Key Points
Question
What are the underlying pathophysiologic processes of autoimmune skin toxic effects induced by anti–PD-1 therapy in patients with non–small cell lung cancer?
Findings
In this cohort study of 73 patients with non–small cell lung cancer who received anti–PD-1 therapy, T cells that reacted to antigens shared in non–small cell lung cancers and the skin mediated autoimmune skin toxic effects. These T cells may also have mediated the tumor regression in patients who responded to therapy.
Meaning
Autoimmune toxic effects associated with checkpoint blockade may serve as biomarkers for clinical responses and provide opportunities to identify cancer T-cell targets.
Abstract
Importance
Immunotherapy with checkpoint inhibitors targeting the PD-1 (programmed cell death 1) axis has brought notable progress in patients with non–small cell lung cancer (NSCLC) and other cancers. However, autoimmune toxic effects are frequent and poorly understood, making it important to understand the pathophysiologic processes of autoimmune adverse effects induced by checkpoint inhibitor therapy.
Objective
To gain mechanistic insight into autoimmune skin toxic effects induced by anti–PD-1 treatment in patients with non–small cell lung cancer.
Design, Setting, and Participants
This prospective cohort study was conducted from July 1, 2016, to December 31, 2018. Patients (n = 73) with non–small cell lung cancer who received anti–PD-1 therapy (nivolumab or pembrolizumab) were recruited from 4 different centers in Switzerland (Kantonsspital St Gallen, Spital Grabs, Spital Wil, and Spital Flawil). Peripheral blood mononuclear cells, tumor biopsy specimens and biopsies from sites of autoimmune skin toxic effects were collected over a 2-year period, with patient follow-up after 1 year.
Main Outcomes and Measures
Response to treatment, overall survival, progression-free survival, and development of autoimmune toxic effects (based on standard laboratory values and clinical examinations).
Results
Of the cohort of 73 patients with NSCLC (mean [SD] age, 68.1 [8.9] years; 44 [60%] men), 25 (34.2% [95% CI, 24.4%-45.7%]) developed autoimmune skin toxic effects, which were more frequent in patients with complete remission or partial remission (68.2% [95% CI, 47.3%-83.6%]) than those with progressive or stable disease (19.6% [95% CI, 11.0%-32.5%]) (χ2 = 14.02, P < .001). Nine T-cell antigens shared between tumor tissue and skin were identified. These antigens were able to stimulate CD8+ and CD4+ T cells in vitro. Several of the antigen-specific T cells found in blood samples were also present in autoimmune skin lesions and lung tumors of patients who responded to anti–PD-1 therapy.
Conclusions and Relevance
These findings highlight a potential mechanism of checkpoint inhibitor–mediated autoimmune toxic effects and describe the association between toxic effects and response to therapy; such an understanding will help in controlling adverse effects, deciphering new cancer antigens, and further improving immunotherapy.
This cohort study examines the pathophysiologic processes of autoimmune tissue and tumor toxic effects in patients with non–small cell lung cancer.
Introduction
Cancer immunotherapy with checkpoint inhibitors has been reported to improve survival for patients with non–small cell lung cancer (NSCLC).1,2,3 However, the autoimmune toxic effects of checkpoint inhibitors are frequent, with immune-mediated adverse effects of any grade occurring in approximately 30% of patients and toxic effects of grade 3, 4, or 5 occurring in up to 10% of patients.1 The causes of these events remain elusive. We studied a prospective cohort of 73 patients with NSCLC receiving anti–PD-1 (programmed cell death 1) therapy to gain insight into the pathophysiologic processes of autoimmune skin toxic effects.
Methods
Clinical Sample Collection
A prospective cohort study of patients with NSCLC who received anti–PD-1 treatment was conducted in 4 different centers in Switzerland (Kantonsspital St Gallen, Spital Grabs, Spital Wil, and Spital Flawil) from July 1, 2016, to December 31, 2018. The study received ethical approval from the Ethikkommission Ostschweiz. Informed consent was obtained from all patients. Peripheral blood mononuclear cells (PBMCs) and lung tumor and skin samples were collected for analyses, with patient follow-up after 1 year.
T-Cell Stimulation Assays and T-Cell Receptor Analysis
Peripheral blood mononuclear cells were stimulated with peptide pools at a concentration of 2 ug/mL/peptide. After 10 days of culture, cells were restimulated with peptide pools for 6 hours and stained for CD3, CD4, CD8, CD45RA, IFN-γ (immune interferon). For 2 patients, CD4+ and CD8+ IFN-γ+ T cells were sorted. DNA from the sorted PBMCs, skin lesions, and lung tumors was used to carry out T-cell receptor (TCR) β sequencing (ImmunoSEQ Assays, survey resolution; Adaptive Biotechnologies).
Statistical Analysis
Kaplan-Meier plots were generated for overall survival and progression-free survival using the survminer package in R, version 3.5.0 (R Foundation for Statistical Computing). The association between the development of autoimmune skin toxic effects and overall survival or progression-free survival was examined using the log-rank test. Hazard ratios (HRs) and 95% CIs were calculated using univariate Cox proportional hazards regression model with the survival package in R; we selected the development of autoimmune skin toxic effects as the covariate and overall survival or progression-free survival as the outcome. The χ2 test was used to test for associations between categorical variables.
Logistic regression was used to test the association between frequencies of IFN-γ+ T-cell response after stimulations with the peptide pools and development of skin toxic effects. Results from 21 patients with skin toxic effects and 18 patients without skin toxic effects were included in the analysis. Statistical significance was defined at the level of P = .05. Additional methods information can be found in the eMethods in the Supplement.
Results
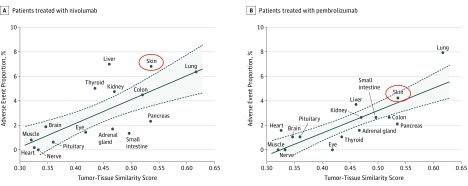
The organ-specific immune-related adverse effects analysis revealed a statistically significant association between the tumor-to-tissue similarity level and the frequency of autoimmune toxic effects (Figure 1). Skin was the second most similar organ to NSCLC (after lung) and had the second highest proportion of autoimmune adverse effects. Of the cohort of 73 patients with NSCLC (mean [SD] age, 68.1 [8.9] years; 44 [60%] men), 25 (34.2% [95% CI, 24.4%-45.7%]) developed autoimmune skin toxic effects, which were more frequent in patients with complete remission or partial remission (68.2% [95% CI, 47.3%-83.6%]) than those with progressive or stable disease (19.6% [95% CI, 11.0%-32.5%]) (χ2 = 14.02, P < .001). In addition, 15 patients (68.2%; 95% CI, 47.3%-83.6%) with complete remission or partial remission developed autoimmune skin toxic effects, compared with only 6 patients (16.2%; 95% CI, 7.7%-31.1%) with progressive disease (χ2 = 14.06; P < .001) (eFigure 1 in the Supplement).
Figure 1. Tissue Similarity Between Lung Tumors and Organs Affected by Autoimmune Toxic Effects.
Positive associations between tumor-tissue similarity score and organ-specific immune-related adverse effects in patients with non–small cell lung carcinoma treated with nivolumab (A) or pembrolizumab (B). Pearson correlation coefficient was R = 0.71 for nivolumab and R = 0.81 for pembrolizumab, and the P value was P = .003 for nivolumab and P = .001 for pembrolizumab.
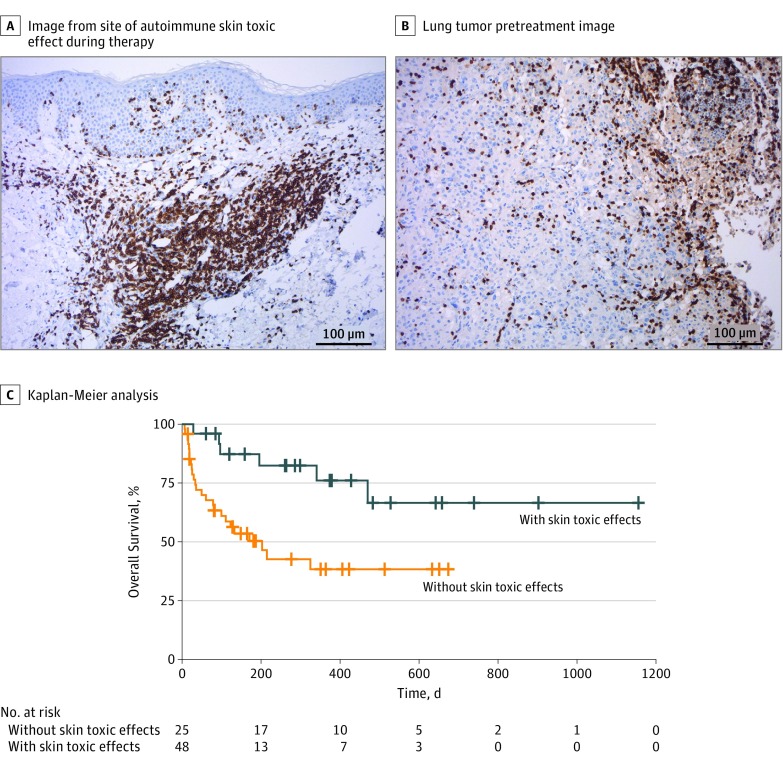
A histologic analysis of lesional biopsy specimens from sites of autoimmune skin toxic effects and lung tumors demonstrated a dense infiltration of T cells (Figure 2A and B; eFigure 2 in the Supplement). Computed tomographic scans taken during therapy showed that most patients with skin toxic effects were good responders (eFigure 3 in the Supplement). Patients with skin toxic effects also showed statistically significantly improved overall survival (HR, 0.29 [95% CI, 0.12-0.71]; P = .004) (Figure 2C) and progression-free survival (HR, 0.22 [95% CI, 0.09-0.49]; P = .001) (eFigure 4 in the Supplement). Patients who responded to therapy were more than 5 times more likely to have experienced autoimmune skin toxic effects compared with those who did not respond (odds ratio, 5.28 [95% CI, 1.78-15.67]; P = .003), a finding that is in line with results of several studies, suggesting that autoimmune adverse effects are associated with response to therapy.4,5,6,7,8,9
Figure 2. Association Between Autoimmune Skin Toxic Effects and Response to Therapy .
Images of a representative patient show autoimmune skin toxic effects (A) and lung tumors (B) characterized by an inflammatory infiltrate shown by CD3 immunohistochemistry (magnification ×100). C, Kaplan-Meier analysis of patients treated with PD-1 inhibitors reveals better outcome for 25 patients who developed skin toxic effects (blue line) compared with 48 other patients who did not develop skin toxic effects (orange line). One-year overall survival rate was 76% in the group with skin toxic effects and 38% in the group without skin toxic effects, with a hazard ratio of 0.29 (95% CI, 0.12-0.71) and log-rank P = .004.
Owing to the T-cell infiltration in the affected skin, we performed a TCR clonotype analysis with patient-matched NSCLC and skin biopsy specimens, revealing identical TCR sequences in the lung tumors and the autoimmune skin lesions (eFigure 5 in the Supplement). This finding indicates that the same T-cell clonotypes infiltrated both sites and suggests that T cells react against shared antigens in the 2 organs.
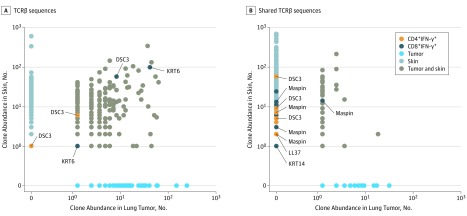
An in silico bioinformatics analysis was subsequently performed to identify potential shared antigens between NSCLC and the skin (eFigure 6 in the Supplement). To be selected, the antigens had to fulfill the following properties: (1) evidence of self-immunogenicity, (2) high expression in NSCLCs, and (3) skin or lung specificity. This analysis identified 9 candidate T-cell antigens (eTable in the Supplement). To determine whether these antigens were recognized by antigen-specific T cells, we stimulated PBMCs from 21 patients with skin toxic effects and 18 patients without skin toxic effects with the antigens in the form of overlapping peptide pools. Regression analysis revealed an association between development of skin toxic effects and high frequencies of IFN-γ+ T-cell response, which was significant for CD8+ IFN-γ+ T-cell response (odds ratio, 1.99 [95% CI, 1.01-3.90]; P = .046), but was not for CD4+ IFN-γ+ T-cell response (odds ratio, 1.05 [95% CI, 0.98-1.13]; P = .146) (eFigure 7 in the Supplement). The stimulated IFN-γ+ T cells were sorted from the PBMCs of 2 patients with skin toxic effects and who showed response to therapy. The TCRs of the sorted IFN-γ+ T cells were then compared with the TCRs found in the NSCLC and skin toxic effects from the same patients. Identical antigen-specific T cells were found in the patients’ PBMCs and tissues, with some clones being present in all 3 compartments. For 1 patient, shared T-cell clones were found specific for desmocollin 3 and keratin 6 (Figure 3A), whereas for another patient, shared T-cell clones were found specific for maspin, LL37, keratin 14, and desmocollin 3 (Figure 3B).
Figure 3. Sharing of T-Cell Clones in Autoimmune Skin Lesions, Lung Tumors, and Antigen-Stimulated Peripheral Blood Mononuclear Cells (PBMCs).
A, TCRβ sequences shared in the non–small cell lung carcinoma (NSCLC), skin, and sorted CD4+/CD8+ interferon (IFN)-γ+ T cells from PBMCs after the latter were stimulated with desmocollin 3 (DSC3) and keratin 6 (KRT6) from 1 patient. In total, 5 unique shared clones were found. The CD4+ clones shared with PBMCs are encircled in orange, and CD8+ clones shared with PBMCs are encircled in dark blue in the dot plots. B, Shared TCRβ sequences in the NSCLC, skin, and sorted CD4+/CD8+ IFN-γ+ T cells from PBMCs after the latter were stimulated with maspin, LL37, keratin 14 (KRT14), and DSC3 from another patient. In total, 31 unique shared clones were found (for this patient, only some of the clones are indicated).
Discussion
Several studies report an association between immune-related adverse effects and response to treatment with checkpoint inhibitors in patients with melanoma.4,5,8 This association also has been hypothesized in patients with NSCLC.6,7,9 To our knowledge, the present study is the first to show a potential mechanism of checkpoint inhibitor–mediated autoimmune skin toxic effects and to describe the association between immune-related adverse effects and treatment efficacy. This study suggests that, during checkpoint inhibitor therapy, T cells recognizing shared lung tumor and skin antigens simultaneously target both organs. This process is associated with the development of autoimmune skin toxic effects and likely with tumor regression as well. A similar association is seen in patients with melanoma treated with anti–PD-1, in which good responders to therapy often develop a skin condition known as vitiligo.10
The identification of antigens shared between tumors and healthy tissues may provide a dual advantage: (1) it helps in anticipating autoimmune toxic effects, and (2) it reveals potential new tumor targets. For effective cancer immunotherapy, many currently favor neoantigens. However, not all tumors harbor potent neoantigens, and tissue-specific antigens can, in principle, also support strong antitumor T-cell responses, with autoimmunity as an adverse effect, as shown in this study. Therefore, it may be necessary to identify shared antigens able to mediate tumor rejection with only limited toxic effects.
Limitations
This study has limitations. First, the sample size is small. Second, we used a limited number of matched non–small cell lung cancer and skin specimens for the TCR clonotype analysis.
Conclusions
This study suggests that specific autoimmune T-cell clones recognize shared self-antigens in lung cancer and the skin. These T-cell clones may mediate the autoimmune skin toxic effects in patients with NSCLC who receive anti–PD-1 therapy as well as the lung cancer regression in patients who respond well to therapy. If this hypothesis can be proven in larger prospective cohorts, relevant antigen-specific T cells may be useful as potential biomarkers of response to treatment. Combined with appropriate patient selection, this knowledge may help improve the efficacy of immunotherapy and anticipate its associated autoimmune toxic effects, thereby fostering its clinical application.
eMethods
eFigure 1. Proportion of Autoimmune Skin Toxicity in Different Response Groups of Anti-PD-1 Treated NSCLC Patients
eFigure 2. T Cell Infiltration in Lung Tumors and Skin Lesions
eFigure 3. CT Scans and Skin Rashes of NSCLC Patients Under Treatment With Anti-PD-1
eFigure 4. Comparison of Progression-free Survival in NSCLC Patients With and Without Skin Toxicity
eFigure 5. Shared TCRs in Skin Lesions and Lung Tumors
eFigure 6. Prediction of Shared NSCLC and Skin Antigens
eFigure 7. Stimulations of PBMCs With Shared NSCLC and Skin Antigens
eTable. Candidate NSCLC Antigens Shared With Skin
References
- 1.Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 2.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi: 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo JA, Fisher DE, Flaherty KT. Prognostic significance of cutaneous adverse events associated with pembrolizumab therapy. JAMA Oncol. 2015;1(9):1340-1341. doi: 10.1001/jamaoncol.2015.2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22(4):886-894. doi: 10.1158/1078-0432.CCR-15-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teraoka S, Fujimoto D, Morimoto T, et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: a prospective cohort study. J Thorac Oncol. 2017;12(12):1798-1805. doi: 10.1016/j.jtho.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 7.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small cell lung cancer. JAMA Oncol. 2018;4(3):374-378. doi: 10.1001/jamaoncol.2017.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773-781. doi: 10.1200/JCO.2014.57.4756 [DOI] [PubMed] [Google Scholar]
- 9.Hasan Ali O, Diem S, Markert E, et al. Characterization of nivolumab-associated skin reactions in patients with metastatic non-small cell lung cancer. Oncoimmunology. 2016;5(11):e1231292. doi: 10.1080/2162402X.2016.1231292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152(1):45-51. doi: 10.1001/jamadermatol.2015.2707 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eFigure 1. Proportion of Autoimmune Skin Toxicity in Different Response Groups of Anti-PD-1 Treated NSCLC Patients
eFigure 2. T Cell Infiltration in Lung Tumors and Skin Lesions
eFigure 3. CT Scans and Skin Rashes of NSCLC Patients Under Treatment With Anti-PD-1
eFigure 4. Comparison of Progression-free Survival in NSCLC Patients With and Without Skin Toxicity
eFigure 5. Shared TCRs in Skin Lesions and Lung Tumors
eFigure 6. Prediction of Shared NSCLC and Skin Antigens
eFigure 7. Stimulations of PBMCs With Shared NSCLC and Skin Antigens
eTable. Candidate NSCLC Antigens Shared With Skin