risk for decreasing bone density while not on sex steroid hormone replacement.
Summary:
Ongoing research is needed to determine the clinical significance of changes in bone density in relation to fracture risk. Screening for osteoporosis should be based on assessment of clinical factors including hormone therapy compliance, gonadal removal, and additional osteoporosis risk factors. Post-pubertal trans children and adolescents on gonadotropin-releasing hormone without sex steroid hormone therapy may be at risk for decreasing bone density. Treatment for osteoporosis in trans men and women follows the same guidelines as cisgender populations and should take into consideration both pharmacologic and non-pharmacologic interventions.
1. Introduction
Transgender children and adults receive gender affirming hormone therapy to improve gender dysphoria and to better align their physical and emotional characteristics with their affirmed gender (1). Gender affirming hormone therapy includes the sex steroid hormones in transgender men and women and medications to lower testosterone such as spironolactone in transgender women (2). Gonadotropin-releasing hormone (GnRH) agonists can also be used to delay puberty in youth and lowers testosterone in transgender women. Both these therapies can impact bone health which has been a concern in transgender populations.
The most recent United States population studies estimate that approximately 0.6% of adults, or 1.4 million Americans, identify as transgender (3). There are no current estimates of the prevalence of osteoporosis or low bone bass in transgender individuals in the United States. Furthermore, there is a paucity of data regarding fracture rates in these patients.
Both sex steroids estrogen and testosterone have been shown to be important factors in bone formation during puberty and bone turnover during adulthood (4). During puberty, males attain a larger, but not denser, bone mass than females due to greater periosteal apposition from testosterone stimulation (5). There is no difference in peak volumetric bone mineral density (BMD), or the amount of bone within the periosteal envelope (quantified by gm/cm3), between sexes (5). Estrogen plays an important role in the acquisition of healthy bone in males (6). Males with inactivating mutations in the estrogen receptor and aromatase genes have low bone mass despite normal or high levels of testosterone (7, 8, 9). Both men and women in hypogonadal states have higher rates of osteoporosis and treatment with hormone supplementation has been shown to improve bone density (10, 11, 12, 13, 14).
This review focuses on the clinical aspects of screening and diagnosis of osteoporosis and the treatment of low bone mass in transgender males and females. Topics regarding bone mass in children and adolescents will also be covered.
2. Diagnosis and Screening
2.1. Diagnosis of osteoporosis in trans persons
There are no studies that have evaluated whether clinicians should use birth assigned sex or affirmed gender for determination of T or Z-score. T-score is a value calculated by comparing bone density in postmenopausal women or men over the age of 50 to the bone density of a healthy gender matched adult at the time of peak bone mass (15). A T-score value of −2.5 or below is considered osteoporosis; a T-score between −1 to −2.5 is considered low bone mass (osteopenia) (15). Z-score is used for patients under the age of 50 and is a value calculated by comparing an individual’s bone density to age-, sex-, and ethnicity-matched controls (15).
The International Society for Clinical Densitometry recommends use of a “Caucasian (non-race adjusted) female reference for cisgender men and women of all ethnic groups” (16). This recommendation is primarily based on the availability of fracture data by bone density measurement. Thus, using the Caucasian female reference range would better reflect fracture risk in both transgender women and men. Finally, the presence of fragility fractures or the occurrence of hip or vertebral fracture in the absence of major trauma independent of bone mineral density would constitute a diagnosis of osteoporosis (17).
2.2. Screening in trans women
The recommended screening modality for osteoporosis is dual energy x-ray absorptiometry (DXA) of the lumbar spine, total hip and femoral neck (18). There are few studies that examine the prevalence of osteoporosis and low bone density in transgender women based on DXA. A study from Belgium found that in hormone-treatment naive transgender women, the prevalence of osteoporosis and low bone density in the lumbar spine was 16% and 32%, respectively (based on male reference range) (19). The authors postulated that the high prevalence of osteoporosis and low bone density in young transgender women was due to a less active lifestyle as determined by a physical activity questionnaire (19).
Hormone therapy is associated with increases in BMD in trans women. A recent meta-analysis demonstrated a statistically significant increase in BMD in transgender women at 12 and 24 months in the lumbar spine compared to baseline across nine studies (20). The clinical significance of this change in BMD on fracture risk is unknown; however, estrogen has been shown to increase bone density and reduce fracture risk in postmenopausal cisgender women (21). There has been no observed difference in fracture rates between trans women compared to control men (22, 23).
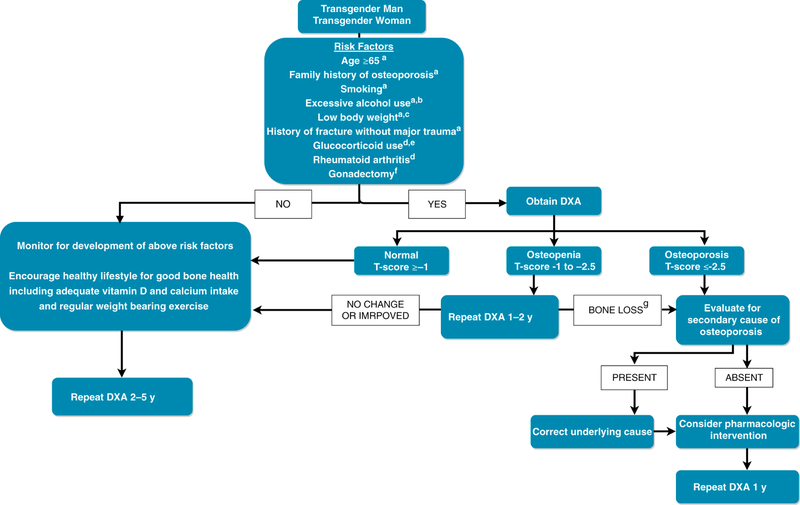
The Endocrine Society Practice Guidelines (2) recommend that clinicians obtain BMD for screening purposes in trans women when risk factors for low bone density exist, such as smoking, low body weight, chronic corticosteroid use, heavy alcohol use (24), and particularly in patients who stop hormone therapy after undergoing gonadectomy. Various risk factors to consider are presented in Figure 1.
Figure 1.
Suggested approach to screening and the treatment of adult transgender patients
a American Association of Clinical Endocrinology/American College of Endocrinology Guidelines. 39 b Three or more drinks per day. c Weighing more than 127 lbs or a body mass index (BMI) of less than 20 kg/m. d Risk factors included in the FRAX tool40. e Prednisolone 5 mg/d or more for 3 months or more, current or past. f Endocrine Society Clinical Practice Guidelines.2 g Bone loss greater than least significant change.
2.3. Screening in trans men
There are a handful of studies that provide data on bone density in transgender men prior to the start of hormone therapy. In one study, the bone density at lumbar, femoral neck and total hip (reported in g/cm2) of 16 trans men compared to control women was similar (25). In another study, a slightly higher bone density was seen in the femoral neck in trans men compared to control cisgender females, 1.02 vs 0.95 g/cm2 (p 0.02), respectively (26).
During treatment, a recent meta-analysis found no statistically significant changes in BMD at 12 and 24 months after initiation of treatment compared to baseline values in testosterone treated trans men (20). One study included in the meta-analysis did find a positive effect of testosterone treatment of bone density with an increase in mean BMD of 7.8% at the femoral neck over a 2 year period (27). There is little data regarding fracture rates in transgender men. In a 1-year prospective study, 53 trans men receiving testosterone therapy over a 1 year period had no observed osteoporotic fractures (28). Adequate levels of testosterone therapy are needed to maintain bone density, and luteinizing hormone (LH) levels in the normal range have been shown to be a useful marker in determining sufficient levels of testosterone therapy to prevent bone loss (inverse relationship between BMD and serum LH levels) (29).
Screening for osteoporosis in trans men is appropriate in patients who stop or are intermittently compliant with testosterone therapy, especially in those who have had gonadectomy (2). In one study done in Singapore, post-gonadectomy trans men both on regular testosterone treatment and those off testosterone treatment or intermittently compliant with treatment were found to have lower BMD as compared to values in trans men without gonadectomy (30). In concordance with the Endocrine Society Clinical Practice guidelines (2), screening for osteoporosis is appropriate when risks for bone loss are present and potentially in those patients who have increased LH values. Other features to consider are presented in Figure 1.
4. Non-pharmacologic therapy in transgender persons for bone health
Vitamin D, calcium and weight bearing activity should be encouraged for all transgender persons to ensure optimal bone health. Studies (19, 31) examining vitamin D status in transgender adults have demonstrated mean serum 25-hydroxyvitamin D (25(OH)D) concentrations below the optimal level of 30 ng/mL (75 nmol/L) as suggested by the Endocrine Society (32) and American Association of Clinical Endocrinologists (33). Van Caenegem et al reported that the low vitamin D status in transgender women could be due to decreased physical activity (19). Indeed, Jones et al reported that transgender persons engage in less physical activity than cisgender people which may limit sunlight exposure and weight bearing activities (34). Furthermore, lower dietary intake of vitamin D (35) and increased adiposity (36), as reported in transgender women, is associated with lower vitamin D status.
All transgender persons should be encouraged to ingest at least 1,000 mg of calcium and 800– 1,000 IU of vitamin D from the diet and engage in regular weight bearing activity (33). Those with risk factors for vitamin D deficiency such as BMI >27 or inadequate dietary intake of vitamin D should have a serum 25(OH)D measurement. Individuals with serum 25(OH)D concentrations below 30 ng/mL should attempt correction of the level to above 30 ng/mL with vitamin D supplements and/or increased intake of vitamin D containing foods. Other considerations to improve bone health include limiting alcohol intake, tobacco cessation, adequate sex steroid hormone intake, and normal BMI.
5. Bone mass in children and adolescents
Very few studies have examined the impact of gender affirming hormone therapy in transgender children. The largest study examined 34 transgender children who were treated with gonadotropin-releasing hormone (GnRH) agonists at the earliest stages of puberty for approximately 1–2 years followed by gender affirming hormone therapy for 5 years (37). They reported no change in absolute bone mineral density (aBMD) of the spine in trans girls during GnRH therapy and slight increase in aBMD after the initiation of gender affirming hormone therapy. In trans boys, they reported significant decreases in aBMD of the spine at the start of GnRH therapy and stabilization of aBMD of the spine after the initiation of gender affirming hormone therapy. However, in both groups of trans boys and trans girls, the Z-scores of the spine were less than 0.
This small study raises concerns regarding prolonged GnRH therapy on bone health without sex steroid hormone replacement in transgender children and adults. Further studies should investigate the timing and duration of GnRH therapy that may impact bone health in post-pubertal children and adults.
6. Treatment of osteoporosis in transgender persons
Most of the studies that have been conducted in transgender populations have been of individuals under the age of 50. According to WHO criteria, osteoporosis cannot be diagnosed using T-scores alone in individuals less than the age of 50 (38). There are no published reports examining the safety and efficacy of pharmacologic agents such as bisphosphonates in the treatment of osteoporosis in transgender populations. Therefore, in the absence of any transgender specific data, pharmacologic therapy should be based on criteria put forth by the National Osteoporosis foundation in cisgender populations (17).
7. Conclusion
There has been a recent increasing interest in both the short and long term effects of sex steroid hormones on bone health in transgender persons. Based on the available data, hormone therapy appears to maintain or improve bone density in transgender adults in short term follow-up. For transgender children and adolescents, there is concern that GnRH agonist use prior to the initiation of sex steroid hormones may put patients at risk for worsening bone density. Both pharmacologic and non-pharmacologic treatment for transgender persons follows the same guidelines as in cisgender persons. See Figure 1 for one suggested approach to screening and treatment of adult transgender patients.
More studies are needed to assess the long term effects of hormone therapy on bone density and the clinical impact of these changes on fracture rates. As the population of transgender persons on hormone therapy ages, this will become especially important. Further studies are also required to assess the timing and duration of GnRH agonists in transgender youth and the associated effects on bone density.
Acknowledgments
Funding sources: Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Coleman E, Bockting W, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, Fraser L, Green J, Knudson G, Meyer WJ,Monstrey S, Adler RK, Brown GR, Devor AH, Ehrbar R, Ettner R, Eyler E, Garofalo R, Karasic DH, Lev AI, Mayer G, Meyer-Bahlburg H, Hall BP, Pfaefflin F, Rachlin K, Robinson B, Schechter LS, Tangpricha V, van Trotsenburg M, Vitale A, Winter S, Whittle S, Wylie KR, Zucker K. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int J Transgenderism 2012;13:165–232. [Google Scholar]
- 2.Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema S, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T’Sjoen G.Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: an Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism 2017; 102 (11): 3869–3903. [DOI] [PubMed] [Google Scholar]
- 3.Flores AR, Herman JL, Gates GJ, Brown TNT. (2016). How Many Adults Identify as Transgender in the United States? Los Angeles, CA: The Williams Institute. [Google Scholar]
- 4.Turner RT, Riggs BL, Spelsberg TC. Skeletal effects of estrogen. Endocrine Review 1994; 15:275–300 [DOI] [PubMed] [Google Scholar]
- 5.Seeman E Sexual Dimoprhism in Skeletal Size, Density, and Strength. J Clin Endocrinol Metab 2001; 89 (10):4576–4584. [DOI] [PubMed] [Google Scholar]
- 6.Gennari L , Khosla S , Bilezikian JP 2008. Estrogen and fracture risk in men. J Bone Miner Res 23:1548–1551. [DOI] [PubMed] [Google Scholar]
- 7.Smith EP , Boyd J , Frank GR , Takahashi H , Cohen RM , Specker B , Williams TC , Lubahn DB, Korach KS 1994. Estrogen resistance caused by a mutation in the estrogen receptor gene in a man. N Engl J Med 331:1056–1061 [DOI] [PubMed] [Google Scholar]
- 8.Morishima A , Grumbach MM , Simpson ER , Fisher C , Qin K 1995. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80:3689–3698 [DOI] [PubMed] [Google Scholar]
- 9.Carani C , Qin K , Simoni M , Faustini-Fustini M , Serpente S , Boyd J , Korach KS , Simpson ER 1997. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 337:91–95 [DOI] [PubMed] [Google Scholar]
- 10.Behre HM, Kliesch S, Leifke E, et al. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. J Clin Endocrinol Metab 1997; 82: 2386. [DOI] [PubMed] [Google Scholar]
- 11.Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, et al. Effect of Testosterone Treatment on Volumetric Bone Density and Strength in Older Men With Low Testosterone: A Controlled Clinical Trial. JAMA Intern Med 2017; 177:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkelstein JS, Klibanski A, Neer RM, et al. Increases in bone density during treatment of men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 1989; 69:776. [DOI] [PubMed] [Google Scholar]
- 13.Estrogen Pacifici R., cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res 1996;11:1043–1051 [DOI] [PubMed] [Google Scholar]
- 14.Wells G, Tugwell P, Shea B, Guyatt G, Peterson J, Zytaruk N, Robinson V, Henry D, O’Connell D, Cranney A. Meta-analyses of therapies for postmenopausal osteoporosis. V. Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women. Endocr Rev 2002. August;23(4):529–39. [DOI] [PubMed] [Google Scholar]
- 15.International Society for Clinical Densitometry. 2013. Official Positions—Adult https://www.iscd.org/official-positions/2013-iscd-official-positions-adult/. Accessed March 2018
- 16.Shepherd JA, Schousboe JT, Broy SB, Engelke K, Leslie WD. Executive Summary of the 2015 ISCD Position Development Conference on Advanced Measures From DXA and QCT: Fracture Prediction Beyond BMD. J Clin Densitom 2015. Jul-Sep;18(3):274–86. [DOI] [PubMed] [Google Scholar]
- 17.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporosis International 2014;25(10):2359–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. (1994). Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group [meeting held in Rome from 22 to 25 June 1992] Geneva: World Health Organization. [PubMed] [Google Scholar]
- 19.Van Caenegem E et al. Low bone mass is prevalent in male-to-female transsexual persons before the start of cross-sex hormonal therapy and gonadectomy. Bone 2013; 54 (1): 92–97. [DOI] [PubMed] [Google Scholar]
- 20.Singh-Ospina N, Maraka S, Rodriguez-Gutierrez R, Davidge-Pitts C, Nippoldt TB, Prokop LJ, et al. Effect of Sex Steroids on the Bone Health of Transgender Individuals: A Systematic Review and Meta- Analysis. J Clin Endocrinol Metab, November 2017, 102(11):3904–3913 [DOI] [PubMed] [Google Scholar]
- 21.Jackson RD, Wactawski-Wende J, LaCroix AZ, Pettinger M, Yood RA, Watts NB, Robbins JA, Lewis CE, Beresford SA, Ko MG, et al. Women’s Health Initiative Investigators: Effects of conjugated equine estrogen on risk of fractures and BMD in postmenopausal women with hysterectomy: Results from the women’s health initiative randomized trial. J Bone Miner Res 2006;21:817–828. [DOI] [PubMed] [Google Scholar]
- 22.Lapauw B, Taes Y, Simoens S, et al. Body composition, volumetric and areal bone parameters in male-to-female transsexual persons. Bone 2008; 43:1016–1021. [DOI] [PubMed] [Google Scholar]
- 23.Sosa M, Jódar E, Arbelo E, et al. Bone mass, bone turnover, vitamin D, and estrogen receptor gene polymorphisms in male to female transsexuals: effects of estrogenic treatment on bone metabolism of the male. J Clin Densitom 2003; 6:297–304. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. (2003). Prevention and Management of Osteoporosis: Report of a WHO Scientific Group Geneva: World Health Organization. [Google Scholar]
- 25.Van Caenegem E, Wierckx K, Taes Y, Dedecker D, Van de Peer F, Toye K, Kaufman JM, T’Sjoen G. Bone mass, bone geometry, and body composition in female-to-male transsexual persons after longterm cross-sex hormonal therapy. J Clin Endocrinol Metab 2012; 97(7):2503–2511. [DOI] [PubMed] [Google Scholar]
- 26.Haraldsen IR, Haug E, Falch J, et al. Cross-sex pattern of bone mineral density in early onset gender identity disorder. Horm Behav 2007; 52:334–343. [DOI] [PubMed] [Google Scholar]
- 27.Turner A, Chen TC, Barber TW, Malabanan AO, Holick MF, Tangpricha V. Testosterone increases bone mineral density in female-to-male transsexuals: a case series of 15 subjects. Clin Endocrinol (Oxf) 2004;61(5):560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wierckx K, Van Caenegem E, Schreiner T, Haraldsen I, Fisher AD, Toye K, Kaufman JM, T’Sjoen G. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence J Sex Med 2014;1:1999–2011 [DOI] [PubMed] [Google Scholar]
- 29.van Kesteren P, Lips P, Gooren LJ, Asscheman H, Megens J. Long- term follow-up of bone mineral density and bone metabolism in transsexuals treated with cross-sex hormones. Clin Endocrinol (Oxf) 1998;48(3):347–354. [DOI] [PubMed] [Google Scholar]
- 30.Goh HH, Ratnam SS. Effects of hormone deficiency, androgen therapy and calcium supplementation on bone mineral density in female transsexuals. Maturitas 1997; 26:45–52. [DOI] [PubMed] [Google Scholar]
- 31.Wiepjes CM, Vlot MC, Klaver M, Nota NM, de Blok CJ, de Jongh RT, Lips P, Heijboer AC, Fisher AD, Schreiner T, T’Sjoen G, den Heijer M. Bone Mineral Density Increases in Trans Persons After 1 Year of Hormonal Treatment: A Multicenter Prospective Observational Study. J Bone Miner Res 2017. June;32(6):1252–1260. [DOI] [PubMed] [Google Scholar]
- 32.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011. July;96(7):1911–30. [DOI] [PubMed] [Google Scholar]
- 33.Hurley DL, Binkley N, Camacho PM, Diab DL, Kennel KA, Malabanan A, Tangpricha V. The Use of vitamins and minerals in skeletal health: American Association of Clinical Endocrinology (AACE/ACE) Position Statement. Endocr Pract 2018. July 23. doi: 10.4158/PS-2018-0050. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Jones BA, Haycraft E, Bouman WP, Arcelus J. The Levels and Predictors of Physical Activity Engagement Within the Treatment-Seeking Transgender Population: A Matched Control Study. J Phys Act Health 2018. February 1;15(2):992–107. [DOI] [PubMed] [Google Scholar]
- 35.Vilas MVA, Rubalcava G, Becerra A, Para MCM. Nutritional Status and Obesity Prevalence in People with Gender Dysphoria. AIMS Public Health 2014. August 6;1(3):137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klaver M, de Blok CJM, Wiepjes CM, Nota NM, Dekker MJHJ, de Mutsert R, Schreiner T, Fisher AD, T’Sjoen G, den Heijer M. Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: results from a multicenter prospective study. Eur J Endocrinol 2018. February;178(2):165–173. [DOI] [PubMed] [Google Scholar]
- 37.Klink D, Caris M, Heijboer A, van Trotsenburg M, Rotteveel J. Bone mass in young adulthood following gonadotropin-releasing hormone analog treatment and cross-sex hormone treatment in adolescents with gender dysphoria. J Clin Endocrinol Metab 2015; 100(2):E270–E275. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech Rep Ser 1994;843:1–129. [PubMed] [Google Scholar]
- 39.Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, Kleerekoper M, Lewiecki EM, Miller PD, Narula HS, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis - 2016. Endocr Pract 2016;22:1–42 [DOI] [PubMed] [Google Scholar]
- 40.Kanis JA, Oden A, Johansson H et al. (2009) FRAX and its applications to clinical practice. Bone 44:734–743. [DOI] [PubMed] [Google Scholar]