Abstract
BACKGROUND
The United States is undergoing a crippling opioid epidemic, spurred in part by overuse of prescription opioids by adults 25 to 64 years of age. Of concern are long-duration and high-dose initial prescriptions, which place the patients and their friends and relatives at heightened risk for long-term opioid use, misuse, overdose, and death.
METHODS
We estimated the incidence of initial opioid prescriptions in each month between July 2012 and December 2017 using administrative-claims data from across the United States (accessed through Blue Cross–Blue Shield [BCBS] Axis); monthly incidence was estimated as the percentage of enrollees who received an initial opioid prescription among those who had not used opioids (i.e., no opioid pre-scription or a diagnosis of opioid use disorder in the 6 months before a given month). We then estimated the percentage of enrollees initiating opioid therapy who received a long-duration or high-dose initial opioid prescription in each month during this period. We also calculated the number of providers who initiated opioid therapy in any patient who had not used opioids in each month and examined monthly trends in the duration and dose of initial opioid prescriptions in prescriber and patient subgroups. Our study sample included 63,817,512 enrollees who had not used opioids (mean, 15,897,673 per month).
RESULTS
The monthly incidence of initial opioid prescriptions among enrollees who had not used opioids declined by 54%, from 1.63% in July 2012 to 0.75% in December 2017. This decline was accompanied by a decreasing number of providers (from 114,043 in July 2012 to 80,462 in December 2017) who initiated opioid therapy in any patient who had not used opioids. Nonetheless, among the shrinking sub-group of physicians who initiated opioid therapy in such patients, high-risk prescribing (i.e., prescriptions for more than a 3-day supply or for a dose of 50 morphine milligram equivalents per day or higher) persisted at a monthly rate of 115,378 prescriptions per 15,897,673 enrollees who had not used opioids.
CONCLUSIONS
As the opioid crisis progressed between July 2012 and December 2017, many providers stopped initiating opioid therapy. Although the number of initial opioid prescriptions declined, a subgroup of providers continued to write high-risk initial opioid prescriptions. (Funded by the National Institute on Aging and a gift from Owen and Linda Robinson.)
The United States is gripped by a crippling opioid epidemic. In 2016, more than 2 million Americans had an opioid use disorder, and overdoses claimed more than 115 lives daily.1 Most who died were adults 25 to 64 years of age.2 Although the exact cause of the crisis is not known with certainty, one probable contributing factor is an increasing reliance on prescription opioids for pain management.3,4 Although recent increases in mortality from opioid overdose have been driven by illicit drug use,5 the majority of current heroin users began with prescription opioids.6 Initial opioid prescriptions given to patients who have not used opioids are important drivers of the epidemic: longer-duration and higher-dose initial opioid prescriptions are associated with a higher likelihood of long-term opioid use, misuse, and overdose.7–13 Even prescription durations as short as 6 days are associated with a substantially increased risk of longer-term opioid use.10 Perhaps more consequential is that the greater availability of opioids that are intended for medical use has facilitated misuse by persons obtaining unused pills from friends and relatives,14 which in turn has been associated with the use of illicit opioids.15
Recognizing that minimizing initial exposure to prescription opioids helps prevent long-term opioid use, the Centers for Disease Control and Prevention (CDC) issued draft prescribing guidelines in December 2015 and final guidelines in March 2016 that emphasized limiting the use, duration, and dose of opioids for first-time users.16 Specifically, the guidelines called on medical providers to forgo opioid therapy when the benefits are unlikely to outweigh the risks or, otherwise, to give the lowest effective dose for the shortest therapeutic duration — a 3-day supply is typically sufficient, and more than a 7-day supply is rarely needed for nonsurgical, nontraumatic acute pain. The CDC also advised caution when increasing doses above 50 morphine milligram equivalents (MME) per day (equivalent to 10 tablets of 5-mg hydrocodone per day) and avoidance of doses higher than 90 MME per day for chronic pain.
Despite this emphasis by the CDC, little is known about recent trends in initial opioid prescriptions in the United States, particularly among commercially insured patients, who are the large majority of adults 18 to 64 years of age — the age group most affected by the opioid crisis. Previous research has focused on general opioid prescribing by physicians.17–27 The limited evidence regarding initial opioid prescriptions has come from subpopulations, such as Medicare beneficiaries,13,28 certain geographic regions,7,9 specific sites of care,13,28 and patients with specific medical indications.8,12 We provide a national portrait of monthly trends in initial opioid prescriptions among commercially insured patients, overall and for subgroups of providers and patients, from July 2012 through December 2017, a period that includes the release of the CDC prescribing guidelines.
METHODS
DATA SOURCES AND STUDY SAMPLES
Using the secure data portal, we accessed a limited data set of administrative claims from Blue Cross–Blue Shield (BCBS) Axis (BCBS Association), the largest source of commercial insurance claims in the United States. Our overall study sample included 86,258,528 enrollees (mean, 24,277,964 per month) who were 15 years of age or older, resided in the United States, and had primary medical and prescription drug coverage in any month between July 2012 and December 2017; a total of 19,911,193 of these enrollees received at least one opioid prescription during the study period. We further identified 63,817,512 enrollees (mean, 15,897,673 per month) who had not used opioids (defined as those who had been continuously enrolled for at least 6 months and had no recorded opioid prescription or diagnosis of opioid use disorder in the 6 months before a given month between July 2012 and December 2017); a total of 10,893,788 of these enrollees received their first opioid prescription during the study period. When we analyzed trends in duration and dose, we further excluded prescription records that were missing duration or dose information, had a duration greater than 365 days for a single prescription, or had a duration or dose that was entered as zero. After applying these restrictions, there were 10,874,869 initial opioid prescriptions.
As compared with the U.S. privately insured population, the population of all eligible enrollees in BCBS Axis had the same sex distribution, but the data we used included fewer who were 65 years of age or older because we purposefully omitted persons with secondary commercial insurance coverage (Table S1 in Supplementary Appendix, available with the full text of this article at NEJM.org). The BCBS Axis population also included more enrollees who resided in the South and fewer who resided in the West, a reflection of its geographic coverage. The characteristics of the sample of enrollees who had not used opioids were similar to those of all eligible enrollees. Enrollees who received an opioid prescription (new or continued) were more likely to be female and between 45 and 64 years of age.
We obtained information on provider specialty from the National Plan and Provider Enumeration System. Drug descriptions were obtained from the Food and Drug Administration (FDA), the National Center for Injury Prevention and Control (NCIPC), and the MarketScan 2016 Red Book and Red Book Online (as of August 8, 2018) (Truven Health Analytics).
KEY VARIABLE DEFINITIONS
We determined that a drug was an opioid if the National Drug Code on the prescription claim was identified as an opioid by the FDA29 or the NCIPC30 or in the MarketScan 2016 Red Book or Red Book Online. We excluded cough syrups and injectable opioids typically given during inpatient stays. When computing the incidence of initial opioid prescriptions (as opposed to the prevalence of any opioid use), we further excluded opioids that are used primarily for the treatment of opioid use disorder (i.e., buprenorphine, levomethadyl, and methadone) and their combinations. We identified 89 million opioid prescriptions during our study period, of which nearly 11 million were new prescriptions.
The duration of an opioid prescription was expressed as the number of days of supply, as obtained from the claim record. The strength of a prescription was expressed as the MME-per-day dose (computed as the product of drug strength per dose unit, number of units per day, and an MME conversion factor) to standardize potency across different opioid substances or dose forms (e.g., tablet or patch). We obtained information on drug strength per dose unit from the FDA, the National Center for Injury Prevention and Control, and the Red Book directories. The number of units per day was calculated by dividing the total number of units by the number of days of supply. MME conversion factors were obtained from the Centers for Medicare and Medicaid Services, the CDC, the NCIPC, and selected literature (see the Supplementary Appendix).27,30–32
We identified the prescriber from the National Provider Identifier (NPI) listed on the pharmacy claim and used the NPI taxonomy file in the National Plan and Provider Enumeration System to classify prescribers as primary care physicians, specialists, dentists, and nonphysician practitioners. When the NPI referred to an organization rather than a person, we classified the prescriber as a provider organization. Finally, we classified the prescriber as unknown when the NPI was either missing, contained the pharmacy NPI rather than the prescriber NPI, or — in a small fraction of cases — was not in the National Plan and Provider Enumeration System.
Because prescription claims do not include diagnosis codes, we identified the medical indications for opioid therapy from the diagnosis and type of service codes associated with any office or hospital visit that occurred on the day of or the day before the date the prescription was filled. This time window was chosen to identify visits that were most likely to be related to the indication for the opioid prescription. Specifically, we assigned patients an indication of surgery-related pain if the patient had undergone surgery. If there was no surgery, we checked whether the patient had one or more diagnoses of an acute or chronic medical condition that typically results in pain severe enough to warrant treatment with prescription-strength analgesics (see the Supplementary Appendix).33 We assigned the prescription an indication of dental pain if the opioid was prescribed by a dentist; we accessed claims for prescriptions ordered by dentists but not dental procedure claims. If there was no recorded pain-related diagnosis, the indication was classified as not known to be pain related. If there was no same-day or day-before medical visit, the prescription was coded as having no concurrent medical claim.
STATISTICAL ANALYSIS
We estimated the monthly incidence of initial opioid prescriptions as the percentage of enrollees who received an opioid prescription in a given month among those who had not used opioids; we also calculated the number of providers who initiated opioid therapy in any patient who had not used opioids in each month. We estimated the monthly prevalence of opioid prescriptions as the percentage of all eligible enrollees who received any opioid prescription (new or continued) in a given month. We also estimated the mean duration (number of days of supply) and daily potency (MME-per-day dose) of the initial opioid prescription in each month, as well as the percentage of enrollees initiating opioid therapy who received a long-duration (>3-day and >7-day supply) or high-dose (≥50 and ≥90 MME per day) opioid prescription, as defined by the CDC in March 2016.
RESULTS
MONTHLY TRENDS IN PRESCRIPTION INCIDENCE
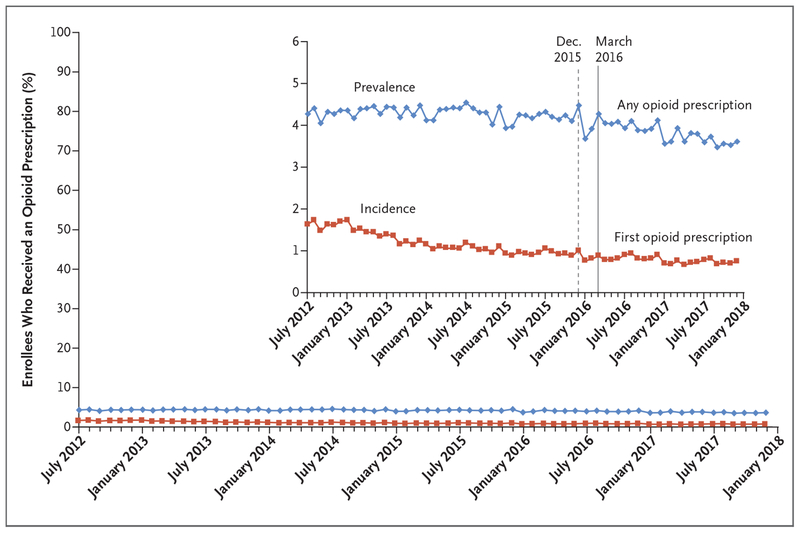
The monthly incidence of initial opioid prescriptions among enrollees who had not used opioids fell from 1.63% in July 2012 to 0.75% in December 2017 — a relative decline of 54% (Fig. 1). In comparison, the monthly prevalence of any opioid prescription held steady at approximately 4.3% between July 2012 and March 2016 (when the CDC issued opioid prescribing guidelines) and fell thereafter, reaching 3.6% in December 2017 — a relative decline of 16%.
Figure 1. Prevalence and Incidence of Opioid Prescriptions (July 2012–December 2017).
Prevalence was estimated as the percentage of enrollees who received any opioid prescription in each month during the study period (July 2012 through December 2017). The denominator was 86,258,528 enrollees (mean, 24,277,964 per month) who were 15 years of age or older, resided in the United States, and had primary medical and prescription coverage in any month. Incidence was estimated as the percentage of enrollees who received an initial opioid prescription in each month during the study period, among those who had not used opioids (defined as those who satisfied the above criteria and also had been continuously enrolled for at least 6 months and had no recorded opioid prescription or diagnosis of opioid use disorder during those 6 months). The denominator was 63,817,512 enrollees (mean, 15,897,673 per month), among whom 10,893,788 were identified as having received an initial opioid prescription during this period. The dashed and solid vertical lines represent when the Centers for Disease Control and Prevention (CDC) issued opioid-prescribing guidelines in draft form and final version, respectively. The inset shows the same data on an enlarged y axis.
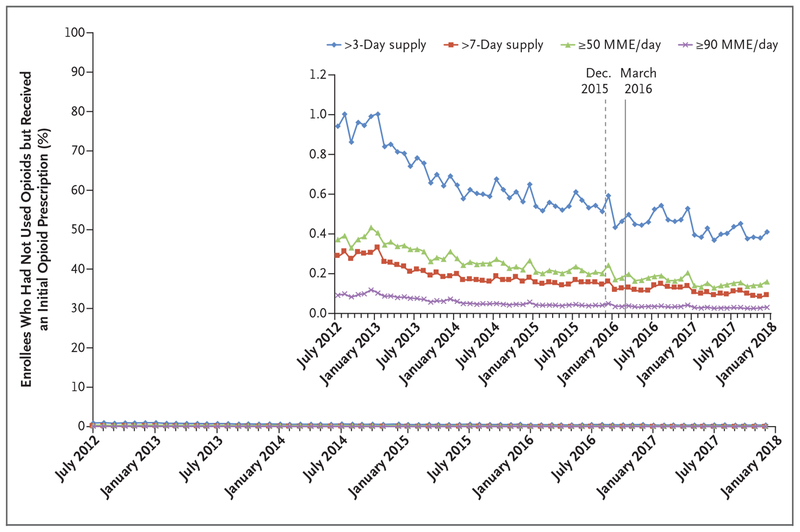
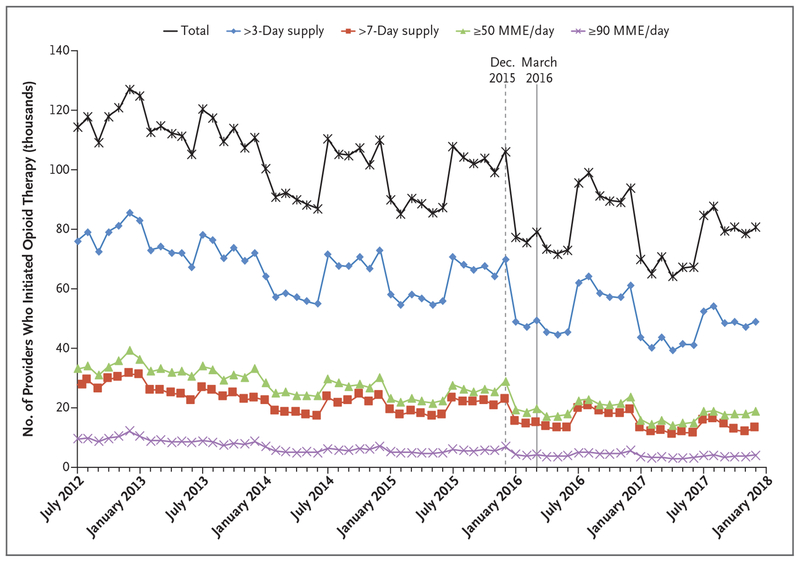
As overall incidence declined, the incidence of long-duration and high-dose initial opioid prescriptions also declined by similar percentages. The incidence of initial prescription durations of more than a 3-day supply fell by 57%, and initial prescription durations of more than a 7-day supply fell by 68%. The incidence of initial prescription doses of more than 50 MME per day and more than 90 MME per day declined by 57% and 67%, respectively (Fig. 2). These reductions were accompanied by a 29% reduction in the number of providers who initiated opioid therapy in any patient who had not used opioids, from 114,043 in July 2012 to 80,462 in December 2017 (Fig. 3). Notably, these downward trends began long before the release of the CDC guidelines in March 2016 (or even the draft guidelines in December 2015), indicating that the CDC guidelines were not the sole precipitating force behind the declines (Figs. 2 and 3).
Figure 2. Incidence of Long-Duration or High-Dose Initial Opioid Prescriptions (July 2012–December 2017).
Shown is the percentage of enrollees who received a long-duration (>3-day and >7-day supply) or high-dose (≥50 and ≥90 morphine milligram equivalents [MME] per day) initial opioid prescription in each month from July 2012 through December 2017. The denominator was 63,817,512 enrollees (mean, 15,897,673 per month) who had not used opioids, among whom 10,874,869 were identified as having received an initial opioid prescription during this period. The dashed and solid vertical lines represent when the CDC issued opioid-prescribing guidelines in draft form and final version, respectively. The inset shows the same data on an enlarged y axis.
Figure 3. Providers Who Initiated Opioid Therapy in Any Patient Who Had Not Used Opioids (July 2012–December 2017).
Shown are the numbers of providers who initiated opioid therapy and who initiated long-duration or high-dose initial opioid therapy in any patient who had not used opioids. The sample comprised 710,698 providers (mean, 95,449 per month) with identifiable National Provider Identifiers who wrote initial opioid prescriptions to patients who had not used opioids. The dashed and solid vertical lines represent when the CDC issued opioid-prescribing guidelines in draft form and final version, respectively.
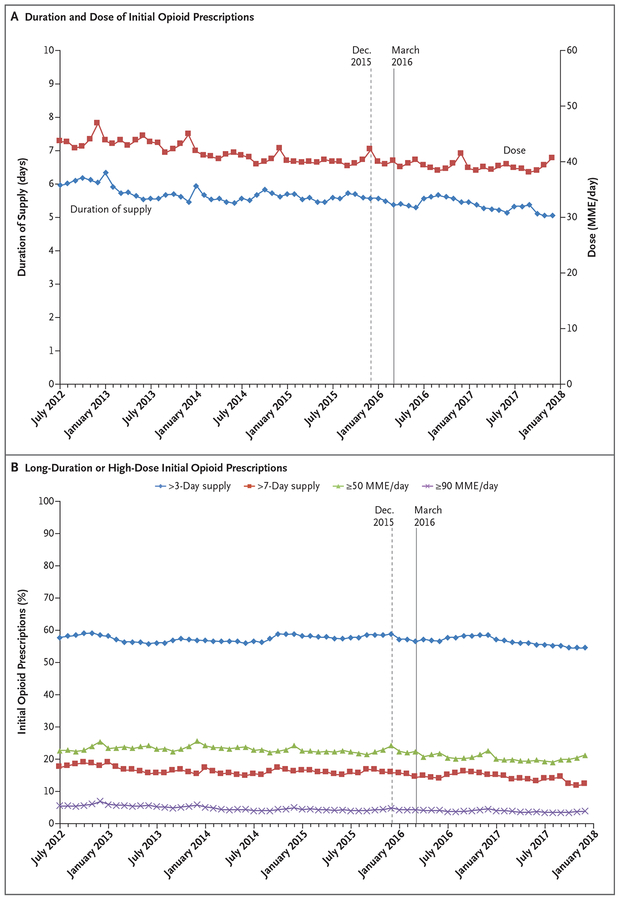
Although the incidence of initial prescriptions declined as fewer providers chose to initiate opioid therapy in any patient who had not used opioids, among the providers who initiated opioid therapy, the mean prescription duration increased from a 6.0-day supply in July 2012 to a 6.3-day supply by January 2013 and then declined to a 5.5-day supply by December 2013, where it approximately remained until declining to a 5.1-day supply by December 2017 (Fig. 4A). The mean daily potency declined slightly between July 2012 and April 2014 and remained at approximately 40 MME per day thereafter, with seasonal increases every December. Strikingly, the percentage of long-duration or high-dose initial prescriptions remained high over the period, even after the release of the CDC guidelines (Fig. 4B). Throughout the study period, approximately 57% of initial prescriptions were for more than a 3-day supply, which is greater than the amount recommended by the CDC to control acute pain, and nearly 16% exceeded a 7-day supply, an amount the CDC deemed rarely necessary. Although the CDC advised caution when increasing doses above 50 MME per day, the doses in more than 22% of initial prescriptions exceeded 50 MME per day. Nearly 5% of the doses exceeded 90 MME per day, which the CDC said should be rarely needed, if at all. These numbers suggest that from July 2012 through December 2017, high-risk initial opioid prescriptions (with a supply duration of >3 days or a dose of ≥50 MME per day) were issued at a mean monthly rate of 0.73%, or 115,378 prescriptions per 15,897,673 enrollees who had not used opioids.
Figure 4. Duration and Dose of Initial Opioid Prescriptions and Percentage of Long-Duration or High-Dose Initial Opioid Prescriptions (July 2012–December 2017).
The sample comprised 10,874,869 patients who had not used opioids but received an initial opioid prescription during the study period. The dashed and solid vertical lines represent when the CDC issued opioid-prescribing guidelines in draft form and final version, respectively.
MONTHLY TRENDS ACCORDING TO PROVIDER AND PATIENT SUBGROUPS
The overall decline in the monthly incidence of initial opioid prescriptions among enrollees who had not used opioids occurred across all prescriber specialties and medical indications, with seasonal increases in December among those who were prescribed opioids by specialists and those who were prescribed opioids for surgery-related pain. Throughout the study period, patients were more likely to receive an initial opioid prescription if they resided in the South than in other census regions and if they were female. Although incidence increased with age at the beginning of the study period, incidence rates have since converged. (Further details on trends in the monthly incidence of initial opioid prescriptions are provided in Figs. S1 and S2 in the Supplementary Appendix.)
Among the patients who initiated opioid therapy, there was a slight decline in the mean duration of the initial prescription in all provider and patient subgroups. Similarly, in all groups, the mean daily potency gradually declined, with seasonal increases in December among younger patients 15 to 24 years of age. None of the subgroups showed much change in the percentage of long-duration or high-dose initial prescriptions, even after the release of the CDC guidelines. Throughout the study period, primary care physicians were most likely of all provider types to issue long-duration prescriptions when they initiated opioid therapy — approximately 80% of their initial prescriptions exceeded a 3-day supply, and approximately 40% were for more than a 7-day supply. Dentists were the least likely to issue long-duration opioid prescriptions. Initial prescriptions for more than a 7-day supply were most common for the indication of chronic non-cancer pain. The percentage of long-duration prescriptions was highest among patients who resided in the South, increased with age, and was similar among male and female patients. High-dose initial prescriptions were most likely to be issued by specialists and were most common for indications of surgery-related pain and chronic cancer pain and among patients who resided in the West; the percentage of high-dose initial prescriptions did not differ according to sex or age. (Further details on trends in the duration and strength of initial opioid prescriptions are provided in Figs. S3 through S5 in the Supplementary Appendix.)
DISCUSSION
Initial opioid prescriptions are important drivers of the current opioid epidemic.7–13 Our study provides a snapshot of trends in the rate at which opioid therapy was initiated among commercially insured patients during a period of heightened attention to the dangers of opioids and the prominent release of prescribing guidelines by the CDC. We found that opioid therapy was initiated in fewer patients who had not used opioids in December 2017 than in July 2012, amounting to a 54% decline in the incidence of initial opioid prescriptions. These findings are consistent with recent statistics showing a gradual decrease in the total number of opioid prescriptions in the United States.22
As the incidence of initial opioid prescriptions fell, the numbers of both high-risk and low-risk opioid prescriptions declined proportionately. Analyses at the provider level revealed that the decline in incidence was accompanied by a 29% reduction in the number of providers who initiated opioid therapy in any patient who had not used opioids. This finding suggests that large numbers of providers have responded to the opioid crisis by ceasing to prescribe opioids to patients who had not used opioids, rather than prescribing opioids when indicated but at safer doses and durations. At the same time, an important subgroup of providers continued to issue long-duration or high-dose initial opioid prescriptions even after the CDC guidelines were issued in March 2016.
On average, high-risk initial opioid prescriptions (with a supply duration of >3 days or a dose of ≥50 MME per day) were issued at a monthly rate of 115,378 prescriptions per 15,897,673 patients who had not used opioids. Moreover, the dose in 7745 of these prescriptions exceeded 90 MME per day, which is particularly problematic because these patients had not used opioids and were therefore at risk for respiratory depression from high-potency opioids. It is difficult to ascertain the severity of pain from claims data alone, and prescriptions that deviate from the CDC guidelines are not necessarily medically inappropriate, although, as the CDC notes, they are associated with a higher risk of adverse outcomes.
We observed seasonal peaks in the incidence and dose of initial opioid prescriptions each December. Although untested, the seasonal peaks could be due to lower out-of-pocket costs for health care at the end of each deductible year, seasonal changes in case mix (e.g., more accidents such as motor vehicle crashes and falls related to the weather and mental health or substance use episodes), or limited provider availability during the holidays.
Our study has several limitations. First, receipt of an opioid prescription may not reflect actual opioid consumption. Some persons may consume less than prescribed, whereas others may access extra pills from family, friends, or illicit drug markets. Nevertheless, understanding trends in the prescription of opioids to persons who have not used opioids is an important step in understanding the contribution of provider behavior to the opioid problem. Second, our data included outliers with respect to the duration and dose of opioid prescriptions. Following the method used by Brat and colleagues,12 we re-estimated the percentage of prescriptions according to duration and dose using a truncated sample that excluded patients who received an opioid prescription with a supply duration of more than 91 days or a dose higher than 350 MME per day. Similar conclusions were obtained after the outliers were excluded. Third, because there is no definitive list of National Drug Codes for opioids, it was not possible to verify that our list was complete; however, we consulted multiple sources to be as comprehensive as possible. Finally, although we attempted to infer the indication for the opioid prescription from the diagnoses rendered in a contemporaneous medical encounter, we could not determine the reasons for the opioid prescription with certainty and hence could not categorize as medically inappropriate any prescription that exceeded the dose and duration guidelines of the CDC.
Our findings illuminate some aspects of opioid policy in the United States. First, it appears that a large number of providers have responded to the opioid crisis by ceasing to prescribe opioids to patients who had not used opioids. Although this trend suggests that many providers have internalized the message that opioids should be avoided, it also indicates that patients may be increasingly unable to access safe amounts of opioids when needed for pain control. Second, an important subgroup of providers continues to issue long-duration or high-dose opioid prescriptions to patients who had not used opioids. Not all of these prescriptions are necessarily inappropriate; without more detailed data describing the clinical context, in particular the severity of pain, it is difficult to accurately determine medical appropriateness. Nonetheless, these high-risk prescriptions warrant further attention. Understanding the clinical circumstances in which long-duration or high-dose opioid prescriptions are issued to patients who have not used opioids would help inform the design of opioid control policies that attempt to strike a balance between preserving access to pain control when needed and mitigating the risk of inappropriate use.
Supplementary Material
Acknowledgments
Supported by grants (P01AG005842 and R01AG026290) from the National Institute on Aging and a monetary gift from Owen and Linda Robinson.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. Harvard Medical School participates in the Blue Cross–Blue Shield Alliance for Health Research.
REFERENCES
- 1.What is the U.S. opioid epidemic? Washington, DC: Department of Health and Human Services, 2018. (https://www.hhs.gov/opioids/about-the-epidemic/). [Google Scholar]
- 2.Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants — United States, 2015–2016. MMWR Morb Mortal Wkly Rep 2018; 67: 349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain 2008; 24: 521–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 2009; 18: 1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999–2016. NCHS Data Brief 2017; 294: 1–8. [PubMed] [Google Scholar]
- 6.Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry 2014; 71: 821–6. [DOI] [PubMed] [Google Scholar]
- 7.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010; 152: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med 2015; 175: 608–15. [DOI] [PubMed] [Google Scholar]
- 9.Deyo RA, Hallvik SE, Hildebran C, et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naïve patients: a statewide retrospective cohort study. J Gen Intern Med 2017; 32: 21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use — United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66: 265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah A, Hayes CJ, Martin BC. Factors influencing long-term opioid use among opioid naïve patients: an examination of initial prescription characteristics and pain etiologies. J Pain 2017; 18:1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ 2018; 360: j5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnett ML, Olenski AR, Jena AB. Opioid-prescribing patterns of emergency physicians and risk of long-term use. N Engl J Med 2017; 376: 663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann Intern Med 2017; 167: 293–301. [DOI] [PubMed] [Google Scholar]
- 15.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med 2016; 374: 154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep 2016; 65(1): 1–49. [DOI] [PubMed] [Google Scholar]
- 17.Jena AB, Goldman D, Weaver L, Karaca-Mandic P. Opioid prescribing by multiple providers in Medicare: retrospective observational study of insurance claims. BMJ 2014; 348: g1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morden NE, Munson JC, Colla CH, et al. Prescription opioid use among disabled Medicare beneficiaries: intensity, trends, and regional variation. Med Care 2014; 52: 852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007–2012. Am J Prev Med 2015; 49: 409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuo YF, Raji MA, Chen NW, Hasan H, Goodwin JS. Trends in opioid prescriptions among Part D Medicare recipients from 2007 to 2012. Am J Med 2016; 129(2): 221.e21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pezalla EJ, Rosen D, Erensen JG, Haddox JD, Mayne TJ. Secular trends in opioid prescribing in the USA. J Pain Res 2017; 10: 383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep 2017; 66: 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Department of Health and Human Services. Opioids in Medicare Part D: concerns about extreme use and questionable prescribing. Report no. OEI-02-17-00250. Washington, DC: Office of the Inspector General, July 2017. [Google Scholar]
- 24.Cao S, Karmouta R, Li DG, Din RS, Mostaghimi A. Opioid prescribing patterns and complications in the dermatology Medicare population. JAMA Dermatol 2018; 154: 317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tehrani AB, Henke RM, Ali MM, Mutter R, Mark TL. Trends in average days’ supply of opioid medications in Medicaid and commercial insurance. Addict Behav 2018; 76: 218–22. [DOI] [PubMed] [Google Scholar]
- 26.Axeen S, Seabury SA, Menchine M. Emergency department contribution to the prescription opioid epidemic. Ann Emerg Med 2018; 71(6): 659–667.e3. [DOI] [PubMed] [Google Scholar]
- 27.Jeffery MM, Hooten WM, Henk HJ, et al. Trends in opioid use in commercially insured and Medicare Advantage populations in 2007–16: retrospective cohort study. BMJ 2018; 362: k2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jena AB, Goldman D, Karaca-MandicP. Hospital prescribing of opioids to Medicare beneficiaries. JAMA Intern Med 2016; 176: 990–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Food and Drug Administration. National Drug Code database file (https://www.fda.gov/Drugs/InformationOnDrugs/ucm142438.htm).
- 30.CDC compilation of benzodiazepines, muscle relaxants, stimulants, zolpidem, and opioid analgesics with oral morphine milligram equivalent conversion factors, 2016 version. Atlanta: Centers for Disease Control and Prevention, 2016. [Google Scholar]
- 31.Calculating total daily dose of opioids for safer dosage. Atlanta: Centers for Disease Control and Prevention; (https://www.cdc.gov/drugoverdose/pdf/calculating_total_daily_dose-a.pdf). [Google Scholar]
- 32.Opioid oral morphine milligram equivalent (MME) conversion factors. Baltimore: Centers for Medicare & Medicaid Services; (https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-April-2017.pdf). [Google Scholar]
- 33.Sherry TB, Sabety A, Maestas N. Documented pain diagnoses in adults prescribed opioids: results from the National Ambulatory Medical Care Survey, 2006–2015. Ann Intern Med 2018; 169: 892–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.