Abstract
Age-related changes in cognition are linked to decreased expression of somatotropins, GHRH and IGF-1. Mild cognitive impairment (MCI) and Alzheimer’s disease (AD) are heterogeneous conditions. The loss of GHRH signaling in the brain may be mechanistically involved in AD pathogenesis. The consequent need to identify AD at an early and perhaps more treatable stage has fueled research into blood-based, exosome biomarkers. Plasma exosomes from participants enrolled in a randomized, double-blind, placebo-controlled 20-week trial of GHRH administration, were isolated, precipitated, and enriched by immuno-absorption with anti-L1CAM antibody (neural adhesion protein) from adults with MCI and age-matched, cognitively normal controls (CNC). Extracted protein cargo from neuronally-derived exosomes (NDEs) were assessed by ELISAs for protein levels implicated in AD neuropathology and for synaptic proteins altered by AD. Plasma NDE concentrations of Aβ1-42 were significantly increased while plasma NDE concentrations of NRGN, synaptophysin, synaptotagmin, and synaptopodin were significantly decreased in patients with MCI, independent of GHRH treatment. Plasma NDE concentrations of ptau-S396 and GAP43 were not affected by cognitive status (CNC versus MCI) or by GHRH treatment. Aβ1-42, neurogranin (NRGN), synaptophysin, synaptotagmin, and synaptopodin demonstrated the highest diagnostic accuracy for distinguishing between CNC and MCI patients, while synaptophysin and synaptotagmin demonstrated moderate accuracy in distinguishing between placebo-treated and GHRH-treated, MCI patients.
Keywords: Amyloid, biomarker, GHRH, growth hormone, mild cognitive impairment, neuronal exosomes, synapse, tau
INTRODUCTION
Somatotropin hormones play an important role in normal brain development and healthy brain function [1]. Activation of the somatotropin axis begins with the release of growth hormone-releasing hormone (GHRH) from the hypothalamus. Subsequently, GHRH promotes increased circulating levels of insulin-like growth factor 1 (IGF-1). Circulating IGF-1 stimulates neurite outgrowth, promote neuronal survival, regulate tau phosphorylation [2], and attenuates amyloid-β (Aβ) toxicity in vivo [3]. GHRH and IGF-1 decline with advancing age [4] and may be mechanistically involved in AD pathogenesis [3, 5–8]. Reduced hormone levels of GHRH and IGF-1 are associated with poorer executive function [9], short-term memory impairments [10], and increased Alzheimer’s disease (AD) pathology in humans [11] and in mice [12, 13].
The accumulation of neuropathological hallmarks such as toxic Aβ oligomers, neurofibrillary tangle formation of hyperphosphorylated tau protein, and widespread synapse loss are well characterized hallmarks of AD [14–17]. The interest in exosomes biology has grown dramatically in recent years, specifically regarding their role as potential biomarkers [18–29]. We recently reported that abnormal levels of AD-related and synaptic-related proteins contained within blood based, neuronally-derived exosomes (NDEs) can accurately predict the conversion of mild cognitive impairment (MCI) to AD [22, 30]. Plasma NDE concentrations of Aβ1-42, ptau-T181, ptau-S396, and synaptic-related proteins neurogranin (NRGN), synaptophysin, synaptotagmin, synaptopodin, and GAP43 were previously reported to be highly diagnostic in distinguishing cognitively normal controls (CNC) from patients diagnosed with AD, frontotemporal dementia [22, 26, 30, 31], and traumatic brain injury [32]. Other studies have analyzed additional cargo proteins contained within plasma NDEs and further demonstrated that exosome cargo levels can differentiate patients based on cognitive status and severity [19, 21, 24, 26, 27, 30, 33].
It is not well understood how somatotropic signaling contributes to AD pathogenesis; however, the positive effects of GHRH on cognition has hypothetically been linked to the hormone’s effect on modulating AD neuropathophysiology. Baker and colleagues reported that twenty-week (20 w) administration of GHRH improved cognitive function in healthy older adults [34] and individuals with MCI [35]. In the current study, we examined whether the cargo of plasma NDEs could accurately distinguish healthy older adults (hereafter, CNC) from patients with MCI. Specifically, we investigated whether the beneficial effects of GHRH treatment could modulate plasma concentrations of NDE cargo proteins, including traditional AD-related proteins, Aβ42 and ptau-S396; and several pre- and post-synaptic proteins, including NRGN, synaptophysin, synaptotagmin, synaptopodin, and GAP43.
Plasma exosomes from participants enrolled in the Baker et al. [35], randomized, double-blind, placebo-controlled, twenty-week study of GHRH administration, were isolated, and enriched by immuno-absorption with anti-L1CAM antibody (neural adhesion protein, hereafter L1CAM) from CNC and patients with MCI (both 55–87 years old). Protein cargo contained within L1CAM positive exosomes (NDEs) were quantified by human-specific enzyme-linked immunosorbent assays (ELISAs). We found that plasma NDE concentrations for Aβ1-42, were significantly increased while plasma NDE concentrations of NRGN, synaptophysin, synaptotagmin, and synaptopodin were significantly deceased in MCI patients as compared to CNC. Collectively, these biomarkers were highly diagnostic in distinguishing MCI patients from CNC; however, these biomarkers were poor diagnostic markers for distinguishing between placebo-treated and GHRH-treated, MCI patients. Plasma NDE concentrations of ptau-S396 and GAP43 were not significantly modulated by cognitive status (CNC versus MCI) or GHRH treatment. When all seven biomarkers were considered together, plasma NDE concentrations for synaptophysin and synaptotagmin, were moderately sensitive in distinguishing between placebo-treated and GHRH-treated, MCI patients.
METHODS
Baseline characteristics of GHRH study completers
Specimens from CNC and adults with MCI (55–87 years of age) were obtained from a randomized, double-blind, placebo-controlled trial of GHRH versus placebo [35]. A total of 137 adults (CNC, n = 76; MCI, n = 61) successfully completed the original study [35]. MCI was diagnosed using the National Institute on Aging (NIA) standard guidelines. Plasma samples were collected from all participants at baseline and following 20 weeks of treatment and stored at −80°C. Baseline characteristics for age, education, body composition, cognitive status, diagnosis, and circulating levels of IGF-1, insulin, and glucose were comparable across treatment groups. Participants were assigned to one of two groups, by cognitive status: Placebo – CNC (n = 36) or Placebo – MCI (n = 31); GHRH – CNC (n = 40) or GHRH – MCI (n = 30). Cognitive status was assessed based on the Mini-Mental State Examination (MMSE) and baseline performance in executive function and episodic memory tasks. Tests of executive function included a computer-administered version of Stroop Color-Word Interference, Task Switching, the Self-Ordered Pointing Test, and Word Fluency. Tests of episodic memory included total recall and the Hopkins Verbal Learning Test assess verbal memory, and the Visual-Spatial Learning Test, and Delayed Match-to-Sample, to assess visual memory [35]. Information regarding ApoE genotype was not available for these participants. Plasma specimens from 12 participants were randomly selected per treatment group for exosome isolation, neural enrichment, and protein cargo analysis. Table 1 summarizes baseline characteristics of the study participants [35] that were randomly selected to participate in the current study.
Table 1.
Baseline characteristics of participants who completed the study [35]
| Characteristic | Controls | Adults w/ MCI |
|---|---|---|
| Agea | 67.8 ± 2.3 | 70.2 ± 2.3 |
| Years of Education | 16.9 ± 0.69 | 15.6 ± 0.69 |
| %Female | 61.5 | 63.6 |
| % Male | 38.5 | 36.4 |
| MMSE Scorea | 29.1 ± 0.33 | 27.9 ± 0.64 |
| Story recall scoreb | 55.6 ± 12.2 | 42.8 ± 15.7 |
| Depression Scalec | 3.9 ± 1.10 | 5.09 ± 1.36 |
GHRH, growth hormone–releasing hormone; IGF-1, insulinlike growth factor 1; MCI, mild cognitive impairment; MMSE, Mini-Mental Status Examination (maximum score, 30).
p < 0.05 (baseline difference by diagnosis).
Immediate + delayed memory score on Story Recall, a test of verbal memory for thematic information (maximum score, 80).
Self report measurement of depression in older adults. A score >5 points is suggestive of depression. A score ≥10 points is almost always indicative of depression. A score >5 points should warrant a follow-up comprehensive assessment.
Isolation and purification of neuronal exosomes from human plasma
250 μL of plasma were incubated with 2.5 μL of purified thrombin [System Biosciences, Inc., Mountain View, CA) at room temperature for 5 min. After centrifugation at 10,000 rpm for 5 min, supernatants were incubated with 63 μL ExoQuick Exosome Precipitation solution (EXOQ; System Biosciences, Inc.), and resultant suspensions were centrifuged at 3,000× g for 15 min at 4°C.
Each pellet was suspended in 300 μl of 1X phosphate buffer saline (PBS) with 1X Halt protease and phosphatase inhibitor cocktail EDTA-free (100 μL of 100X Halt protease and phosphatase inhibitor (PIC) was diluted in 10 mL of 1x PBS; ThermoScientific) followed by immunochemical enrichment of exosomes from neural sources [22].
Total exosome suspensions were incubated with 2 μg of mouse anti-human CD171 (L1CAM neural adhesion protein) biotinylated antibody (clone 5G3, eBioscience, San Diego, CA) in 50 μL of 3% BSA for 60 min at 20°C followed by addition of 10 μl of Streptavidin-Plus UltraLink resin (Pierce-Thermo Scientific, Inc.) in 40 μL of 3% BSA and further incubation for 60 min. After centrifugation at 400× g for 5 min at 4°C, pellets were resuspended in 100 μl of 0.05 M glycine-HCl (pH 3.0), incubated at 4°C for 10 min, and re-centrifuged at 4,000× g for 10 min at 4°C. Each supernate was transferred to a new Eppendorf tube containing 10 μL of 1 M Tris-HCl (pH 8.0) and 40 μL of 3% BSA, mixed and received 400 μL of M-PER mammalian protein extraction reagent (Thermo Scientific, Inc.) containing protease and phosphatase inhibitors, mixed and stored at −80°C.
L1CAM-positive NDE cargo proteins were quantified by human-specific ELISAs for Aβ1-42 (Cusabio, American Research Products, Waltham, MA), ptau-S396 (Life Technologies/Invitrogen, Camarillo, CA), neurogranin (Cloud Clone Corp, American Research Products, Katy, TX), synaptophysin (Cusabio, American Research Products, Waltham, MA), synaptotagmin-2 (Biomatik, Wilmington, DE), synaptopodin, GAP43 (Cusabio, American Research Products, Waltham, MA), and tetraspanning exosome marker CD81 (Cusabio, American Research Products, Waltham, MA) with verification of the CD81 antigen standard curve using purified human recom-binant CD81 antigen (Origene Technologies, Inc., Rockville, MD), according to suppliers’ directions [26]. The mean value for all determinations of CD81 in each assay group was set at 1.00, and the relative values for each sample were used to normalize their recovery [22].
Statistical analyses
Statistical significance of differences between groups (placebo – MCI versus GHRH – MCI) and between each patient group and their respective control group (placebo – CNC versus placebo – MCI; GHRH – CNC versus GHRH – MCI) was determined with by one-way ANOVA with Newman–Keuls Multiple Comparison post hoc test (GraphPad Prism 6, La Jolla, CA). Discriminant classifier analyses were conducted by the Wilks’ Lambda method to assess the performance of each NDE protein and the combined set in patient classification as described. Receiver operating characteristic (ROC) analyses were conducted under the non-parametric distribution assumption for standard error of area to determine the performance of classifier models (SPSS v21.0, IBM).
RESULTS
Plasma NDE concentrations of Aβ1-42, NRGN, synaptophysin, synaptotagmin, and synaptopodin accurately identify MCI patients based on protein cargo content
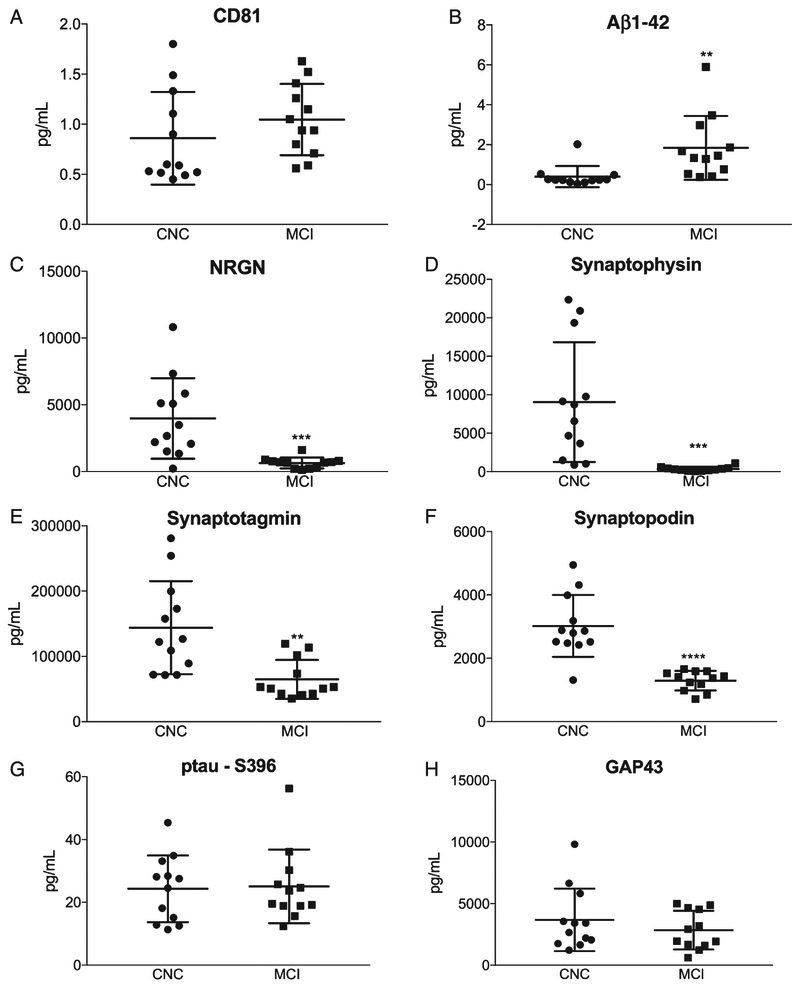
Plasma NDEs were isolated and protein cargo were extracted and analyzed by human-specific ELISAs, as previously described [22]. All NDE concentrations for biomarkers were normalized against the exosome membrane protein marker, CD81. CD81 normalized concentrations of plasma NDEs were not statistically different between CNC and MCI patients (Fig. 1A, 1289 ± 200.3 pg/ml versus 1567 ± 153.8 pg/ml). Plasma NDE concentrations for Aβ1-42 (Fig. 1B, 1.838 ± 0.462 pg/ml, versus 0.398 ± 0.153 pg/ml, p < 0.01) were significantly increased, while plasma NDE concentrations for NRGN (Fig. 1C, 658.5 ± 111,6 pg/ml versus 3978 ± 869.5 pg/ml, p < 0.001), synaptophysin (Fig. 1D, 355.6 ± 82.81 pg/ml versus 9046 ± 2465 pg/ml, p < 0.001), synaptotagmin (Fig. 1E, 65750 ± 8644 pg/ml versus 143914 ± 20548 pg/ml, p < 0.0001), and synaptopodin (Fig. 1F, 1293 ± 89.28 pg/ml versus 3019 ± 282.1 pg/ml, p < 0.01) were significantly decreased in MCI patients as compared to CNC. There was no significant difference in plasma NDE concentrations for ptau-S396 (Fig. 1G, 25.08 ± 3.394 pg/ml versus 24.31 ± 3.065 pg/ml) and GAP43 (Fig. 1H, 2848 ± 453.2 pg/ml versus 3685 ± 733 pg/ml) between both patient groups.
Fig. 1.
Plasma NDE concentrations for Aβ1-42, NRGN, synaptophysin, synaptotagmin, and synaptopodin accurately identify MCI patients based on protein cargo content. Plasma NDE concentrations for AD-related and synaptic-related proteins were quantified using ELISA. Plasma NDE concentrations for CD81 were not statistically different between CNC and MCI patients (A). Plasma NDE concentrations for Aβ1-42 (B) were significantly higher while plasma NDE concentrations for NRGN (D), synaptophysin (E), synaptotagmin (F), and synaptopodin (G), were significantly lower in MCI patients as compared to CNC patients. We found no significant difference in plasma NDE concentrations for ptau-S396 (C) and GAP43 (H) between CNC and MCI patients. Unpaired t-test: **p < 0.01 versus CNC, ***p < 0.001 versus CNC, ***p < 0.0001 versus CNC.
Plasma NDE concentrations of AD-related and synaptic-related proteins were not modulated with GHRH treatment
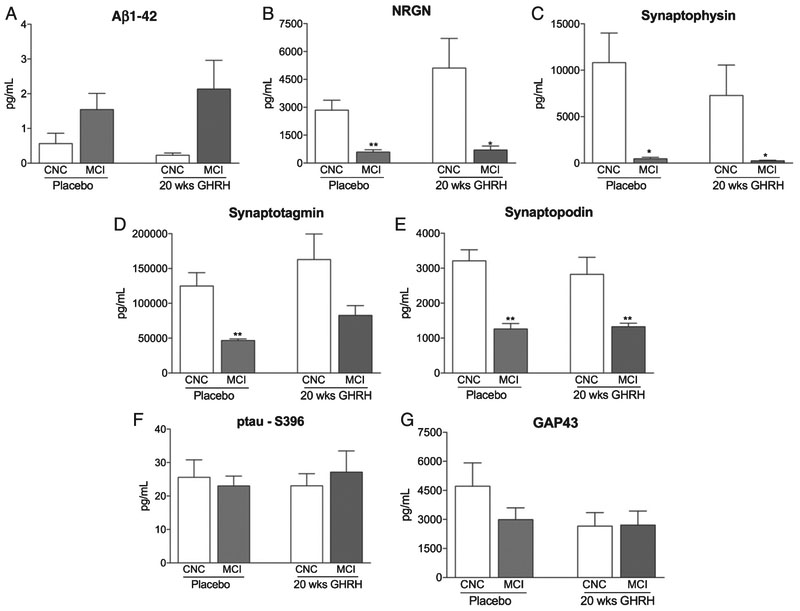
Plasma NDE concentrations were measured in four patient groups (placebo – CNC; placebo – MCI; GHRH – CNC, and GRHR – MCI) that were identified from the twenty-week GHRH study [35]. Plasma NDE concentrations for Aβ1-42 (Fig. 2A, 1.542 ± 0.465 pg/ml versus 0.567 ± 0.296 pg/ml) were increased while plasma NDE concentrations for NRGN (Fig. 2B, 592.2 ± 121.9 pg/ml versus 2848 ± 528.1 pg/ml, p < 0.05), synaptophysin (Fig. 2C, 467.7 ± 146.4 pg/ml versus 10817 ± 3187 pg/ml, p < 0.05), synaptotagmin (Fig. 2D, 46806 ± 2120 pg/ml versus 124899 ± 19165 pg/ml, p < 0.01), and synaptopodin (Fig. 2E, 1263 ± 146.9 pg/ml versus 3212 ± 316.9 pg/ml, p < 0.01) were significantly decreased in placebo-treated, MCI patients as compared to placebo-treated, CNC patients. Similarly, plasma NDE concentrations for Aβ1-42 (Fig. 2A, 2.133 ± 0.829 pg/ml), NRGN (Fig. 2B, 704.7 ± 208.6 pg/ml versus 5107 ± 1593 pg/ml, p < 0.05), synaptophysin (Fig. 2C, 243.5 ± 60.9 pg/ml versus 7275 ± 3285 pg/ml, p < 0.05), synaptotagmin (Fig. 2D, 82694 ± 13982 pg/ml versus 162928 ± 36687 pg/ml), and synaptopodin (Fig. 2E, 1324 ± 100.5 pg/ml versus 2826 ± 484.6 pg/ml, p < 0.01) were significantly decreased in GHRH-treated, MCI patients as compared to GHRH-treated, CNC patients. Following twenty-weeks of GHRH administration, inter-group analysis determined that there was no significant difference in plasma NDE concentrations for all biomarkers tested in GHRH-treated, MCI patients as compared to placebo-treated, MCI patients (Fig. 2A-E).
Fig. 2.
Plasma NDE concentrations of Aβ1-42, NRGN, synaptophysin, synaptotagmin, and synaptopodin are not modulated with GHRH treatment while plasma NDE concentrations of p-tauS396 and GAP43 are not modulated by cognitive status or GHRH treatment. Plasma NDE concentrations for Aβ1-42 (A) were increased while plasma NDE concentrations for NRGN (B), synaptophysin (C), synaptotagmin (D), and synaptopodin (E) were significantly reduced in MCI patients as compared to CNC patients, independent of GHRH treatment. Plasma NDE concentrations for ptau-S396 (F) and GAP43 (G) were not significantly different between both patient groups (CNC versus MCI), independent of GHRH treatment. There was no significant difference in plasma NDE concentrations for all biomarkers (A-G) in placebo-treated, MCI patients as compared to GHRH-treated, MCI patients. 1-way ANOVA with Tukey’s multiple comparisons post-hoc test; *p < 0.05 versus placebo-treated, CNC, **p < 0.01 versus GHRH-treated, CNC.
Conversely, plasma NDE concentrations for ptau-S396 (Fig. 2F, 23.02 ± 2.948 pg/ml versus 25.58 ± 5.229 pg/ml) and GAP43 (Fig. 2G, 2990 ± 608.8 pg/ml versus 4718 ± 1201 pg/ml) were not significantly different in placebo-treated, MCI patients as compared to placebo-treated, CNC patients. Additionally, we observed no statistically significant difference in plasma NDE concentrations for ptau-S396 (Fig. 2F, 27.15 ± 6.346 pg/ml versus 23.02 ± 3.653 pg/ml) and GAP43 (Fig. 2G, 2707 ± 724.7 pg/ml versus 2652 ± 702.7 pg/ml) in GHRH-treated, MCI patients as compared to GHRH-treated, CNC patients. Plasma NDE concentrations for GAP43 were moderately reduced in GHRH-treated, MCI patients as compared to placebo-treated, CNC patients (Fig. 2G); however these data failed to reach significance. Intergroup analysis determined that there was no significant difference in plasma NDE concentrations for ptau-S396 (Fig. 2F) and GAP43 (Fig. 2G) in GHRH-treated, MCI patients and GHRH, CNC patients. Together, plasma NDE concentrations of ptau-S396 and GAP43 were not modulated by cognitive status (CNC versus MCI) or GHRH treatment (Fig. 2H, I).
Receiver operating characteristic curve (ROC) analyses for patient characterization and treatment effect following 20 weeks GHRH administration
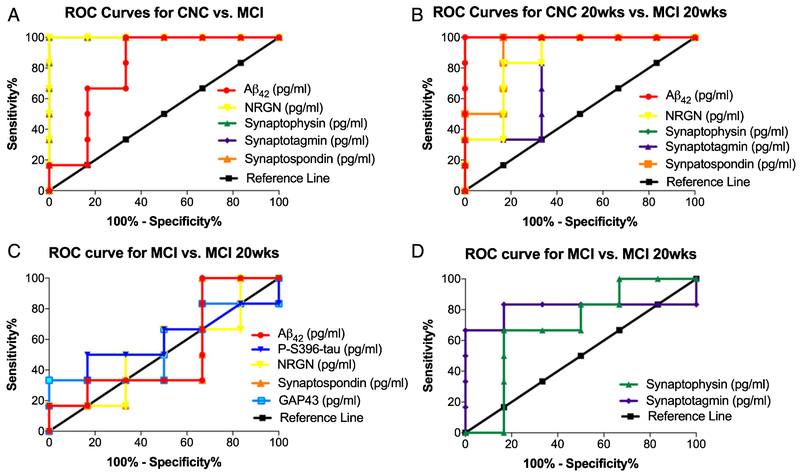
The diagnostic accuracy of the seven biomarkers was assessed by ROC analysis [22]. ROC analysis was conducted to determine if the diagnostic sensitivity of the extracted NDE cargo proteins, individually or collectively, increased their predictive ability in distinguishing two patient populations. ROC analysis revealed that plasma NDE concentrations for Aβ1-42, NRGN, synaptophysin, synaptotagmin, and synaptopodin demonstrated the highest diagnostic accuracy for distinguishing between MCI and CNC patients, independent of GHRH treatment (Table 2). The sensitivity for distinguishing placebo-treated, CNC patients from placebo-treated, MCI was 81.4 ± 0.092% (CI: 63.4% to 99.5%) (Fig. 3A) while the while the sensitivity for distinguishing GHRH-treated, CNC patients from GHRH-treated, MCI was 86.1 ± 0.116% (CI: 63.4 to 100%) (Fig. 3B).
Table 2.
Individual diagnostic accuracy readings for plasma NDE biomarkers as determined by ROC analysis
| Plasma NDE Biomarker | Placebo CNC vs. Placebo MCI |
GHRH CNC vs. GHRH MCI |
Placebo MCI vs. GHRH MCI |
|||
|---|---|---|---|---|---|---|
| Sensitivity (%) | CI (%) | Sensitivity (%) | CI (%) | Sensitivity (%) | CI (%) | |
| Aβ1-42 | 80.6 ± 0.078 | 53.18 to 100 | 100 | 100 to 100 | 52.8 ± 0.184 | 16.8 ± 88.8 |
| ptau-S396 | 52.8 ± 0.187 | 16.1 to 89.5 | 52.8 ± 0.873 | 18.2 to 87.4 | 55.9 ± 0.176 | 22.5 to 91.49 |
| NRGN | 100 | 100 to 100 | 86.1 ± 0.037 | 63.4 to 100 | 52.8 ± 0.177 | 18.2 to 87.4 |
| Synaptophysin | 100 | 100 to 100 | 100 | 100 to 100 | 69.4 ± 0.166 | 36.9 to 100 |
| Synaptotagmin | 100 | 100 to 100 | 77.8 ± 0.109 | 48.7 to 100 | 80.6 ± 0.153 | 50.6 to 100 |
| Synaptopodin | 100 | 100 to 100 | 91.2 ±0.016 | 74.2 to 100 | 50.0 ± 0.184 | 13.9 to 86.1 |
| GAP43 | 72.2 ± 0.200 | 41.2 to 100 | 50.0 ± 0.177 | 15.3 to 84.7 | 55.6 ± 0.178 | 20.7 to 90.5 |
CI, confidence interval.
Fig. 3.
Plasma NDE concentrations of Aβ1-42, NRGN, synaptophysin, synaptotagmin, and synaptopodin demonstrated the highest diagnostic accuracy for distinguishing between MCI and CNC patients as measured by ROC analysis. Representative ROC curves for plasma NDE concentrations for Aβ1-42, NRGN, synaptophysin, synaptotagmin, and synaptopodin for placebo-treated, MCI patients as compared to placebo-treated, CNC patients (A) and GHRH-treated, MCI patients as compared to GHRH-treated, CNC patients (B). Aβ1-42, NRGN, synaptophysin, synaptotagmin, and synaptopodin demonstrate the highest diagnostic accuracy for distinguishing CNC and MCI patients, independent of GHRH treatment. Plasma NDE concentrations of Aβ1-42, ptau-S396, NRGN, synaptopodin, and GAP43 (C) demonstrated the lowest diagnostic accuracy for distinguishing between placebo-treated and GHRH-treated, MCI patients. Plasma NDE concentrations for a synaptophysin and synaptotagmin demonstrated moderate diagnostic accuracy for distinguishing between placebo-treated and GHRH-treated, MCI patients (D).
Conversely, inter-group analysis determined that plasma NDE concentrations for Aβ1-42, ptau-S396, NRGN, synaptopodin, and GAP43, lacked the sensitivity to distinguish placebo-treated and GHRH-treated, MCI patients (52.8 ± 0.177%, CI: 18.2% to 87.4%, Fig. 3C), while plasma NDE concentrations for synaptophysin and synaptotagmin were moderately accurate in distinguishing placebo-treated and GHRH-treated, MCI patients (80.1 ± 0.153%, CI: 50.6% to 100%, Fig. 3D).
Individually, the sensitivity for distinguishing placebo-treated, CNC patients from placebo-treated, MCI patients was 80.6 ± 0.078% for Aβ1-42 (Confidence Interval (CI): 53.18% to 100%); 52.8 ± 0.187% for ptau-S396 (CI: 16.1% to 89.5%); 100% for NRGN (CI: 100% to 100%); 100% for synaptophysin (CI: 100% to 100%); 100% for synaptotagmin (CI: 100% to 100%); 100% for synaptopodin (CI: 100% to 100%); and 72.2 ± 0.200% for GAP43 (CI: 41.2% to 100%) (Table 2).
The sensitivity for distinguishing GHRH-treated, CNC patients from GHRH-treated, MCI patients was 100% for Aβ1-42 (CI: 100% to 100%); 52.8 ± 0.873% for ptau-S396 (CI: 18.2% to 87.4%); 86.1 ± 0.037% for NRGN (CI: 63.4% to 100%); 100% for synaptophysin (CI: 100% to 100%); 77.8 ± 0.109% for synaptotagmin (CI: 48.7% to 100%); 91.2 ± 0.016% for synaptopodin (CI: 74.2% to 100%); and 50 ± 0.177% for GAP43 (CI: 15.3% to 84.7%) (Table 2).
The sensitivity for distinguishing placebo-treated, MCI patients from GHRH-treated MCI patients was 52.8 ± 0.184% for Aβ1-42 (CI: 16.8% to 88.8%); 52.8 ± 0.176% for ptau-S396 (CI: 22.5% to 91.49%); 52.8 ± 0.177% for NRGN (CI: 18.2% to 87.4%); 69.4 ± 0.166% for synaptophysin (CI: 36.9% to 100%); 80.6 ± 0.153% for synaptotagmin (CI: 50.6% to 100%); 50 ± 0.184% for synaptopodin (CI: 13.9% to 86.1%); and 55.6 ± 0.178% for GAP43 (CI: 20.7% to 90.5%) (Table 2).
DISCUSSION
Validating the clinical utility of plasma NDEs will be critical for the optimization of patient recruitment and outcome monitoring in future clinical trials. Abnormal plasma NDE concentrations of AD-related and synaptic-related proteins can predict the conversion of MCI to AD [22] and accurately distinguish between cognitively normal controls from patients diagnosed with AD [26]. Here, we determined that plasma NDEs can distinguish between cognitively normal controls and patients with MCI. Plasma NDE concentrations of Aβ1-42, pre- and post-synaptic proteins, NRGN, synaptophysin, synaptotagmin, and synaptopodin accurately differentiated between MCI and CNC patients (Fig. 1A-E). Furthermore, ROC analysis confirmed that the combination of Aβ1-42, NRGN, synaptophysin, synaptotagmin, and synaptopodin demonstrated the highest diagnostic accuracy for distinguishing between these two patient populations (Fig. 3A, B). Unfortunately, inter-group analysis determined that twenty-week administration of GHRH did not modulate plasma NDE concentrations of these biomarkers, despite patients exhibiting improved cognition (Fig. 3).
Previously, plasma NDE concentrations of GAP43 and ptau-S396 were reported to be reduced in AD patients as compared to CNC; however, we found that plasma NDE concentrations for GAP43 and ptau-S396 were not influenced by cognitive status or GHRH treatment (Fig. 3A-D). Reduced expression of GAP43 tends to not be as robust in early AD patients as compared to other synaptic proteins [31, 36]. It is probably that there is a temporal order associated with the phosphorylation of tau species and a differential loss of synaptic-related proteins as cognitive dysfunction worsens. Similarly, ptau-S396 may be associated with advanced cases of dementia while other epitopes of tau, such as ptau-T181 is associated early dementia (i.e., MCI) [22]. Given our patient population, an early stage p-tau isoform, such as ptau-T181 would have been more appropriate for this study. Because of this, the lack of diagnostic value of p-tau in MCI patients is a recognized limitation of the study. All future analyses will include multiple p-tau isoforms which are known to be associated with early and late stages of AD. Together, these data suggest that loss of ptau-S396 and GAP43 expression may be associated with more advanced cases of AD while loss of synaptophysin, synaptotagmin, and NRGN are associated with MCI and early dementia.
The lack of diagnostic value of p-tau in our study could also be attributed to the similarities in MMSE scores between the MCI and CNC patients. The MMSE is the most commonly used mental status test, however it has significant limitations. Study participants were classified by MMSE and a series of executive function and verbal memory tasks, including task switching accuracy, Hopkins Verbal Learning Test and Total Story Recall [35]. Unfortunately, the individual measurements for the executive function and verbal memory tasks were not available. While examining the individual MMSE scores, we found that the cognitive measurements do not directly correlate with plasma NDEs concentrations of NRGN, synaptotagmin, and synaptophysin in CNC patients. The varying levels of plasma NDE concentrations for these proteins could reveal a subset of the CNC patients are a pre-MCI group with a risk to convert and warrants further investigation. Approximately 46% of our MCI and CNC patients reported MMSE scores >29. While we expected to see in an increase in plasma NDE concentrations for ptau-S396 and Aβ1-42, here, we found plasma NDE concentrations were either sustained (ptau-S396) and/or reduced (Aβ1-42) in MCI patients. The diagnostic accuracy of the plasma NDE biomarkers we investigated in the current study could have be improved if there was a greater separation in MMSE scores between the CNC and MCI patients.
The mechanisms associated with exosome trafficking are not fully understood. We are actively investigating why pre- and post-synaptic markers were significantly reduced while GAP43 and the AD- related markers were sustained (ptau-s396) and/or increased (Aβ1-42) in plasma NDEs of MCI patients. Exosomes have been described as “molecular trash cans;” aiding in the clearance of cellular debris and toxins while the cell is still viable. It is plausible that as the cell becomes terminal, the rate of exosomes packaging increases to expedite protein clearance.
Overall, GHRH appeared to have no effect on modulating the plasma NDE concentrations of all the biomarkers tested. However, ROC analysis revealed that the combination of synaptophysin and synaptotagmin was moderately accurate in distinguishing between GHRH-treated and placebo-treated, MCI patients (Fig. 3D). The synergistic effect of these markers provides preliminary, mechanistic insight into the cognitive benefit of GHRH in MCI patients. However, further investigations are needed to elucidate those specific mechanisms in greater detail. Although GHRH appeared to have no effect on modulating plasma concentrations of individual NDE cargo proteins; analyzing changes in plasma NDE concentrations from multiple protein signatures, simultaneously, could improve the diagnostic accuracy and clinical utility of plasma NDEs for patient recruitment and outcome monitoring in clinical trials. The development of a high throughput screening tool and protein algorithm to assess the clinical utility plasma NDE protein cargo is needed to further this aim.
Modulating somatotropin expression via hormone supplementation has been investigated as a potential therapeutic for neurodegenerative diseases including amyotrophic lateral sclerosis [37–39] and AD [7, 8, 13, 40]. Unfortunately, these earlier studies have been inconsistent and inconclusive. Although our data suggest that somatotropic supplementation does not modulate intermediates involved in AD neuropathology in blood, somatotropins may still have a role in AD pathogenesis. In 2008, MK-677, a drug that enhances IGF-1 secretion, similarly to GHRH, was reported to have no clinical benefit for people with mild to moderate AD over a one-year trial [41]. However, the small clinical trial found that MK-677 provided some benefit to those in the study who did not carry the apolipoprotein E (ApoE4) ε4 allele, the strongest genetic risk factor for developing AD [42]. These finding suggest that there is a beneficial outcome to stimulating IGF-1 signaling in those suffering from mild to moderate AD, yet cognitive status and genetic influence must also be accounted for when designing therapeutic interventions [40]. The ApoE status of the participants in this study was not available. The lack of ApoE status and the short duration of GHRH administration (20 weeks) could explain the poor diagnostic accuracy of the plasma NDE biomarkers in this study. Detectable changes in plasma NDE concentrations for these biomarkers in response to GHRH treatment may be better identified in patients who were treated and followed over an extended period of time. Lastly, our less than expected results could be attributed to our relatively small sample size.
In conclusion, it is unclear whether GHRH impacts the clearing mechanisms associated with reducing AD neuropathology in the brain or if the difference in engagement of these intermediates is due to the early stage of disease and subsequent disease progression in these patients. The underlying mechanism for exosome protein packaging and exosome trafficking from the CNS to periphery are grossly understudied and not well understood. Nonetheless, blood-based biomarkers have the potential to revolutionize the way patients are identified and monitored during clinical trials. In lieu of conducting a battery of lengthy and exhausting neurophysiological tests, developing a single method that analyzes multiple disease-related protein combinations in blood would enhance the diagnostic accuracy of plasma NDEs for patient selection and outcome monitoring during clinical trials. Early determination of the beneficial and/or deleterious effects of certain drug treatments could also give pharmaceutical companies and other researchers preliminary insight into drug affects prior to the cessation of the clinical trial.
ACKNOWLEDGMENTS
The authors thank the members of the Rissman lab for technical assistance with this project and the assembly of the manuscript. This work was supported by NIH Grants AG051848, BX003040, AG0051839, AG005131 and AG018440 to RAR.
Footnotes
DISCLOSURE STATEMENT
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0302r2).
REFERENCES
- [1].Thornton PL, Ingram RL, Sonntag WE (2000) Chronic [D-Ala2]-growth hormone-releasing hormone administration attenuates age-related deficits in spatial memory. J Gerontol A Biol Sci Med Sci 55, B106–112. [DOI] [PubMed] [Google Scholar]
- [2].Hong M, Lee VM (1997) Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem 272, 19547–19553. [DOI] [PubMed] [Google Scholar]
- [3].Doré S, Kar S, Quirion R (1997) Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proc Natl Acad Sci U S A 94, 4772–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sonntag WE, Ramsey M, Carter CS (2005) Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev 4, 195–212. [DOI] [PubMed] [Google Scholar]
- [5].Westwood AJ, Beiser A, Decarli C, Harris TB, Chen TC, He XM, Roubenoff R, Pikula A, Au R, Braverman LE, Wolf PA, Vasan RS, Seshadri S (2014) Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy. Neurology 82, 1613–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].de la Monte SM (2012) Contributions of brain insulin resistance and deficiency in amyloid-related neurodegeneration in Alzheimer’s disease. Drugs 72, 49–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].de la Monte SM, Tong M, Schiano I, Didsbury J (2017) Improved brain insulin/IGF signaling and reduced neuroin-flammation with T3D-959 in an experimental model of sporadic Alzheimer’s disease. J Alzheimers Dis 55, 849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gómez JM (2008) Growth hormone and insulin-like growth factor-I as an endocrine axis in Alzheimer’s disease. Endocr Metab Immune Disord Drug Targets 8, 143–151. [DOI] [PubMed] [Google Scholar]
- [9].Bellar D, Glickman EL, Juvancic-Heltzel J, Gunstad J (2011) Serum insulin like growth factor-1 is associated with working memory, executive function and selective attention in a sample of healthy, fit older adults. Neuroscience 178, 133–137. [DOI] [PubMed] [Google Scholar]
- [10].Aleman A, de Vries WR, de Haan EH, Verhaar HJ, Samson MM, Koppeschaar HP (2000) Age-sensitive cognitive function, growth hormone and insulin-like growth factor 1 plasma levels in healthy older men. Neuropsychobiology 41, 73–78. [DOI] [PubMed] [Google Scholar]
- [11].Watanabe T, Miyazaki A, Katagiri T, Yamamoto H, Idei T, Iguchi T (2005) Relationship between serum insulin-like growth factor-1 levels and Alzheimer’s disease and vascular dementia. J Am Geriatr Soc 53, 1748–1753. [DOI] [PubMed] [Google Scholar]
- [12].Trueba-Sáiz A, Cavada C, Fernandez AM, Leon T, González DA, Fortea Ormaechea J, Lleó A, Del Ser T, Nuñez A, Torres-Aleman I (2013) Loss of serum IGF-I input to the brain as an early biomarker of disease onset in Alzheimer mice. Transl Psychiatry 3, e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I (2002) Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med 8, 1390–1397. [DOI] [PubMed] [Google Scholar]
- [14].Masliah E, Terry RD, Alford M, DeTeresa R, Hansen LA (1991) Cortical and subcortical patterns of synaptophysin-like immunoreactivity in Alzheimer’s disease. Am J Pathol 138, 235–246. [PMC free article] [PubMed] [Google Scholar]
- [15].Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R (1991) Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol 30, 572–580. [DOI] [PubMed] [Google Scholar]
- [16].DeKosky ST, Scheff SW, Styren SD (1996) Structural correlates of cognition in dementia: Quantification and assessment of synapse change. Neurodegeneration 5, 417–421. [DOI] [PubMed] [Google Scholar]
- [17].Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Morris JC (2001) Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology 56, 127–129. [DOI] [PubMed] [Google Scholar]
- [18].H Rashed M, Bayraktar E, K Helal G, Abd-Ellah MF, Amero P, Chavez-Reyes A, Rodriguez-Aguayo C (2017) Exosomes: From garbage bins to promising therapeutic targets. Int J Mol Sci 18, E538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hamlett ED, Goetzl EJ, Ledreux A, Vasilevko V, Boger HA, LaRosa A, Clark D, Carroll SL, Carmona-Iragui M, Fortea J, Mufson EJ, Sabbagh M, Mohammed AH, Hartley D, Doran E, Lott IT, Granholm AC (2017) Neuronal exosomes reveal Alzheimer’s disease biomarkers in Down syndrome. Alzheimers Dement 13, 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K (2006) Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A 103, 11172–11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stern RA, Tripodis Y, Baugh CM, Fritts NG, Martin BM, Chaisson C, Cantu RC, Joyce JA, Shah S, Ikezu T, Zhang J, Gercel-Taylor C, Taylor DD (2016) Preliminary study of plasma exosomal tau as a potential biomarker for chronic traumatic encephalopathy. J Alzheimers Dis 51, 1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Winston CN, Goetzl EJ, Akers JC, Carter BS, Rockenstein EM, Galasko D, Masliah E, Rissman RA (2016) Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst) 3, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Feneberg E, Steinacker P, Lehnert S, Schneider A, Walther P, Thal DR, Linsenmeier M, Ludolph AC, Otto M (2014) Limited role of free TDP-43 as a diagnostic tool in neurodegenerative diseases. Amyotroph Lateral Scler Frontotemporal Degener 15, 351–356. [DOI] [PubMed] [Google Scholar]
- [24].Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, Kapogiannis D (2015) Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 85, 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, Carlson OD, Mustapic M, Kapogiannis D (2015) Low neural exosomal levels of cellular survival factors in Alzheimer’s disease. Ann Clin Transl Neurol 2, 769–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Goetzl EJ, Kapogiannis D, Schwartz JB, Lobach IV, Goetzl L, Abner EL, Jicha GA, Karydas AM, Boxer A, Miller BL (2016) Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J 30, 4141–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mullins RJ, Mustapic M, Goetzl EJ, Kapogiannis D (2017) Exosomal biomarkers of brain insulin resistance associated with regional atrophy in Alzheimer’s disease. Hum Brain Mapp 38, 1933–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, Li Y, Aro P, Dator R, He C, Hipp MJ, Zabetian CP, Peskind ER, Hu SC, Quinn JF, Galasko DR, Banks WA, Zhang J (2014) Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol 128, 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stuendl A, Kunadt M, Kruse N, Bartels C, Moebius W, Danzer KM, Mollenhauer B, Schneider A (2016) Induction of α-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain 139(Pt 2), 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ (2015) Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement 11, 600–607.e601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Goetzl EJ, Abner EL, Jicha GA, Kapogiannis D, Schwartz JB (2018) Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer’s disease. FASEB J 32, 888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Goetzl L, Merabova N, Darbinian N, Martirosyan D, Poletto E, Fugarolas K, Menkiti O (2018) Diagnostic potential of neural exosome cargo as biomarkers for acute brain injury. Ann Clin Transl Neurol 5, 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, Frassetto L, Petersen RC, Miller BL, Goetzl EJ (2015) Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J 29, 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vitiello MV, Moe KE, Merriam GR, Mazzoni G, Buchner DH, Schwartz RS (2006) Growth hormone releasing hormone improves the cognition of healthy older adults. Neurobiol Aging 27, 318–323. [DOI] [PubMed] [Google Scholar]
- [35].Baker LD, Barsness SM, Borson S, Merriam GR, Friedman SD, Craft S, Vitiello MV (2012) Effects of growth hormone–releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: Results of a controlled trial. Arch Neurol 69, 1420–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W, Kaye J, Manczak M (2005) Differential loss of synaptic proteins in Alzheimer’s disease: Implications for synaptic dysfunction. J Alzheimers Dis 7, 103–117; discussion 173-180. [DOI] [PubMed] [Google Scholar]
- [37].Borasio GD, Robberecht W, Leigh PN, Emile J, Guiloff RJ, Jerusalem F, Silani V, Vos PE, Wokke JH, Dobbins T (1998) A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. European ALS/IGF-I Study Group. Neurology 51, 583–586. [DOI] [PubMed] [Google Scholar]
- [38].Lai EC, Felice KJ, Festoff BW, Gawel MJ, Gelinas DF, Kratz R, Murphy MF, Natter HM, Norris FH, Rudnicki SA (1997) Effect of recombinant human insulin-like growth factor-I on progression of ALS. A placebo-controlled study. The North America ALS/IGF-I Study Group. Neurology 49, 1621–1630. [DOI] [PubMed] [Google Scholar]
- [39].Sorenson EJ, Windbank AJ, Mandrekar JN, Bamlet WR, Appel SH, Armon C, Barkhaus PE, Bosch P, Boylan K, David WS, Feldman E, Glass J, Gutmann L, Katz J, King W, Luciano CA, McCluskey LF, Nash S, Newman DS, Pascuzzi RM, Pioro E, Sams LJ, Scelsa S, Simpson EP, Subramony SH, Tiryaki E, Thornton CA (2008) Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology 71, 1770–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Craft S, Asthana S, Cook DG, Baker LD, Cherrier M, Purganan K, Wait C, Petrova A, Latendresse S, Watson GS, Newcomer JW, Schellenberg GD, Krohn AJ (2003) Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: Interactions with apolipoprotein E genotype. Psychoneuroendocrinology 28, 809–822. [DOI] [PubMed] [Google Scholar]
- [41].Sevigny JJ, Ryan JM, van Dyck CH, Peng Y, Lines CR, Nessly ML, Group M-PS (2008) Growth hormone secreta-gogue MK-677: No clinical effect on AD progression in a randomized trial. Neurology 71, 1702–1708. [DOI] [PubMed] [Google Scholar]
- [42].Ashford JW (2004) APOE genotype effects on Alzheimer’s disease onset and epidemiology. J Mol Neurosci 23, 157–165. [DOI] [PubMed] [Google Scholar]