Abstract
Adaptive radiotherapy emerged over 20 years ago and is now an established clinical practice in a number of organ sites. No one solution for adaptive therapy exists. Rather, adaptive radiotherapy is a process which combines multiple tools for imaging, assessment of need for adaptation, treatment planning, and quality assurance of this process. Workflow is therefore a critical aspect to ensure safe, effective, and efficient implementation of adaptive radiotherapy. In this work, we discuss the tools for online and offline adaptive radiotherapy and introduce workflow concepts for these types of adaptive radiotherapy. Common themes and differences between the workflows are introduced and controversies and areas of active research are discussed.
Introduction
Adaptive radiotherapy (ART), a process to control for anatomical and functional variation over the treatment course, is in active clinical use in a variety of organ sites.1, 2, 11, 3–10 Research in this field remains very active as the underlying technology -- including imaging, tools for adapting the treatment plan, and image registration – continues to evolve and through clinical trials to test the fundamental concepts of ART.
ART emerged over 20 years ago as a process to initially control for day-to-day setup error using megavoltage portal imaging and repeat computed tomography (CT) imaging.12 The term ‘adaptive’ was employed due to the similarity to adaptive control in feedback control theory. However, as ART has evolved and expanded beyond these modalities and techniques, the definition has broadened. Currently, ART can be more formally defined as radiotherapy where the delivered dose is monitored for clinical acceptability during the course of treatment and modified as needed with the goal of improving clinical outcomes. Adaptive radiotherapy allows modification of the treatment plan to account for changes in target and normal organs (size, shape, function, and response) and patient contours (weight) ultimately with the goal of accurately delivering dose to minimize normal tissue exposure and maximize dose to target.
ART can be applied at three timescales: offline between fractions; online immediately prior to a fraction; and inline, or real-time, during a fraction. Because ART is basically a process incorporating a variety of different tools, all three of these types of ART require highly efficient workflows due to the still laborious nature and high complexity of ART implementation. The goal of this work is to introduce and describe the ART process and clinical workflow for offline and online ART (realtime ART is discussed elsewhere in this issue). We begin with the basic ART process and tools, then describe the detailed ART online and offline workflows, and end with open questions and future directions for offline and online ART.
Adaptive Radiotherapy Process and Tools
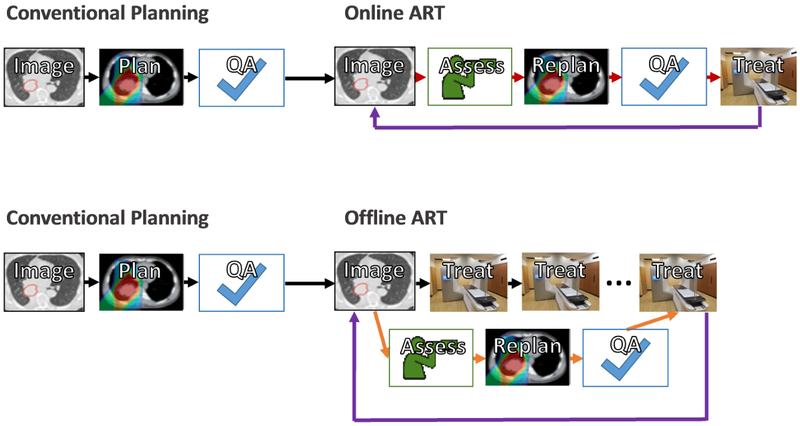
Before discussing the timescales of ART in detail, it is best to understand the basic ART process and tools. At its heart, ART requires four underlying key technologies: imaging, assessment, replanning, and quality assurance (Figure 1). Since ART focuses on adapting to the changing patient anatomy and/or physiology, an accurate means to measure such changes is required. Imaging can be performed either: a) in the treatment room with cone beam CT13–15, magnetic resonance imaging (MRI)16, or other means17, 18 or b) with a CT or MRI simulator or diagnostic imaging. Assessment is the process by which imaging is used to decide whether to adapt the plan or not. This process can range in complexity from a straightforward manual evaluation to highly automated review of cumulative dose. For example, manual review of in-room imaging employing a decision protocol with fixed rules19, re-calculation of the daily dose distribution on the new image16, 20, or deformable dose mapping and estimation of cumulative dose21–24 have all been employed for ART assessment. Assessment may therefore include tools for image review, delineation of targets and organs at risk, re-calculation and review of dose, and image registration (either rigid or deformable). These tools may be manual, semi-automated, or fully-automated, depending on the timescale of ART.
Figure 1.
Workflow and tools for the offline and online adaptive radiation therapy processes. The offline and online ART workflows differ in time of and between processes. Red arrows for the online process represent a time scale of minutes. Orange arrows for the offline process represent a timescale of hours to days. Black arrows, in the traditional planning process, represent a time scale of days. Purple arrows represent repetition of the entire workflow over multiple treatment days.
Once the decision to re-plan the treatment has been made, tools to enable this re-planning must be available. While the standard treatment planning system can be employed for offline ART, online ART requires a highly-integrated, specialized system due to the compressed timeline. For example, replanning tools integrated into the treatment delivery unit may also reduce the quality assurance burden as it can reduce the need to send and receive data between different systems. Finally, but crucially, quality assurance must be integrated throughout the ART process. ART can be a highly complex process25 and robust and efficient quality assurance is therefore critical to ensure accurate, consistent, and safe delivery of ART.
The selection of a particular timescale of ART depends on the clinical goal, available technology, and resources available. Each of these implementations has advantages and disadvantages that must be considered. Offline ART, being performed generally during the time between fractions, allows a more conventional treatment planning workflow and set of tools to be employed, albeit in a compressed timeframe. One advantage of offline ART is that in-room imaging such as cone beam CT or MRI may be used, or conventional CT or MRI on a simulator or diagnostic scanner used as the basis for replanning the treatment, if sufficient image quality cannot be achieved with the in-room imaging. Due to the lack of functional imaging systems currently available in the treatment room to enable either online or realtime functional ART, offline ART is most commonly used when functional changes in tumor10, 11 or normal tissue26 are incorporated into the ART process. Offline ART is also most amenable to using existing clinical tools used in the conventional radiotherapy process, having the fewest requirements for specialized tools. However, the downside of offline ART is the potential to be ‘chasing’ anatomical changes.27 If the frequency of anatomical change is high (for example, changes occur within a fraction or frequently between fractions), then offline ART may not be capable of responding to such changes rapidly enough, and could induce, rather than correct, geometric error.
Online ART occurs by imaging the patient in the treatment position immediately prior to delivery, assessing the need for ART, and then replanning and performing quality assurance all while the patient remains in the treatment position. This process must therefore be highly efficient, and requires specialized, well-integrated tools for assessment, replanning, and quality assurance. However, the advantage of online ART is that observed changes can be immediately adapted to and treatment delivered. Many anatomical changes are relatively large inter-fraction (day-to-day) but smaller intra-fraction, including reconfiguration of the anatomy within the abdomen28, baseline variation in the lung29–31, and tumor response32–37, and are therefore well-suited to online ART. Furthermore, in recent years well-integrated systems have been developed specifically for online ART, improving efficiency of this process38, 39. One challenge to online ART is large, rapid, or unpredictable intra-fraction change in patient anatomy. Some intra-fraction changes after the plan has been adapted can be managed through standard motion management techniques such as patient surveillance or beam gating. However, large anatomical changes that occur between the time of in-room imaging and beam delivery can decrease the accuracy of online ART. For example, bladder filling or stomach emptying can occur during the minutes it takes to evaluate, re-optimize, and verify an online plan. Another key challenge, discussed below, is the inability to perform patient-specific quality assurance measurements (e.g., with a delivery to phantom), as the patient is not removed from the treatment position. Therefore, conventional quality assurance workflows must be modified for online ART.
Finally, realtime ART uses realtime or near-realtime imaging of anatomy or surrogates to adapt the plan in realtime during delivery. Realtime ART is capable of reacting to all anatomical changes that are measurable or predictable in realtime, and is therefore potentially the most accurate form of ART. However, realtime ART comes with a higher quality assurance burden and requires substantially more automation than offline or online ART. Currently, no realtime functional imaging modalities are in clinical use so online, or, more likely, offline ART must be used for functional adaptation. Strategies and workflows for realtime ART are discussed elsewhere in this issue.
ART Workflow
For either offline or online ART, the starting point is the identification of the clinical necessity for adaptation. The subsequent workflow depends on the speed with which this necessity is to be addressed, but in general the steps are analogous, with the main differences manifesting in implementation. The first step – the identification of a need for change – typically depends on detection of anatomic shift via imaging. Other scenarios include a significant weight loss or gain, observation of changes in the comfort or fitting of immobilization devices, or outside procedures done to the patient that alter their internal anatomy. The general ART workflow steps are described below.
Physician Orders and Radiation Therapy Prescription
Changes that necessitate plan adaptation may be expected (as in the case of daily internal organ motion or gradual tumor volume changes) or unexpected. If the change is anticipated, such as with treatment to intra-abdominal sites, where inter-fraction anatomic shifts in organs-at-risk are expected to occur, the ART process is triggered with some frequency (e.g., daily for online ART, weekly or once mid-treatment for offline ART). While a new plan is created at each of these determined intervals, it may not necessarily be implemented, as the plan changes may be too small to produce clinically-significant dosimetric changes. The most important part of this step is therefore clear communication on the part of the physician as to the clinical threshold of necessity for adaptation. For online ART, it may be a predetermined level of improvement in target coverage due to more favorable distribution of organs at risk (OAR) that day, or a violation of stated OAR constraints of predetermined severity that must be reversed. For offline ART, it may be improvement of normal tissue sparing due to tumor volume reduction or need to regain intended target coverage that has been lost secondary to significant anatomic shifts as with patient weight loss.
Simulation
Simulation and immobilization considerations for offline ART seldom differ from the standard clinical workflow and are determined primarily by the site to be treated. Online ART considerations arise from the fact that it is currently only commonly performed on MRI-guided systems, therefore the immobilization must take into account the MRI receiver coils and the enclosed bore of the machine.1, 16, 28 Another important consideration is the comfort of the patient for the duration of both the adaptive process - which has been reported to be about 25 minutes, in addition to imaging and treatment.1 For example, it is typical to position the patient’s arms up for abdominal treatments, in which case custom immobilization with shoulder support may improve patient comfort and reduce patient motion within the MRI bore. Given the longer on-table times with online-adaptive processes, one might also consider unique allowances, like placing the patient’s contralateral arm down, to improve comfort and positional stability. It is critical to attain adequate patient comfort so that the patient is immobile once the adaptation process is underway, as any motion due to discomfort may necessitate further adaptive re-plans in response to motion, and therefore further prolong the treatment time.
Additionally, the type of re-planning approach that will be used for online adaptation must be considered prior to simulation. While most users implementing online ART at this time (typically with MRI-guided radiotherapy systems) use a unique plan derived based on the anatomy-of-the-day revealed by daily volumetric setup imaging, a plan library approach can also be considered. A plan library approach is based upon predictable changes in specific organs-at-risk that impact the location of or overlap with the target, such as with changes in bladder or rectal filling for pelvic targets. In this case, the initial simulation processes should include multiple simulation images emulating these predicted anatomic changes, like simulation with bladder full, partially fully, and empty, so that the plan library can be created. Then, during daily treatment, the best matched plan can be selected based on the visualized daily, on-table anatomy. In contrast, if the intent is to perform online adaptation without a plan library (creating plans “on the fly” based on the anatomy-of-the-day) multiple simulation images are unnecessary.
For offline-adaptation, simulation processes typically mirror those of standard treatment simulation. Given that re-plans do not occur while the patient is on the treatment table in this approach, unique positioning and comfort considerations are less critical than with online adaptation. Similarly, multiple simulation images to create a plan library are also not needed.
Treatment Planning Considerations
Offline reoptimization is often unplanned; therefore it is difficult to anticipate what changes may be required partway through treatment at the time of creation of the initial plan. The necessity for online ART, however, should be determined in advance, as it requires both greater coordination of the treatment team as well as a longer time slot for treatment itself. For the plan library approach, a treatment plan must be created, checked, and prepared for implementation for each simulation scan obtained. For example, in the most common site for this approach (bladder cancer40, 41), three plans are created - one with bladder full, one mid-fill, and one empty. Each plan must independently meet all requisite constraints and achieve target coverage goals. It is also advantageous to evaluate the robustness of each plan in the library for small changes to the volume. Even with three or more plans, the daily shape of the organ will seldom perfectly match what was observed at time of simulation. Therefore, the allowable margin of error must be determined and guidelines regarding appropriate plan selection should be clearly communicated to the therapists (or other staff members who will be responsible for plan selection).42, 43
Online ART also requires a robust plan, but has the additional challenge of needing to account for unpredictable changes in organ position relative to the target, or the target itself. For example, when creating a treatment plan for an abdominal lesion, the possibility of gastrointestinal OARs moving variably close to the target on any given treatment day must be considered. Whereas during the non-adaptive planning process the dosimetrist will place higher importance on OARs closest to the target based on the simulation scan, when a plan is to be used as the starting point for online adaptation, the dosimetrist must instead enable the plan to place equal importance on any OAR that may move closer on different treatment days. For example, it is typical to observe significant daily motion of the bowel, as well as stomach filling changes, and the adapted plan must be able to correspondingly change.44, 45 Such initial plan robustness is required, because current implementations of online-adaption maintain the initial treatment plan’s beam angles and isocenter position, changing only the fluence and segment shapes, to minimize replanning times and enable efficient and optimized quality assurance (see below).
There are several published approaches for robust plan creation for MRI-guided online ART. One uses an expansion approach, where 1-cm-thick portions of OARs at distances of 1, 2, and 3 cm from the edge of the PTV are recreated each time after recontouring the OARs on a daily basis, and these portions are then used to control the high and mid-doses to the OARs while attempting to maintain PTV coverage.45 Alternatively, one may simply use the OAR contours directly in the optimizer, eliminating the need for recreating optimization structures at each fraction.46 Whatever the approach, the goal is to reduce the necessity to change optimization structures or their weights and other planning parameters to minimize patient on-table time.
Localization
Patient positioning for both online and offline ART should adhere as closely as possible to the patient’s original position during simulation. Although it may be argued that for online ART this is not as crucial because the adaptation process can account for a variety of anatomic changes, we would like to suggest (based on institutional experience) that reproducing the patient’s habitus as close as possible makes for a more efficient treatment time as the only changes that need to be addressed are the internal, uncontrollable ones. This is an important factor, given that the beam angles for adaptive plans typically match those of the simulation plan, as discussed above. In most current online-adaptive paradigms, daily localization is to the centroid of the target volume.1, 45 However, the choice may also be made to align to bony anatomy, to minimize the requirement to account for daily changes in electron density.
Imaging
Detection of changes necessitating either online or offline ART often requires high quality imaging. While some changes may be observed without imaging at all -- for example, weight loss or volume changes in superficial tumors -- internal anatomy changes require imaging for visualization. Changes observable with standard cone beam CT technology available on most modern medical linear accelerators include examples like lung tumor volume changes (where the lower density of the surrounding normal lung tissue allows for adequate contrast), changes in exophytic lesions such as anal cancer, or particularly pronounced tumor responses of bulky lesions. Other examples of offline adaptation triggers include fiducial marker motion that may be identified with either planar X-ray imaging or auxiliary detector systems.
Online adaptation is most commonly chosen for internal changes that occur on relatively rapid timescales, like the constant peristaltic motion of bowel, and therefore necessitates an imaging modality with adequate soft-tissue contrast to enable identification of individual organs-at-risk, as well as changes in the target itself. While cone beam CT technology has improved greatly, MRI guidance even with fields lower than typical diagnostic strengths (e.g., 0.35T vs 1.5T) often provides superior image quality in a variety of sites, especially in soft tissue sites of the abdomen and pelvis.47 It is important to note that daily imaging does not have to be of diagnostic quality to be sufficient to enable online adaptation. It is sufficient if imaging is of high enough quality that the target and OARs can be clearly identified and delineated, either manually or automatically.
Selection of the specific imaging techniques used to acquire images for replanning should consider not only the necessity to see the individual organs well, but also to account for potential intrafraction motion, for example due to breathing. For example, a typical cone beam CT image takes about one minute to acquire and is therefore less suited for abdominal lesions where blurring of low contrast boundaries due to breathing motion will occur, but may be adequate for thoracic, pelvic, or extremity sites. These issues can be mitigated with advanced techniques such as 4D acquisition and reconstruction or multiple breath hold acquisition. Conversely, on the commercially-available low-field MRI-guided radiotherapy system, a short (17-second) MRI scan may be used with a single breath-hold technique to acquire a large field-of-view image that both mitigates blurring due to breathing motion and provides sufficient anatomic information for replanning. Users of MRI-guided systems should also ensure adequate spatial integrity throughout the entire field-of-view of the image, as all tissues from the skin to the tumor will be accounted for during treatment planning to accurately calculate the deposition of dose in the body. This approach is fundamentally different from diagnostic MRI scans, where only a narrow region around the lesion may be important for its identification - in online ART, the complete body contour is required.
Assessment
As discussed above, a key component of both offline and online adaptation is assessment of the need to adapt. For offline adaptation, this need can only be assessed during the course of treatment. Typically, an anatomic shift is identified by either surrogate, such as poor fit of a thermoplastic mask, measured weight loss, or fiducial marker motion, or visualization of systematic changes like gross tumor response on daily imaging. Identification of anatomic change typically prompts re-assessment of performance of the original plan upon the current anatomy, such as with re-calculation of the original plan upon a daily image like cone beam CT, a pre-planned interval CT ‘verify’ scan (as is often obtained weekly during proton beam treatments48), or a requested re-simulation scan prompted by the visualized anatomic change. If the original plan performance meets some threshold of poor performance selected by the physician, offline adaptation is prompted. This is often a subjective judgment, based on a variety of factors such as the intent of treatment, dosimetric change observed, the proportion of the overall treatment course remaining to be delivered, and patient performance status. Depending on the severity of change, the original plan may continue to be used for treatment while the offline adaptive plan is prepared, or treatment may be halted until the offline adaptive plan is ready.
In contrast with offline adaptation, the impetus for online adaptation should be selected in advance of the treatment course initiation. This is due to the need to pre-plan for treatment plan robustness (see treatment planning considerations above) and to allocate necessary time and resources like physician and physics presence at the time of each treatment delivery. Online adaptation is therefore best used in situations where the need to adapt is predictable and known ahead of the initiation of the treatment course. The classic example is for delivery of ablative doses, such as with stereotactic body radiotherapy, within the abdomen and pelvis. In this scenario, daily anatomic changes occur reliably due to bowel filling and peristalsis. Any motion of adjacent OARs or the target itself will occur within a high dose gradient, substantially affecting the projected dose. Thus, the physician can predict ahead of treatment initiation that daily unplanned organ-at-risk constraint violations will occur unless online-adaptive planning is used.
Additionally, the dosimetric thresholds to determine whether the original non-adapted plan or the daily adaptive plan should be delivered on a given treatment day should also be pre-determined based on specific criteria. This is due to the need to minimize on-table treatment time for the patient as well as to optimize resource allocation, like machine time and physician/physicist efforts, within the clinic. In one original prospective implementation of online ART1, patients were evaluated for adaptation on a pre-planned daily basis based on pre-specified strict organ-at-risk constraints. If the initial treatment plan violated OAR constraints when applied to the daily anatomy, adaptive planning was performed. Non-adaptive and adaptive plans were then compared by dose-volume histogram analysis to evaluate OAR sparing and target coverage, strictly prioritizing the sparing of organs-at-risk. Target coverage was sacrificed as needed to meet constraints, but could also be increased at subsequent fractions if favorable daily OAR anatomy permitted dose increase to the target.1 While the clinical threshold to adapt can only be determined by the treating physician and is likely to vary by treatment site and type of treatment course, criteria for online adaptation should be similarly pre-specified to minimize treatment time and resource demands and to avoid indecision at the time of treatment delivery. This need for pre-specification of adaptation thresholds and evaluation criteria applies equally to plan-of-the-day and plan library approaches.
Replanning
The replanning process for any approach starts with imaging. This step may be implemented in several ways: 1) the acquisition of a new image just prior to on-table replanning; 2) the transferring of on-board images that originally indicated the need for a replan to the treatment planning system; 3) a re-simulation CT or MRI; 4) acquisition of additional diagnostic imaging. For online adaptation, the first option is used. For offline ART, several of these means may need to be utilized to obtain necessary information for adaptation, depending on the clinical scenario, and whether anatomical or functional ART are employed.
The next step is image registration and contour propagation. For online ART, the original image of the baseline (initial simulation) plan is fused either rigidly or deformably to the image of the day during the patient positioning process. In the current implementation of the low-field MRI-guided radiotherapy system, the existing contours and underlying relative electron density are registered in the same way as the original image, after which their accuracy must be confirmed and may be edited manually for any discrepancies. The manual editing of these is an often time consuming step in the most common online ART workflow.1, 49 It is also the step most prone to error.25
While it is possible to transfer the image of the day to a separate treatment planning system for purposes of replanning, it is most advantageous in terms of both time and quality assurance (to reduce the number of possible failure modes) to have an integrated system for online ART. For offline ART, any standard treatment planning process is acceptable for the creation of the new plan, as the patient is not waiting on-table during the re-planning process. Similarly, offline ART allows additional flexibility for the dosimetrist to take more time to create the best possible plan by varying optimization objectives and other parameters, as there is the time and flexibility to perform standard quality assurance and process control on any updated offline ART plan. In contrast, a lean workflow approach is favored for online ART, in which the attending physicist (typically) re-optimizes the plan and the only major changes are the contours and relative electron densities.
One of the ongoing challenges (see below) of both offline and online ART is the calculation of dose accumulation to ascertain the actual total delivered dose, accounting for both anatomic and dosimetric changes. It is especially challenging in online ART, where a new plan may be created for every single fraction of a patient's treatment (so far reported up to 15).28 The challenge arises from the fact that, especially for the malleable GI tract, it is (currently) impossible to reliably identify and track correspondences of point volumes of tissue (such as a constraint volume of 0.5cc) between treatment days. Such accounting is comparatively straightforward in more immobile tissues like the spinal cord, but most OARs remain inadequately tracked to permit day-by-day dose accumulation using current technology. Therefore, a common and conservative method is to use a combination of an isotoxicity and ‘parameter adding’50 approach. In parameter adding, the maximum point dose to an OAR is summed over all treatment days, rather than trying to estimate the location of this maximum point dose region between days. In some scenarios, dose accumulation for the gross tumor volume alone can be estimated, if the contour for the gross tumor volume has not been altered during the course of therapy.1
In the conservative isotoxicity approach, each new daily plan is evaluated de novo, as if it were to be delivered for all the fractions past and present. In other words, no consideration is given to the previously delivered dose in the current treatment fraction. OAR constraints are strict and are not violated. In this manner, even if it is the same portion of an OAR that receives the constraint dose each day, the cumulative dose in this “worse case” scenario will still not exceed the aggregate dose constraint50. This approach does require the physician to also consider the minimum acceptable PTV coverage, as the proximity of OARs on a given day may reduce PTV coverage substantially in order to maintain OAR constraints.
Quality Assurance
The quality assurance process for a plan created in the offline setting follows the same steps as any standard new plan, including thorough plan review and a measurement-based test of the dose to be delivered, albeit usually on a compressed timescale. In online ART, the patient must stay on-table in the treatment position. Therefore, pre-treatment, measurement-based patient-specific quality assurance (e.g., delivery of the plan onto a phantom) is not practical. However, given that the majority of errors in a radiotherapy process are not those caught during patient-specific measurement-based quality assurance51, an independent dose calculation method may be used instead of this step.52 In fact, if the treatment planning system and treatment device are integrated and one has carefully and comprehensively commissioned the treatment planning system and treatment device, the accuracy of predicted dose delivery is not the most important safety concern when considering the online ART process. Instead, fidelity and reliability of the pre-treatment image, recontouring sufficient to capture the new positions of the OARs and/or target, and accurate representation of the relative electron density are the fundamental concerns for a safe, robust online ART replan. Therefore, a comprehensive quality assurance program for online ART would benefit most from a well-trained, well-prepared team, as well as automation of checks (via either built-in or standalone software) for potential errors in contouring and plan parameters.49, 52
Another aspect of quality assurance for all three modes addressed in this issue (offline, online, and realtime) is the verification that the individual machine components performed as expected. For both offline and online ART, this may be done by analyzing the machine delivery log files after each treatment53, including after the patient-specific phantom-based delivery for offline. It is also good practice to establish a correlation between the independent dosimetric quality assurance for online ART and the phantom-based delivery by performing additional quality assurance after each online-adapted fraction for the first set of patients to build confidence in the process. For realtime ART, where the radiotherapy delivery system must respond to changes in real-time, the machine log analysis should also be done in real time, and preferably combined with another method of near-instantaneous feedback (e.g., via portal dosimetry and exit dose analysis). Such realtime methods may also be useful for both offline and online ART deliveries (as well as standard treatments).
Delivery
The delivery of a new plan created offline does not differ from standard processes, unless the physician chooses to change the method of delivery at the time of replanning. Intra-fraction motion is similarly not a unique concern, with management following standard processes. In contrast, for an online adapted plan, intra-fraction motion management considerations can be of increased importance if smaller target margins or planning risk volume margins are used. For example, if a breath hold approach is used to acquire a quality volumetric image to be used for online-replanning, and that image is used to delineate OARs that move with respiration, the accuracy of the dose projected on the breath-hold replanning image cannot be guaranteed unless the treatment is delivered with motion management to replicate the planning image position. Although this is similarly true for standard processes, ignoring these issues can have a more detrimental impact when coupled with the tighter margins often employed in ART. Lack of motion management in this scenario can result in both target underdosing and potential OAR overdosing, particularly if sharp dose gradients are used, such as with stereotactic methods. Therefore, care must be taken to select a motion management approach that enables delivery only when the patient’s anatomy is in the same position as it was during the re-planning process. An example of this may be treating with breath-hold32, 54, where a patient is asked to hold their breath in the same position as during volumetric image acquisition for repeated short periods. Another example may be to use an exhale breath hold setup, and subsequently gate the delivery using either fiducial tracking or realtime MRI guidance to beam-on only when the patient’s breathing is at exhale.55
The last step of either adaptive process is to ensure delivery was performed as intended by the physician. For an online plan, it is beneficial for the efficiency of workflow and a full understanding of the delivered dose to document for every fraction the reason why adaptation was necessary, what structures had to be recontoured and/or recreated, if any significant changes to the relative electron density had to be accounted for, the passing rate of the independent dose calculation, the starting plan for the next fraction, and any other such relevant parameters. This information allows all team members, whether or not they attended a given fraction of the patient's treatment, to quickly have the necessary information for efficient and safe delivery of any subsequent fraction. Similar ‘hand-off procedures may be beneficial for offline ART, where different care team members may interact with the patient, patient’s chart, and patient’s plan at different stages of the adaptive process. Clear documentation of the indication for adaptive replanning, information collected (e.g., imaging), changes made, and reasons for such changes can help alleviate communication issues and prevent errors.
Open Questions and Future Directions
Although both offline and online ART are in routine clinical use, a number of open questions remain to be addressed for each. One very active area of research involves improving ART assessment methods and tools through enhanced decision support. Moving towards more quantitative, automated or assisted methods to decide how and when to adapt is crucial to reduce variability and ensure ART is implemented consistently and correctly as it expands beyond high-volume centers. Typically, this has involved the use of deformable image registration to map delivered or estimated daily dose from in-room imaging to a reference anatomy (such as a baseline planning CT) where these daily doses are then accumulated to estimate cumulative, delivered dose.22, 23 High-quality commercial tools are available to enable deformable registration of single and multi-modality imaging, which are required for cumulative dose generation. However, key questions remain in this process such as how to manage cumulative dose in regressing or growing tumors56–59 and development of quality assurance processes for deformable dose mapping. Critically, the use of cumulative dose to guide ART assumes that cumulative dose is a better predictor of outcome than either daily dose or the originally-planned dose, but this question remains relatively unanswered to date24.
Other means to support assessment and ART decision making are emerging from the groundswell of machine learning and deep learning algorithms entering many fields related to image analysis, including radiotherapy. Such developing approaches produce a model to predict when to adapt, based on learning optimal time points to adapt with supervised data (though, for example, physician determination of whether a fraction should be adapted or not)60, 61. Deep learning has recently shown very promising results in autosegmentation of organs at risk and even targets, which if clinically deployed could help improve the efficiency of ART.
Motion during treatment remains a key challenge for online ART, both in achieving high-quality in-room imaging and for ensuring adapted plans are relevant to the anatomy during delivery. Four-dimensional imaging solutions for in-room cone beam CT62–65 and MRI66 continue to evolve to improve image quality in motion-influenced sites, which is critical to move ART from clearly-defined tumors to more challenging stages of disease and sites where it may be critically needed, such as locally-advanced lung cancer.2, 67 Merging advances in realtime ART, such as motion tracking, with efficient pre-treatment online ART tools may help take advantage of the merits of both approaches for managing anatomical changes on multiple timescales.68
Adaptation with functional imaging has traditionally been performed with offline ART.11, 69 However, with the advent of MRI-guided radiotherapy and the availability of in-room MRI, functional MRI-guided online ART becomes feasible. Relatedly, a system to enable online ART with PET is in commercial development currently. To fully enable functional online ART, tools for interpretation, target identification, and assessment will need to be developed to establish how to adapt to both functional and anatomical change simultaneously online.
Finally, although both offline and online ART are in routine clinical use, evidence of the efficacy of these techniques is crucial to help guide more widespread deployment and justify the additional resources and tools required for these techniques. Several phase I and single-arm, phase II studies of anatomical or functional ART have been conducted in bladder cancer70, abdominal malignancies1, liver tumors10, and non-small cell lung cancer11, among others. However, phase III randomized clinical trials to compare ART to standard radiotherapy are ongoing, including RTOG-1106 (locally-advanced non-small cell lung cancer, offline ART with FDG-PET/CT) in the US and ARTFORCE (locally-advanced head and neck cancer, offline ART with FDG-PET) in Europe.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henke L, Kashani R, Robinson C, et al. , Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen, Radiother. Oncol. 126(3), 519–526 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Sonke J-J and Belderbos J, Adaptive radiotherapy for lung cancer, Semin. Radiat. Oncol. 20(2), 94–106 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Lim-Reinders S, Keller BM, Al-Ward S, Sahgal A, and Kim A, Online Adaptive Radiation Therapy, Int. J. Radiat. Oncol. Biol. Phys. 99(4), 994–1003 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann L, Alber M, Jensen MF, Holt MI, and Møller DS, Adaptation is mandatory for intensity modulated proton therapy of advanced lung cancer to ensure target coverage, Radiother. Oncol. 122(3), 400–405 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Møller DS, Holt MI, Alber M, et al. , Adaptive radiotherapy for advanced lung cancer ensures target coverage and decreases lung dose, Radiother. Oncol. 121(1), 32–38 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Chen AM, Daly ME, Cui J, Mathai M, Benedict S, and Purdy JA, Clinical outcomes among patients with head and neck cancer treated by intensity-modulated radiotherapy with and without adaptive replanning, Head Neck 36(11), 1541–1546 (2014). [DOI] [PubMed] [Google Scholar]
- 7.van Elmpt W, De Ruysscher D, van der Salm A, et al. , The PET-boost randomised phase II dose-escalation trial in non-small cell lung cancer, Radiother. Oncol. (2012). [DOI] [PubMed] [Google Scholar]
- 8.Schwartz DL, Garden AS, Thomas J, et al. , Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial., Int. J. Radiat. Oncol. Biol. Phys. 83(3), 986–93 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grootjans W, de Geus-Oei L-F, Troost EGC, Visser EP, Oyen WJG, and Bussink J, PET in the management of locally advanced and metastatic NSCLC., Nat. Rev. Clin. Oncol. 12(7), 395–407 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Feng M, Suresh K, Schipper MJ, et al. , Individualized Adaptive Stereotactic Body Radiotherapy for Liver Tumors in Patients at High Risk for Liver Damage, JAMA Oncol. 4(1), 40 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong F-M, Ten Haken RK, Schipper M, et al. , Effect of Midtreatment PET/CT-Adapted Radiation Therapy With Concurrent Chemotherapy in Patients With Locally Advanced Non–Small-Cell Lung Cancer, JAMA Oncol. 3(10), 1358 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan D, Vicini F, Wong J, and Martinez A, Adaptive radiation therapy, Phys. Med. Biol. 42(1), 123–132 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Schreibmann E, Li T, Wang C, and Xing L, Evaluation of on-board kV cone beam CT (CBCT)-based dose calculation, Phys. Med. Biol. 52(3), 685–705 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Nijkamp J, Pos FJ, Nuver TT, et al. , Adaptive radiotherapy for prostate cancer using kilovoltage cone-beam computed tomography: first clinical results, Int J Radiat Oncol Biol Phys 70(1), 75–82 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Wu Q, Liang J, and Yan D, Application of dose compensation in image-guided radiotherapy of prostate cancer, Phys. Med. Biol. 51(6), 1405–1419 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Acharya S, Fischer-Valuck BW, Kashani R, et al. , Online Magnetic Resonance Image Guided Adaptive Radiation Therapy: First Clinical Applications, Int. J. Radiat. Oncol. Biol. Phys. 94(2), 394–403 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Morin O, Aubin M, Bucci MK, Chuang CF, and Pouliot J, Dose-guided radiation therapy with megavoltage cone-beam CT, Br. J. Radiol. 79 Spec No, S87––––98 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Court LE, Dong L, Lee AK, et al. , An automatic CT-guided adaptive radiation therapy technique by online modification of multileaf collimator leaf positions for prostate cancer, Int. J. Radiat. Oncol. Biol. Phys. 62(1), 154–163 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Kwint M, Conijn S, Schaake E, et al. , Intra thoracic anatomical changes in lung cancer patients during the course of radiotherapy., Radiother. Oncol. 113(3), 392–7 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Berkovic P, Paelinck L, Lievens Y, et al. , Adaptive radiotherapy for locally advanced non-small cell lung cancer, can we predict when and for whom?, Acta Oncol. 54(9), 1438–44 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Yan D, Jaffray DA, and Wong JW, A model to accumulate fractionated dose in a deforming organ, Int J Radiat Oncol Biol Phys 44(3), 665–675 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Dial C, Weiss E, Siebers JV, and Hugo GD, Benefits of adaptive radiation therapy in lung cancer as a function of replanning frequency, Med. Phys. 43(4), 1787–1794 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim K, Stewart J, Kelly V, et al. , Dosimetrically Triggered Adaptive Intensity Modulated Radiation Therapy for Cervical Cancer, Int. J. Radiat. Oncol. Biol. Phys. 90(1), 147–154 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Jaffray DA, Lindsay PE, Brock KK, Deasy JO, and Tomé WA, Accurate Accumulation of Dose for Improved Understanding of Radiation Effects in Normal Tissue, Int. J. Radiat. Oncol. Biol. Phys. 76(3), S135–S139 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noel CE, Santanam L, Parikh PJ, and Mutic S, Process-based quality management for clinical implementation of adaptive radiotherapy, Med. Phys. 41(8Part1), 081717 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kipritidis J, Hugo G, Weiss E, Williamson J, and Keall PJ, Measuring interfraction and intrafraction lung function changes during radiation therapy using four-dimensional cone beam CT ventilation imaging, Med. Phys. 42(3), 1255–1267 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de la Zerda A, Armbruster B, and Xing L, Formulating adaptive radiation therapy (ART) treatment planning into a closed-loop control framework, Phys. Med. Biol. 52(14), 4137–4153 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Henke LE, Contreras JA, Green OL, et al. , Magnetic Resonance Image-Guided Radiotherapy (MRIgRT): A 4.5-Year Clinical Experience, Clin. Oncol. 30(11), 720–727 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonke J-JJ, Lebesque J, and van Herk M, Variability of four-dimensional computed tomography patient models, Int J Radiat Oncol Biol Phys 70(2), 590–598 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Hugo G, Vargas C, Liang J, Kestin L, Wong JW, and Yan D, Changes in the respiratory pattern during radiotherapy for cancer in the lung., Radiother. Oncol. 78(3), 326–31 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Balik S, Weiss E, Jan N, et al. , Evaluation of 4-dimensional computed tomography to 4-dimensional cone-beam computed tomography deformable image registration for lung cancer adaptive radiation therapy., Int. J. Radiat. Oncol. Biol. Phys. 86(2), 372–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glide-Hurst CK, Gopan E, and Hugo GD, Anatomic and pathologic variability during radiotherapy for a hybrid active breath-hold gating technique, Int J Radiat Oncol Biol Phys 77(3), 910–917 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nijkamp J, de Jong R, Sonke J-J, Remeijer P, van Vliet C, and Marijnen C, Target volume shape variation during hypo-fractionated preoperative irradiation of rectal cancer patients, Radiother. Oncol. 92(2), 202–209 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Woodford C, Yartsev S, Dar AR, Bauman G, and Van Dyk J, Adaptive radiotherapy planning on decreasing gross tumor volumes as seen on megavoltage computed tomography images, Int J Radiat Oncol Biol Phys 69(4), 1316–1322 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Kupelian PA, Ramsey C, Meeks SL, et al. , Serial megavoltage CT imaging during external beam radiotherapy for non-small-cell lung cancer: Observations on tumor regression during treatment, Int. J. Radiat. Oncol. Biol. Phys. 63(4), 1024–1028 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Potter R, Kirisits C, Fidarova EF, et al. , Present status and future of high-precision image guided adaptive brachytherapy for cervix carcinoma, Acta Oncol. (Madr). 47(7), 1325–1336 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Mencarelli A, van Kranen SR, Hamming-Vrieze O, et al. , Deformable Image Registration for Adaptive Radiation Therapy of Head and Neck Cancer: Accuracy and Precision in the Presence of Tumor Changes, Int. J. Radiat. Oncol. Biol. Phys. 90(3), 1–8 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Lagendijk JJW, Raaymakers BW, and van Vulpen M, The Magnetic Resonance Imaging–Linac System, Semin. Radiat. Oncol. 24(3), 207–209 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Mutic S and Dempsey JF, The ViewRay System: Magnetic Resonance–Guided and Controlled Radiotherapy, Semin. Radiat. Oncol. 24(3), 196–199 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Hafeez S, Warren-Oseni K, McNair HA, et al. , Prospective Study Delivering Simultaneous Integrated High-dose Tumor Boost (≤70 Gy) with Image Guided Adaptive Radiation Therapy for Radical Treatment of Localized Muscle-Invasive Bladder Cancer, Int. J. Radiat. Oncol. Biol. Phys. 94(5), 1022–1030 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Murthy V, Masodkar R, Kalyani N, et al. , Clinical outcomes with dose-escalated adaptive radiation therapy for urinary bladder cancer: A prospective study, Int. J. Radiat. Oncol. Biol. Phys. 94(1), 60–66 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Vestergaard A, Muren LP, Lindberg H, et al. , Normal tissue sparing in a phase II trial on daily adaptive plan selection in radiotherapy for urinary bladder cancer, Acta Oncol. (Madr). 53(8), 997–1004 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Heijkoop ST, Langerak TR, Quint S, et al. , Clinical implementation of an online adaptive plan-of-the-day protocol for nonrigid motion management in locally advanced cervical cancer IMRT, Int. J. Radiat. Oncol. Biol. Phys. 90(3), 673–679 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Tyran M, Jiang N, Cao M, et al. , Retrospective evaluation of decision-making for pancreatic stereotactic MR-guided adaptive radiotherapy, Radiother. Oncol. (2018). [DOI] [PubMed] [Google Scholar]
- 45.Bohoudi O, Bruynzeel AME, Senan S, et al. , Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer, Radiother. Oncol. 125(3), 439–444 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Olberg S, Green O, Cai B, et al. , Optimization of treatment planning workflow and tumor coverage during daily adaptive magnetic resonance image guided radiation therapy (MR-IGRT) of pancreatic cancer, Radiat. Oncol. 13(1), 51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noel CE, Parikh PJ, Spencer CR, et al. , Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy, Acta Oncol. (Madr). 54(9), 1474–1482 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Veiga C, Janssens G, Teng C-L, et al. , First Clinical Investigation of Cone Beam Computed Tomography and Deformable Registration for Adaptive Proton Therapy for Lung Cancer, Int. J. Radiat. Oncol. Biol. Phys. 95(1), 549–559 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Lamb J, Cao M, Kishan A, et al. , Online Adaptive Radiation Therapy: Implementation of a New Process of Care, Cureus 9(8), e1618 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teo A-K, Bonner Millar LP, Ding X, and Lin LL, Assessment of cumulative external beam and intracavitary brachytherapy organ doses in gynecologic cancers using deformable dose summation., Radiother. Oncol. 115(2), 195–202 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Ford EC, Terezakis S, Souranis A, Harris K, Gay H, and Mutic S, Quality control quantification (QCQ): A tool to measure the value of quality control checks in radiation oncology, Int. J. Radiat. Oncol. Biol. Phys. 84(3), e263–e269 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Cai B, Green OL, Kashani R, Rodriguez VL, Mutic S, and Yang D, A practical implementation of physics quality assurance for photon adaptive radiotherapy, Z. Med. Phys. 28(3), 211–223 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Litzenberg DW, Moran JM, and Fraass BA, Verification of dynamic and segmental IMRT delivery by dynamic log file analysis., J. Appl. Clin. Med. Phys. 3(2), 63–72 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tetar S, Lagerwaard F, Palacios M, et al. , Magnetic Resonance Imaging-Guided Delivery of Lung Stereotactic Radiotherapy Using Patient-Controlled Visual Guidance, J. Thorac. Oncol. 12(1), S420–S421 (2017). [Google Scholar]
- 55.Green OL, Rankine LJ, Cai B, et al. , First clinical implementation of real-time, real anatomy tracking and radiation beam control, Med. Phys. 45(8), 3728–3740 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Zhong H and Chetty IJ, Caution Must Be Exercised When Performing Deformable Dose Accumulation for Tumors Undergoing Mass Changes During Fractionated Radiation Therapy, Int. J. Radiat. Oncol. Biol. Phys. 97(1), 182–183 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Hugo GD, Dial C, and V Siebers J, In Regard to Zhong and Chetty, Int. J. Radiat. Oncol. Biol. Phys. 99(5), 1308–1310 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Zhong H and Chetty IJ, Adaptive radiotherapy for NSCLC patients: utilizing the principle of energy conservation to evaluate dose mapping operations, Phys. Med. Biol. 62(11), 4333–4345 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dial CW, Adaptive radiation therapy for lung cancer (Virginia Commonwealth University, 2014). [Google Scholar]
- 60.van den Bosch M, Öllers M, Reymen B, and van Elmpt W, Automatic selection of lung cancer patients for adaptive radiotherapy using cone-beam CT imaging, Phys. Imaging Radiat. Oncol. 1, 21–27 (2017). [Google Scholar]
- 61.Guidi G, Maffei N, Meduri B, et al. , A machine learning tool for re-planning and adaptive RT: A multicenter cohort investigation, Phys. Medica 32(12), 1659–1666 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Riblett MJ, Christensen GE, Weiss E, and Hugo GD, Data-Driven Respiratory Motion Compensation for Four-Dimensional Cone-Beam Computed Tomography (4D-CBCT) Using Groupwise Deformable Registration., Med. Phys. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li T, Xing L, Munro P, et al. , Four-dimensional cone-beam computed tomography using an on-board imager, Med. Phys. 33(10), 3825–3833 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Rit S, Wolthaus JWH, van Herk M, and Sonke J-J, On-the-fly motion-compensated cone-beam CT using an a priori model of the respiratory motion, Med. Phys. 36(6), 2283–2296 (2009). [DOI] [PubMed] [Google Scholar]
- 65.Sonke JJ, Zijp L, Remeijer P, and van Herk M, Respiratory correlated cone beam CT, Med. Phys. 32(4), 1176–1186 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Cai J, Chang Z, Wang Z, Paul Segars W, and Yin F-F, Four-dimensional magnetic resonance imaging (4D-MRI) using image-based respiratory surrogate: A feasibility study, Med. Phys. 38(12), 6384 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guy CL, Weiss E, Christensen GE, Jan N, and Hugo GD, CALIPER: A deformable image registration algorithm for large geometric changes during radiotherapy for locally advanced non-small cell lung cancer, Med. Phys. 45(6), 2498–2508 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ge Y, O’Brien RT, Shieh CC, Booth JT, and Keall PJ, Toward the development of intrafraction tumor deformation tracking using a dynamic multi-leaf collimator, Med. Phys. 41(6), 061703 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kong FP, Ten Haken RK, Schipper M, et al. , A Phase 2 Trial of Midtreatment FDG-PET Adaptive, Individualized Radiation Therapy Plus Concurrent Chemotherapy in Patients With Non-Small Cell Lung Cancer (NSCLC), Int. J. Radiat. Oncol. Biol. Phys. 87(2), S76 (2013). [Google Scholar]
- 70.Huddart R, Henry A, Staffurth J, et al. , OC-0058: Clinical outcomes of the first rct of adaptive radiotherapy in bladder cancer (HYBRID CRUK/12/055), Radiother. Oncol. 127, S25–S26 (2018). [Google Scholar]