Abstract
Background:
Patients with opioid use disorder (OUD) show memory deficiencies and impaired treatment outcomes. Emerging evidence suggests that opioid abuse activates proinflammatory processes by increasing cytokine production and impairing neuroprotection, which damages the memory function in OUD patients. Therefore, we investigated whether plasma inflammatory and neurotrophic markers correlate with memory function in OUD patients.
Method:
OUD patients undergoing methadone maintenance therapy (MMT) were investigated and followed up for 12 weeks. Plasma tumor necrosis factor (TNF)-α, C-reactive protein (CRP), interleukin (IL)-6, transforming growth factor (TGF)-β1, brain-derived neurotrophic factor (BDNF) levels, and Wechsler memory scale-revised (WMS-R) scores were assessed at baseline and after 12 weeks of MMT. Multiple linear regressions and generalized estimating equations (GEEs) were used to examine the correlation between cytokines and memory performance.
Results:
We enrolled 89 patients at baseline; 47 patients completed the end-of-study assessments. Although Pearson correlations showed that CRP and TGF-β1 levels were significantly negatively associated with some memory indices, the results were not significant after correction. The GEE results, controlled for several confounding factors and multiple testing, showed that changes in TNF-α levels were negatively correlated with changes in the visual memory index (P = 0.01), and that changes in IL-6 levels were negatively correlated with changes in the verbal memory index (P = 0.009).
Conclusion:
Memory performance, TNF-α, and IL-6 levels in OUD patients were negative correlated. Additional studies on regulating TNF-α and IL-6 expression to improve memory function in OUD patients might be warranted.
Keywords: opioid use disorder, cytokines, brain-derived neurotrophic factor, memory, cognitive function
1. Introduction
The lifetime prevalence rate of opioid use disorder (OUD) is high—2.1% in the US (Grant et al., 2016) and 0.22% globally (Degenhardt et al., 2014)—and it causes substantial public health problems in the world (Degenhardt et al., 2014). The development of OUD is associated with multiple genetic, environmental, and developmental risk factors (Volkow et al., 2016). In addition to the classical etiology for OUD, emerging evidence also suggests that dysregulated neuroinflammation is involved in the development of addiction (Coller and Hutchinson, 2012). Larger doses and long-term heroin use are associated with dysregulation of the immune system, which damages neurons and triggers additional glial cells to upregulate the proinflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-6 (Chan et al., 2015; Dyuizen and Lamash, 2009; Pacifici et al., 2000; Wang et al., 2012). This drug-induced activation of central immune signaling contributes to increases in the engagement of classical mesolimbic dopamine reward pathways and withdrawal centers (Coller and Hutchinson, 2012; Narita et al., 2006), and it is associated with developing OUD. The overactive microglial and inflammatory responses cause neurotoxicity in the hippocampus and amygdala via neuronal apoptotic pathways (Atici et al., 2004; Hu et al., 2002). Postmortem data also show that progressive neuron loss occurs in the brains of long-term heroin users (Li et al., 2005). Other findings suggest that chronic heroin users express lower levels of brain-derived neurotrophic factor (BDNF) (Angelucci et al., 2007). Collectively, these findings indicate that opioid abuse activates microglia and causes neurotoxicity by inducing the production of inflammatory cytokines and impairing neuroprotection, which consequently damages the brain in OUD patients.
The prolonged use of opioids, such as heroin, is associated with impaired attention, concentration, visual and verbal recall, and visual-spatial skills (Gruber et al., 2007). Methadone maintenance therapy (MMT) is the most-studied and it is an effective treatment for OUD (Soyka et al., 2011). However, changes in neuropsychological functioning persist in patients undergoing MMT (Prosser et al., 2006), especially in the domains of episodic (Curran et al., 2001), visual (Prosser et al., 2006), and working and long-term memory (Darke et al., 2000). Maladaptive memories associated with drug abuse might lead to relapses into drug-seeking behavior (Ross and Peselow, 2009). Therefore, increasing our understanding of the underlying mechanisms of the associated memory deficits in OUD patients undergoing MMT might help us design better treatment strategies.
The immune system modulate learning, memory, and neural plasticity (Yirmiya and Goshen, 2011). Manipulating individual cytokines can also modulate learning, memory, and synaptic plasticity (Donzis and Tronson, 2014). Some proinflammatory cytokines have been implicated in memory and plasticity deficits. Overexpression of TNF-α in neurons and glial cells impairs passive avoidance memory (Fiore et al., 2000), inhibits long-term potentiation (LTP) and synaptic plasticity (Butler et al., 2004; Cunningham et al., 1996), and hinders cerebellar learning (Paredes et al., 2010). TNF-α also mediates memory deficits after chronic lipopolysaccharide (LPS) treatment (Belarbi et al., 2012), which is consistent with the memory impairing effect of TNF-α. Overexpression of IL-6 or application of IL-6 cause broad memory impairments (Heyser et al., 1997; Wei et al., 2012), diminished LTP (Li et al., 1997), and a reduction of neurogenesis in the hippocampal dentate gyrus (Vallieres et al., 2002). Increased C-reactive protein (CRP) levels have been associated with a wide range of brain dysfunctions, including age-related cognitive decline (Laurin et al., 2009), all-cause dementia (Kravitz et al., 2009), and memory impairment in schizophrenia (Bulzacka et al., 2016). In contrast, cytokines that modulate the inflammation process might be neuroprotective and improve the cognitive deficits caused by inflammation. Transforming growth factor (TGF)-β1 is normally present at low levels under physiological conditions, but it is rapidly upregulated after a brain injury (Zhu et al., 2000). TGF-β1 protects neurons against different kinds of insults, including excitotoxic injury and neurotoxins (Qian et al., 2008), and preserves both cognitive function and memory (Li et al., 2013; Matias et al., 2017). In addition, the production and signaling of BDNF has been implicated in almost every aspect of neural and behavioral plasticity, including hippocampal-dependent memory (Barnes and Thomas, 2008; Heldt et al., 2007), LTP (Lu et al., 2008), and neurogenesis (Li et al., 2008). Increased BDNF signaling underlies the influence of immune processes on learning, memory, LTP, and neurogenesis (Yirmiya and Goshen, 2011). Therefore, investigating the potential impacts of cytokines and BDNF on the memory function in OUD patients might reveal the underlying mechanism for opioid-induced memory deficits.
Few studies have investigated the association between the plasma levels of cytokines, BDNF, and memory function in OUD patients undergoing MMT. Because studies have reported that memory function might change after two months of MMT (Gruber et al., 2006), a longitudinal study controlled for potential confounding factors is more suitable for assessing the association between changes in memory function and in plasma levels of cytokines and BDNF. We therefore used a longitudinal study to assess the correlation between cytokines and BDNF levels and changes in the memory performance of OUD patients after 12 weeks of MMT.
2. Methods
2.1. Patients
The research protocol was approved by the Institutional Review Board for the Protection of Human Subjects at National Cheng Kung University Hospital. The study was done in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. The procedures were fully explained to each participant before they were asked to sign the informed consent. OUD patients were recruited from the MMT program. Each patient was initially interviewed by an attending psychiatrist and then by a research team member well trained in using the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) (American Psychiatric Association, 1994) criteria and the Chinese Version of the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998). The MINI was used because completing 4–6 h of structured interviews, such as the Chinese Version of the Modified Schedule of Affective Disorder and Schizophrenia-Lifetime (SADS-L) (Endicott and Spitzer, 1978), is difficult for OUD patients. The MINI has good reliability and has been widely used in clinical trials and epidemiological studies (Ritchie et al., 2004), and its inter-rater reliability in Chinese Version was approximately 0.75 in previous studies (Kuo et al., 2003; Lung et al., 2008). Inclusion criteria were being an adult male or female between 18 and 65 years old who met the DSM-IV criteria for current opioid dependence and who used opioids daily. Exclusion criteria were having a cognitive disorder, being pregnant or nursing an infant, having taken any anti-inflammatory medications within 1 week before the study, or having a history of one or more uncontrolled major physical conditions such as diabetes mellitus or hypertension.
We asked patients whether they had taken any anti-inflammatory agents, such as: steroids, nonsteroidal anti-inflammatory drugs (NSAIDs), or other immune modulating agents (disease-modifying anti-rheumatic drugs, DMARDs) before enrollment. If they had taken these agents, we would not enroll them in our study until they had stopped taking these medications for at least one week. We also informed our patients that we would follow up their cytokine expression levels during the follow-up and that taking any anti-inflammatory agents would interfere with the results. If they reported taking these agents during follow up, we excluded their cytokine data. Glucocorticoids inhibit the expression and action of most cytokines, including IL-1β, TNF-α, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12 and IFN-γ (Tsuboi et al., 1995). NSAIDs might also affect the responses of IL-2, IL-6, IL-8, and monocyte chemoattractant protein-1 (Lisboa et al., 2017). DMARDs inhibit the induction of cytokines in monocytes and macrophages (Bondeson, 1997) and interfere with IL-6 and TNF-α expression (Mangoni et al., 2017). Therefore, the current study excluded patients taking anti-inflammatory agents.
We recruited 89 OUD patients at the beginning of our study. At baseline, each patient was assessed for peripheral inflammatory cytokines and BDNF levels, and for memory function using the Wechsler Memory Scale-Revised (WMS-R). Their MMT and psychosocial interventions were maintained during the follow-up. After 12 weeks of follow-up, we assessed their plasma levels of inflammatory cytokines, and BDNF, and their WMS-R performance again. At the endpoint of 12 weeks, 47 OUD patients completed the assessment.
2.2. Blood samples and cytokine analysis
After 20 mL of blood had been drawn from each participant, the plasma was isolated from the whole blood after it had been centrifuged at 3000 g for 15 min at 4°C, and then it was immediately stored at −80°C. Cytokine and BDNF levels were quantified using an antibody pair assay system (Flexia; BioSource Intl., Camarillo, CA). Sample processing and data analysis were done according to the manufacturer’s instructions. The immunological parameters—TNF-α, CRP, IL-6, TGF-β1, and BDNF levels—were measured. All laboratory procedures were performed double-blinded and all assays were done in duplicate.
2.3. Memory function assessment
The WMS-R is designed to understand all factors of memory, and it is considered a major assessment of cognitive function (Wechsler, 1987). The WMS-R consists of 13 subtests and five indices (General Memory, Verbal Memory, Visual Memory, Attention/Concentration, and Delayed Recall). The Information and Orientation Questions (the first subtest) were used to screen for disorientation and intact sensory functioning. Attention and working memory were assessed using the Attention/Concentration index subtests (Kent, 2013). The index-score reliability coefficients range from 0.70 to 0.90 (Elwood, 1991). The test-retest reliability after 2 to 12 weeks is 0.62–0.82 (Wechsler, 1987). Inter-rater reliability is more than 0.9 (O’Carroll and Badenoch, 1994).
2.4. Statistical analysis
Pearson χ2 analysis was used to examine the categorical variables. Fisher’s exact test was substituted for the χ2 test when values were smaller than expected (< 5). Pearson correlations were used initially to test the correlation between WMS-R index scores and the cytokine and BDNF levels. However, because there were repeated assessments, the generalized estimating equation (GEE) method (Zeger et al., 1988) was used for multiple linear regression in repeated-measures analyses that accommodate randomly missing data (Shen and Chen, 2012). In the current study, GEE analysis was used to investigate the changes in cytokine levels and WMS-R individual index scores from baseline to endpoint, and the correlations of changes in plasma cytokine and BDNF levels, and in WMS-R scores. Time effects (treatment period from baseline to week 12), age, sex, methadone dose, disease duration, psychiatric comorbidities, and morphine-positive urine tests were covarying. Significance was set at P < 0.05. SPSS 18.0 was used for all statistical analyses.
3. Results
At baseline, there were 89 OUD patients (mean age: 35.99 ± 7.24 years old; male: 83.2%; and mean duration using heroin: 6.20 ± 4.87 years). All of them used intravenously injected heroin. The participants had a high rate (71.6%) of morphine-positive urine tests, and were undergoing MMT (mean dose: 34.61 ± 27.75 mg/day). Almost a quarter (24.7%) of the patients had psychiatric comorbidities other than OUD (Table 1). Their baseline plasma levels of cytokines and BDNF, and WMS-R scores are listed in Table 1.
Table 1.
Demographic data, cytokine levels, and mean WMS-R index scores in OUD patients before and after 12 weeks of MMT
| Variable | Baseline | After 12 weeks | B | P | ||
|---|---|---|---|---|---|---|
| Number of cases | 89 | 47 | ||||
| Age | 35.99 ± 7.24 | 37.19 ± 7.96 | ||||
| Sex (Male/Female) | 74/15 | 41/6 | ||||
| Disease duration (years) | 6.20 ± 4.87 | 6.72 ± 5.84 | ||||
| Methadone dose (mg) | 34.61 ± 27.75 | 42.91 ± 25.60 | ||||
| Psychiatric comorbidities (+/−) | 22/67 | 11/36 | ||||
| Morphine-positive urine tests (+/−) | 63/25a | 22/24b | ||||
| Plasma cytokines | ||||||
| TNF-α | 3.81 ± 3.67 | 3.60 ± 4.29 | −0.76 | <0.001* | ||
| CRP | 3.51 ± 3.18 | 2.66 ± 2.63 | −0.79 | < 0.001* | ||
| IL-6 | 2.48 ± 2.10 | 1.96 ± 1.44 | −0.43 | <0.001* | ||
| TGF-β1 | 26.41 ± 14.73 | 22.80 ± 16.94 | −2.75 | 0.02* | ||
| BDNF | 11.09 ± 7.41 | 9.07 ± 5.83 | −1.73 | <0.001* | ||
| Wechsler memory scale-revised | ||||||
| Verbal Memory Index | 92.30 ± 12.86 | 99.55 ± 19.40 | 6.83 | <0.001* | ||
| Visual Memory Index | 98.07 ± 13.27 | 105.40 ± 15.91 | 7.59 | <0.001* | ||
| General Memory Index | 93.78 ± 17.49 | 102.04 ± 18.91 | 7.96 | <0.001* | ||
| Attention/Concentration Index | 104.36 ± 13.30 | 109.02 ± 12.44 | 1.97 | 0.06 | ||
| Delayed Recall Index | 96.97 ± 17.18 | 104.51 ± 19.81 | 7.24 | <0.001* | ||
WMS-R: Wechsler memory scale-revised; OUD: opioid use disorder; MMT: methadone maintenance therapy; TNF-α: tumor necrosis factor α; CRP: C-reactive protein; IL-6: interleukin-6; TGF-β1: transforming growth factor-β1; BDNF: brain-derived neurotrophic factor.
Covarying for age, sex, methadone dose, disease duration, psychiatric comorbidities, and morphine-positive urine tests in GEE analysis.
n = 88;
n = 46;
P < 0.05.
After 12 weeks of MMT, 47 patients had completed the assessment. The endpoint plasma levels of TNF-α, CRP, IL-6, TGF-β1, and BDNF levels were significantly lower than at baseline (Table 1). The endpoint verbal, visual, and general memory and delayed recall indices were significantly higher than at baseline (Table 1). Pearson correlations showed that CRP levels were significantly negatively associated with all five indices (Table 2). IL-6 levels were significantly negatively correlated with the verbal, general memory, and delayed recall indices (Table 2). TGF-β1 levels were significantly negatively correlated with the general memory and attention/concentration indices (Table 2).
Table 2.
Pearson correlations (r) of plasma cytokine and BDNF levels and WMS-R index scores
| Variable | Verbal Memory Index | Visual Memory Index | General Memory Index | Attention/Concentration Index | Delayed Recall Index |
|---|---|---|---|---|---|
| TNF-α | −0.04 | −0.15 | −0.08 | 0.07 | −0.01 |
| CRP | −0.19* | −0.16* | −0.22** | −0.25** | −0.21* |
| IL-6 | −0.28** | −0.10 | −0.24* | 0.01 | −0.22* |
| TGF-β1 | −0.12 | −0.10 | −0.16* | −0.05* | −0.16 |
| BDNF | −0.07 | −0.09 | −0.10 | −0.02 | −0.12 |
WMS-R: Wechsler memory scale-revised; BDNF: brain-derived neurotrophic factor; TNF-α: tumor necrosis factor α; CRP: C-reactive protein; TGF-β1: transforming growth factor-β1; IL-6: interleukin-6.
P < 0.05;
P < 0.01.
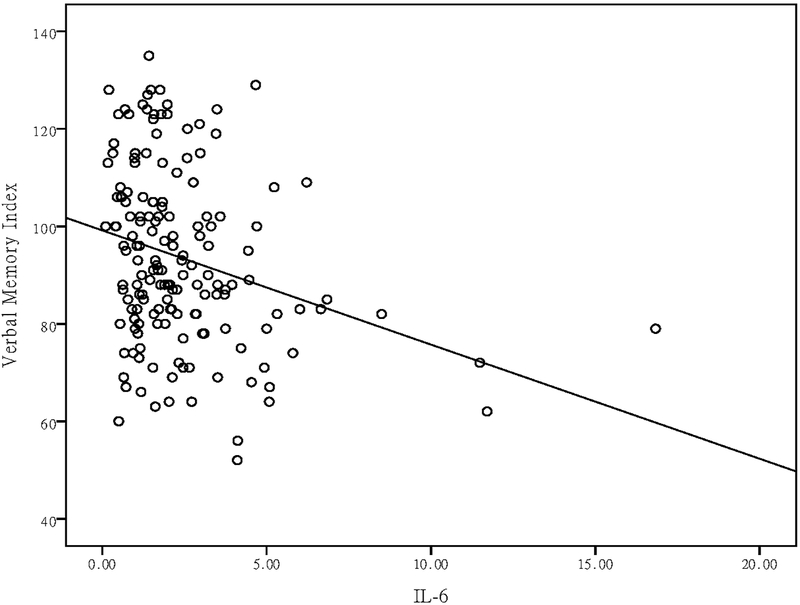
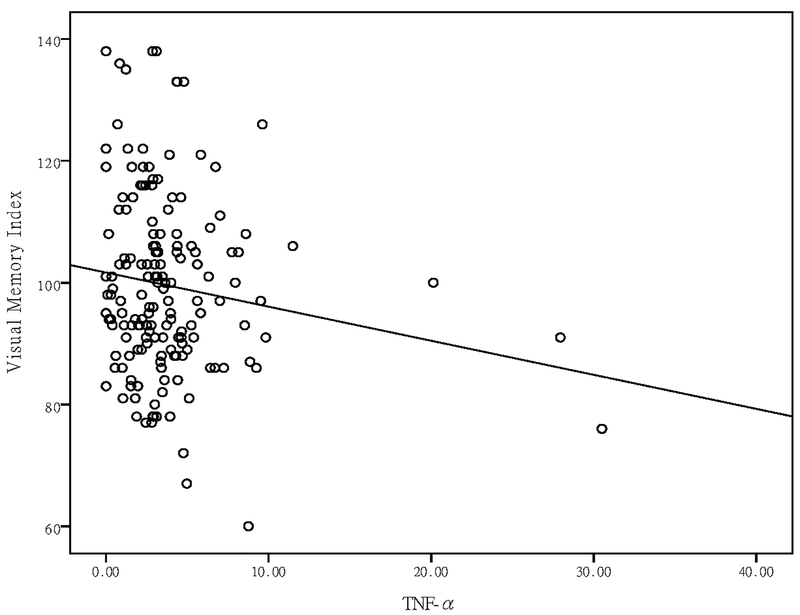
After covarying for multiple possible confounding factors (age, sex, methadone dose, disease duration, time effects, psychiatric comorbidities, and morphine-positive urine tests), a GEE analysis showed that changes in TNF-α levels were negatively correlated with changes in the visual memory index (P = 0.01) (Table 3), and that changes in IL-6 levels were negatively correlated with changes in the verbal memory (P = 0.009) and delayed recall indices (P = 0.03) (Table 3). After a Bonferroni correction for multiple testing, the negative correlations between TNF-α levels and the visual memory index, and IL-6 levels, and the verbal memory index, were still significant (Table 3).
Table 3.
Correlation of changes in WMS-R scores and in plasma cytokine and BDNF levels before and after 12 weeks of MMT
| Indices: | Verbal Memory | Visual Memory | General Memory | Attention/Concentration | Delayed Recall | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | B | Wald χ2 | P | B | Wald χ2 | P | B | Wald χ2 | P | B | Wald χ2 | P | B | Wald χ2 | P | ||||
| TNF-α | 0.02 | 0.01 | 0.92 | −0.52 | 6.69 | 0.01** | −0.25 | 0.83 | 0.36 | −0.14 | 0.46 | 0.50 | −0.06 | 0.07 | 0.79 | ||||
| CRP | −0.40 | 0.72 | 0.40 | −0.47 | 0.99 | 0.32 | −0.78 | 2.21 | 0.14 | −0.66 | 3.62 | 0.06 | −0.73 | 1.54 | 0.22 | ||||
| IL-6 | −1.92 | 6.79 | 0.009** | 0.50 | 0.65 | 0.42 | −1.19 | 2.16 | 0.14 | 1.02 | 3.21 | 0.07 | −1.83 | 4.60 | 0.03* | ||||
| TGF-β1 | −0.05 | 0.45 | 0.50 | 0.02 | 0.04 | 0.84 | −0.06 | 0.41 | 0.52 | −0.02 | 0.07 | 0.79 | −0.02 | 0.03 | 0.87 | ||||
| BDNF | 0.13 | 0.35 | 0.56 | 0.18 | 0.57 | 0.45 | 0.18 | 0.69 | 0.41 | 0.02 | 0.02 | 0.90 | 0.21 | 1.09 | 0.30 | ||||
WMS-R: Wechsler memory scale-revised; BDNF: brain-derived neurotrophic factor; MMT: methadone maintenance treatment; TNF-α: tumor necrosis factor α; CRP: C-reactive protein; TGF-β1: transforming growth factor-β1; IL-6: interleukin-6.
Covarying for age, sex, methadone dose, disease duration, time effects, psychiatric comorbidities, and morphine-positive urine tests.
P < 0.05;
P < 0.01.
4. Discussion
This is the first study that shows the relationship between memory test scores and peripheral inflammatory cytokines in OUD patients. We found a significantly negative correlation between TNF-α levels and the visual memory index, and IL-6 levels and the verbal memory index after controlling for multiple factors. We also found that memory performance and inflammatory status in OUD patients might improve during MMT treatment. Although CRP and TGF-β1 were correlated with memory test scores in the initial analysis, the results were not significant after controlling for multiple confounding factors. These findings indicated that TNF-α- and IL-6-related pathways are involved in memory processes in opioid-addicted patients.
It has been hypothesized that immune processes in the brain, including microglial activation and inflammatory cytokine production, have a complex dual role in learning, memory, and neural plasticity and excitability (Yirmiya and Goshen, 2011). Locally controlled and properly timed activation of immune processes (Schneider et al., 1998; Stellwagen and Malenka, 2006) might increase the neural excitability that underlies neural plasticity and memory consolidation. However, excessive immune activation and “cytokine storms” can hyperexcite neural circuits and disturb both memory and neural plasticity (Yirmiya and Goshen, 2011). If the immune processes are severely or chronically overactivated, excitotoxicity, apoptosis, and neurodegeneration might occur, which will reduce neural excitability and increase impairments in learning, memory, and neural plasticity (Yirmiya and Goshen, 2011). Our study results partly support this hypothesis and show that greater levels of inflammation might have negative effects on memory test scores.
The detrimental effect of TNF-α on memory was first reported in learning-impaired transgenic mice (Fiore et al., 1996) that overexpressed TNF-α in the brain in a passive-avoidance paradigm. Chronic ventricular administration of TNF-α for a week before water-maze training consistently impaired spatial learning and memory in mice (Bjugstad et al., 1998). After TNF-α had been intrahippocampally administered for 10 days in rats, their hippocampal-dependent working memory was also impaired (Matsumoto et al., 2002). Human studies have also shown that elevated TNF-α levels are associated with reduced verbal memory test scores in patients with psychotic disorders (Hoseth et al., 2016) and with breast cancer (Kesler et al., 2013), and are associated with short- and medium-term cognitive dysfunction after coronary artery surgery (Hudetz et al., 2011). Our study showed that TNF-α levels were negatively correlated with visual memory test scores in OUD patients, which partially supported the notion that the negative influence of TNF-α might be dose-dependent, and was restricted to the performance of tasks that depend on normal hippocampal functioning (Yirmiya and Goshen, 2011).
The role of IL-6 in memory is quite complex and in opposite ways under various conditions. Balschun et al. (Balschun et al., 2004) reported that LTP upregulated IL-6 gene expression in rats, but that treatment with anti-IL-6 antibodies 90 min prolonged the LTP. Thus, they suggested that endogenous IL-6 regulated or fine-tune the consolidation of long-term synaptic plasticity and hippocampal-dependent learning and memory during physiological conditions. However, chronic and pathological IL-6 overproduction might mediate age-related memory impairments (Weaver et al., 2002). Elevated IL-6 levels have also been associated with memory deficiencies in dementia (Engelhart et al., 2004), and in various pathologies accompanied by an increase production of the cytokine, e.g., cardiovascular and liver diseases and major depression (Grassi-Oliveira et al., 2011; Hudetz et al., 2011; Luo et al., 2012; Trapero and Cauli, 2014). After combining the findings of all these studies with our findings, we hypothesize that the negative effects of IL-6 and TNF-α on memory performance are the same in addicted patients as in those with other pathological conditions, and that they illustrate the pivotal role of IL-6 and TNF-α in memory function.
The efficacy of MMT has been widely investigated, and showed that OUD patients undergoing MMT will markedly reduce illicit opioid use, drug-related health consequences, crimes, and transmission of HIV and hepatitis (Marsch, 1998; Shi et al., 2007) and have improvement in social productivities, personal relationships and health status (Chou et al., 2013; Hsiao et al., 2015). Our study results suggested an additional benefit in OUD patients undergoing MMT. They may have improvement in memory function and inflammatory status. Although past studies also showed significant improvements in verbal learning and memory, visuospatial memory, and psychomotor speed in opiate-dependent patients after 2 months of MMT, the study sample size was small (Gruber et al., 2006). Our study enrolled more OUD patients, adjusted more confounding factors and followed longer to reduce the learning effect of WMS-R, which will provide stronger evidence. However, further longer follow up studies and compared with controls may be required to examine how long the improvement will last and control for the learning effects.
Consistent with our finding, studies have shown that a dysregulated immune function in heroin abusers might be normalized by switching to long-term MMT (McLachlan et al., 1993; Sacerdote et al., 2008). Several possible factors have been proposed to explain the changes in the immune function during MMT. First, lifestyle changes like reducing how much heroin is injected, and reducing the risks of infectious diseases, malnutrition, stress, reversed sleep-wake patterns, and sleep deprivation may all attribute to restore immune function (McLachlan et al., 1993). Second, long lasting treatment with methadone normalizes the hypothalamic-pituitary-adrenal (HPA) axis, which is altered by withdrawal-induced stress (Gerra et al., 2007), and restores the disturbed immune function in heroin abusers (Kapadia et al., 2005; Kreek, 2001). Additionally, both preclinical and clinical studies suggest that not all opioid receptor agonists have the same immune-modulating properties (Sacerdote et al., 2008). Short-acting opioid drugs like heroin and morphine can markedly change the immune system (Pacifici et al., 2000), and long-acting opioid drugs like methadone can progressively restore the immune function and cytokine expression (Neri et al., 2002; Sacerdote et al., 2008). It is possible that improved immune responses require the constant activation of μ opioid receptors (MOR) provided by methadone and buprenorphine treatment (Sacerdote et al., 2008).
However, we found that BDNF levels and TGF-β1 levels decreased during MMT. Some studies (Heberlein et al., 2011; Nabati et al., 2013; Zhang et al., 2014) reported that BDNF and TGF-β1 levels were higher in OUD patients than in controls, partly because greater BDNF and TGF-β1 expression occurs as a compensatory response to neuronal insults (Zhang et al., 2014; Zhu et al., 2000). Though previous study did not find the difference in serum BDNF levels among heroin addicts at baseline and one month after heroin cessation (Zhang et al., 2014), we supposed that the compensatory elevation of BDNF and TGF-β1 may be normalized when reducing heroin injection a longer period of time.
Some researchers may argue that peripheral cytokines levels are irrelevant for the brain. However, inflammatory cytokines pass through the blood-brain barrier (BBB) and influence brain function through three pathways (Miller and Raison, 2016). The first pathway is the humoral pathway, which involves cytokine passage through leaky regions in the BBB, such as the circumventricular organs, and the binding of cytokines to active transport molecules on the BBB (Erickson et al., 2012; Miller and Raison, 2016). The second pathway is the neural pathway, which involves the binding of cytokines to peripheral afferent vagus nerve fibers that in turn stimulate ascending catecholaminergic fibers in the brain and then are translated back into central cytokine signals (Erickson et al., 2012; Miller and Raison, 2016). The last one is the cellular pathway, which involves the trafficking of activated immune cells, typically monocytes, to the brain vasculature and parenchyma (D’Mello et al., 2009). Therefore, it is possible that peripheral inflammatory responses drive inflammation in the brain and influence its cognitive function.
We found no significant associations between CRP, TGF-β1, BDNF and memory test scores in WMS-R after we had controlled for several possible confounders and corrected for multiple testing. Although the initial correlation analysis showed a significant negative association between memory test scores and CRP, IL-6, and TGF-β1 levels, the findings for CRP and TGF-β1 did not remain significant after correction. Because we controlled for several factors, including age, sex, methadone dose, disease duration, time effects, psychiatric comorbidities and urine morphine-positive urine tests, our results differ from those of studies that found significant associations between CRP, TGF-β1, and BDNF levels and memory function (Bulzacka et al., 2016; Laurin et al., 2009; Li et al., 2013; Zhang et al., 2014). In addition, we analyzed OUD patients undergoing MMT, which were also different from the types of patients in some of those other studies (Bulzacka et al., 2016; Laurin et al., 2009; Zhang et al., 2014). This suggests that these molecules may not have common pathways for impairing memory function in different psychopathologies.
Our study has some limitations. This is the first study that tests the hypothesis that peripheral inflammation markers are associated with memory performance in OUD patients. A larger sample size would provide more convincing results. Although we have tested five potential factors in this study, there are still many other proinflammatory and anti-inflammatory cytokines and neurotrophic factors that might also affect memory function. However, our study results underline the initial and important linkage between memory function and inflammation, which will provide a new perspective in addiction research. Although we tried to control for factors that might affect changes in plasma cytokine and BDNF levels, other factors, e.g., metabolic profiles and smoking, might also have affected our findings. Most of our participants smoked, which might interfere with cytokine expression levels. Additionally, we did not check urine screen tests for non-morphine opioids or other drug classes, like marijuana, cocaine, etc. It is less common to abuse other non-morphine opioids in Taiwan. The most commonly abused opioid in Taiwan are heroin (14,685 positive specimens in 2017) and codeine (10,699 positive specimens in 2017) (Taiwan Food and Drug Administration [TFDA] annual reports for drug abuse examination and statistics <https://www.fda.gov.tw/TC/site.aspx?sid=1578>). Because heroin and codeine will both metabolize into morphine and be excreted in urine, we did urinary morphine tests for each OUD patient. We hypothesized that the results would reveal their opioid abuse levels. In Taiwan, marijuana and cocaine use are not as common as in America or Europe (TFDA); thus, few Taiwanese OUD patients use them concurrently. However, in this study, we did not control for their concurrent methamphetamine and alcohol use, which is more common in Taiwan OUD patients. Our patients were not severely intoxicated when their memory function was assessed: they passed the Information and Orientation Questions in the first subset of the WMS-R, which indicated that their orientation and sensory functions were intact. But subtle effects from using alcohol or methamphetamine on WMS-R performance still cannot be excluded. These factors should be controlled for in future research. Also, all our patients were undergoing MMT, which might affect their memory function. We tried to control for the methadone dose in our analysis to reduce the effect, but to generalize our results to abstinent former opioid abusers who did not undergo MMT might require additional research. Therefore, our findings should be interpreted with caution.
5. Conclusion
We found a significantly negative correlation between memory function and TNF-α and IL-6 levels in OUD patients. Our findings provide preliminary evidence that changes in inflammation levels might be involved in memory function in OUD patients. Given that effective treatments for cognitive deficiency in OUD patients are scarce and that MMT might cause cognitive impairment (Verdejo et al., 2005), further studies are required to test the efficacy of agents that regulate TNF-α and IL-6 expression to improve memory function in OUD patients.
Figure 1.
Scatter plot of plasma TNF-α levels and visual memory index in patients with OUD undergoing MMT.
Figure 2.
Scatter plot of plasma IL-6 levels and verbal memory index in patients with OUD undergoing MMT.
References
- Angelucci F, Ricci V, Pomponi M, Conte G, Mathe AA, Attilio Tonali P, Bria P, 2007. Chronic heroin and cocaine abuse is associated with decreased serum concentrations of the nerve growth factor and brain-derived neurotrophic factor. J. Psychopharmacol 21, 820–825. [DOI] [PubMed] [Google Scholar]
- Atici S, Cinel L, Cinel I, Doruk N, Aktekin M, Akca A, Camdeviren H, Oral U, 2004. Opioid neurotoxicity: comparison of morphine and tramadol in an experimental rat model. Int. J. Neurosci 114, 1001–1011. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO, 2004. Interleukin-6: a cytokine to forget. FASEB J. 18, 1788–1790. [DOI] [PubMed] [Google Scholar]
- Barnes P, Thomas KL, 2008. Proteolysis of proBDNF is a key regulator in the formation of memory. PLoS One 3, e3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belarbi K, Jopson T, Tweedie D, Arellano C, Luo W, Greig NH, Rosi S, 2012. TNF-alpha protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J. Neuroinflammation 9, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjugstad KB, Flitter WD, Garland WA, Su GC, Arendash GW, 1998. Preventive actions of a synthetic antioxidant in a novel animal model of AIDS dementia. Brain Res. 795, 349–357. [DOI] [PubMed] [Google Scholar]
- Bondeson J, 1997. The mechanisms of action of disease-modifying antirheumatic drugs: a review with emphasis on macrophage signal transduction and the induction of proinflammatory cytokines. Gen. Pharmacol 29, 127–150. [DOI] [PubMed] [Google Scholar]
- Bulzacka E, Boyer L, Schurhoff F, Godin O, Berna F, Brunel L, Andrianarisoa M, Aouizerate B, Capdevielle D, Chereau-Boudet I, Chesnoy-Servanin G, Danion JM, Dubertret C, Dubreucq J, Faget C, Gabayet F, Le Gloahec T, Llorca PM, Mallet J, Misdrahi D, Rey R, Richieri R, Passerieux C, Roux P, Yazbek H, Leboyer M, Fond G, Group F-S, 2016. Chronic peripheral inflammation is associated with cognitive impairment in schizophrenia: results from the multicentric FACE-SZ dataset. Schizophr. Bull 42, 1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, O’Connor JJ, Moynagh PN, 2004. Dissection of tumor-necrosis factor-alpha inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early-but not late-phase LTP. Neuroscience 124, 319–326. [DOI] [PubMed] [Google Scholar]
- Chan YY, Yang SN, Lin JC, Chang JL, Lin JG, Lo WY, 2015. Inflammatory response in heroin addicts undergoing methadone maintenance treatment. Psychiatry Res. 226, 230–234. [DOI] [PubMed] [Google Scholar]
- Chou YC, Shih SF, Tsai WD, Li CS, Xu K, Lee TS, 2013. Improvement of quality of life in methadone treatment patients in northern Taiwan: a follow-up study. BMC Psychiatry 13, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller JK, Hutchinson MR, 2012. Implications of central immune signaling caused by drugs of abuse: mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol. Ther 134, 219–245. [DOI] [PubMed] [Google Scholar]
- Cunningham AJ, Murray CA, O’Neill LA, Lynch MA, O’Connor JJ, 1996. Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci. Lett 203, 17–20. [DOI] [PubMed] [Google Scholar]
- Curran HV, Kleckham J, Bearn J, Strang J, Wanigaratne S, 2001. Effects of methadone on cognition, mood and craving in detoxifying opiate addicts: a dose-response study. Psychopharmacology (Berl) 154, 153–160. [DOI] [PubMed] [Google Scholar]
- D’Mello C, Le T, Swain MG, 2009. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci 29, 2089–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Sims J, McDonald S, Wickes W, 2000. Cognitive impairment among methadone maintenance patients. Addiction 95, 687–695. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Charlson F, Mathers B, Hall WD, Flaxman AD, Johns N, Vos T, 2014. The global epidemiology and burden of opioid dependence: results from the global burden of disease 2010 study. Addiction 109, 1320–1333. [DOI] [PubMed] [Google Scholar]
- Donzis EJ, Tronson NC, 2014. Modulation of learning and memory by cytokines: signaling mechanisms and long term consequences. Neurobiol. Learn. Mem 115, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyuizen I, Lamash NE, 2009. Histo- and immunocytochemical detection of inducible NOS and TNF-alpha in the locus coeruleus of human opiate addicts. J. Chem. Neuroanat 37, 65–70. [DOI] [PubMed] [Google Scholar]
- Elwood RW, 1991. The Wechsler Memory Scale-Revised: psychometric characteristics and clinical application. Neuropsychol. Rev 2, 179–201. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, 1978. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch. Gen. Psychiatry 35, 837–844. [DOI] [PubMed] [Google Scholar]
- Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM, 2004. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch. Neurol 61, 668–672. [DOI] [PubMed] [Google Scholar]
- Erickson MA, Dohi K, Banks WA, 2012. Neuroinflammation: a common pathway in CNS diseases as mediated at the blood-brain barrier. Neuroimmunomodulation 19, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M, Angelucci F, Alleva E, Branchi I, Probert L, Aloe L, 2000. Learning performances, brain NGF distribution and NPY levels in transgenic mice expressing TNF-alpha. Behav. Brain Res. 112, 165–175. [DOI] [PubMed] [Google Scholar]
- Fiore M, Probert L, Kollias G, Akassoglou K, Alleva E, Aloe L, 1996. Neurobehavioral alterations in developing transgenic mice expressing TNF-alpha in the brain. Brain Behav. Immun 10, 126–138. [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Raggi MA, Moi G, Branchi B, Moroni M, Brambilla F, 2007. Experimentally induced aggressiveness in heroin-dependent patients treated with buprenorphine: comparison of patients receiving methadone and healthy subjects. Psychiatry Res. 149, 201–213. [DOI] [PubMed] [Google Scholar]
- Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, Zhang H, Smith SM, Pickering RP, Huang B, Hasin DS, 2016. Epidemiology of DSM-5 drug use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry 73, 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi-Oliveira R, Bauer ME, Pezzi JC, Teixeira AL, Brietzke E, 2011. Interleukin-6 and verbal memory in recurrent major depressive disorder. Neuro. Endocrinol. Lett 32, 540–544. [PubMed] [Google Scholar]
- Gruber SA, Silveri MM, Yurgelun-Todd DA, 2007. Neuropsychological consequences of opiate use. Neuropsychol. Rev 17, 299–315. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Tzilos GK, Silveri MM, Pollack M, Renshaw PF, Kaufman MJ, Yurgelun-Todd DA, 2006. Methadone maintenance improves cognitive performance after two months of treatment. Exp. Clin. Psychopharmacol 14, 157–164. [DOI] [PubMed] [Google Scholar]
- Heberlein A, Dursteler-MacFarland KM, Lenz B, Frieling H, Grosch M, Bonsch D, Kornhuber J, Wiesbeck GA, Bleich S, Hillemacher T, 2011. Serum levels of BDNF are associated with craving in opiate-dependent patients. J. Psychopharmacol 25, 1480–1484. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ, 2007. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry 12, 656–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH, 1997. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc. Natl. Acad. Sci. U. S. A 94, 1500–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoseth EZ, Westlye LT, Hope S, Dieset I, Aukrust P, Melle I, Haukvik UK, Agartz I, Ueland T, Ueland T, Andreassen OA, 2016. Association between cytokine levels, verbal memory and hippocampus volume in psychotic disorders and healthy controls. Acta Psychiatr. Scand 133, 53–62. [DOI] [PubMed] [Google Scholar]
- Hsiao CY, Chen KC, Lee LT, Tsai HC, Chang WH, Lee IH, Chen PS, Lu RB, Yang YK, 2015. The reductions in monetary cost and gains in productivity with methadone maintenance treatment: one year follow-up. Psychiatry Res. 225, 673–679. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK, 2002. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology 42, 829–836. [DOI] [PubMed] [Google Scholar]
- Hudetz JA, Gandhi SD, Iqbal Z, Patterson KM, Pagel PS, 2011. Elevated postoperative inflammatory biomarkers are associated with short- and medium-term cognitive dysfunction after coronary artery surgery. J. Anesth 25, 1–9. [DOI] [PubMed] [Google Scholar]
- Kapadia F, Vlahov D, Donahoe RM, Friedland G, 2005. The role of substance abuse in HIV disease progression: reconciling differences from laboratory and epidemiologic investigations. Clin. Infect. Dis 41, 1027–1034. [DOI] [PubMed] [Google Scholar]
- Kent P, 2013. The evolution of the Wechsler Memory Scale: a selective review. Appl. Neuropsychol. Adult [DOI] [PubMed] [Google Scholar]
- Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, Dhabhar FS, 2013. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav. Immun 30 Suppl, S109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz BA, Corrada MM, Kawas CH, 2009. Elevated C-reactive protein levels are associated with prevalent dementia in the oldest-old. Alzheimers. Dement 5, 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, 2001. Drug addictions. Molecular and cellular endpoints. Ann. N. Y. Acad. Sci 937, 27–49. [PubMed] [Google Scholar]
- Kuo CJ, Tang HS, Tsay CJ, Lin SK, Hu WH, Chen CC, 2003. Prevalence of psychiatric disorders among bereaved survivors of a disastrous earthquake in taiwan. Psychiatr. Serv 54, 249–251. [DOI] [PubMed] [Google Scholar]
- Laurin D, David Curb J, Masaki KH, White LR, Launer LJ, 2009. Midlife C-reactive protein and risk of cognitive decline: a 31-year follow-up. Neurobiol. Aging 30, 1724–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AJ, Katafuchi T, Oda S, Hori T, Oomura Y, 1997. Interleukin-6 inhibits long-term potentiation in rat hippocampal slices. Brain Res. 748, 30–38. [DOI] [PubMed] [Google Scholar]
- Li L, Lu G, Yao H, Zhao Y, Feng Z, Yew DT, 2005. Postmortem changes in the central nervous system and adrenal medulla of the heroin addicts. Int. J. Neurosci 115, 1443–1449. [DOI] [PubMed] [Google Scholar]
- Li LY, Li JL, Zhang HM, Yang WM, Wang K, Fang Y, Wang Y, 2013. TGFbeta1 treatment reduces hippocampal damage, spontaneous recurrent seizures, and learning memory deficits in pilocarpine-treated rats. J. Mol. Neurosci 50, 109–123. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF, 2008. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 59, 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisboa FA, Bradley MJ, Hueman MT, Schobel SA, Gaucher BJ, Styrmisdottir EL, Potter BK, Forsberg JA, Elster EA, 2017. Nonsteroidal anti-inflammatory drugs may affect cytokine response and benefit healing of combat-related extremity wounds. Surgery 161, 1164–1173. [DOI] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B, 2008. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol. Learn. Mem 89, 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung FW, Cheng CT, Chang WT, Shu BC, 2008. Anxiety and mood disorder in young males with mitral valve prolapse. J. Multidiscip. Healthc 1, 89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Li L, Yang EN, Cao WK, 2012. Relationship between interleukin-6 and ammonia in patients with minimal hepatic encephalopathy due to liver cirrhosis. Hepatol. Res 42, 1202–1210. [DOI] [PubMed] [Google Scholar]
- Mangoni AA, Zinellu A, Sotgia S, Carru C, Piga M, Erre GL, 2017. Protective effects of methotrexate against proatherosclerotic cytokines: a review of the evidence. Mediators Inflamm. 2017, 9632846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, 1998. The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior and criminality: a meta-analysis. Addiction 93, 515–532. [DOI] [PubMed] [Google Scholar]
- Matias I, Diniz LP, Buosi A, Neves G, Stipursky J, Gomes FCA, 2017. Flavonoid hesperidin induces synapse formation and improves memory performance through the astrocytic TGF-beta1. Front. Aging Neurosci. 9, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Watanabe S, Suh YH, Yamamoto T, 2002. Effects of intrahippocampal CT105, a carboxyl terminal fragment of beta-amyloid precursor protein, alone/with inflammatory cytokines on working memory in rats. J. Neurochem 82, 234–239. [DOI] [PubMed] [Google Scholar]
- McLachlan C, Crofts N, Wodak A, Crowe S, 1993. The effects of methadone on immune function among injecting drug users: a review. Addiction 88, 257–263. [DOI] [PubMed] [Google Scholar]
- Miller AH, Raison CL, 2016. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol 16, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabati S, Asadikaram G, Arababadi MK, Shahabinejad G, Rezaeian M, Mahmoodi M, Kennedy D, 2013. The plasma levels of the cytokines in opium-addicts and the effects of opium on the cytokines secretion by their lymphocytes. Immunol. Lett 152, 42–46. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Narita M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y, Suzuki T, 2006. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology 31, 2476–2488. [DOI] [PubMed] [Google Scholar]
- Neri S, Bruno CM, Abate G, Ierna D, Mauceri B, Cilio D, Bordonaro F, Pulvirenti D, Italiano C, Caruso L, 2002. Controlled clinical trial to assess the response of recent heroin abusers with chronic hepatitis C virus infection to treatment with interferon alpha-n2b. Clin. Ther 24, 1627–1635. [DOI] [PubMed] [Google Scholar]
- O’Carroll RE, Badenoch LD, 1994. The inter-rater reliability of the Wechsler Memory Scale-Revised Visual Memory test. Br. J. Clin. Psychol 33 ( Pt 2), 208–210. [DOI] [PubMed] [Google Scholar]
- Pacifici R, di Carlo S, Bacosi A, Pichini S, Zuccaro P, 2000. Pharmacokinetics and cytokine production in heroin and morphine-treated mice. Int. J. Immunopharmacol 22, 603–614. [DOI] [PubMed] [Google Scholar]
- Paredes D, Acosta S, Gemma C, Bickford PC, 2010. Role of TNFα Induced Inflammation in Delay Eyeblink Conditioning in Young and Aged Rats. Aging Dis. 1, 191–198. [PMC free article] [PubMed] [Google Scholar]
- Prosser J, Cohen LJ, Steinfeld M, Eisenberg D, London ED, Galynker II, 2006. Neuropsychological functioning in opiate-dependent subjects receiving and following methadone maintenance treatment. Drug Alcohol Depend. 84, 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Wei SJ, Zhang D, Hu X, Xu Z, Wilson B, El-Benna J, Hong JS, Flood PM, 2008. Potent anti-inflammatory and neuroprotective effects of TGF-beta1 are mediated through the inhibition of ERK and p47phox-Ser345 phosphorylation and translocation in microglia. J. Immunol 181, 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K, Artero S, Beluche I, Ancelin ML, Mann A, Dupuy AM, Malafosse A, Boulenger JP, 2004. Prevalence of DSM-IV psychiatric disorder in the French elderly population. Br. J. Psychiatry 184, 147–152. [DOI] [PubMed] [Google Scholar]
- Ross S, Peselow E, 2009. The neurobiology of addictive disorders. Clin. Neuropharmacol 32, 269–276. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Franchi S, Gerra G, Leccese V, Panerai AE, Somaini L, 2008. Buprenorphine and methadone maintenance treatment of heroin addicts preserves immune function. Brain Behav. Immun 22, 606–613. [DOI] [PubMed] [Google Scholar]
- Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO, 1998. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc. Natl. Acad. Sci. U. S. A 95, 7778–7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 Suppl 20, 22–33;quiz 34–57. [PubMed] [Google Scholar]
- Shen CW, Chen YH, 2012. Model selection for generalized estimating equations accommodating dropout missingness. Biometrics 68, 1046–1054. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao LY, Epstein DH, Zhao C, Shuai Y, Yan B, Jin J, Lu L, 2007. The effect of methadone maintenance on illicit opioid use, human immunodeficiency virus and hepatitis C virus infection, health status, employment, and criminal activity among heroin abusers during 6 months of treatment in china. J. Addict. Med 1, 186–190. [DOI] [PubMed] [Google Scholar]
- Soyka M, Kranzler HR, van den Brink W, Krystal J, Moller HJ, Kasper S, Wfsbp Task Force on Treatment, G.f.S.U.D., 2011. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of substance use and related disorders. Part 2: Opioid dependence. World J. Biol. Psychiatry 12, 160–187. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC, 2006. Synaptic scaling mediated by glial TNF-alpha. Nature 440, 1054–1059. [DOI] [PubMed] [Google Scholar]
- Trapero I, Cauli O, 2014. Interleukin 6 and cognitive dysfunction. Metab. Brain Dis. 29, 593–608. [DOI] [PubMed] [Google Scholar]
- Tsuboi I, Tanaka H, Nakao M, Shichijo S, Itoh K, 1995. Nonsteroidal anti-inflammatory drugs differentially regulate cytokine production in human lymphocytes: up-regulation of TNF, IFN-gamma and IL-2, in contrast to down-regulation of IL-6 production. Cytokine 7, 372–379. [DOI] [PubMed] [Google Scholar]
- Vallieres L, Campbell IL, Gage FH, Sawchenko PE, 2002. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J. Neurosci 22, 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT, 2016. Neurobiologic advances from the brain disease model of addiction. N. Engl. J. Med 374, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H, 2012. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc. Natl. Acad. Sci. U. S. A 109, 6325–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE, 2002. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology 59, 371–378. [DOI] [PubMed] [Google Scholar]
- Wechsler D, 1987. WMS-R: Wechsler Memory Scale-Revised. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Wei H, Chadman KK, McCloskey DP, Sheikh AM, Malik M, Brown WT, Li X, 2012. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochim. Biophys. Acta 1822, 831–842. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I, 2011. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun 25, 181–213. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS, 1988. Models for longitudinal data: a generalized estimating equation approach. Biometrics 44, 1049–1060. [PubMed] [Google Scholar]
- Zhang J, Zhang X, Su H, Tao J, Xie Y, Han B, Lu Y, Wei Y, Sun H, Wang Y, Wu W, Zou S, Liang H, Zoghbi AW, Tang W, He J, 2014. Increased serum brain-derived neurotrophic factor levels during opiate withdrawal. Neurosci. Lett 571, 61–65. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Roth-Eichhorn S, Braun N, Culmsee C, Rami A, Krieglstein J, 2000. The expression of transforming growth factor-beta1 (TGF-beta1) in hippocampal neurons: a temporary upregulated protein level after transient forebrain ischemia in the rat. Brain Res. 866, 286–298. [DOI] [PubMed] [Google Scholar]
Web references
Taiwan Food and Drug Administration annual reports for drug abuse examination and statistics. https://www.fda.gov.tw/TC/site.aspx?sid=1578 <accessed on [May 7, 2018]