Abstract
Ulcerative colitis is a chronic inflammatory disease affecting the colon, and its incidence is rising worldwide. The pathogenesis is multifactorial, involving genetic predisposition, epithelial barrier defects, dysregulated immune responses, and environmental factors. Patients with ulcerative colitis have mucosal inflammation starting in the rectum that can extend continuously to proximal segments of the colon. Ulcerative colitis usually presents with bloody diarrhoea and is diagnosed by colonoscopy and histological findings. The aim of management is to induce and then maintain remission, defined as resolution of symptoms and endoscopic healing. Treatments for ulcerative colitis include 5-aminosalicylic acid drugs, steroids, and immunosuppressants. Some patients can require colectomy for medically refractory disease or to treat colonic neoplasia. The therapeutic armamentarium for ulcerative colitis is expanding, and the number of drugs with new targets will rapidly increase in coming years.
Introduction
Ulcerative colitis is a chronic, idiopathic inflammatory disease that affects the colon, most commonly affiicting adults aged 30–40 years and resulting in disability.1,2 It is characterised by relapsing and remitting mucosal inflammation, starting in the rectum and extending to proximal segments of the colon. The aim of therapy is to induce and maintain clinical and endoscopic remission.3 Aminosalicylates are the main choice of treatment for mild to moderate ulcerative colitis, topical and systemic steroids can be used to treat ulcerative colitis flares, while immunosuppressants and biological drugs are used in moderate to severe disease. Colectomy is needed in up to 15% of patients with ulcerative colitis.4 The annual direct and indirect costs related to ulcerative colitis are estimated to be as high as €12.5–29.1 billion in Europe and US$8.1–14.9 billion in the USA.5
Epidemiology
No sex predominance exists in ulcerative colitis.6–8 The peak age of disease onset is between ages 30 years and 40 years.7,9 The incidence and prevalence of ulcerative colitis have been increasing over time worldwide (figure 1).10 The highest incidences of ulcerative colitis have been reported in northern Europe (24.3 per 100 000), Canada (19.2 per 100 000), and Australia (17.4 per 100 000).6,10,11 Prevalence rates are highest in Europe (505 per 100 000), Canada (248 per 100 000), and the USA (214 per 100 000).7,10,12,13 Within Europe, there appears to be differences in ulcerative colitis incidence, with countries located in the western and northern regions having higher incidences than eastern countries.14 The risk of developing ulcerative colitis in children of migrants from low-incidence to high-incidence countries is similar to non-immigrants.15–17 Less data is available from developing countries; however, recognition of ulcerative colitis is increasing in Asia, the Middle East, and South America.18–21
Figure 1. Increase in worldwide incidence of ulcerative colitis over time.
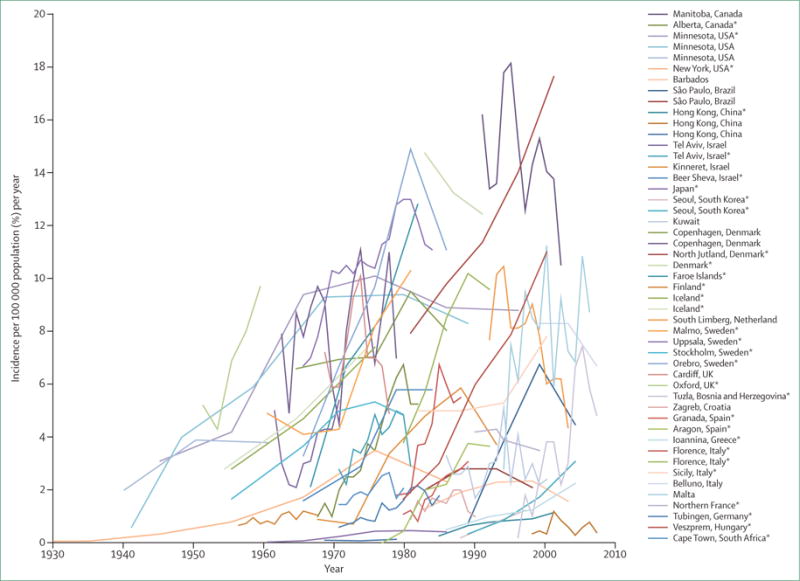
*Statistically significant increase in incidence over time (p<0.05). Reproduced and adapted with permission from Molodecky and colleagues.10
Risk factors
8–14% of patients with ulcerative colitis have a family history of inflammatory bowel disease and first-degree relatives have four times the risk of developing the disease.22,23 Jewish populations have higher rates of ulcerative colitis than other ethnicities.24,25 Genome-wide association studies have identified 200 risk loci for inflammatory bowel disease to date, with most genes contributing to both ulcerative colitis and Crohn’s disease phenotypes.26,27 Examples of loci associated with increased ulcerative colitis susceptibility include human leukocyte antigen and genes associated with barrier function, such as HNF4A and CDH1.27,28 However, genetics only explain 7.5% of disease variance, have little predictive capacity for phenotype, and currently are of limited clinical use.27,28
The rising incidence of ulcerative colitis worldwide suggests the importance of environmental factors in its development. Former cigarette smoking is one of the strongest risk factors associated with ulcerative colitis (odds ratio [OR] 1.79, 95% CI 1.37–2.34), while active smokers are less likely to develop ulcerative colitis compared with former and non-smokers (OR 0.58, 95% CI 0.45–0.75) and have a milder disease course.24,29–32 Appendectomy appears to confer a protective effect against developing ulcerative colitis, especially when done for acute appendicitis in young patients.33 Patients newly diagnosed with ulcerative colitis are more likely than matched controls to have a history of gastroenteritis.34,35 Drugs, such as oral contraceptives, hormone replacement therapy, and non-steroidal anti-inflammatory drugs, have all been associated with an increased risk of ulcerative colitis, while antibiotic exposure has not.36–42 Breastfeeding appears to decrease the risk of ulcerative colitis, while urban living can increase the risk.43,44 Certain ulcerative colitis risk factors that are significant in developed countries might not have the same effect in developing Asian or Middle Eastern populations. For example, smoking might not have as strong an effect, appendectomy does not appear to decrease risk, and antibiotics have been found to be protective when comparing developed countries with developing Asian or Middle Eastern countries.40,41 Pooled data from 11 European prospective studies did not find an association between stress and new onset ulcerative colitis.45
Pathophysiology
Although existing literature often describes the pathogenesis of ulcerative colitis alongside that of Crohn’s disease, important differences exist. Overview of the intestinal immune system in the healthy state and during ulcerative colitis is shown in figure 2. Colonic epithelial cells (colonocytes), and mucous barrier and epithelial barrier defects are strongly implicated in the pathogenesis of ulcerative colitis. The expression of peroxisome proliferator-activated receptor gamma (PPAR-γ), a negative regulator of NF-κB-dependent inflammation, is reduced in the colonocytes of patients with ulcerative colitis, suggesting a causal link.46,47 Existing PPAR-γ agonists are restricted by cardiac and metabolic toxicity. However, novel 5-aminosalicylic acid (5-ASA) analogues with greater PPAR-γ agonistic activity are being developed.48 Autoantibodies against colonocyte-associated tropomyosins have been described in ulcerative colitis,49 but conclusive evidence classifying ulcerative colitis as an autoantibody-mediated disease is scarce. Colonocyte-associated defects within XBP1, a key component of the endoplasmic reticulum stress response pathway, have been reported in ulcerative colitis.50
Figure 2. Overview of the intestinal immune system in the healthy state and for ulcerative colitis with a focus on proven and promising therapeutic targets.
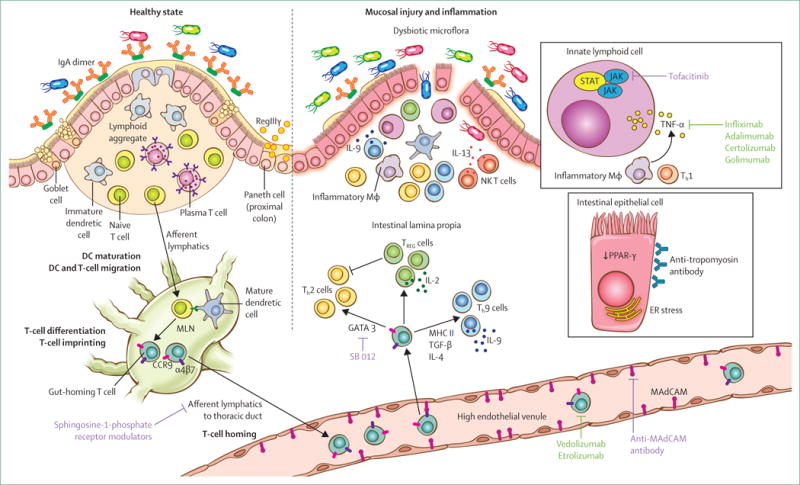
During the healthy state, barrier function is maintained by the mucus layer and epithelial cells bound across tight junctions. Additionally, IgA and antimicrobial factors such as RegIIIγ sequester luminal microflora away from the mucosal immune system. Specialised antigen-presenting cells such as dendritic cells process and present antigen to T and B cells within the draining lymph nodes, defaulting to a tolerising phenotype. Intestinal DCs also imprint T and B lymphocytes to express gut-homing molecules α4β7 and CCR9. Lymphocytes thus imprinted within the gastrointestinal tract enter the systemic circulation and upon reaching intestinal high endothelial venules, the gut-imprinted, α4β7-expressing lymphocytes engage locally expressed MAdCAM and egress the circulation to enter into the intestinal lamina propria. Coordinated activity of innate and adaptive immune cells maintains homoeostasis within the intestinal mucosa at steady state. Ulcerative colitis is associated with damage to the mucosal barrier (inset), allowing the luminal microflora to trigger a sustained and uninhibited inflammatory response. Among the inflammatory cells, TH9 cells perpetuate enterocyte apoptosis and inhibit mucosal healing. IL-13, produced by NK T cells, also contributes to epithelial injury. Additionally, innate lymphoid cells (inset), homeostatic at steady state, contribute to the cytokine production, perpetuating inflammation. Mucosal injury and damage is associated with dysbiosis, which perhaps contributes to the inflammatory cascade. An increasing understanding of the mucosal immune system has led to an expanding array of therapeutic targets. Of these, TNF-α antagonists and homing inhibitors are currently in clinical practice (green text), while the others are in early to advanced stages of clinical development (purple text). Illustration by Jill Gregory. Printed with permission of ©Mount Sinai Health System. IgA=immunoglobulin A. DC=dendritic cell. MAdCAM=mucosal addressin cell associated molecule. IL=interleukin. Th=T-helper cell. TREG=regulatory T cell. IFN=interferon, Mϕ=macrophage. TGF=transforming growth factor. ER=endoplasmic reticulum. MHC=major histocompatibility complex. NK T cell=natural killer T cell. MLN=mesenteric lymph node.
Alterations in trefoil factors, a family of goblet cell-derived proteins that are produced in response to mucosal injury and contribute to the integrity of the mucosal barrier, have been described in patients with ulcerative colitis.51,52 The contention that barrier function defects are the primary drivers of disease is supported by the fact that patients with active ulcerative colitis have depleted colonic goblet cells and a permeable mucus barrier.53
Dysbiosis is seen in patients with ulcerative colitis, although to a lesser degree than in patients with Crohn’s disease.54 Decreased biodiversity, with a lower proportion of Firmicutes and increased Gamma-proteobacteria and Enterobacteriaceae, has been reported in patients with ulterative colitis.55 Additionally, patients with the disease have increased sulphite-reducing Deltaproteobacteria in the colon.56 However, it is unclear if dysbiosis is the cause or effect of mucosal inflammation.
The expression levels of Toll-like receptors 2 (TLR2) and TLR4 are increased in colonocytes and the lamina propria in active ulcerative colitis, although it is unclear if the increase in expression is a cause or consequence of mucosal inflammation.57 Similarly, TLR4 polymorphisms have been reported in patients with ulcerative colitis and Crohn’s disease but their implications for disease pathogenesis are unclear.58 Activated neutrophils accumulate in the blood and colonic tissue of patients with active ulcerative colitis compared with normal volunteers.59 Dendritic cells in patients with ulcerative colitis have enhanced expression of costimulatory molecules and are likely to be first responders in the setting of a breach in barrier integrity.60
Innate lymphoid cells (ILCs) might be central in the pathogenesis of inflammatory bowel disease. ILC3 are major mediators of chronic intestinal inflammation.61 Furthermore, ILCs isolated from patients with active ulcerative colitis show increased gene expression of key ILC3 cytokines (IL17A and IL22), transcription factors (RORC and AHR), and cytokine receptors (including IL23R).62 The possibility that ILCs might be drivers of disease pathogenesis has led to a number of potential novel therapeutic targets.
Although elevated IgM, IgA, and IgG concentrations are reported in inflammatory bowel disease, there is a disproportionate increase in IgG1 antibodies in patients with ulcerative colitis. It is not known whether B cells are drivers of disease pathogenesis or merely responsive to barrier disruption.
Current evidence implicates both innate and adaptive cellular immunity as key to disease pathogenesis. Earlier evidence suggested that ulcerative colitis is a modified T-helper-2 (Th2) disease, while Crohn’s disease is Th1 driven. In support, colonic lamina propria cells from patients with ulcerative colitis were found to contain Th2-polarised T cells that produce interleukin-5 (IL-5).63 Additionally, IL-4 and IL-13 mRNA levels were significantly increased in rectal biopsies from patients with ulcerative colitis compared with patients in the control group.64 Subsequent data have further implicated IL-13 in the pathogenesis of ulcerative colitis. IL-13, produced by non-classical natural killer T cells (perhaps a member of the ILC family), is a key mediator of epithelial cytotoxicity and barrier dysfunction in ulcerative colitis.65,66
Extending the T-helper Th1/Th2 paradigm for Crohn’s disease versus ulcerative colitis, data from 2014 show that a novel population of CD4-positive Th cells, which produce IL-9, are identified by the transcription factor PU.1 and contribute to the development of ulcerative colitis.67 Th9 cells develop after undifferentiated Th (Th0) cells encounter MHC class II-antigen complexes in the presence of the cytokines transforming growth factor-β and IL-4. IL-9 produced by Th9 cells inhibits cellular proliferation and repair, and has a negative effect on intestinal barrier function. Additionally, IL-9 modestly but significantly increases tissue concentrations of tumour necrosis factor-α (TNF-α).
Naive lymphocytes are imprinted during activation with specific trafficking programmes. Dendritic cells play a central part in this process by integrating environmental cues and inducing expression of specific integrins and chemokine receptors. For example, dendritic cells residing in Peyer’s patches or small bowel draining lymph nodes metabolise vitamin A to produce retinoic acid and induce the expression of integrin α4β7 and CCR9 on T and B lymphocytes. Therefore, imprinted cells enter into circulation, and upon re-entering the gut vasculature they engage their respective ligands—MAdCAM-1 (for α4β7) and CCL25 (for CCR9). While defects in mucosal homing have not yet been shown in patients with ulcerative colitis, therapeutic strategies targeting α4β7 interaction with MAdCAM have become major tools in the management of ulcerative colitis.68
Clinical presentation and differential diagnosis
Ulcerative colitis is a chronic disease affecting the colonic mucosa that most commonly presents with blood in the stool and diarrhoea. Up to 15% of patients can initially present with severe disease.69 Symptoms can include urgency, incontinence, fatigue, increased frequency of bowel movements, mucus discharge, nocturnal defecations, and abdominal discomfort (cramps), although abdominal pain tends to be less of a hallmark feature than in Crohn’s disease.70 Fevers and weight loss can also be present in severe disease. Ulcerative colitis is classified by the extent of colonic involvement (figure 3).71 Clinical presentation might vary on the basis of disease extent. Patients with proctitis might predominantly have urgency and tenesmus (sensation of incomplete evacuation), while in pancolitis, bloody diarrhoea and abdominal pain might be more prominent. Up to 10% of patients with proctitis or left-sided colitis can suffer from paradoxical constipation. Physical examination might reveal signs of anaemia, abdominal tenderness, and blood on rectal exam. Abdominal distention and tympany on percussion might indicate colonic dilatation, requiring prompt radiological assessment. Patients with ulcerative colitis might have anal fissures or skin tags due to irritation from diarrhoea, but the presence of anal or perianal fistulas should raise suspicion for Crohn’s disease. Clostridium diffi cile is an important precipitant of flares and is associated with an increased risk of surgery and mortality, and should be ruled out at diagnosis and flare-ups.72,73 The panel lists the differential diagnoses.
Figure 3. Ulcerative colitis phenotypes by Montreal Classification71.
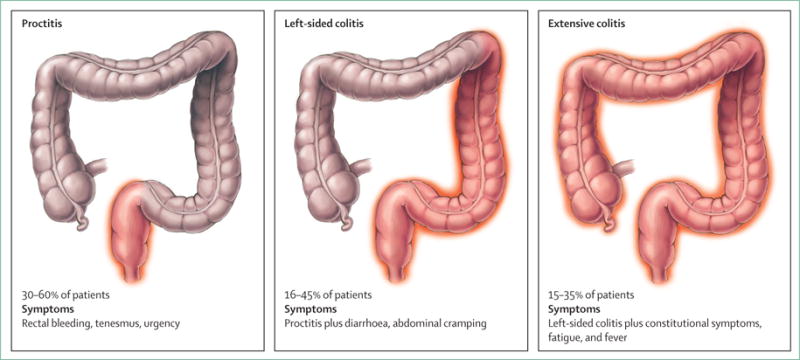
Symptoms and treatment strategy can differ based on extent of disease. Illustration by Jill Gregory. Printed with permission of ©Mount Sinai Health System.
Extraintestinal manifestations can occur in about a third of patients with ulcerative colitis, and up to a quarter might have extraintestinal manifestations before inflammatory bowel disease diagnosis (appendix p 6).75,76 Peripheral arthritis appears to be the most common extraintestinal manifestation; primary sclerosing cholangitis and pyoderma gangrenosum are more common in ulcerative colitis than in Crohn’s disease.75,76 The risk of venous thromboembolism in patients with inflammatory bowel disease is increased three to four times, and is greater when the patient is admitted with a flare or being treated with corticosteroids.77–80 Clinicians should have a high index of suspicion for venous thromboembolism, and hospitalised patients with ulcerative colitis should be prescribed venous thromboembolism prophylaxis.81
Diagnostic investigations
The diagnosis of ulcerative colitis is based on a combination of symptoms, endoscopic findings, histology, and the absence of alternative diagnoses.69,82 All patients with possible ulcerative colitis should have stool assessments (stool culture and Clostridium difficile assay) to rule out enteric superimposed infections. Patients might have anaemia, iron deficiency, leucocytosis, or thrombocytosis. Hypoalbuminaemia can be observed in severe disease, in which it is a predictor of colectomy and poor response to biological drugs.83,84 Markers of inflammation, such as ESR and C-reactive protein, can be elevated (severe ulcerative colitis) or normal (mild to moderate disease). Perinuclear antineutrophil cytoplasmic antibodies can be elevated in ulcerative colitis, but are non-specific and have low sensitivity (0.55%, 95% CI 0.53-0.58) so are not recommended as a diagnostic test.69,71,82,85 Non-invasive stool biomarkers are more specific for intestinal inflammation.86 Fecal calprotectin, a protein detectable in stool that correlates with increased neutrophils in the intestine, can be helpful in ruling out inflammatory bowel disease, since patients with low fecal calprotectin have a less than 1% chance of having inflammatory bowel disease.86–88 However, fecal calprotectin does not distinguish between various causes of intestinal inflammation so cannot be used as a definitive diagnostic tool in ulcerative colitis.69
Endoscopy with biopsies is the only way to establish the diagnosis of ulcerative colitis. Colonoscopy with intubation of the terminal ileum is recommended for patients with suspected inflammatory bowel disease. Classic endoscopic findings in ulcerative colitis include erythema, loss of normal vascular pattern, granularity, erosions, friability, bleeding, and ulcerations (appendix p 17).82,89 The disease generally begins in the rectum, extending proximally in a continuous, circumferential pattern. Rectal sparing or patchy disease can be the result of topical or systemic medications and should not necessarily be interpreted as evidence of Crohn’s disease.90 Mucosal inflammation often has a clear demarcation between inflamed and normal mucosa, although histological inflammation can be found in normal appearing mucosa.82,89 Ulcers in ulcerative colitis are always associated with mucosal inflammation in contrast with Crohn’s disease, in which the surrounding mucosa can appear uninflamed.69 Up to 75% of patients with ulcerative colitis with distal disease also have an isolated area of inflammation around the appendiceal orifice, commonly known as a cecal patch.91,92 Up to 20% of patients with pancolitis can have mild inflammatory changes in the terminal ileum called backwash ileitis.89,93 Ileitis that is severe or seen in the absence of pancolitis should raise suspicion of Crohn’s disease. An esophagogastroduodenoscopy should be performed in patients with symptoms of upper tract involvement to rule out Crohn’s disease.93 At least two biopsies should be taken from six different areas (terminal ileum, ascending, transverse, descending, sigmoid colon, and rectum), including normal appearing areas, since inflammatory changes could become evident on microscopy.89 Suggestive histological findings include distortion of crypt architecture, crypt shortening, increased lymphocytes and plasma cells in the lamina propria (basal plasmacytosis), mucin depletion, and paneth cell metaplasia (appendix p 18).82,94 Generally, imaging studies are of limited use in establishing the diagnosis. In patients with acute severe ulcerative colitis, assessment for toxic megacolon (defined as mid-transverse colon dilation >5.5 cm) should be performed with a plain upright abdominal film.95 CT and MRI might show a thickened, ahaustral colon, but are not sensitive or specific enough to be diagnostic tools.95
Disease severity assessment
Determining the severity and extent of ulcerative colitis is important for selecting the most appropriate treatment. Disease severity is typically classified as remission, mild, moderate, or severe. Numerous ulcerative colitis severity indices exist, but of the more commonly used are the Mayo score, Lichtiger score, and Simple Clinical Colitis Activity Index (appendix p 6).69,96–98 Endoscopy is essential in assessing disease severity since endoscopic healing is associated with improved remission rates and decreased risk of colectomy.99 Frequently used endoscopic ulcerative colitis scores include the endoscopy subscore of the Mayo score and the Ulcerative Colitis Endoscopic Index of severity (appendix p 9).96,100 Additionally, histological disease activity can be classified on the basis of histological scores such as the Robarts Histopathology index or Nancy index (appendix p 19).101,102 Ulcerative colitis severity scores account for disease activity at a single timepoint and do not take into account the entirety of the effect of ulcerative colitis. There is, therefore, a push to redefine disease severity using composite criteria that incorporate (1) disease effect on patient symptoms, quality of life, and disability; (2) measureable inflammatory burden using objective markers of disease activity and extent; and (3) disease course, including structural damage, number of flares, and extraintestinal manifestations.103
Natural history
At presentation, 30–60% of patients with ulcerative colitis have proctitis, 16–45% have left-sided colitis, and 14–35% have extensive pancolitis in population-based studies (figure 3).4 Ulcerative colitis can progress proximally in 10–19% of patients after 5 years, and in up to 28% of patients at 10 years.4 Most patients with ulcerative colitis have a relapsing and remitting disease course with periodic flares.7 When ulcerative colitis flares are associated with proximal disease extension, patients are more likely to need immunosuppressants, biological drugs, or surgery.104,105 Age of onset appears to affect the disease course, since patients with disease onset after age 60 years tend to have milder disease compared with younger patients.106 Primary sclerosing cholangitis-associated ulcerative colitis could be a distinct phenotype, because it is more likely to be extensive, milder, and associated with rectal sparing and so-called backwash ileitis compared with patients with ulcerative colitis without primary sclerosing cholangitis.107 Risk factors for aggressive or complicated disease include a younger age at onset (<40 years), pancolitis, lack of endoscopic healing while in clinical remission, deep ulcerations, and high concentrations of perinuclear antineutrophil cytoplasmic antibodies.104 A small number of patients (5–10%) initially classified with ulcerative colitis might eventually have their diagnosis changed to Crohn’s disease.7 Patients with ulcerative colitis can develop structural and functional damage to the colon, including benign strictures, colonic dysmotility, and anorectal dysfunction.2 Patients with the disease are at increased risk of colorectal cancer, but over time this risk has decreased and might be approaching the general population; however, the risk remains elevated in certain populations, such as those with long duration of disease, primary sclerosing cholangitis, and uncontrolled inflammation.108,109 The risk for surgery in ulcerative colitis has decreased over past decades, but is still substantial with the chance of needing surgery at 5 years being 11.6%, and at 10 years being 15.6%.110 Risk factors for colectomy have been incorporated into a prediction model that includes age less than 40 years at diagnosis, extensive disease, need for systemic steroids, and elevated inflammatory markers.111 Patients with ulcerative colitis do not appear to have an overall increased mortality compared with the general population, but are more likely to have a disability preventing them from working.1,112,113
Management
The primary aim of medical management is to induce and maintain remission with the long-term goals of preventing disability, colectomy, and colorectal cancer. Targets for remission include resolution of clinical symptoms, defined as cessation of rectal bleeding and improvement in bowel habits, and endoscopic healing, which is frequently defined as an endoscopic Mayo score of zero or one.3,114 Patient symptoms and physician assessment can fail to correlate with the endoscopic activity of ulcerative colitis.115–117 It is important to directly assess mucosal and histological inflammation with colonoscopy, since endoscopic healing has been shown to greatly improve long-term clinical remission, decrease risk of colectomy, and limit corticosteroid use.99 The selection of medications is guided by disease severity and extent. A rapid step-up approach based on ulcerative colitis severity and treatment response while closely monitoring intestinal inflammation is recommended (figure 4, appendix). Early use of biological drugs should be considered in patients admitted to hospital with acute severe ulcerative colitis, as well as in steroid-refractory ulcerative colitis. Once remission is induced, medications can be continued or added to maintain remission. Disease extent can help inform therapeutic choices because patients with proctitis might only require topical therapy, such as suppositories, whereas patients with more extensive disease benefit from systemic therapy. Several guidelines are available to guide decision making.82,114,118
Figure 4. Suggested treatment approach algorithm for mild to moderate ulcerative colitis.
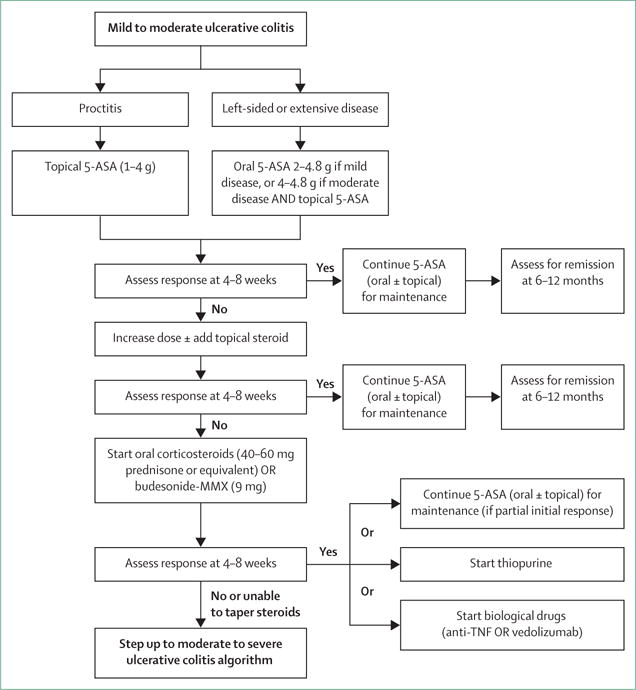
Based on Toronto Consensus and European Crohn’s and Colitis Organisation guidelines.114,118 For patients needing and responding to steroids, the choice for maintenance medication can be either 5-ASA, thiopurine, or a biological drug. 5-ASA can be considered if partial initial response and first course of steroids. Thiopurines can be used if no response to 5-ASA, low risk of complications, and first course of steroids. Biological drugs should be used if unable to taper steroids, second course of steroids, or higher risk of complications. 5-ASA=5-aminosalicylic acid.
Mild to moderate disease
First-line therapy in mild to moderate disease is the 5-ASA drugs, which can be administered as suppositories, enemas, or oral formulations (figure 4). There does not appear to be any difference in efficacy or safety between different 5-ASA formulations.119 Sulfasalazine, which is metabolised to 5-ASA, appears to have similar efficacy to 5-ASA drugs, but tends to be less well tolerated.114 Patients with proctitis should be treated initially with 5-ASA suppositories since they directly target the site of inflammation and appear to be more effective than oral 5-ASA.114,118,120 In left-sided colitis, 5-ASA should be administered as an enema instead of a suppository in order to reach the splenic flexure. For patients with left-sided or extensive disease, it is recommended that oral 5-ASA be used in combination with topical 5-ASA to induce remission.114,118 Oral 5-ASA doses of 2 g or higher per day are more effective than lower doses at inducing and maintaining remission.121–123 5-ASA can be started at a dose of 2.0–2.4 g per day and increased up to 4.8 g, if needed.114,123 Dosing of 5-ASA once a day has similar efficacy to divided doses and could increase adherence.114,123 Patients typically see a response within 14 days, but this response might take up to 8 weeks for symptomatic remission.114 5-ASA drugs have also been shown to be effective at maintaining remission, and patients who achieve remission with 5-ASA should continue on the same medication.114
Patients who do not respond or do not achieve remission on 5-ASA drugs can be treated with corticosteroids. Rectal corticosteroids can be tried as a second-line add-on therapy to induce remission in proctitis or left-sided ulcerative colitis. Topical 5-ASA is superior to topical corticosteroids at inducing remission (OR 2.01, 95% CI 1.41–2.88).114 However, clinical and endoscopic improvement could be higher when combining rectal 5-ASA and corticosteroids.124 Additionally, rectal corticosteroids can be administered as foam formulations that are often better tolerated than enemas by patients with active distal ulcerative colitis.114 Oral corticosteroids are needed to induce remission in patients with mild to moderate disease who are not benefiting from 5-ASA treatment. Oral steroids with minimal systemic activity (due to high first-pass liver metabolism) such as budesonide-multimatrix and prolonged release beclomethasone dipropionate are effective at inducing remission in ulcerative colitis.125–127 Given the lower risk for systemic side-effects, these drugs should be considered as alternative first-line induction drugs for mild to moderate ulcerative colitis, failing 5-ASA. Systemic glucocorticoids are effective at inducing remission in ulcerative colitis with a number needed to treat of three.128 The typical starting dose is 40–60 mg prednisone daily, or the equivalent oral steroid.82 Response should be seen within 2 weeks and then steroids can be tapered. No defined tapering schedule exists, but a common approach is to taper by 5–10 mg per week until reaching 20 mg, then decrease by 2.5–5 mg per week until completed.82,118 Corticosteroids should not be used for maintenance of remission because of a lack of long-term efficacy and risk of side-effects.114 If remission is achieved using corticosteroids, 5-ASA can be considered for maintenance in patients with a mild flare who were recently diagnosed or are naive to 5-ASA. However, patients with poor prognostic factors (young age of disease onset, extensive colitis, deep ulcerations), who require two or more courses of steroids in a year or are unable to effectively taper off steroids, should step-up therapy and start treatment with drugs such as thiopurines or biological drugs (anti-TNF-α or anti-integrin therapy).114
Moderate to severe disease
Patients with moderate to severe colitis should be managed with thiopurines or biological drugs, or both (figure 5). Thiopurines (azathioprine or 6-mercaptopurine) can be used in patients with steroid-dependent moderate to severe disease to maintain remission. Several small studies129,130 reported the modest efficacy of methotrexate in ulcerative colitis, but results of a clinical trial131 were mixed; therefore, its role in ulcerative colitis treatment is still being investigated.
Figure 5. Suggested treatment approach algorithm for moderate to severe ulcerative colitis.
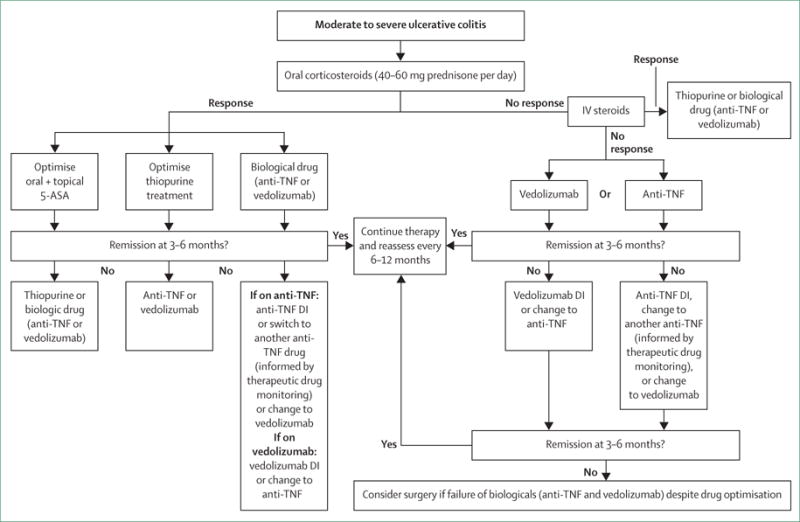
Based on Toronto Consensus and European Crohn’s and Colitis Organisation guidelines.114,118 5-ASA=5-aminosalicylic acid. IV=intravenous. DI=dose intensification.
Anti-TNF-α drugs, such as infliximab, adalimumab, and golimumab, are effective at inducing and maintaining remission in moderate to severe disease.132–135 Infliximab can also be used in patients admitted to hospital with severe ulcerative colitis and remains the most widely used biological for ulcerative colitis.132,133 Azathioprine alone is less effective than in combination with infliximab to achieve both clinical remission and endoscopic healing, while the difference is statistically significant only for endoscopic healing between azathioprine alone and infliximab monotherapy (SUCCESS trial).136
A new class of biological drugs, anti-adhesion molecule inhibitors, are now available.68 Vedolizumab blocks the gut-homing α4β7 integrin and is approved for moderate to severe ulcerative colitis, refractory to standard medications. On the basis of efficacy and safety data, vedolizumab could be considered as a first-line biological for ulcerative colitis.137
Acute severe ulcerative colitis
Patients with acute severe ulcerative colitis, defined as six or more bloody bowel movements per day and at least one of the following: pulse rate >90 beats per min, temperature >37.8°C, haemoglobin count <10.5 g/dL, or ESR >30 mm/h, should be admitted to a tertiary care centre.69 Acute severe ulcerative colitis is associated with significant morbidity and mortality of approximately 1%.138 Patients are initially treated with intravenous corticosteroids to which approximately 65% will respond.139 For patients not responding to intravenous corticosteroids within 3 to 5 days, rescue medical therapy with either ciclosporin or infliximab can be attempted. Both drugs are equally efficacious in acute severe ulcerative colitis.140,141 Delays in surgery can increase postoperative complications and mortality increases significantly after 7 days.142,143 If there is no response to one of these drugs, colectomy should be performed. Further discussion of acute severe ulcerative colitis is in the appendix.
Surgery
Absolute indications for surgery include uncontrolled haemorrhage, perforation, and colorectal carcinoma or dysplastic lesions not amenable to endoscopic removal.82,144 Surgery is also indicated in refractory acute severe ulcerative colitis or medically refractory disease.82 The most commonly performed surgery for ulcerative colitis is restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA). When surgery is emergent or urgent, it is typically done in two or three stages starting with a subtotal colectomy and creation of a temporary ileostomy (first stage) to decrease the risk of immediate postoperative complications such as anastomotic leak or pelvic sepsis.145 The ileal pouch is then created and anastomosed to the anal canal with a diverting ileostomy (second stage), which is eventually taken down to restore intestinal continuity (third stage). IPAA surgery should be done in high-volume referral centres where pouch failure rates are lower.146
Early postoperative complications following IPAA can occur in up to 33% of patients.147 Excluding pouchitis, late complications, such as bowel obstructions and strictures, can occur in as many as 30% of patients with pouch failure rates up to 5%.145,146 A common concern related to IPAA is decreased fertility and increased sexual dysfunction.148 Laparoscopic restorative proctocolectomy with IPAA is associated with a significantly higher prevalence of pregnancy than open surgery, and has a similar prevalence of infertility compared with controls who had an appendectomy.149,150 Up to 25% of men might experience erectile dysfunction or retrograde ejaculation following IPAA, but satisfaction with sexual life might not be different or could even improve following surgery because of the negative effects of active ulcerative colitis on sexuality.151
Pouchitis is a non-specific inflammatory condition of the ileal pouch and is the most commonly encountered postoperative issue following IPAA.152 Up to 46% of patients who had IPAA will have at least one episode of pouchitis, showing that colectomy should not be presented as a cure for ulcerative colitis.153 At baseline, patients can have four to seven daily bowel movements, but pouchitis typically presents with increased frequency, urgency, incontinence, or abdominal discomfort.153 Most episodes can be successfully treated by 2–4 weeks of ciprofloxacin (1000 mg daily) or metronidazole (20 mg/kg daily); one small trial154 suggested that ciprofloxacin could be more effective than metronidazole.152,154 10–15% of patients can develop chronic pouchitis with frequent relapses or symptoms persisting beyond 4 weeks of treatment.152 Patients might often have residual rectal tissue, referred to as a rectal cuff, at the anastomosis between the ileum and anal canal. This area can become inflamed leading to cuffitis, which, in contrast with pouchitis, typically presents with bleeding and can usually be successfully treated with 5-ASA suppositories.152
Treat to target, disease monitoring, and long-term management
The treatment strategy in ulcerative colitis has evolved into a treat to target approach, in which patients are regularly assessed to ensure they are meeting strict targets for disease control. The targets for ulcerative colitis are resolution of patient reported outcomes (rectal bleeding and diarrhoea) and endoscopic remission.3 Given the importance of endoscopic healing, the colon should be directly assessed 3–6 months following the initiation of a new treatment.3 Flexible sigmoidoscopy is sufficient for assessing endoscopic healing.155 Patients should have a regular follow-up at a minimum of every 3 months until symptom resolution, and then at least every 6–12 months with the goal of maintaining tight control.3 Once patients are in remission, non-invasive markers, such as fecal calprotectin, can be used to monitor disease activity. In a post-hoc analysis of a clinical trial,156 a fecal calprotectin cutoff of 150 mg/kg was best for endoscopic remission (sensitivity of 0.79 and specificity of 0.75).
When patients have symptoms suggestive of an ulcerative colitis flare, infection should be excluded and objective assessments, such as sigmoidoscopy, fecal calprotectin, or stool lactoferrin should be done. Fecal calprotectin appears to have the highest sensitivity and specificity for active inflammation.157,158 If there is objective evidence of inflammation, medications should be optimised by reviewing dosage, administration, and adherence. Therapeutic drug monitoring can ensure adequate dosing. For example, patients given azathioprine or 6-mercaptopurine can have the blood concentrations of the active metabolite, 6-thioguanine, checked to ensure an adequate therapeutic amount.159 Assessment of anti-TNF-α drug concentrations can similarly be of clinical use (appendix p 21). Higher serum concentrations of infliximab and adalimumab during induction and at trough are associated with endoscopic healing and clinical remission.160,161 The results of a randomised trial showed that dosing of infliximab based on a target trough of 3–7 μL/mL did not improve remission at 1 year, but did lead to more efficient drug use and a decreased risk of relapse.162 Additionally, assays for biological concentrations provide data on the development of antidrug antibodies, which have been associated with decreased drug concentrations and loss of response.163
The major elements of chronic care for patients with ulcerative colitis are colon cancer surveillance and health maintenance. Patients with ulcerative colitis should undergo regular surveillance colonoscopy to detect dysplasia and early cancer. Patients with extensive colitis and left-sided disease should undergo a colonoscopy every 1–2 years starting 8 years after diagnosis.82 Proctitis confers no increased risk of colorectal cancer so these patients should follow standard colorectal cancer screening guidelines.89 The risk of colorectal cancer in patients with ulcerative colitis and primary sclerosing cholangitis is up to five times greater than other patients with the disease, so surveillance should begin at the time of diagnosis and continue annually.164,165 Guidelines on surveillance vary among international societies (appendix p 10). Dysplasia and neoplastic lesions in ulcerative colitis can often be non-polypoid, flat, ill-defined, or multifocal, so a prevalent strategy has been to do four random biopsies every 10 cm in the colon to increase detection of neoplasia.89 Enhanced visualisation using chromo-endoscopy, by spraying the colon with methylene blue or indigo carmine and doing targeted biopsies, is recommended by the SCENIC (Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus Recommendations) statement.144 Colectomy is recommended if colorectal cancer or high-grade dysplasia that is not endoscopically resectable is detected. Patients with multifocal low-grade dysplasia, dense pseudo-polyposis, or strictures limiting effective surveillance might also require surgery.109
Patients should have their vaccination status reviewed regularly. Live vaccines are contraindicated while immunosuppressed.82 Annual influenza vaccine, tetanus and diphtheria boosters, and pneumococcal vaccine every 5 years are recommended.166 Hepatitis B status should be checked before initiating treatment with anti-TNF-α drugs and those who are not immune should be vaccinated. Patients should be screened for osteoporosis if exposed to at least 3 months of corticosteroids, are malnourished, or have typical risk factors (postmenopausal women, family history, smoking). Thiopurines increase the risk of non-melanoma skin cancer (hazard ratio 5.9, 95% CI 2.1–16.4) and biological drugs are associated with increased rates of melanoma (OR 1.88, 95% CI 1.08–3.29).167–169 Patients on these medications should limit sun exposure and have annual dermatological assessments. A checklist for routine health maintenance and preventive care in inflammatory bowel disease is available for reference (appendix p 13).
Future directions and controversies
The number of drugs modulating different disease pathways is expected to expand in the near future. There are at least 27 new drugs for ulcerative colitis with either recently completed or active trials.170 One example is the oral pan-janus kinase inhibitor tofacitinib, which has shown higher rates of clinical remission than placebo in phase 2 studies.171 Etrolizumab, a subcutaneous monoclonal antibody that blocks the β7 subunit of the heterodimeric integrins α4β7 and αEβ7 achieved higher clinical remission rates than placebo in a phase 2 trial.172 An oral anti-α4 integrin therapy (AJM300) significantly increased clinical remission and endoscopic healing in a phase 2 trial.173 An oral drug inhibiting sphingosine-1-phosphate receptors that blocks lymphocyte egress from lymph nodes has also shown efficacy.174 In a small trial of 5-ASA non-responders, curcumin increased endoscopic remission in mild to moderate ulcerative colitis as an add-on therapy.175 Biosimilar biological drugs should decrease the cost of therapy. Results from initial studies with an infliximab biosimilar, CT-P13, have shown efficacy at inducing endoscopic healing in ulcerative colitis.176 However, immunogenicity and efficacy remains a concern particularly in patients switching from the originator to the biosimilar.177
Studies on the efficacy of fecal microbiota transplantation (FMT) in ulcerative colitis have yielded conflicting results. While results from one study showed no improvement in clinical and endoscopic remission at 12 weeks following two infusions of FMT product from healthy donors via a nasogastric tube, a second study showed higher endoscopic remission at 7 weeks in patients treated with weekly FMT enemas.178,179 Results from the largest randomised FMT study to date, FOCUS,180 showed a higher rate of clinical and endoscopic remission or response at 8 weeks post-FMT first administered through colonoscopy followed by enemas five times a week. Although these findings are intriguing, evidence to recommend FMT for ulcerative colitis is still insufficient. Notably, FMT for treatment of recurrent Clostridium difficile in patients with inflammatory bowel disease (including those on immunosuppression) appears to be safe but less efficacious.181
Another area that requires further research is determining ideal targets for treatment. The optimum level of symptom control and mucosal healing that is needed to prevent long-term complications remains to be fully understood. Histological remission could ultimately become the target for therapy in ulcerative colitis.182,183
The need for precision medicine in ulcerative colitis will be greater than ever, as clinicians will have to choose which drug to use and which molecular pathway to target. An increased understanding of pharmacogenomics, biomarkers, and clinical features that identify subpopulations of patients who will best respond to specific medications will be needed to tailor therapy to individual patients. Other future research directions include combining biological therapies and head-to-head trials to determine the most optimal therapies and how to best position new medications.
Supplementary Material
Search strategy and selection criteria.
We searched for relevant manuscripts in PubMed/MEDLINE, Embase, and Cochrane Central from their inception until March 1, 2016. The search combined the MeSH terms “ulcerative colitis” and “inflammatory bowel disease” with the subheadings “epidemiology”, “etiology”, “physiopathology”, “innate and adaptive immunity”, “diagnosis”, “genetics”, “diagnosis”, “endoscopy”, “therapy”, “surveillance”, and “complications”. Bibliographies of included articles were searched and experts in inflammatory bowel disease were consulted to identify additional studies. Relevant articles and abstracts published in English were critically reviewed. Priority was given to manuscripts published in the past 5 years, randomised placebo-controlled trials, and meta-analyses.
Panel: Major differential diagnoses in diagnostic examination of ulcerative colitis74.
Infectious colitis: bacterial, viral, fungal (histoplasmosis), mycobacterial, and Clostridium difficile
Ischaemic colitis
Segmental colitis associated with diverticulitis
Radiation-induced colitis or proctitis
Medication-induced colitis (in particular non-steroidal anti-inflammatory drugs)
Crohn’s disease
Sexually transmitted diseases (particularly in patients with proctitis who have engaged in anal intercourse): Chlamydia trachomatis, Neisseria gonorrhoeae, herpes, and syphilis
If predominant symptom is diarrhoea and not bleeding: coeliac disease, microscopic colitis, lactose or other food intolerances, and irritable bowel syndrome
Acknowledgments
We would like to acknowledge and thank academic medical illustrator Jill Gregory for her wonderful help with figure conception and design. We would like to thank Jerome Waye, Mabel Ko, and Aude Marchal-Bressenot for their help in providing endoscopic and histological images. This Seminar was supported in part by the SUCCESS (Sinai Ulcerative Colitis Clinical, Experimental and System Studies) grant (GCO14-0560).
Footnotes
Contributors
RCU, SM, and PBA did the literature search, wrote the manuscript and drafted the figures. LP-B and J-FC wrote and revised manuscript. All authors critically revised the manuscript and approved the final version of the manuscript.
Declaration of interests
RCU has no conflicts of interest. SM has served as a consultant for Pfizer Inc and receives research funding support from Takeda Pharmaceuticals. PBA has received Speaker fees for MSD, AbbVie, Allergen, Ferring, Warner Chilcott, and Napp. LP-B has received consulting fees from Merck, AbbVie, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Therakos, Pharmacosmos, Pilège, Bristol-Myers Squibb, Union Chimique Belge (UCB) Pharmaceuticals, Hospira, Celltrion, Takeda, Biogaran, Boerhinger-Ingelheim, Lilly, Pfizer, HAC Pharma, Index Pharmaceuticals, Amgen, Sandoz, Forward Pharma GmbH, Celgene, Biogen, Lycera, and Samsung Bioepis, and lecture fees from Merck, AbbVie, Takeda, Janssen, Takeda, Ferring, Norgine, Tillots, Vifor, Therakos, Mitsubishi, and HAC Pharma. J-FC has served as consultant or advisory board member for AbbVie, Amgen, AstraZeneca, ABScience, Boehringer, Bristol-Meyers Squibb, Celgene, Celltrion, Danone, Enterome, Evidera, Ferring, Genentech, Giuliani SPA, Given Imaging, Janssen & Janssen, Immune Pharmaceuticals, Intestinal Biotech Development, Kyowa Kirin Pharma, Lilly, Medimmune, Merck Sharp Dohme, Merck & Co, Millennium Pharmaceuticals Inc, Navigant Consulting, Neovacs, Nestle Nutrition Sciences Partner, Nutrition Science Partners Ltd, Pfizer, Prometheus Laboratories, Protagonist Therapies, Receptos, Sanofi, Schering Plough Corporation, Second Genome, Shire, Takeda, Teva Pharmaceuticals, Tigenix, UCB, UEGW AbbVie Advisory Board, UEGW AbbVie Symposium, Vertex, and Dr August Wolff GmbH Co.
References
- 1.Høivik ML, Moum B, Solberg IC, et al. Work disability in inflammatory bowel disease patients 10 years after disease onset: results from the IBSEN Study. Gut. 2013;62:368–75. doi: 10.1136/gutjnl-2012-302311. [DOI] [PubMed] [Google Scholar]
- 2.Torres J, Billioud V, Sachar DB, Peyrin-Biroulet L, Colombel J-F. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis. 2012;18:1356–63. doi: 10.1002/ibd.22839. [DOI] [PubMed] [Google Scholar]
- 3.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–38. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 4.Magro F, Rodrigues A, Vieira AI, et al. Review of the disease course among adult ulcerative colitis population-based longitudinal cohorts. Inflamm Bowel Dis. 2012;18:573–83. doi: 10.1002/ibd.21815. [DOI] [PubMed] [Google Scholar]
- 5.Cohen RD, Yu AP, Wu EQ, Xie J, Mulani PM, Chao J. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther. 2010;31:693–707. doi: 10.1111/j.1365-2036.2010.04234.x. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol. 2006;101:1559–68. doi: 10.1111/j.1572-0241.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 7.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–94. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 8.Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro JM, Zoega H, Shah SA, et al. Incidence of Crohn’s disease and ulcerative colitis in Rhode Island: report from the Ocean State Crohn’s and Colitis Area Registry. Inflamm Bowel Dis. 2016;22:1456–61. doi: 10.1097/MIB.0000000000000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Shivananda S, Lennard-Jones J, Logan R, et al. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD) Gut. 1996;39:690–97. doi: 10.1136/gut.39.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bengtson M-B, Solberg C, Aamodt G, et al. Familial aggregation in Crohn’s disease and ulcerative colitis in a Norwegian population-based cohort followed for ten years. J Crohns Colitis. 2009;3:92–99. doi: 10.1016/j.crohns.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Loftus CG, Loftus EV, Harmsen WS, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm Bowel Dis. 2007;13:254–61. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 14.Burisch J, Pedersen N, Čuković-Čavka S, et al. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut. 2014;63:588–97. doi: 10.1136/gutjnl-2013-304636. [DOI] [PubMed] [Google Scholar]
- 15.Benchimol EI, Mack DR, Guttmann A, et al. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am J Gastroenterol. 2015;110:553–63. doi: 10.1038/ajg.2015.52. [DOI] [PubMed] [Google Scholar]
- 16.Benchimol EI, Manuel DG, To T, et al. Asthma, type 1 and type 2 diabetes mellitus, and inflammatory bowel disease amongst South Asian immigrants to Canada and their children: a population-based cohort study. PLoS One. 2015;10:e0123599. doi: 10.1371/journal.pone.0123599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr I, Mayberry JF. The effects of migration on ulcerative colitis: a three-year prospective study among Europeans and first- and second- generation South Asians in Leicester (1991–1994) Am J Gastroenterol. 1999;94:2918–22. doi: 10.1111/j.1572-0241.1999.01438.x. [DOI] [PubMed] [Google Scholar]
- 18.Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158–65. doi: 10.1053/j.gastro.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Sood A, Midha V, Sood N, Bhatia AS, Avasthi G. Incidence and prevalence of ulcerative colitis in Punjab, North India. Gut. 2003;52:1587–90. doi: 10.1136/gut.52.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tozun N, Atug O, Imeryuz N, et al. Clinical characteristics of inflammatory bowel disease in Turkey: a multicenter epidemiologic survey. J Clin Gastroenterol. 2009;43:51–57. doi: 10.1097/MCG.0b013e3181574636. [DOI] [PubMed] [Google Scholar]
- 21.Victoria CR, Sassak LY, de C Nunes HR. Incidence and prevalence rates of inflammatory bowel diseases, in midwestern of São Paulo State, Brazil. Arq Gastroenterol. 2009;46:20–25. doi: 10.1590/s0004-28032009000100009. [DOI] [PubMed] [Google Scholar]
- 22.Halme L, Paavola-Sakki P, Turunen U, Lappalainen M, Farkkila M, Kontula K. Family and twin studies in inflammatory bowel disease. World J Gastroenterol. 2006;12:3668–72. doi: 10.3748/wjg.v12.i23.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moller FT, Andersen V, Wohlfahrt J, Jess T. Familial risk of inflammatory bowel disease: a population-based cohort study 1977–2011. Am J Gastroenterol. 2015;110:564–71. doi: 10.1038/ajg.2015.50. [DOI] [PubMed] [Google Scholar]
- 24.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–17. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein CN, Rawsthorne P, Cheang M, Blanchard JF. A population-based case control study of potential risk factors for IBD. Am J Gastroenterol. 2006;101:993–1002. doi: 10.1111/j.1572-0241.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979–86. doi: 10.1038/ng.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.UK IBD Genetics Consortium. Barrett JC, Lee JC, et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41:1330–34. doi: 10.1038/ng.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81:1462–71. doi: 10.4065/81.11.1462. [DOI] [PubMed] [Google Scholar]
- 30.Odes HS, Fich A, Reif S, et al. Effects of current cigarette smoking on clinical course of Crohn’s disease and ulcerative colitis. Dig Dis Sci. 2001;46:1717–21. doi: 10.1023/a:1010609722315. [DOI] [PubMed] [Google Scholar]
- 31.Beaugerie L, Massot N, Carbonnel F, Cattan S, Gendre JP, Cosnes J. Impact of cessation of smoking on the course of ulcerative colitis. Am J Gastroenterol. 2001;96:2113–16. doi: 10.1111/j.1572-0241.2001.03944.x. [DOI] [PubMed] [Google Scholar]
- 32.Birrenbach T, Böcker U. Inflammatory bowel disease and smoking: a review of epidemiology, pathophysiology, and therapeutic implications. Inflamm Bowel Dis. 2004;10:848–59. doi: 10.1097/00054725-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Sahami S, Kooij IA, Meijer SL, Van den Brink GR, Buskens CJ, Te Velde AA. The link between the appendix and ulcerative colitis: clinical relevance and potential immunological mechanisms. Am J Gastroenterol. 2016;111:163–69. doi: 10.1038/ajg.2015.301. [DOI] [PubMed] [Google Scholar]
- 34.García Rodríguez LA, Ruigómez A, Panés J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology. 2006;130:1588–94. doi: 10.1053/j.gastro.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Gradel KO, Nielsen HL, Schønheyder HC, Ejlertsen T, Kristensen B, Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology. 2009;137:495–501. doi: 10.1053/j.gastro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Cornish JA, Tan E, Simillis C, Clark SK, Teare J, Tekkis PP. The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta-analysis. Am J Gastroenterol. 2008;103:2394–400. doi: 10.1111/j.1572-0241.2008.02064.x. [DOI] [PubMed] [Google Scholar]
- 37.Ananthakrishnan AN, Higuchi LM, Huang ES, et al. Aspirin, nonsteroidal anti-inflammatory drug use, and risk for Crohn disease and ulcerative colitis: a cohort study. Ann Intern Med. 2012;156:350–59. doi: 10.1059/0003-4819-156-5-201203060-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalili H, Higuchi LM, Ananthakrishnan AN, et al. Hormone therapy increases risk of ulcerative colitis but not Crohn’s disease. Gastroenterology. 2012;143:1199–206. doi: 10.1053/j.gastro.2012.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ungaro R, Bernstein CN, Gearry R, et al. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: a meta-analysis. Am J Gastroenterol. 2014;109:1728–38. doi: 10.1038/ajg.2014.246. [DOI] [PubMed] [Google Scholar]
- 40.Ng SC, Tang W, Leong RW, et al. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut. 2015;64:1063–71. doi: 10.1136/gutjnl-2014-307410. [DOI] [PubMed] [Google Scholar]
- 41.Ko Y, Kariyawasam V, Karnib M, et al. Inflammatory bowel disease environmental risk factors: a population-based case-control study of Middle Eastern migration to Australia. Clin Gastroenterol Hepatol. 2015;13:1453–63.e1. doi: 10.1016/j.cgh.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 42.Hviid A, Svanström H, Frisch M. Antibiotic use and inflammatory bowel diseases in childhood. Gut. 2011;60:49–54. doi: 10.1136/gut.2010.219683. [DOI] [PubMed] [Google Scholar]
- 43.Soon IS, Molodecky NA, Rabi DM, Ghali WA, Barkema HW, Kaplan GG. The relationship between urban environment and the inflammatory bowel diseases: a systematic review and meta-analysis. BMC Gastroenterol. 2012;12:51. doi: 10.1186/1471-230X-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klement E, Cohen RV, Boxman J, Joseph A, Reif S. Breastfeeding and risk of inflammatory bowel disease: a systematic review with meta-analysis. Am J Clin Nutr. 2004;80:1342–52. doi: 10.1093/ajcn/80.5.1342. [DOI] [PubMed] [Google Scholar]
- 45.Heikkilä K, Madsen IEH, Nyberg ST, et al. Job strain and the risk of inflammatory bowel diseases: individual-participant meta-analysis of 95,000 men and women. PLoS One. 2014;9:e88711. doi: 10.1371/journal.pone.0088711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 47.Dubuquoy L, Jansson EA, Deeb S, et al. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology. 2003;124:1265–76. doi: 10.1016/s0016-5085(03)00271-3. [DOI] [PubMed] [Google Scholar]
- 48.Rousseaux C, Lefebvre B, Dubuquoy L, et al. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J Exp Med. 2005;201:1205–15. doi: 10.1084/jem.20041948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geng X, Biancone L, Dai HH, et al. Tropomyosin isoforms in intestinal mucosa: production of autoantibodies to tropomyosin isoforms in ulcerative colitis. Gastroenterology. 1998;114:912–22. doi: 10.1016/s0016-5085(98)70310-5. [DOI] [PubMed] [Google Scholar]
- 50.Kaser A, Lee AH, Franke A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–56. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–65. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 52.Podolsky DK, Isselbacher KJ. Glycoprotein composition of colonic mucosa. Specific alterations in ulcerative colitis. Gastroenterology. 1984;87:991–98. [PubMed] [Google Scholar]
- 53.Johansson MEV, Gustafsson JK, Holmén-Larsson J, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–91. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andoh A, Imaeda H, Aomatsu T, et al. Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn’s disease using terminal restriction fragment length polymorphism analysis. J Gastroenterol. 2011;46:479–86. doi: 10.1007/s00535-010-0368-4. [DOI] [PubMed] [Google Scholar]
- 55.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–85. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roediger WE, Moore J, Babidge W. Colonic sulfide in pathogenesis and treatment of ulcerative colitis. Dig Dis Sci. 1997;42:1571–79. doi: 10.1023/a:1018851723920. [DOI] [PubMed] [Google Scholar]
- 57.Hausmann M, Kiessling S, Mestermann S, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 58.Senhaji N, Diakité B, Serbati N, Zaid Y, Badre W, Nadifi S. Toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms: New data and a meta-analysis. BMC Gastroenterol. 2014;14:206. doi: 10.1186/s12876-014-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanai H, Takeuchi K, Iida T, et al. Relationship between fecal calprotectin, intestinal inflammation, and peripheral blood neutrophils in patients with active ulcerative colitis. Dig Dis Sci. 2004;49:1438–43. doi: 10.1023/b:ddas.0000042243.47279.87. [DOI] [PubMed] [Google Scholar]
- 60.Hart AL, Al-Hassi HO, Rigby RJ, et al. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 61.Buonocore S, Ahern PP, Uhlig HH, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–75. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geremia A, Arancibia-Cárcamo CV, Fleming MPP, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127–33. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fuss IJ, Neurath M, Boirivant M, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–70. [PubMed] [Google Scholar]
- 64.Inoue S, Matsumoto T, Iida M, et al. Characterization of cytokine expression in the rectal mucosa of ulcerative colitis: correlation with disease activity. Am J Gastroenterol. 1999;94:2441–46. doi: 10.1111/j.1572-0241.1999.01372.x. [DOI] [PubMed] [Google Scholar]
- 65.Fuss IJ, Heller F, Boirivant M, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490–97. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–64. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Gerlach K, Hwang Y, Nikolaev A, et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat Immunol. 2014;15:676–86. doi: 10.1038/ni.2920. [DOI] [PubMed] [Google Scholar]
- 68.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 69.Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965–90. doi: 10.1016/j.crohns.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–57. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 71.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(suppl A):5A–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 72.Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut. 2008;57:205–10. doi: 10.1136/gut.2007.128231. [DOI] [PubMed] [Google Scholar]
- 73.Nguyen GC, Kaplan GG, Harris ML, Brant SR. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:1443–50. doi: 10.1111/j.1572-0241.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 74.Abreu MT, Harpaz N. Diagnosis of colitis: making the initial diagnosis. Clin Gastroenterol Hepatol. 2007;5:295–301. doi: 10.1016/j.cgh.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 75.Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106:110–19. doi: 10.1038/ajg.2010.343. [DOI] [PubMed] [Google Scholar]
- 76.Vavricka SR, Rogler G, Gantenbein C, et al. Chronological order of appearance of extraintestinal manifestations relative to the time of IBD diagnosis in the Swiss inflammatory bowel disease cohort. Inflamm Bowel Dis. 2015;21:1794–800. doi: 10.1097/MIB.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 77.Harbord M, Annese V, Vavricka SR, et al. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis. 2016;10:239–54. doi: 10.1093/ecco-jcc/jjv213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Higgins PDR, Skup M, Mulani PM, Lin J, Chao J. Increased risk of venous thromboembolic events with corticosteroid vs biologic therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2015;13:316–21. doi: 10.1016/j.cgh.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen GC, Bernstein CN, Bitton A, et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146:835–48.e6. doi: 10.1053/j.gastro.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 80.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375:657–63. doi: 10.1016/S0140-6736(09)61963-2. [DOI] [PubMed] [Google Scholar]
- 81.Ananthakrishnan AN, Cagan A, Gainer VS, et al. Thromboprophylaxis is associated with reduced post-hospitalization venous thromboembolic events in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12:1905–10. doi: 10.1016/j.cgh.2014.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kornbluth A, Sachar DB, Practice Parameters Committee of the American College of Gastroenterology Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–23. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 83.Lennard-Jones JE, Ritchie JK, Hilder W, Spicer CC. Assessment of severity in colitis: a preliminary study. Gut. 1975;16:579–84. doi: 10.1136/gut.16.8.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ho GT, Mowat C, Goddard CJ, et al. Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment Pharmacol Ther. 2004;19:1079–87. doi: 10.1111/j.1365-2036.2004.01945.x. [DOI] [PubMed] [Google Scholar]
- 85.Reese GE, Constantinides VA, Simillis C, et al. Diagnostic precision of anti-Saccharomyces cerevisiae antibodies and perinuclear antineutrophil cytoplasmic antibodies in inflammatory bowel disease. Am J Gastroenterol. 2006;101:2410–22. doi: 10.1111/j.1572-0241.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 86.Sands BE. Biomarkers of inflammation in inflammatory bowel disease. Gastroenterology. 2015;149:1275–85.e2. doi: 10.1053/j.gastro.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 87.Røseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. 1999;34:50–54. doi: 10.1080/00365529950172835. [DOI] [PubMed] [Google Scholar]
- 88.Menees SB, Powell C, Kurlander J, Goel A, Chey WD. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol. 2015;110:444–54. doi: 10.1038/ajg.2015.6. [DOI] [PubMed] [Google Scholar]
- 89.Annese V, Daperno M, Rutter MD, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 90.Bernstein CN, Shanahan F, Anton PA, Weinstein WM. Patchiness of mucosal inflammation in treated ulcerative colitis: a prospective study. Gastrointest Endosc. 1995;42:232–37. doi: 10.1016/s0016-5107(95)70097-8. [DOI] [PubMed] [Google Scholar]
- 91.D’Haens G, Geboes K, Peeters M, Baert F, Ectors N, Rutgeerts P. Patchy cecal inflammation associated with distal ulcerative colitis: a prospective endoscopic study. Am J Gastroenterol. 1997;92:1275–79. [PubMed] [Google Scholar]
- 92.Park SH, Loftus EV, Yang S-K. Appendiceal skip inflammation and ulcerative colitis. Dig Dis Sci. 2014;59:2050–57. doi: 10.1007/s10620-014-3129-z. [DOI] [PubMed] [Google Scholar]
- 93.Simpson P, Papadakis KA. Endoscopic evaluation of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1287–97. doi: 10.1002/ibd.20398. [DOI] [PubMed] [Google Scholar]
- 94.Magro F, Langner C, Driessen A, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7:827–51. doi: 10.1016/j.crohns.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 95.Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556–85. doi: 10.1016/j.crohns.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 96.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–29. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 97.Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841–45. doi: 10.1056/NEJM199406303302601. [DOI] [PubMed] [Google Scholar]
- 98.Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shah SC, Colombel JF, Sands BE, Narula N. Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1245–55. doi: 10.1016/j.cgh.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 100.Travis SPL, Schnell D, Krzeski P, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) Gut. 2012;61:535–42. doi: 10.1136/gutjnl-2011-300486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mosli MH, Feagan BG, Zou G, et al. Development and validation of a histological index for UC. Gut. 2015 doi: 10.1136/gutjnl-2015-310393. published online Oct 16. [DOI] [PubMed] [Google Scholar]
- 102.Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, et al. Development and validation of the Nancy histological index for UC. Gut. 2015 doi: 10.1136/gutjnl-2015-310187. published online Oct 13. [DOI] [PubMed] [Google Scholar]
- 103.Siegel CA, Whitman CB, Spiegel BM, et al. Development of an index to define overall disease severity in IBD. Gut. 2016 doi: 10.1136/gutjnl-2016-312648. published online Oct 25. [DOI] [PubMed] [Google Scholar]
- 104.Reinisch W, Reinink AR, Higgins PDR. Factors associated with poor outcomes in adults with newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13:635–42. doi: 10.1016/j.cgh.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 105.Etchevers MJ, Aceituno M, García-Bosch O, et al. Risk factors and characteristics of extent progression in ulcerative colitis. Inflamm Bowel Dis. 2009;15:1320–25. doi: 10.1002/ibd.20897. [DOI] [PubMed] [Google Scholar]
- 106.Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut. 2014;63:423–32. doi: 10.1136/gutjnl-2012-303864. [DOI] [PubMed] [Google Scholar]
- 107.Loftus EV, Harewood GC, Loftus CG, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–96. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choi CHR, Rutter MD, Askari A, et al. Forty-year analysis of colonoscopic surveillance program for neoplasia in ulcerative colitis: an updated overview. Am J Gastroenterol. 2015;110:1022–34. doi: 10.1038/ajg.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;372:1441–52. doi: 10.1056/NEJMra1403718. [DOI] [PubMed] [Google Scholar]
- 110.Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. doi: 10.1053/j.gastro.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 111.Solberg IC, Høivik ML, Cvancarova M, Moum B, IBSEN Study Group Risk matrix model for prediction of colectomy in a population-based study of ulcerative colitis patients (the IBSEN study) Scand J Gastroenterol. 2015;50:1456–62. doi: 10.3109/00365521.2015.1064991. [DOI] [PubMed] [Google Scholar]
- 112.Siebert U, Wurm J, Gothe RM, et al. Predictors of temporary and permanent work disability in patients with inflammatory bowel disease: results of the swiss inflammatory bowel disease cohort study. Inflamm Bowel Dis. 2013;19:847–55. doi: 10.1097/MIB.0b013e31827f278e. [DOI] [PubMed] [Google Scholar]
- 113.Gower-Rousseau C, Sarter H, Savoye G, et al. the International Programme to Develop New Indexes for Crohn’s Disease (IPNIC) group, International Programme to Develop New Indexes for Crohn’s Disease IPNIC group Validation of the Inflammatory Bowel Disease Disability Index in a population-based cohort. published online Dec 8. Gut. 2015 doi: 10.1136/gutjnl-2015-310151. [DOI] [Google Scholar]
- 114.Bressler B, Marshall JK, Bernstein CN, et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology. 2015;148:1035–58.e3. doi: 10.1053/j.gastro.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 115.Jharap B, Sandborn WJ, Reinisch W, et al. Randomised clinical study: discrepancies between patient-reported outcomes and endoscopic appearance in moderate to severe ulcerative colitis. Aliment Pharmacol Ther. 2015;42:1082–92. doi: 10.1111/apt.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Falvey JD, Hoskin T, Meijer B, et al. Disease activity assessment in IBD: clinical indices and biomarkers fail to predict endoscopic remission. Inflamm Bowel Dis. 2015;21:824–31. doi: 10.1097/MIB.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 117.Regueiro M, Rodemann J, Kip KE, et al. Physician assessment of ulcerative colitis activity correlates poorly with endoscopic disease activity. Inflamm Bowel Dis. 2011;17:1008–14. doi: 10.1002/ibd.21445. [DOI] [PubMed] [Google Scholar]
- 118.Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991–1030. doi: 10.1016/j.crohns.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 119.Feagan BG, Chande N, MacDonald JK. Are there any differences in the efficacy and safety of different formulations of oral 5-ASA used for induction and maintenance of remission in ulcerative colitis? Evidence from cochrane reviews. Inflamm Bowel Dis. 2013;19:2031–40. doi: 10.1097/MIB.0b013e3182920108. [DOI] [PubMed] [Google Scholar]
- 120.Gionchetti P, Rizzello F, Venturi A, et al. Comparison of oral with rectal mesalazine in the treatment of ulcerative proctitis. Dis Colon Rectum. 1998;41:93–97. doi: 10.1007/BF02236902. [DOI] [PubMed] [Google Scholar]
- 121.Lie MRKL, Kanis SL, Hansen BE, van der Woude CJ. Drug therapies for ulcerative proctitis: systematic review and meta-analysis. Inflamm Bowel Dis. 2014;20:2157–78. doi: 10.1097/MIB.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 122.Ford AC, Achkar JP, Khan KJ, et al. Efficacy of 5-aminosalicylates in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:601–16. doi: 10.1038/ajg.2011.67. [DOI] [PubMed] [Google Scholar]
- 123.Feagan BG, Macdonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:CD000544. doi: 10.1002/14651858.CD000544.pub3. [DOI] [PubMed] [Google Scholar]
- 124.Mulder CJ, Fockens P, Meijer JW, van der Heide H, Wiltink EH, Tytgat GN. Beclomethasone dipropionate (3 mg) versus 5-aminosalicylic acid (2 g) versus the combination of both (3 mg/2 g) as retention enemas in active ulcerative proctitis. Eur J Gastroenterol Hepatol. 1996;8:549–53. doi: 10.1097/00042737-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 125.Van Assche G, Manguso F, Zibellini M, et al. Oral prolonged release beclomethasone dipropionate and prednisone in the treatment of active ulcerative colitis: results from a double-blind, randomized, parallel group study. Am J Gastroenterol. 2015;110:708–15. doi: 10.1038/ajg.2015.114. [DOI] [PubMed] [Google Scholar]
- 126.Sandborn WJ, Danese S, D’Haens G, et al. Induction of clinical and colonoscopic remission of mild-to-moderate ulcerative colitis with budesonide MMX 9 mg: pooled analysis of two phase 3 studies. Aliment Pharmacol Ther. 2015;41:409–18. doi: 10.1111/apt.13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Danese S, Siegel CA, Peyrin-Biroulet L. Review article: integrating budesonide-MMX into treatment algorithms for mild-to-moderate ulcerative colitis. Aliment Pharmacol Ther. 2014;39:1095–103. doi: 10.1111/apt.12712. [DOI] [PubMed] [Google Scholar]
- 128.Ford AC, Bernstein CN, Khan KJ, et al. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:590–99. doi: 10.1038/ajg.2011.70. [DOI] [PubMed] [Google Scholar]
- 129.Kozarek RA, Patterson DJ, Gelfand MD, Botoman VA, Ball TJ, Wilske KR. Methotrexate induces clinical and histologic remission in patients with refractory inflammatory bowel disease. Ann Intern Med. 1989;110:353–56. doi: 10.7326/0003-4819-110-5-353. [DOI] [PubMed] [Google Scholar]
- 130.Wang Y, MacDonald JK, Vandermeer B, Griffiths AM, El-Matary W. Methotrexate for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2015(8):CD007560. doi: 10.1002/14651858.CD007560.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carbonnel F, Colombel JF, Filippi J, et al. Methotrexate is not superior to placebo for inducing steroid-free remission, but induces steroid-free clinical remission in a larger proportion of patients with ulcerative colitis. Gastroenterology. 2016;150:380–88.e4. doi: 10.1053/j.gastro.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 132.Järnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005;128:1805–11. doi: 10.1053/j.gastro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 133.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 134.Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780–87. doi: 10.1136/gut.2010.221127. [DOI] [PubMed] [Google Scholar]
- 135.Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85–95. doi: 10.1053/j.gastro.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 136.Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392–400.e3. doi: 10.1053/j.gastro.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 137.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2016 doi: 10.1136/gutjnl-2015-311079. published online Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lynch RW, Lowe D, Protheroe A, Driscoll R, Rhodes JM, Arnott IDR. Outcomes of rescue therapy in acute severe ulcerative colitis: data from the United Kingdom inflammatory bowel disease audit. Aliment Pharmacol Ther. 2013;38:935–45. doi: 10.1111/apt.12473. [DOI] [PubMed] [Google Scholar]
- 139.Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007;5:103–10. doi: 10.1016/j.cgh.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 140.Laharie D, Bourreille A, Branche J, et al. for the Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012;380:1909–15. doi: 10.1016/S0140-6736(12)61084-8. [DOI] [PubMed] [Google Scholar]
- 141.Duijvis NW, Ten Hove AS, Ponsioen CIJ, et al. Similar short- and long-term colectomy rates with ciclosporin and infliximab treatment in hospitalised ulcerative colitis patients. J Crohns Colitis. 2016;10:821–27. doi: 10.1093/ecco-jcc/jjw031. [DOI] [PubMed] [Google Scholar]
- 142.Kaplan GG, McCarthy EP, Ayanian JZ, Korzenik J, Hodin R, Sands BE. Impact of hospital volume on postoperative morbidity and mortality following a colectomy for ulcerative colitis. Gastroenterology. 2008;134:680–87. doi: 10.1053/j.gastro.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 143.Randall J, Singh B, Warren BF, Travis SPL, Mortensen NJ, George BD. Delayed surgery for acute severe colitis is associated with increased risk of postoperative complications. Br J Surg. 2010;97:404–09. doi: 10.1002/bjs.6874. [DOI] [PubMed] [Google Scholar]
- 144.Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology. 2015;148:639–51.e28. doi: 10.1053/j.gastro.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 145.Øresland T, Bemelman WA, Sampietro GM, et al. European evidence based consensus on surgery for ulcerative colitis. J Crohns Colitis. 2015;9:4–25. doi: 10.1016/j.crohns.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 146.Burns EM, Bottle A, Aylin P, et al. Volume analysis of outcome following restorative proctocolectomy. Br J Surg. 2011;98:408–17. doi: 10.1002/bjs.7312. [DOI] [PubMed] [Google Scholar]
- 147.Fazio VW, Kiran RP, Remzi FH, et al. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg. 2013;257:679–85. doi: 10.1097/SLA.0b013e31827d99a2. [DOI] [PubMed] [Google Scholar]
- 148.Waljee A, Waljee J, Morris AM, Higgins PDR. Threefold increased risk of infertility: a meta-analysis of infertility after ileal pouch anal anastomosis in ulcerative colitis. Gut. 2006;55:1575–80. doi: 10.1136/gut.2005.090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bartels SAL, D’Hoore A, Cuesta MA, Bensdorp AJ, Lucas C, Bemelman WA. Significantly increased pregnancy rates after laparoscopic restorative proctocolectomy: a cross-sectional study. Ann Surg. 2012;256:1045–48. doi: 10.1097/SLA.0b013e318250caa9. [DOI] [PubMed] [Google Scholar]
- 150.Beyer-Berjot L, Maggiori L, Birnbaum D, Lefevre JH, Berdah S, Panis Y. A total laparoscopic approach reduces the infertility rate after ileal pouch-anal anastomosis: a 2-center study. Ann Surg. 2013;258:275–82. doi: 10.1097/SLA.0b013e3182813741. [DOI] [PubMed] [Google Scholar]
- 151.Kani HT, Shen B. Male issues of the ileal pouch. Inflamm Bowel Dis. 2015;21:716–22. doi: 10.1097/MIB.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 152.Biancone L, Michetti P, Travis S, et al. European evidence-based consensus on the management of ulcerative colitis: special situations. J Crohns Colitis. 2008;2:63–92. doi: 10.1016/j.crohns.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 153.Shen B. Pouchitis: what every gastroenterologist needs to know. Clin Gastroenterol Hepatol. 2013;11:1538–49. doi: 10.1016/j.cgh.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 154.Shen B, Achkar JP, Lashner BA, et al. A randomized clinical trial of ciprofloxacin and metronidazole to treat acute pouchitis. Inflamm Bowel Dis. 2001;7:301–05. doi: 10.1097/00054725-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 155.Colombel JF, Ordás I, Ullman T, et al. Agreement between rectosigmoidoscopy and colonoscopy analyses of disease activity and healing in patients with ulcerative colitis. Gastroenterology. 2016;150:389–95.e3. doi: 10.1053/j.gastro.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 156.Sandborn WJ, Panés J, Zhang H, Yu D, Niezychowski W, Su C. Correlation between concentrations of fecal calprotectin and outcomes of patients With ulcerative colitis in a phase 2 trial. Gastroenterology. 2016;150:96–102. doi: 10.1053/j.gastro.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 157.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis. 2013;19:332–41. doi: 10.1097/MIB.0b013e3182810066. [DOI] [PubMed] [Google Scholar]
- 158.Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:802–19. doi: 10.1038/ajg.2015.120. [DOI] [PubMed] [Google Scholar]
- 159.Yarur AJ, Abreu MT, Deshpande AR, Kerman DH, Sussman DA. Therapeutic drug monitoring in patients with inflammatory bowel disease. World J Gastroenterol. 2014;20:3475–84. doi: 10.3748/wjg.v20.i13.3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Roblin X, Marotte H, Rinaudo M, et al. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12:80–84.e2. doi: 10.1016/j.cgh.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 161.Adedokun OJ, Sandborn WJ, Feagan BG, et al. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology. 2014;147:1296–1307.e5. doi: 10.1053/j.gastro.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 162.Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320–29.e3. doi: 10.1053/j.gastro.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 163.Ben-Horin S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev. 2014;13:24–30. doi: 10.1016/j.autrev.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 164.Annese V, Beaugerie L, Egan L, et al. European evidence-based consensus: inflammatory bowel disease and malignancies. J Crohns Colitis. 2015;9:945–65. doi: 10.1093/ecco-jcc/jjv141. [DOI] [PubMed] [Google Scholar]
- 165.Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48–54. doi: 10.1067/mge.2002.125367. [DOI] [PubMed] [Google Scholar]
- 166.Sinclair JA, Wasan SK, Farraye FA. Health maintenance in the inflammatory bowel disease patient. Gastroenterol Clin North Am. 2012;41:325–37. doi: 10.1016/j.gtc.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 167.Singh S, Nagpal SJS, Murad MH, et al. Inflammatory bowel disease is associated with an increased risk of melanoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:210–18. doi: 10.1016/j.cgh.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 168.Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143:390–99.e1. doi: 10.1053/j.gastro.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621–28.e1. doi: 10.1053/j.gastro.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 170.Khanna R, Jairath V, Vande Casteele N, et al. Efficient early drug development for ulcerative colitis. Gastroenterology. 2016;150:1056–60. doi: 10.1053/j.gastro.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 171.Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–24. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 172.Vermeire S, O’Byrne S, Keir M, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384:309–18. doi: 10.1016/S0140-6736(14)60661-9. [DOI] [PubMed] [Google Scholar]
- 173.Yoshimura N, Watanabe M, Motoya S, et al. safety and efficacy of ajm300, an oral antagonist of α4 integrin, in induction therapy for patients with active ulcerative colitis. Gastroenterology. 2015;149:1775–83.e2. doi: 10.1053/j.gastro.2015.08.044. [DOI] [PubMed] [Google Scholar]
- 174.Sandborn WJ, Feagan BG, Wolf DC, et al. ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med. 2016;374:1754–62. doi: 10.1056/NEJMoa1513248. [DOI] [PubMed] [Google Scholar]
- 175.Lang A, Salomon N, Wu JCY, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13:1444–49.e1. doi: 10.1016/j.cgh.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 176.Farkas K, Rutka M, Golovics PA, et al. Efficacy of infliximab biosimilar CT-P13 induction therapy on mucosal healing in ulcerative colitis. J Crohns Colitis. 2016 doi: 10.1093/ecco-jcc/jjw085. published online April 21. [DOI] [PubMed] [Google Scholar]
- 177.Danese S, Gomollon F, Governing Board and Operational Board of ECCO ECCO position statement: the use of biosimilar medicines in the treatment of inflammatory bowel disease (IBD) J Crohns Colitis. 2013;7:586–89. doi: 10.1016/j.crohns.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 178.Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015;149:110–18.e4. doi: 10.1053/j.gastro.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 179.Moayyedi P, Surette MG, Kim PT, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–09.e6. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 180.Paramsoth S, Kamm M, Walsh A, et al. Multi-donor intense faecal microbiota transplantation is an effective treatment for resistant ulcerative colitis: a randomised placebo-controlled trial. 11th Congress of European Crohn’s and Colitis Organisation; Amsterdam. March 16–19, 2016; p. OP017. [Google Scholar]
- 181.Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109:1065–71. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zenlea T, Yee EU, Rosenberg L, et al. Histology grade is independently associated with relapse risk in patients with ulcerative colitis in clinical remission: a prospective study. Am J Gastroenterol. 2016;111:685–90. doi: 10.1038/ajg.2016.50. [DOI] [PubMed] [Google Scholar]
- 183.Peyrin-Biroulet L, Bressenot A, Kampman W. Histologic remission: the ultimate therapeutic goal in ulcerative colitis? Clin Gastroenterol Hepatol. 2014;12:929–34.e2. doi: 10.1016/j.cgh.2013.07.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


