Abstract
Purpose
Frailty and atherosclerotic diseases are prevalent among the older people and usually present the same pathogenesis and risk factors. Therefore, the aim of this study was to determine the association between frailty and atherosclerosis.
Patients and methods
The enrolled participants were 171 patients aged 60–96 years in Beijing Tongren Hospital. Data that were collected included sex, age, height, weight, calculated body mass index (BMI), past medical history, comorbidities (including hypertension, coronary heart disease [CHD], and diabetes), ability to perform activities of daily living (ADL) as measured using the Barthel index, handgrip strength, 15-feet (4.57 m) walking speed, body composition features determined by bioelectrical impedance analysis, the ankle–brachial index (ABI), and atherosclerosis determined by the cardio-ankle vascular index (CAVI). Patients were divided into frail, pre-frail, and non-frail groups using Fried’s frailty index. ANOVA was used to assess the differences among these groups. Linear correlation analysis was used to examine the relationship between the CAVI and frailty phenotype. Ordinal multivariate logistic regression analysis was used to examine the factors affecting frailty and the relationship between frailty and atherosclerosis.
Results
The population was categorized as 21.3% frail, 38.4% pre-frail, and 40.3% non-frail. Patients in the frail group were older, had lower handgrip strength, slower walking speed, and a lower ABI and a higher proportion of carotid intima-media thickening with values of at least 1 mm compared with those in the pre-frail and non-frail groups. The CAVI score was higher in the frail group than that in the other two groups. There were significant inverse linear correlations between grip strength, walking speed, and the CAVI. CAVI showed an independent risk factor for frailty (OR: 2.013, 95% CI 1.498–2.703, p<0.001).
Conclusion
Our study shows that arterial stiffness is associated with frailty in older patients, even when adjusting for multiple factors.
Keywords: frailty, atherosclerosis, CAVI, age
Introduction
In 2001, Dr Fried (Johns Hopkins University School of Medicine, USA) described frailty as a clinical syndrome characterized by decreased physiological reserves, multiple system disorders, and increased susceptibility to stress events.1 Frailty may lead to adverse health outcomes, such as physical disability, poor quality of life, mortality, and extra financial expenditure.2
Additionally, older people may have some adverse health outcomes because of vascular aging, such as increased arterial stiffness.3 These may lead to the development of cardiovascular and cerebrovascular diseases, with patients becoming increasingly bedridden. This in turn results in reduced activity and increased comorbidity.
A growing body of evidence has shown that frailty and atherosclerosis have common pathogenesis, such as inflammaging. Frailty often coincides with comorbidities. When aging leads to comorbidity, the net health outcome is the erosion of homeostasis of multiple organs that become unable to support each other. Frailty can be interpreted as a state of multi-organ functional decline.4 The Healthy Aging and Body Composition Study showed that frailty was a risk factor for the development of incident atherosclerotic disease.5
Frailty and atherosclerosis share the same pathogenesis and have reciprocal causation, but the association between them remains unclear. In clinical practice, we have observed that the degree of atherosclerosis is serious in older people with worsening mobility or bradykinesia. We hypothesize that frailty is associated with atherosclerosis.
The aim of this cross-sectional study was to investigate the relationship between frailty and atherosclerosis as measured by the cardio-ankle vascular index (CAVI). Controlling atherosclerosis may delay the progress of frailty and thus may improve the prognosis.
Patients and methods
Patient population
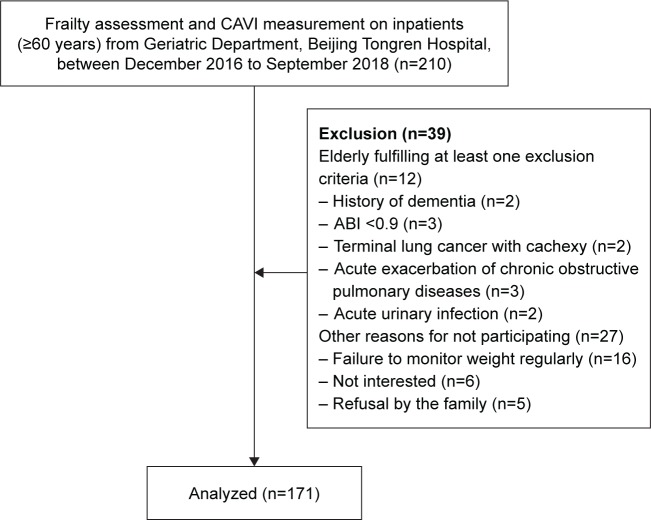
The study participants were patients from geriatric wards in Beijing Tongren Hospital. They met the following inclusion criteria: 1) aged 60 years or older and 2) approved to participate. Exclusion criteria for all the study participants were as follows: 1) no autonomic activity, 2) patients with clinically definite diagnosis of dementia, 3) patients with terminal cancer and cachexy, 4) acute exacerbation of chronic obstructive pulmonary disease and uncontrolled acute heart failure, 5) other diseases affecting limb muscle strength, eg, myasthenia gravis, postoperative fracture, and acute infection, and 6) peripheral artery disease, which was identified as an ankle–brachial index (ABI) of less than 0.9 and more than 1.4 (at least one limb). Data were collected from December 2016 to September 2018. The study protocol was approved by the medicine ethics committee of Beijing Tongren Hospital, Capital Medical University, conducted in accordance with the Declaration of Helsinki. All patients volunteered to participate in the assessment and signed the informed consent. Figure 1 shows the recruitment procedure.
Figure 1.
Recruitment of the participants.
Abbreviations: ABI, ankle–brachial index; CAVI, cardio-ankle vascular index.
Methods
Assessment of frailty
The participants were divided into three groups using Fried’s frailty phenotype,6 including the following five components: weight loss, slowness (time to cover 4.57 m), handgrip strength (kg), low physical activity, and exhaustion. Participants were considered to be “frail” if they had three or more frailty components, “pre-frail” if they had one or two frailty criteria, and “non-frail” if they had none. The criteria used to define frailty are summarized in Table 1.
Table 1.
Frailty phenotype model and Fried’s criteria
| Male | Female | |
|---|---|---|
| Weight loss | Greater than 10 lbs (4.5 kg) or 5% of weight loss in the last year | |
| Slowness (4.57 m) | Height ≤173 cm: ≥7 s Height >173 cm: ≥6 s |
Height ≤159 cm: ≥7 s Height >159 cm: ≥6 s |
| Dominant handgrip strength (kg) | BMI ≤24.0: ≤29 BMI 24.1–26.0: ≤30 BMI 26.1–28.0: ≤30 BMI >28.0: ≤32 |
BMI ≤23.0: ≤17 BMI 23.1–26.0: ≤17.3 BMI 26.1–29.0: ≤18 BMI >29.0: ≤21 |
| Physical activity (MLTA) | <383 kcal/week (about 2.5 h walk) | <270 kcal/week (about 2 h walk) |
| Exhaustion | A score of 2 or 3 on either question on the CES-D How often in the last week did you feel this way? (a) I felt everything I did was an effort. (b) I could not get going. 0= less than 1 day, 1=1–2 days, 2=3–4 days, 3= more than 4 days |
|
Abbreviations: BMI, body mass index; CES-D, Center for Epidemiologic Studies Depression Scale; MLTA, Minnesota Leisure Time Activity Questionnaire.
Assessment of atherosclerosis
The CAVI was measured by a VaSera VS-1000 (Fukuda Denshi Co., Tokyo, Japan) vascular screening system, and subjects rested in the supine position. The CAVI examination was carried out strictly in line with the operational procedure: in a quiet environment, and both ankles and brachium were secured with cuffs. Electrodes for electrocardiography were placed on both wrists, and a microphone was placed on the sternum for phonocardiography. The patient information was entered and measurement began. Finally, a scale conversion was carried out using the following formula:
where “ρ” is blood density, “Ps” is systolic blood pressure, “Pd” is diastolic blood pressure, “ΔP” = Ps−Pd, “PWV” is pulse wave velocity, and “a” and “b” are constants. Mean right and left CAVI values were used for analysis.7 While acquiring CAVI, we also acquired the ABI.
Other indicators
We also assessed the following factors: we performed a physical examination by using the ruler and the scale to measure height and body weight. Body mass index (BMI, kg/m2) was calculated by body weight and height. A standard handgrip dynamometer and a stopwatch were used to assess the handgrip and walking time, respectively. Blood pressure was recorded with a mercury sphygmomanometer. Data of age and sex were also collected at the same time. Physical status was assessed including the ability to perform activities of daily living (ADL), using the Barthel index.8
For laboratory tests, blood samples were collected when participants who had already signed the protocol were in a 10 h fasting state. We measured the levels of hemoglobin, albumin, fasting blood glucose, high-sensitivity C-reactive protein (hs-CRP), creatinine (Cr), triglycerides (TGs), cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and the estimated glomerular filtration rate (eGFR).
Color Doppler ultrasound was used to measure the left or right carotid arterial intima–media thickness of at least 1 mm. We also determined whether patients had other chronic diseases such as hypertension, diabetes, chronic obstructive pulmonary diseases (COPD), coronary heart disease (CHD), chronic kidney disease (CKD), or malignant tumor. Whether the participant was taking more than five types of medication was determined.9 The history of smoking and drinking was recorded.
Two technicians measured CAVI, and two nurses performed the assessment of frailty according to Fried’s criteria. Two statisticians entered and sorted the data. In addition, our geriatric team screened the personnel.
Statistical analyses
SPSS22.0 for Windows (IBM Corporation, Armonk, NY, USA) was used for statistical analysis. Among the three groups, comparisons of continuous variables with normal distribution were carried out by ANOVA, and least significance difference (LSD) test was used in ANOVA to create CIs for all pairwise differences. The Kruskal–Wallis test was used for the comparison of continuous variables with non-normal distribution among three groups. Comparison of categorical variables was performed using the chi-square test. The Pearson correlation coefficient was used to identify linear relations between the CAVI and frailty phenotypes. Ordinal multivariate logistic regression was used to investigate whether CAVI was an independent risk factor for frailty. Significance was considered as p<0.05, with p<0.01 as highly significant.
Results
A total of 171 subjects participated in this study (age: 60–96 years, mean age: 78.52±9.15 years), including men (122, 70%) and women (49, 30%). In total, 70 (41%) patients were considered non-frail, 65 (38%) were pre-frail, and 36 (21%) were frail.
Patients in the frail group were generally older (p<0.001) and had a lower ADL score (p<0.001) compared with those in the pre-frail and non-frail groups. Their BMI in the frail group was significantly lower than that in the non-frail group (p<0.05). The frail group also had significantly poorer physical functioning, such as low grip strength (p<0.001) and low walking speed (p<0.001) than did the other two groups.
Several variables were significantly lower in the frail group than in the other two groups, including hemoglobin levels (p=0.007), albumin levels (p=0.001), and the eGFR (p=0.009). Levels of hs-CRP (p=0.02) were significantly higher in the frail group than in the non-frail group. CHD in the frail group was significantly more prevalent (p=0.008) than that in the pre-frail and non-frail groups. Patients in the frail group had significantly more polypharmacy than those in the non-frail group (p<0.05). There were no significant differences in sex, prevalence of hypertension, diabetes, COPD, CKD, cancer, levels of fasting blood glucose, TG, TC, HDL-C, history of smoking and alcohol consumption among the three groups.
Patients in the frail group had a significantly higher CAVI (p<0.001), lower ABI (p=0.027), and a higher proportion of carotid intima-media thickness (p<0.001) than those in the pre-frail and non-frail groups (Table 2).
Table 2.
Comparison of demographic health and lifestyle characteristics by the level of frailty (n=171)
| Parameter | Frailty | F/H/χ2 | P-value | ||
|---|---|---|---|---|---|
| Non-frail (n=70) | Pre-frail (n=65) | Frail (n=36) | |||
| Age (years)a,b,c | 72.65±8.45 | 82.38±5.59 | 87.72±5.73 | 64.296 | <0.001 |
| Male | 46 (38.0%) | 50 (41.0%) | 25 (21.0%) | 1.372 | =0.503 |
| BMIc (kg/m2) | 24.76±2.36 | 23.71±3.26 | 22.95±3.72 | 3.054 | =0.041 |
| CAVIa,b,c (m/s) | 9.29±1.24 | 10.28±1.23 | 12.13±2.64 | 35.55 | <0.001 |
| ABIa,c | 1.15 (1.09–1.23) | 1.14 (1.07–1.18) | 1.13 (0.98–1.20) | 6.062 | =0.027 |
| CIMT (≥1 mm)a,c | 48 (68.6%) | 62 (95.4%) | 34 (94.4%) | 21.816 | <0.001 |
| ADLa,b,c | 97.21±7.04 | 89.76±12.33 | 75.14±19.91 | 34.969 | <0.001 |
| Handgripa,b,c (kg) | 32.81±9.94 | 23.82±7.54 | 18.78±6.04 | 37.608 | <0.001 |
| Speeda,b,c (m/s) | 1.06±0.18 | 0.85±0.24 | 0.53±0.18 | 15.007 | <0.001 |
| Comorbidity | |||||
| Hypertension | 16 (23.9%) | 9 (14.1%) | 4 (11.1%) | 4.921 | =0.296 |
| Diabetes | 35 (51.5%) | 39 (60.9%) | 23 (63.9%) | 3.128 | =0.537 |
| CHDa,c | 30 (44.1%) | 39 (60.9%) | 27 (75.0%) | 19.775 | =0.008 |
| COPD | 7 (10.3%) | 9 (14.1%) | 7 (19.4%) | 3.299 | =0.509 |
| CKD | 8 (11.8%) | 17 (26.6%) | 8 (22.2%) | 4.767 | =0.092 |
| Tumor | 8 (11.8%) | 4 (6.3%) | 6 (16.7%) | 4.350 | =0.361 |
| Biochemical indicators | |||||
| Albumina,c (g/L) | 38.35 (36.25–41.93) | 36.7 (33.9–38.85) | 35.9 (33.7–38) | 13.518 | =0.001 |
| Hemoglobina,c (g/L) | 130 (118–141) | 121 (108–131) | 124.5 (112.5–132.5) | 9.806 | =0.005 |
| FBG (mmol/L) | 5.22 (4.75–6.28) 5.73±1.40 | 5.46 (4.80–6.38) | 5.02 (4.52–7.54) | 0.235 | =0.889 |
| Cra (mmol/L) | 75.85 (64.33–88.05) | 87.00 (73.55–102.50) | 81.45 (67.93–103.93) | 9.362 | =0.009 |
| eGFRa,c (mL/min) | 80.90±21.06 | 66.63±24.08 | 64.68±20.10 | 9.589 | <0.001 |
| hs-CRPc (mg/L) | 3.80±11.28 | 11.24±32.16 | 24.48±60.13 | 3.99 | =0.02 |
| TG (mmol/L) | 1.10 (0.81–1.54) | 0.97 (0.71–1.49) | 1.15 (0.77–1.48) | 1.852 | =0.396 |
| TC (mmol/L) | 3.72 (3.18–4.36) | 3.34 (2.96–3.99) | 3.91 (3.21–4.29) | 5.222 | =0.073 |
| LDLa,b (mmol/L) | 2.04 (1.69–2.70) | 1.83 (1.48–2.11) | 2.11 (1.70–2.73) | 6.218 | =0.045 |
| HDL (mmol/L) | 1.01 (0.83–1.22) | 1.05 (0.83–1.29) | 0.99 (0.75–1.23) | 0.872 | =0.647 |
| Smoking | 31 (47.0%) | 25 (39.7%) | 14 (40.0%) | 0.831 | =0.660 |
| Alcohol | 12 (18.2%) | 5 (7.9%) | 5 (14.3%) | 2.942 | =0.230 |
| Polypharacyc | 45 (68.2%) | 48 (76.2%) | 32 (91.4%) | 6.819 | =0.033 |
Notes: Values are mean ± SD, median (IQR) vs or valid percentages (n%).
Non-frail and pre-frail, P<0.05.
Pre-frail and frail, P<0.05.
Non-frail and frail, P<0.05. The bold text means that it is statistically significant.
Abbreviations: ABI, ankle–brachial index; ADL, activities of daily living; BMI, body mass index; CAVI, cardio-ankle vascular index; CHD, coronary heart disease; CIMT, carotid intima–media thickness; CKD, chronic kidney disease; Cr, creatinine; FBG, fasting blood glucose; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; TC, cholesterol; TG, triglycerides.
Linear correlation analysis showed that there were significant inverse linear correlations between grip strength (Pearson correlation coefficient =−0.211, p=0.006), walking speed (Pearson correlation coefficient =−0.428, p<0.001), and the CAVI (Figures 2 and 3). Figures 4–6 show walking speed, handgrip, and the CAVI in the non-frail, pre-frail, and frail groups, respectively.
Figure 2.
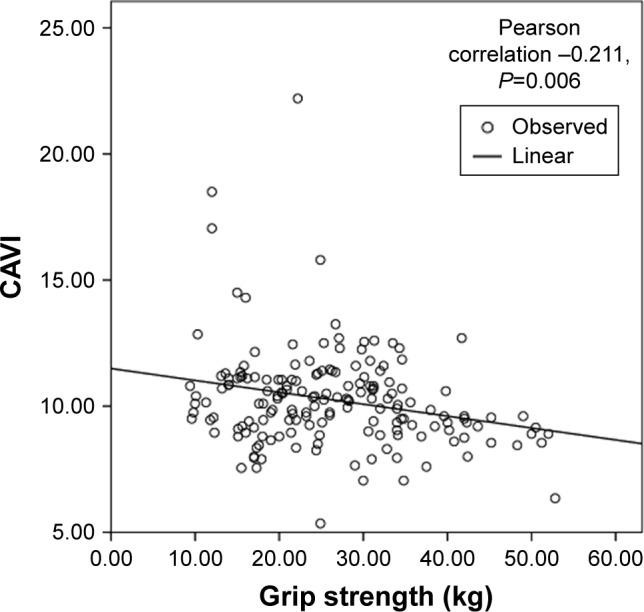
Linear correlation analysis between grip strength and CAVI.
Abbreviation: CAVI, cardio-ankle vascular index.
Figure 3.
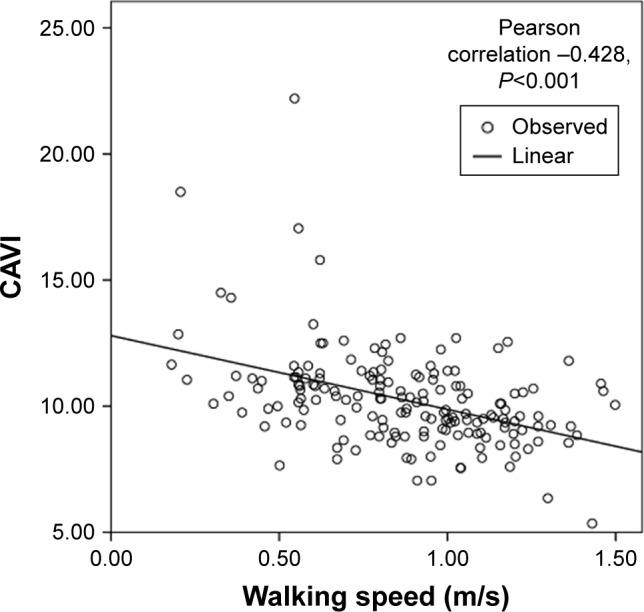
Linear correlation analysis between walking speed and CAVI.
Abbreviation: CAVI, cardio-ankle vascular index.
Figure 4.
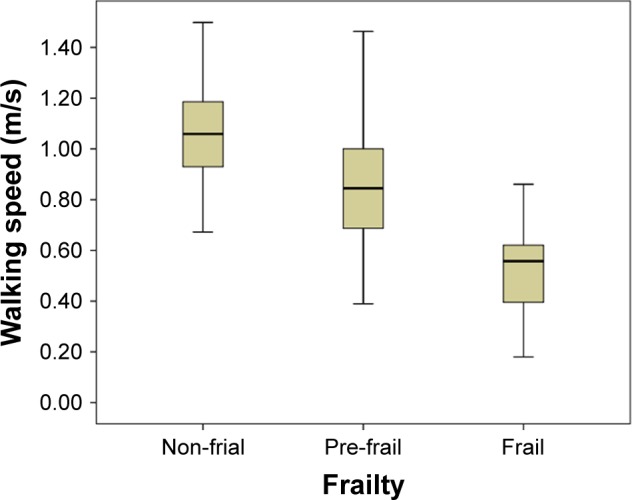
Box–whiskers graph for walking speed of non-frail, pre-frail, and frail. Box–whiskers plot shows the 25th and 75th percentile range (box) and median values (transverse lines in the box).
Figure 5.
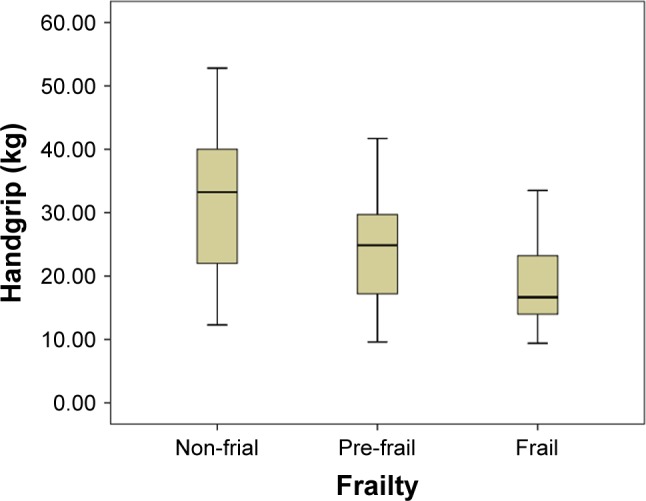
Box–whiskers graph for handgrip of non-frail, pre-frail, and frail.
Figure 6.
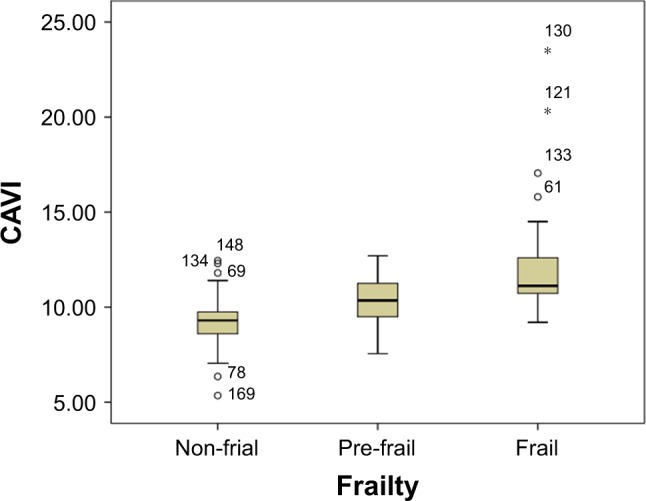
Box–whiskers graph for CAVI of non-frail, pre-frail, and frail.
Note: °Represensts mild outlier; *Represents extreme outlier.
Abbreviation: CAVI, cardio-ankle vascular index.
The frail score (non-frail =0, pre-frail =1, frail =2) was used as the dependent variable, and age, BMI, ADL, the CAVI, the ABI, hemoglobin, albumin, eGFR, hs-CRP, and LDL-C were used as independent variables in ordinal multivariate logistic regression. We found that age and the CAVI were positively associated with an increase in the frailty score, with an OD and 95% CI of 1.159 (1.095–1.228) and 2.013 (1.498–2.703), respectively. The ADL score was negatively associated with an increase in the frailty score, with OD and 95% CI of 0.949 (0.921–0.977; Table 3).
Table 3.
Ordinal multivariate logistic regression
| OR | 95% CI | P-value | |
|---|---|---|---|
| Age | 1.159 | 1.095–1.228 | <0.001 |
| ADL | 0.949 | 0.921–0.977 | 0.001 |
| CAVI | 2.013 | 1.498–2.703 | <0.001 |
Abbreviations: ADL, activities of daily living; CAVI, cardio-ankle vascular index.
Discussion
Prevalence of frailty in older patients
With the development of geriatrics, epidemiological studies on frailty have been conducted in many countries. In this study, we investigated 171 inpatients aged older than 60 years, including 121 men, and grouped them into non-frail (41%), pre-frail (38%), and frail (21%) patients. A previous study that used American Cardiovascular Health Research data, which included Fried’s criterion, showed that the prevalence of frailty was 6.9% among 5,201 people aged 65 years or older.10 The prevalence of frailty in our study population is higher than this rate. The reason for this discrepancy between studies could be because we chose a small population and had a high proportion of older inpatients and a more selected population because of our inclusion and exclusion criteria.
Factors affecting frailty
Numerous studies have shown that ethnicity, comorbidities, lifestyle, malnutritional status, depression, and sarcopenia are risk factors for frailty, and these factors independently or synergistically affect the progression of frailty.11 Our results suggest that the factors affecting frailty can be grouped into four areas.
Atherosclerosis and frailty
In theory, changes in arterial stiffness may mediate the relationship between body composition and cardiovascular risk factors.12 However, whether there is an association between atherosclerosis and frailty is unclear. Sampaio et al13 suggested that muscle blood flow decreases with age and that this is partly related to the degree of atherosclerosis. Arterial hemodynamic dysfunction may have a predictive effect on reduction in muscle mass. This reduction results in a decrease in body mass, grip strength, and walking speed, which is more likely to cause disability, falls, and even death. Therefore, atherosclerosis is a risk factor for comorbidities resulting in frailty.
In the past, pulse wave velocity (PWV) has been used to assess atherosclerosis. PWV is a predictor of prognosis in patients with cardiovascular disease and a marker of the severity of atherosclerotic vascular injury.14 The CAVI is another measurement tool that was developed from PWV by Japanese scholars to evaluate the degree of arterial stiffness. Calculation of the CAVI combines the stiffness parameter and the Bramwell–Hill formula.15 The most important feature of the CAVI is its independence from blood pressure during examinations. This is useful for objectively reflecting the degree of atherosclerosis for those with high blood pressure variability, with recessive hypertension or taking antihypertensive drugs.16 We quantified the degree of atherosclerosis by the CAVI score, using the mean value of the right and left CAVI score for analysis.7 We found that there was a significant difference in the CAVI score among the groups; the frail group had a higher CAVI score than did the other two groups. Ordinal multivariate logistic regression analysis showed that the CAVI was an independent risk factor for frailty.
The ABI is regarded as an indicator of peripheral vascular disease. In our study, the ABI score in the frail group was significantly lower than that in the pre-frail and non-frail groups. This result was also found in a study in Italy, with ABI scores of 1.16±0.13, 1.12±0.14, and 1.09±0.14 in the non-frail, pre-frail, and frail groups, respectively. Therefore, there was a significant correlation between frailty and the risk of cardiovascular disease. Targeting pre-frailty as a potentially reversible risk factor for CVD in older people could have significant implications.17
The results of our study suggest that frail patients are likely to have impaired grip strength and exercise capacity. The walking speed test is designed to test mobility, power, and balance. Impaired mobility, power, and balance will result in more time required for the walking tests. We found that frail patients took significantly longer to walk 4.57 m than did pre-frail and non-frail patients. Linear correlation analysis showed that the CAVI score had an inverse linear correlation with walking speed. This finding suggests that atherosclerosis causes a decrease in walking speed. Other studies have drawn the same conclusions as follows. The LIFE-P study of older people living in the community in USA showed that an increase in pulse pressure was an independent risk factor for slowing gait speed.18 Another study on aerobic exercise showed that the degree of arteriosclerosis in older men who undertook endurance training was lower than that in those who were sedentary. Furthermore, interventions to improve aerobic capacity might mitigate arterial stiffening that accompanies normative aging.19
The other components of the Fried phenotype have a significant correlation with grip strength. First, reduced physical fitness can result in tiredness. Second, unintentional weight loss is associated with decreased muscle strength. Grip strength and slow movement are direct measures of strength, and grip strength is a direct measure of muscle strength. Grip strength is a representative way to assess skeletal muscle strength in the upper limbs and is sensitive to age-related changes in skeletal muscle mass and physiological function.20 In this study, grip strength showed a downward trend as the degree of frailty increased. The J-SHIPP study of 1,593 patients with an average age of 66 years found that brachial–ankle PWV, the brachial artery augmentation index, and pulse pressure increased with decreasing grip strength.21 We also found that there was an inverse linear correlation between the CAVI score and grip strength. This finding suggested that atherosclerosis had an effect on grip strength.
Nutrition
In the Chianti study, which involved 802 people aged 65 years or older, a daily energy intake of less than 21 kcal/kg/day was associated with frailty.22 Lyu et al23 studied 371 people aged older than 65 years and found that as frailty increased hemoglobin and albumin levels gradually decreased and the risk of malnutrition gradually increased. We also found that patients in the frail group had lower hemoglobin levels and albumin levels than did those in the pre-frail and non-frail groups. This finding suggested that the nutritional status was associated with frailty.
Comorbidity
Prospective longitudinal studies by Woods et al24 showed that patients with a history of CHD, COPD, and diabetes had a significantly increased risk of becoming frail within 3 years. A Chinese study showed that frailty was an independent risk predictor in the short-term prognosis of death and unplanned visits to hospital for older patients with CHD.25 Our study also showed that the incidence of CHD was higher in people in the frail group compared with the other two groups. A study in USA suggested that the eGFR was lower in frail people than in non-frail populations.26 We also found a significantly lower eGFR in the frail group than in the non-frail group, which suggested that there was a correlation between reduced kidney function and frailty.
Inflammatory index
As the level of frailty increases, so do inflammatory markers in the blood. A previous study showed that frail older people often show chronic inflammatory status, manifested by an increase in inflammatory molecules such as IL-6, C-reactive protein, and tumor necrosis factor-α.27 Our study showed that participants in the frail and pre-frail groups had elevated circulating hs-CRP levels compared with those in the non-frail group, which suggested that frailty was associated with chronic inflammation. Therefore, inflammatory changes in the body play a role in developing frailty. Levels of hs-CRP have been found to be an independent factor affecting frailty.28
Conclusion
Frailty is associated with aging, higher inflammatory marker levels, and preclinical atherosclerosis. There is an inverse linear correlation between the CAVI and frail phenotypes such as grip strength and walking speed. The CAVI is also an independent risk factor for frailty. Future researchers may investigate whether anti-atherosclerosis drugs can control the progression of frailty.
Acknowledgments
We thank the Department of Geriatrics at Beijing Tongren Hospital for providing the database and equipment. We also thank our geriatric team, nurses, technicians, and statisticians who assisted our research. This work was supported by Municipal Science and Technology Commission Research Project (D121100004912001) and the Beijing Healthcare Research Project (2017–2019).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fried P, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 2.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681–687. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 3.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI) J Atheroscler Thromb. 2006;13(2):101–107. doi: 10.5551/jat.13.101. [DOI] [PubMed] [Google Scholar]
- 4.Theou O, Rockwood MRH, Mitnitski A, Rockwood K. Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr. 2012;55:e1–e8. doi: 10.1016/j.archger.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 6.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 7.Tian G, Wei W, Zhang W, et al. Increasing age associated with elevated cardio-ankle vascular index scores in patients with type 2 diabetes mellitus. J Int Med Res. 2013;41:435–444. doi: 10.1177/0300060513477290. [DOI] [PubMed] [Google Scholar]
- 8.Collin C, Wade DT, Davies S, Horne V. The Barthel ADL index: a reliability study. Int Disabil Stud. 1988;10(2):61–63. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 9.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818–1830. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman AB, Arnold AM, Sachs MC, et al. Long term function in an older cohort – the cardiovascular health study all stars study. J Am Geriatr Soc. 2009;57:432–440. doi: 10.1111/j.1532-5415.2008.02152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarado BE, Zunzunegui MV, Béland F, Bamvita JM. Life course social and health conditions linked to frailty in Latin American older men and women. J Gerontol A Biol Sci Med Sci. 2008;63:1399–1406. doi: 10.1093/gerona/63.12.1399. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira I, Snijder MB, Twisk JWR, et al. Central fat mass versus peripheral fat and lean mass: opposite (adverse versus favorable) associations with arterial stiffness? The Amsterdam growth and health longitudinal study. J Clin Endocrinol Metab. 2004;89:2632–2639. doi: 10.1210/jc.2003-031619. [DOI] [PubMed] [Google Scholar]
- 13.Sampaio RA, Sewo Sampaio PY, Yamada M, et al. Arterial stiffness is associated with low skeletal muscle mass in Japanese community-dwelling older adults. Geriatr Gerontol Int. 2014;14:109–114. doi: 10.1111/ggi.12206. [DOI] [PubMed] [Google Scholar]
- 14.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 15.Namekata T, Suzuki K, Ishizuka N, et al. Baseline criteria of cardio-ankle vascular index as a new indicator of arteriosclerosis: a cross-sectional study. BMC Cardiovasc Disord. 2011;11:51. doi: 10.1186/1471-2261-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirai K, Hiruta N, Song M, et al. Cardio-Ankle Vascular Index (CAVI) as a novel indicator of arterial stiffness: theory, evidence, and perspectives. J Atheroscler Thromb. 2011;18:924–938. doi: 10.5551/jat.7716. [DOI] [PubMed] [Google Scholar]
- 17.Sergi G, Veronese N, Fontana L, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the Pro. V.A. Study. J Am Coll Cardiol. 2015;65:976–983. doi: 10.1016/j.jacc.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 18.Liu CK, Leng X, Hsu FC, et al. The impact of sarcopenia on a physical activity intervention: the lifestyle interventions and independence for elders pilot study (LIFE-P) J Nutr Health Aging. 2014;18:59–64. doi: 10.1007/s12603-013-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaitkevicius PV, Fleg JL, Engel JH, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 20.Sternäng O, Reynolds CA, Finkel D, et al. Factors associated with grip strength decline in older adults. Age Ageing. 2015;44:269–274. doi: 10.1093/ageing/afu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohara M, Kohara K, Tabara Y, et al. Portable indices for sarcopenia are associated with pressure wave reflection and central pulse pressure: the J-SHIPP study. J Hypertens. 2015;33:314–322. doi: 10.1097/HJH.0000000000000394. [DOI] [PubMed] [Google Scholar]
- 22.Bartali B, Frongillo EA, Bandinelli S, et al. Low nutrient intake is an essential component of frailty in older persons. J Gerontol A Biol Sci Med Sci. 2006;61:589–593. doi: 10.1093/gerona/61.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyu W, Wang Q, Qinghua Z, et al. Correlation between nutritional status and frailty in hospitalized elderly patients. J Capital Univ Med. 2017;38:377–380. [Google Scholar]
- 24.Woods NF, Lacroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the women’s health initiative observational study. J Am Geriatr Soc. 2005;53(8):1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 25.Lin K, Minglei Z, Xiaohong L, et al. Correlation between frailty and coronary heart disease in the elderly. Chin J Geriatr. 2015;34:951–955. [Google Scholar]
- 26.Ballew SH, Chen Y, Daya NR, et al. Frailty, kidney function, and polypharmacy: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2017;69:228–236. doi: 10.1053/j.ajkd.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leng S, Chaves P, Koenig K, et al. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50:1268. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- 28.Chang CC, Hsu CY, Huang PH, et al. Association between frailty and carotid intima media thickness and inflammatory marker in an elderly population. Geriatr Gerontol Int. 2017;17:10. doi: 10.1111/ggi.13099. [DOI] [PubMed] [Google Scholar]