Abstract
Purpose
Studies evaluating treatment margins for high grade gliomas (HGG) are limited. We hypothesize patients with HGG treated with GTV to PTV expansion of 1 cm or less will have similar progression-free survival (PFS) and overall survival (OS) as those treated according to standard RTOG or EORTC protocols. Furthermore, PFS and OS of subgroups within the study population, with GTV1 to PTV1 margins of 1.0 cm and 0.4 cm, will have equivalent survival outcomes.
Methods
Treatment plans and outcomes for patients with pathologically confirmed HGG were analyzed (n=267). Survival (PFS and OS) was calculated from first radiation treatment. Chi-square test or Fisher’s exact test was used to calculate associations between margin size and patient characteristics. Survival was estimated using Kaplan-Meier and compared using the log-rank test. All analyses were performed on the univariate level.
Results
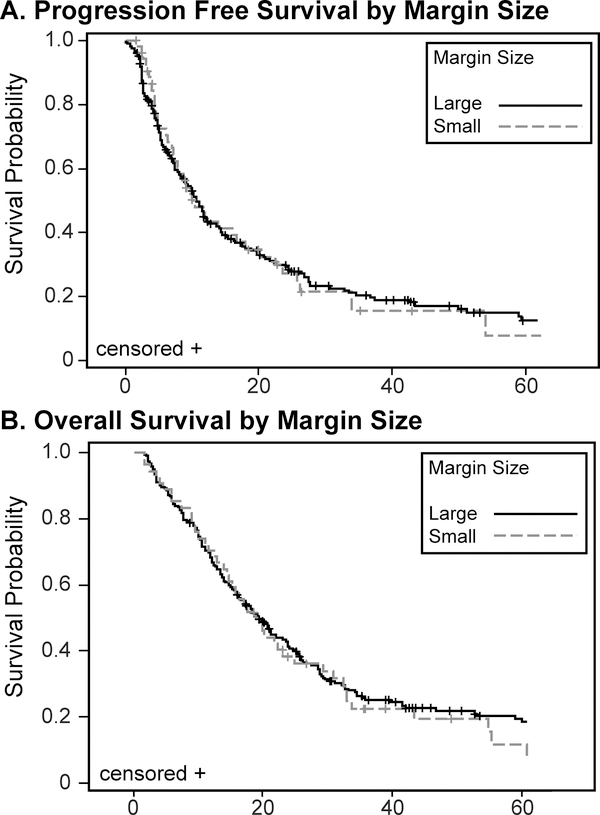
Median PFS and OS was 10.6 and 19.1 months, respectively. By disease, median PFS and OS were 8.6 and 16.1 months for glioblastoma (GBM) and 26.7 and 52.5 months for anaplastic glioma (AG). Median follow-up was 18.3 months. Treatment margin had no effect on outcome, with the 1.0 cm GTV1-PTV1 margin subgroup (n=212) showing a median PFS and OS of 10.7 and 19.1 months and the 0.4 cm margin subgroup (n=55) showing a median PFS and OS of 10.2 and 19.3 months. In comparison with the standard treatment with 2–3 cm margins, there was not a significant difference in outcomes.
Conclusions
There is no apparent difference in survival when utilizing smaller margins compared to larger margins defined by RTOG and EORTC guidelines. While there remains no class I evidence that outcomes after treatment with smaller margins are identical to those after treatment with larger margins, this large series with long-term follow-up suggests reducing margins is safe and further investigation is warranted.
Introduction
An estimated 24,790 new cases of primary malignant brain tumors are expected to be diagnosed in the US in 2016. About 50% of gliomas are glioblastomas (GBM) with 12,120 new cases predicted (1). Glioblastoma is a diagnosis with a 5% five-year survival rate (2). Despite significant advances in radiotherapy, neurosurgery, neuro-imaging, and chemotherapy for HGG, prognosis remains bleak, with median progression-free survival (PFS) of 6.9 and 36 months, and overall survival (OS) of 14.6 and 56.4 months for GBM and anaplastic glioma (AG), respectively (3–5).
Radiation therapy (RT) became standard of care for HGG after randomized trials in the 1970s demonstrated improved survival outcomes (6,7). Although initial studies suggested a need for whole brain radiotherapy (WBRT), subsequent trials conducted with improved imaging showed no advantage of WBRT over partial-brain irradiation (8). These studies also revealed the predominant pattern of treatment failure for HGG was within 2–3 cm of the primary tumor (7). Small studies in the 1980s documented tumor to be histologically present 2–3 cm from the primary tumor volume on CT and infiltrating into the edema volume on MR (5,9). Based on these data, the most standard margins used for conformal irradiation delivered to a target volume (defined by edema volume for the initial field and T1 gadolinium enhanced volume for boost field), now most often identified on MR, is surrounded by a 2–3 cm margin, as outlined by Stupp et al., RTOG 525, RTOG 0825, and other standard treatment protocols (3,10,11). In addition to this margin a set-up error margin is added based on the individual institutions set-up paradigm. Evidence-based guidelines show scant literature evaluating alternative margins (12).
Radiation therapy to the brain is associated with significant acute and late neurotoxic sequelae that are positively correlated with higher treatment dose and larger treatment volume. Advances in image-guided and stereotactic radiation therapy as well as improved image quality support reducing the margin needed and some radiation oncologists have started to adopt smaller treatment margins for HGG in effort to reduce these toxicities and improve quality of life. However, despite this rationale there is a dearth of published data studying specific deviations from standard protocol (13).
Radiation therapy treatment margins used at our institution between 2006 and 2017 are considerably smaller than those defined in the current RTOG and EORTC treatment protocols. These have been consistently applied due to a well-developed frameless stereotactic experience that reduces set-up inaccuracy to .5 mm and availability of high resolution MR imaging (0.6 mm x 0.6mm x 1.0 mm voxel size) within the radiation oncology department. Using smaller margins minimizes irradiation-induced damage of normal brain tissue. Our standard practice uses non-invasive daily stereotactic guidance or image-guided radiation therapy (IGRT) for daily localization unless the patient is unable to cooperate, which is rare. We evaluated outcomes for these patients treated with smaller margins (PFS and OS) compared to patients treated with the standard ~2–3 cm margins adopted by conventional protocols (RTOG and EORTC). Further, one of our staff physicians, generally uses 0.4 cm margins added to the edema and T1 enhanced target volumes while another generally uses 1.0 cm margins for these expansions. We evaluated outcomes of patients treated by each of these two physicians.
Methods
Patient Population
This study was approved by our Institutional Review Board. All adult patients receiving definitive RT for HGG from 2006 to 2017 were reviewed. We included patients for analysis who met the following criteria: (1) age ≥ 18, (2) pathologically confirmed WHO grade III anaplastic glioma (AG) or WHO grade IV glioblastoma (GBM), and (3) received definitive RT (defined as receiving a dose of 45 Gy or more).
This review resulted in 267 evaluable cases. Several patients included in this study were treated according to protocols with specific radiotherapy guidelines, such as RTOG protocol 0525, RTOG protocol 0825, NewLink Genetics protocol NLG-2102 (Indoximod and TMZ for TMZ-refractory malignant brain tumors), and North Central Cancer Treatment Group protocol N0877 (Dasatinib vs placebo combined with standard chemo-radiotherapy) that specify standard margin requirements (n=8) (3,10,14–16). These patients were excluded for analysis of reduced tumor margins. An investigator-initiated trial of intravenous ascorbate used institutional specific margins for planning (n=12) and was therefore included for margin analysis.
All treatment plans and margins were reviewed in the treatment planning system (TPS) (Philips Healthcare, Andover, MA). Patient demographic and outcome data were retrieved from the EMR (EPIC, Verona, WI) including imaging and follow-up for progression and survival.
Treatment
Patients treated for HGG from 2006–2017 most commonly received RT doses and chemotherapy regimens as outlined in the Stupp and RTOG 0825 protocols (10,11). Therapy for HGG includes maximal safe resection followed by RT to 61.2 Gy at 1.8 Gy per fraction with concurrent temozolomide (TMZ) starting within 6 weeks following surgery. The 61.2 Gy dose is split into an initial 45 Gy in 25 fractions followed by a 16.2 Gy boost in 9 fractions. Initial simulation consists of formation of a custom bite-plate and mask for imaging and daily stereotactic positioning (17,18). A contrast-enhanced, high-resolution CT is acquired with 0.6× 0.6×1.0 mm voxel size. Volumetric T2, FLAIR and T1 gadolinium-enhanced MR are acquired on a 3T MR simulator with 1 mm voxel resolution and the thermoplastic mask in place. Image fusion is accomplished in the TPS prior to manual contouring of the treatment volumes. Pre-operative imaging is also fused to aid in tumor cavity and areas at risk identification for targeting.
Typically, the initial 45 Gy is delivered to the edema volume as defined by T2 or FLAIR imaging, consistent with the RTOG definition. An expansion of 0.4 cm or 1.0 cm is then applied to define a planning target volume (PTV1). Barriers to tumor growth such as dura and bone are considered and margins reduced accordingly within the limit of daily set-up. This is inconsistent with RTOG, which expands the edema volume by 2–2.5 cm and adds an additional 0.3–0.5 cm for set-up to define this PTV1. The boost 16.2 Gy is delivered to a cone-down volume encompassing the enhancing tumor volume and/or the resection cavity as outlined on the T1 gadolinium-enhanced scan. This volume is also expanded by 0.4 cm or 1.0 cm to create the PTV2 while similarly considering barriers to tumor growth. This also differs from the RTOG protocol, which adds 2cm to this enhancing tumor volume or cavity plus 0.3–0.5 cm for set-up to create the PTV2.
All RT plans were normalized such that at least 95% of PTV1 and PTV2 received the prescribed dose. The majority of plans used intensity modulated techniques with daily stereotactic positioning described previously.
Follow-up
Post-radiation imaging begins approximately 30 days after completing radiation therapy and then is scheduled after every 2 cycles of TMZ (approximately every 2 months). Patients are scheduled to receive a minimum of 6 cycles of TMZ with some patients electing to continue TMZ after this initial treatment. Although this has not been shown to improve long-term outcomes in HGG, the decision for this additional adjuvant therapy was most often made prior to data availability to the contrary.
Analysis
Survival (PFS and OS) was calculated from the first RT fraction. The date of imaging following RT demonstrating radiographic progression confirmed by the attending radiation oncologist was defined as the date of first progression. If death occurred from suspected progression without imaging confirmation, the date of death was used as the date of tumor progression. The date of death was determined using the EMR and online obituary sources.
Associations between margin size and patient characteristics were calculated using the chi-square test or Fisher’s exact test for small sample sizes where appropriate. Statistical testing was performed using SAS v9.4 (SAS Institute, Cary, NC). Survival probabilities were estimated and plotted using the Kaplan-Meier method. Overall survival is defined to date of death or date of last follow-up. Progression free survival (PFS) is defined until recurrence or date of last follow-up. All testing is performed on the univariate level and unadjusted for multiple comparisons due to the exploratory nature of the project. Differences between survival curves were compared using the log-rank test. All statistical testing is two-sided and assessed for significance at the 5% level.
Results
Patient Characteristics
Two hundred sixty-seven patients met inclusion criteria from January 2006 to May 2017 (Table 1); 114 (42.7%) were female and 153 (57.3%) were male. 191 (71.5%) patients had GBM and 76 (28.5%) patients had AG. Median age was 56 years (58 for GBM and 48.5 for AG). Median PFS and OS for the entire group was 10.6 and 19.1 months, respectively. By disease, median PFS and OS were 8.6 and 16.1 months for glioblastoma (GBM) and 26.7 and 52.5 months for anaplastic glioma (AG). Median follow-up was 18.3 months. Treatment margin had no effect on outcome, with the 1.0 cm margin subgroup (n=212) showing a median PFS and OS of 10.7 and 19.1 months and the 0.4 cm margin subgroup (n=55) showing a median PFS and OS of 10.2 and 19.3 months (Figure 1). There was no effect of margin size within either the GBM or AG group. In comparison with historical standard treatment with 2–3 cm margins, there was not a significant difference in outcomes.
Table 1.
Patient and treatment characteristics (n=267)
| Variable | Level | N = 267 | % |
|---|---|---|---|
| Gender | Female | 114 | 42.7 |
| Male | 153 | 57.3 | |
| Disease | Anaplastic glioma | 76 | 28.5 |
| Glioblastoma | 191 | 71.5 | |
| Physician | A | 48 | 18.0 |
| B | 206 | 77.2 | |
| Other | 13 | 4.9 | |
| Physician (excluding trials) | A | 48 | 19.5 |
| B | 198 | 80.5 | |
| Surgery | biopsy only | 77 | 28.8 |
| gross total | 108 | 40.4 | |
| subtotal resection | 82 | 30.7 | |
| Dose | 45–59.3 Gy | 8 | 3.0 |
| 59.4–61.2 Gy | 259 | 97.0 | |
| Margin Size | Large | 212 | 79.4 |
| Small | 55 | 20.6 | |
| Disease by Margin Size | A6 and Large | 64 | 24.0 |
| A6 and Small | 12 | 4.5 | |
| GBM and Large | 148 | 55.4 | |
| GBM and Small | 43 | 16.1 |
Figure 1.
Progression Free and Overall Survival by Margin Size
All patients received some type of surgery: 108 (40.4%) of the 267 patients had gross total resection (GTR), 82 (30.7%) patients had subtotal resection (STR), and 77 (28.8 %) patients had biopsy only. Two-hundred-and-fifty-nine (97%) patients received a total dose of 59.4–61.2 Gy, while 8 (3%) patients received a total dose of 45–59.3 Gy. Daily stereotactic localization was used in addition to a facemask for 195 (73%) of the patients, while the other 72 (27%) patients had facemask only generally complemented with daily cone beam CT for alignment. Margins were adjusted when localization accuracy was not done using stereotactic techniques or daily image guidance.
Additionally, 243 (91.0%) patients received TMZ chemotherapy as part of their treatment regimen. Of these patients, 223 (83.5%) were treated with concurrent chemotherapy and radiotherapy while 214 (80.1%) received adjuvant chemotherapy following radiation. Patient and treatment characteristics are summarized in Tables 1-5.
As found in previous studies, gender, grade III histology, type of surgery, chemotherapy, and age are significant predictors of PFS and OS at the univariate level. Margin size is not a significant predictor of PFS or OS between the subgroups (0.4cm vs. 1.0 cm margins) in this study (Figure 1), nor is there a difference in PFS or OS when comparing our patients treated with reduced margins (≤ 1.0 cm) to historical reports. This review provides data that not only do patients treated with ≤ 1.0 cm median margins did not have a difference in survival compared to patients treated with 2–3 cm margins, but also that those treated with 0.4 cm margins demonstrate no survival difference as well. The study did not address differences in toxicity between treatment groups although a theoretic benefit of smaller volumes was noted. Similarly, recurrences were reported as within the treatment field in the vast majority of cases with no preponderance of marginal or distant failures noted in any group. A detailed analysis of these recurrence patterns was beyond the scope of this report.
Discussion
High grade gliomas remain associated with extremely poor prognosis. Multiple clinical trials over decades have evaluated varying fractionation, dose, and radiation sensitizers, yet none resulted in significant changes to local recurrence patterns or outcomes (19–21). With advances in technology (both imaging and positioning methods) leading to the improved precision of radiation delivery and the potential to better identify tumor involved regions, the trend in RT has been to decrease treatment margins and hence, treatment volumes. This has potential benefit in HGG treatment, where tumors are surrounded by critical neural structures yet less than certainly identified. Unfortunately, alternative treatment margins have not been carefully investigated (10). Our approach using high resolution MR imaging combined with stereotactic or image-guided daily localization is now readily available at most academic medical centers as well as many community practices and enables better investigation of target definition and margins.
Clinical trials for HGG still typically use 2–3 cm expansion margins as outlined in RTOG or EORTC protocols. Both groups define the ultimate target as the surgical tumor bed plus residual enhancing tumor. The EORTC employs a single-phase technique where this target is expanded by 2–3cm and 5–7mm is also added for daily positioning (PTV). This is treated to 60 Gy in 30 fractions. For RTOG, the sum of the T1 enhancing target and peritumoral edema (defined on T2 or FLAIR images) defines an initial target volume. This is expanded 2cm (or 2.5cm if no edema is present) to create PTV1 which is treated to 46 Gy in 23 fractions. This is followed by a cone-down boost dose of 14 Gy in 7 fractions delivered to the gross residual tumor or tumor cavity on T1 contrast enhanced imaging plus a margin of 2.3–2.5 cm. These guidelines are inconsistent with the smaller margins (≤1 cm) employed at our institution.
Several studies have shown TMZ therapy does not alter HGG treatment failure pattern (22). The same has been shown with use of smaller margins (0.5cm CTV margins plus 0.5cm PTV expansion) and IMRT (20,23). Evidence supporting currently used 2–3cm margin standards in national protocols is limited.
A brief search of the peer-reviewed literature returned papers reporting patient outcomes after treatment with smaller margins. Three institutions treated HGG patients according to the Adult Brain Tumor Consortium (ABTC) guidelines, which expands the contrast enhancing T1 volume or resection cavity by 0.5 cm to create a CTV, which is further expanded 0.3–0.5 cm for the PTV. Investigators at Emory evaluated the pattern of tumor progression when using ABTC guidelines (n=43) (24). They found 93% of recurrences were in field, 5% were marginal, and only 2% were distant. Investigators from Wake Forest evaluated outcomes with varied expansion margins (n=145). They found no significant difference in pattern of failure, PFS, or OS between different margin groups (0.5cm n=29, 1cm n=78, 1–2cm n=38) (20). The University of Alabama reviewed outcomes of patients (n=95) treated per ABTC guidelines and reported 81% experienced in-field failure and only 6% experienced marginal progression (25). While these studies used ABTC margins, which are smaller than RTOG and EORTC margins, the total margin in these studies is 0.8–1.0 cm, which is significantly larger than our institution’s 0.4 cm median margin subgroup (n=55).
Increasing treatment margin size can considerably increase the volume of brain tissue irradiated. For example, treating a spherical tumor with a 5 cm radius with a 0.4 cm margin results in irradiation of a volume of 136.0 cm3 of tissue. In contrast, a 2–3 cm margin results in irradiation of 913.2–1621.1 cm3, representing a 7 to 12-fold increase in irradiated volume compared to the 0.4 cm margin.
Advances in MR imaging may also improve our quality of tumor targeting and potentially reduce or increase the size of targets using a range of techniques. Diffusion weighted imaging (DWI) along with the apparent diffusion coefficient (ADC) have been associated with increased cellularity and potentially with areas of increased tumor infiltration. Perfusion imaging with dynamic susceptibility contrast maps may identify areas of increased blood flow associated with tumor. MR spectroscopy is also evolving rapidly to enable larger volume acquisitions with more facile processing that may make these methods more accessible for tumor targeting based on chemical metabolites. All may substantially change our approach to margins particularly when coupled with precise daily localization methods.
The effects of radiation on local and systemic immune function are worth considering. Grossman et al. conducted a prospective study observing the effects of immunosuppression in patients with HGG treated with radiation and temozolomide (26). Results suggest median survival correlated with CD4 suppression. Furthermore, CD4 suppression was associated with death from tumor progression. It has also been shown that increased exposure of circulating blood volume to radiation can lead to a CD4 T-cell deficit, which can persist after radiation therapy due to disruption of the compensatory cytokine response. Although we did not measure any immune relevant endpoints in our study, one could reasonably hypothesize that reduced volumes irradiated could to reduce lymphopenia severity and hence improve anti-tumor immune response (27).
A review by Wernicke et al. discusses the importance of the radiation-induced antitumor T-effector response (28). Two types of T-cells are at play: tumor-infiltrating lymphocytes (TILs), which suppress the innate antitumor response, and T-effector cells, which can be induced against tumor cells. Most lymphocytes are extremely radiosensitive with an early beneficial radiation effect being elimination of TILs. Radiation also increases release of tumor neo-antigens that induce a T-effector response. Unfortunately, like TILs, T-effector cells are easily killed by radiation. Fractionated treatment over long periods of time and increased radiation treatment volume will cause increased toxicity to T-effector cells, decreasing the body’s ability to attack tumor cells. Preclinical studies have shown improved survival in glioma mouse models treated with T-cell boosting therapies plus radiation vs. radiation alone (29,30). The significance of TILs has also been studied in patients. Han et al showed that a high level of CD4+ TILs combined with a low level of CD8+ TILs was associated with unfavorable prognosis in patients with GBM. The use of checkpoint inhibitors and other cancer immunotherapies is currently being studied in GBM, and may add to a rationale for smaller local fields that enable effector cell activation (31,32). While recognizing these potential benefits are speculative, it does add to the rationale for considering smaller fields as an alternative.
Potential limitations of the study include the potential selection bias associated with any retrospective design as well as confounding variables on OS such as the rigor or nature of salvage therapies. Molecular data such as MGMT methylation, 1p/19q deletion and IDH1 status was not included because it was not performed for the majority of the time period included in the study, and it is not clear these factors alter the impact of margins. Brandes et al. reported the patterns of failure of GBM using RTOG margins and found 85% of patients with unmethylated MGMT had failure within field or marginally compared to 58% of patients with methylated MGMT (33). The present lack of data prevents evaluation of molecular traits as confounding factors in analysis of the planning margins and survival. A recent clinical trial suggests our patient population mimics norms for MGMT and IDH-1 status (34).
In addition this retrospective study was not designed to perform a detailed analysis of histologic subtypes of anaplastic gliomas such as oligodendroglial versus astrocytic variants, potential benefits of reduced toxicity with smaller fields or the detailed analysis of treatment failures in reference to delivered dose distributions registered to recurrence scans. While these would be interesting additions and potentially informative, the retrospective nature and overall goal to correlate outcomes with margins was a valuable initial finding that may spur additional investigation into the impact of treatment volume and margin on management of HGG.
With 259 patients evaluable for outcome after treatment with reduced treatment margins, this review represents the largest to date to evaluate the effect of smaller radiation planning margins on patient survival. A review of literature suggests it is the first study to evaluate patients treated with total PTV margins of 0.4 cm (n=55). Results suggest smaller RT margins in the treatment of high-grade gliomas do not impact survival outcomes. These data coupled with a lack of an evidenced-based standard margin, survival data from ABTC studies, potential rationale from immune mediators of the radiation-induced antitumor response, and reduced neurotoxicity associated with smaller RT fields, suggest the need for further studies to reevaluate treatment margin guidelines for high-grade gliomas.
Conclusion
Using smaller-than-standard margin radiation therapy for high-grade gliomas results in progression-free survival and overall survival consistent with the existing literature and historical controls. Using margins as small as 0.4 cm instead of the 2–3 cm used in conventional protocols significantly reduces the volume of normal brain tissue radiated during treatment. Future studies could potentially study the effect of smaller margins on anti-tumor T-effector response.
Table 2.
Margin Size Associations
| Covariate | Statistics | Level | Large N=212 | Small N=55 | P-value |
|---|---|---|---|---|---|
| Gender | N (Row %) | Female | 88 (77.2) | 26 (22.8) | 0.44 |
| N (Row %) | Male | 124 (81) | 29 (19) | ||
| Disease | N (Row %) | Anaplastic glioma | 64 (84.2) | 12 (15.8) | 0.22 |
| N (Row %) | Glioblastoma | 148 (77.5) | 43 (22.5) | ||
| Physician | N (Row %) | A | 3 (6.3) | 45 (93.8) | <.01 |
| N (Row %) | B | 188 (94.9) | 10 (5.1) | ||
| Surgery | N (Row %) | biopsy only | 60 (77.9) | 17 (22.1) | 0.79 |
| N (Row %) | gross total | 88 (81.5) | 20 (18.5) | ||
| N (Row %) | subtotal resection | 64 (78) | 18 (22) | ||
| Dose | N (Row %) | 45–59.3 Gy | 5 (62.5) | 3 (37.5) | 0.37 |
| N (Row %) | 59.4–61.2 Gy | 207 (79.9) | 52 (20.1) | ||
Table 3.
Anaplastic Glioma (n=76)
| Variable | Large Margin (n=64) | Small Margin (n=12) |
| OS | 52.5 months | 60.3 months |
| PFS | 27.4 months | 17.0 months |
| Median age (median=48.5) | 51 | 32 |
| Below median age | 28 (43.75%) | 10 (83.33%) |
| Above median age | 36 (56.25%) | 2 (16.67%) |
| Surgery type | ||
| GTR | 20 (31.25%) | 1 (8.33%) |
| STR | 20 (31.25%) | 4 (33.3%) |
| Biopsy only | 24 (37.5%) | 7 (58.33%) |
| GBM (n=191) | ||
| Variable | Large Margin (n=148) | Small Margin (n=43) |
| OS | 16.6 months | 16.0 months |
| PFS | 8.2 months | 9.1 months |
| Median age (median=58) | 58 | 58 |
| Below median age | 73 (49.32%) | 20 (46.51%) |
| Above median age | 75 (50.68%) | 20 (46.51%) |
| Equal to median age | 0 (0%) | 3 (6.89%) |
| Surgery type | ||
| GTR | 68 (45.94%) | 19 (44.19%) |
| STR | 44 (29.73%) | 14 (32.56%) |
| Biopsy only | 36 (24.32%) | 10 (23.26%) |
Footnotes
Conflict of Interest:
The authors declare that no actual or potential conflict of interest exists.
References:
- 1.American Brain Tumor Associattion. Glioblastoma and Malignant Astrocytoma. http://www.abta.org/secure/glioblastoma-brochure.pdf Last accessed: 11-17-2017.
- 2.American Cancer Society. Survival Rates for Selected Adult Brain and Spinal Cord Tumors. https://www.cancer.org/cancer/brain-spinal-cord-tumors-adults/detection-diagnosis-staging/survival-rates.html Last accessed: 11-17-2017.
- 3.National Cancer Institute. Temozolomide (TMZ) and Radiation Therapy (RT) With or Without Bevacizumab in Treating Patients With Newly Diagnosed Glioblastoma Multiforme (GBM). https://clinicaltrials.gov/ct2/show/NCT00884741 Last accessed: 10-31-2017.
- 4.Brandes AA, Nicolardi L, Tosoni A, et al. Survival Following Adjuvant PCV or Temozolomide for Anaplastic Astrocytoma. Neuro Oncol 2006;8:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Rhun E, Taillibert S, Chamberlain MC. The Future of High-Grade Glioma: Where we are and where are we going. Surg Neurol Int 2015;6:S9–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker MD, Green SB, Byar DP, et al. Randomized Comparisons of Radiotherapy and Nitrosoureas for the Treatment of Malignant Glioma after Surgery. N Engl J Med 1980;303:1323–9. [DOI] [PubMed] [Google Scholar]
- 7.Wallner KE, Galicich JH, Krol G, et al. Patterns of Failure Following Treatment for Glioblastoma Multiforme and Anaplastic Astrocytoma. Int J Radiat Oncol Biol Phys 1989;16:1405–9. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro WR, Green SB, Burger PC, et al. Randomized Trial of Three Chemotherapy Regimens and Two Radiotherapy Regimens and Two Radiotherapy Regimens in Postoperative treatment of Malignant Glioma. Brain Tumor Cooperative Group Trial 8001. J Neurosurg 1989;71:1–9. [DOI] [PubMed] [Google Scholar]
- 9.Walker MD, Alexander E Jr., Hunt WE, et al. Evaluation of BCNU and/or Radiotherapy in the Treatment of Anaplastic Gliomas. A Cooperative Cinical Trial. J Neurosurg 1978;49:333–43. [DOI] [PubMed] [Google Scholar]
- 10.Radiation Therapy Oncology Group. Radiation Therapy (RT) and Temozolomide (TMZ) in Treating Patients with Newly Diagnosed Glioblastoma or Gliosarcoma. (2006-2016). https://clinicaltrials.gov/ct2/show/NCT00304031 Last accessed: 10-31-2017.
- 11.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy Plus Concomitant and Adjuvant temozolomide for Glioblastoma. N Engl J Med 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- 12.Olson JJ, Fadul CE, Brat DJ, et al. Management of Newly Diagnosed Glioblastoma: Guidelines Development, Value and Application. J Neurooncol 2009;93:1–23. [DOI] [PubMed] [Google Scholar]
- 13.Dhabaan A, Schreibmann E, Siddiqi A, et al. Six Degrees of Freedom CBCT-Based Positioning for Intracranial Targets Treated with Frameless Stereotactic Radiosurgery. J Appl Clin Med Phys 2012;13:3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Institute. A Phase I Trial of High-Dose Ascorbate in Glioblastoma Multiforme. https://clinicaltrials.gov/ct2/show/NCT01752491?term=NCT01752491&rank=1 Last accessed: 11-01-2017.
- 15.National Cancer Institute. Study of IDO Inhibitor and Temozolomide for Adult Patients With Primary Malignant Brain Tumors. https://clinicaltrials.gov/ct2/show/NCT02052648 Last accessed: 11-01-2017.
- 16.National Cancer Institute. Dasatinib or Placebo, Radiation Therapy, and Temozolomide in Treating Patients With Newly Diagnosed Glioblastoma Multiforme. https://clinicaltrials.gov/ct2/show/NCT00869401 Last accessed: 11-01-2017.
- 17.Meeks SL, Tome WA, Willoughby TR, et al. Optically Guided Patient Positioning Techniques. Semin Radiat Oncol 2005;15:192–201. [DOI] [PubMed] [Google Scholar]
- 18.Tome WA, Meeks SL, Buatti JM, et al. A High-Precision System for Conformal Intracranial Radiotherapy. Int J Radiat Oncol Biol Phys 2000;47:1137–43. [DOI] [PubMed] [Google Scholar]
- 19.Deutsch M, Green SB, Strike TA, et al. Results of a Randomized Trial Comparing BCNU Plus Radiotherapy, Streptozotocin Plus Radiotherapy, BCNU Plus Hyperfractionated Radiotherapy, and BCNU Following Misonidazole plus Radiotherapy in the Postoperative Treatment of Malignant Glioma. Int J Radiat Oncol Biol Phys 1989;16:1389–96. [DOI] [PubMed] [Google Scholar]
- 20.Paulsson AK, McMullen KP, Peiffer AM, et al. Limited Margins Using Modern Radiotherapy Techniques Does Not Increase Marginal Failure Rate of Glioblastoma. Am J Clin Oncol 2014;37:177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang TJ, Wu CC, Jani A, et al. Hypofractionated Radiation Therapy Versus Standard Fractionated Radiation Therapy with Concurrent Temozolomide in Elderly Patients With Newly Diagnosed Glioblastoma. Pract Radiat Oncol 2016;6:306–14. [DOI] [PubMed] [Google Scholar]
- 22.Dobelbower MC, Burnett Iii OL, Nordal RA, et al. Patterns of Failure for Glioblastoma Multiforme Following Concurrent Radiation and Temozolomide. J Med Imaging Radiat Oncol 2011;55:77–81. [DOI] [PubMed] [Google Scholar]
- 23.Fuller CD, Choi M, Forthuber B, et al. Standard Fractionation Intensity Modulated Radiation Therapy (IMRT) of Primary and Recurrent Glioblastoma Multiforme. Radiat Oncol 2007;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald MW, Shu HK, Curran WJ Jr., et al. Pattern of Failure After Limited Margin Radiotherapy and Temozolomide for Glioblastoma. Int J Radiat Oncol Biol Phys 2011;79:130–6. [DOI] [PubMed] [Google Scholar]
- 25.Gebhardt BJ, Dobelbower MC, Ennis WH, et al. Patterns of Failure for Glioblastoma Multiforme Following Limited-Margin Radiation and Concurrent Temozolomide. Radiat Oncol 2014;9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossman SA, Ye X, Lesser G, et al. Immunosuppression in Patients with High-Grade Gliomas Treated With Radiation and Temozolomide. Clin Cancer Res 2011;17:5473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yovino S, Grossman SA. Severity, Etiology and Possible Consequences of Treatment-Related Lymphopenia in Patients With Newly Diagnosed High-Grade Gliomas. CNS Oncol 2012;1:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wernicke AG, Smith AW, Taube S, et al. Glioblastoma: Radiation Treatment Margins, How Small is Large Enough? Pract Radiat Oncol 2016;6:298–305. [DOI] [PubMed] [Google Scholar]
- 29.Belcaid Z, Phallen JA, Zeng J, et al. Focal Radiation Therapy Combined with 4–1BB Activation and CTLA-4 Blockade Yields Long-Term Survival and a Protective Antigen-Specific Memory Response in a Murine Glioma Model. PLoS One 2014;9:e101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng J, See AP, Phallen J, et al. Anti-PD-1 Blockade and Stereotactic Radiation Produce Long-Term Survival in Mice with Intracranial Gliomas. Int J Radiat Oncol Biol Phys 2013;86:343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han S, Zhang C, Li Q, et al. Tumour-infiltrating CD4(+) and CD8(+) Lymphocytes as Predictors of Clinical Outcome in Glioma. Br J Cancer 2014;110:2560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JE, Lim M. The Role of Checkpoints in the Treatment of GBM. J Neurooncol 2015;123:413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandes AA, Tosoni A, Franceschi E, et al. Recurrence Pattern After Temozolomide Concomitant with and Adjuvant to Radiotherapy in Newly Diagnosed Patients with Glioblastoma: Correlation with MGMT Promoter Methylation Status. J Clin Oncol 2009;27:1275–9. [DOI] [PubMed] [Google Scholar]
- 34.Schoenfeld JD, Sibenaller ZA, Mapuskar KA, et al. O2•- and H2O2-Mediated Disruption of Fe Metabolism Causes the Differential Susceptibility of NSCLC and GBM Cancer Cells to Pharmacological Ascorbate. Cancer Cell 2017;31:487–500 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]