Key Points
Question
How much would the highly atherogenic lipoprotein(a) have to be lowered to decrease the coronary heart disease outcomes in the same range as observed for a lowering of low-density lipoprotein cholesterol by 38.67 mg/dL?
Findings
In this mendelian randomization analysis, it was estimated that lipoprotein(a) would have to be lowered by 65.7 mg/dL to reach the same potential effect on clinical outcomes as lowering low-density lipoprotein cholesterol by 38.67 mg/dL.
Meaning
Estimations of the required lipoprotein(a)-lowering potential of a drug to be clinically effective might have been overestimated in the past; this might be explained by standardization issues of lipoprotein(a) assays.
Abstract
Importance
Genetic and epidemiologic data suggest that lipoprotein(a) (Lp[a]) is one of the strongest genetically determined risk factors for coronary heart disease (CHD). Specific therapies to lower Lp(a) are on the horizon, but the required reduction of Lp(a) to translate into clinically relevant lowering of CHD outcomes is a matter of debate.
Objective
To estimate the required Lp(a)-lowering effect size that may be associated with a reduction of CHD outcomes compared with the effect size of low-density lipoprotein cholesterol (LDL-C)–lowering therapies.
Design, Setting, and Participants
Genetic epidemiologic study using a mendelian randomization analysis to estimate the required Lp(a)-lowering effect size for a clinically meaningful effect on outcomes. We used the effect estimates for Lp(a) from a genome-wide association study (GWAS) and meta-analysis on Lp(a) published in 2017 of 5 different primarily population-based studies of European ancestry. All Lp(a) measurements were performed in 1 laboratory. Genetic estimates for 27 single-nucleotide polymorphisms on Lp(a) concentrations were used. Odds ratios for these 27 single-nucleotide polymorphisms associated with CHD risk were retrieved from a subsample of the CHD Exome+ consortium.
Exposures
Genetic LPA score, plasma Lp(a) concentrations, and observations of statin therapies on CHD outcomes.
Main Outcomes and Measures
Coronary heart disease.
Results
The study included 13 781 individuals from the Lp(a)-GWAS-Consortium from 5 primarily population-based studies and 20 793 CHD cases and 27 540 controls from a subsample of the CHD Exome+ consortium. Four of the studies were similar in age distribution (means between 51 and 59 years), and 1 cohort was younger; mean age, 32 years. The frequency of women was similar between 51% and 55%. We estimated that the required reduction in Lp(a) effect size would be 65.7 mg/dL (95% CI, 46.3-88.3) to reach the same potential effect on clinical outcomes that can be reached by lowering LDL-C by 38.67 mg/dL (to convert to millimoles per liter, multiply by 0.0259).
Conclusions and Relevance
This mendelian randomization analysis estimated a required Lp(a)-lowering effect size of 65.7 mg/dL to reach the same effect as a 38.67-mg/dL lowering of LDL-C. However, this estimate is determined by the observed effect estimates of single-nucleotide polymorphisms on Lp(a) concentrations and is therefore influenced by the standardization of the Lp(a) assay used. As a consequence, calculations of the required Lp(a)-lowering potential of a drug to be clinically effective might have been overestimated in the past.
This mendelian randomization analysis estimates the required Lp(a)-lowering effect size that might translate into a reduction of coronary heart disease outcomes compared with the effect size of low-density lipoprotein cholesterol–lowering therapies.
Introduction
High lipoprotein(a) (Lp[a]) concentrations are associated with an increased risk for coronary heart disease (CHD).1 The justification to develop drugs lowering Lp(a) concentrations requires a strong support for causality, which came from genetic studies demonstrating that genetic phenotypes and variants that are associated with high Lp(a) concentrations are also associated with CHD risk.2,3,4,5 This was most pronounced in patients receiving statin therapy and low-density lipoprotein cholesterol (LDL-C) levels of 70 mg/dL or less (to convert to millimoles per liter, multiply by 0.0259).6
Until a few years ago, no specific Lp(a)-lowering therapy was available that only and specifically lowers Lp(a) concentrations. This has changed by the introduction of antisense oligonucleotides that lower Lp(a) production by up to 90%.7 An important step for planning interventional studies with such drugs is to estimate the required lowering of Lp(a) to efficiently improve clinical outcomes. First assessments ranged from 50 to 60 mg/dL8 to more than 100 mg/dL,9 to produce similar risk reductions as observed for an LDL-C lowering of 38.67 mg/dL.
As Burgess et al9 did, we used a mendelian randomization approach to estimate the required lowering of Lp(a) that would be expected to show the same association with CHD risk lowering as a 38.67-mg/dL therapeutic reduction in LDL-C levels.
Methods
To perform a mendelian randomization analysis for Lp(a) on CHD risk, genetic association effect estimates from single single-nucleotide polymorphisms (SNPs) on Lp(a) were obtained from our 2017 genome-wide association study meta-analysis on Lp(a)10 in 5 primarily population-based studies (n = 13 781). Each cohort study was approved by the responsible institutional review board and each participant provided written informed consent. The Lp(a) concentration was measured centrally in 1 laboratory using the same method. The median values for Lp(a) in all studies ranged from 11 to 12 mg/dL (except for Finnish patients who are known to have half the concentrations of other white populations).
We used 1000-genome–imputed genotypes and restricted the analysis to SNPs with a minor allele frequency of at least 1%, therefore including only 27 of 43 SNPs reported by Burgess et al.9 Log odds ratios (ORs) for these 27 SNPs on CHD risk were retrieved from eTable 3 in Burgess et al.9 These estimates are based on a subsample of the CHD Exome+ consortium (n = 48 333, including 20 793 CHD cases).
We applied multiplicative random effects models accounting for association between SNPs11 using R package mendelian randomization (The R Foundation).12 The correlation matrix was determined in the Cooperative Health Research in the Region of Augsburg (KORA-F4) study, which was part of the genome-wide association study meta-analysis on Lp(a).10 The required Lp(a)-lowering effect size to be comparable with a reduction of LDL-C by 38.67 mg/dL is obtained by the formula 38.67 × log(OR for LDL-C)/log(OR for Lp[a]), where OR for Lp(a) is the estimate derived from the mendelian randomization analysis described in this paragraph, and OR for LDL-C (in 10 mg/dL) equal to 0.855 (95% CI, 0.818-0.893) is taken from Burgess et al.9 Assuming that the proportion of short-term to lifelong risk reduction is equal for LDL-C and Lp(a), an estimate for short-term trials can be deduced from the genetically predicted estimate.
Results
In a first step, we confirmed the data by Burgess et al9 by using the same calculations and effect estimates from their eTable 39 but only using those 27 common SNPs available in our data set. We estimated an OR for CHD risk of 0.941 for each 10 mg/dL lower genetically predicted Lp(a), which translates to a decrease of Lp(a) levels by 99.8 mg/dL to be comparable with an LDL-C decrease by 38.67 mg/dL (Table). This is similar to what is reported in Burgess et al.9 Therefore, possible differences in our further calculations are not explained by missing these 16 SNPs.
Table. Required Lp(a)-Lowering Effect to Reach the Same Effect of CHD Outcomes as a 38.67-mg/dL Lowering of LDL Cholesterol Using Different Approaches.
| Approach and Data Source | OR for CHD Risk per 10-mg/dL Lower Genetically Predicted Lp(a) | Required Lp(a)-Lowering Effect Size, mg/dL (95% CI)a | |
|---|---|---|---|
| SNPs, No. | Lp(a) Estimates, Source | ||
| 43 | Burgess et al9 | 0.942 (0.933-0.951) | 101.5 (71.0-137.0) |
| 27b | Burgess et al9 | 0.941 (0.932-0.949) | 99.8 (69.8-132.4) |
| 27b | Study data | 0.912 (0.899-0.925) | 65.7 (46.3-88.3) |
Abbreviations: CHD, coronary heart disease; LDL, low-density lipoprotein; Lp(a), lipoprotein(a); OR, odds ratio; SNP, single-nucleotide polymorphism.
SI conversion factor: To convert cholesterol levels to millimoles per liter, multiply by 0.0259.
95% CI was derived from bootstrap sampling (n = 100 000) as described in Burgess et al.9
SNPs that are available in both data sets from Burgess et al9 and our data.
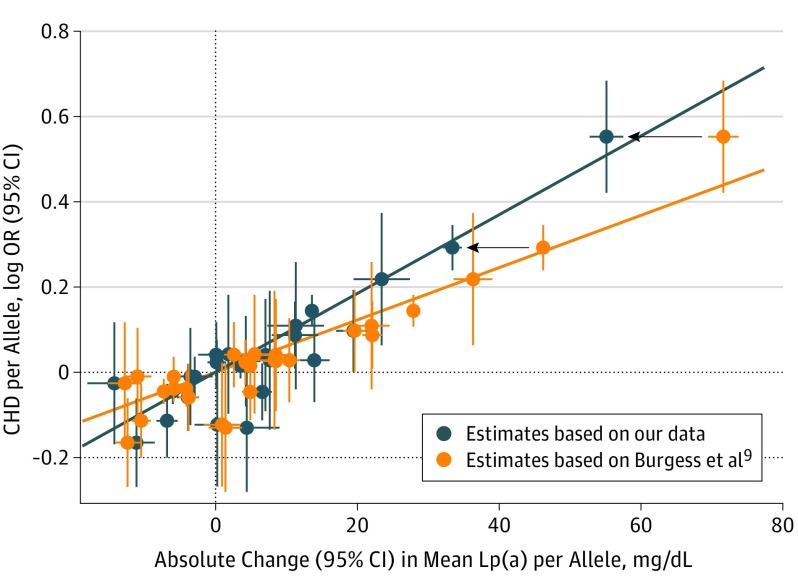
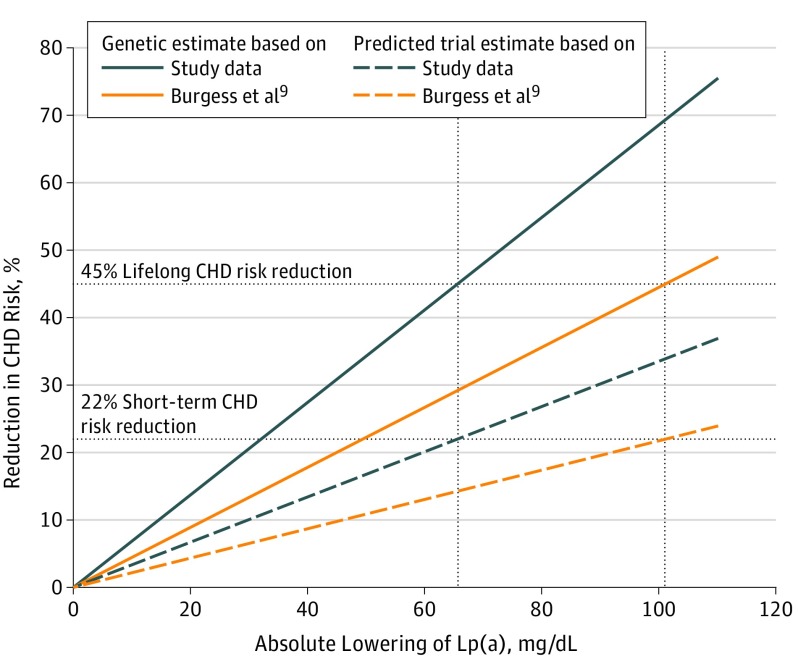
Next, we used the effect estimates from the same 27 SNPs for Lp(a) from our meta-analysis, which were smaller in 22 of 27 SNPs (Figure 1); 13 of them even significantly smaller. With these data, genetically estimated lower Lp(a) levels by 10 mg/dL were associated with an 8.8% (95% CI, 7.5-10.1) lifetime lower risk of CHD, whereas the short-term lower risk was calculated to be 3.7%. This corresponds to a median of 5 years of treatment in the Cholesterol Treatment Trialists’ [CTT] trial.13 This means Lp(a) would have to be reduced by 65.7 mg/dL (95% CI, 46.3-88.3) to be comparable with a 38.67 mg/dL reduction of LDL-C with respect to CHD risk (Table). This would correspond to a CHD risk reduction over the lifetime by 45% and by 22% over a short term (Figure 2).
Figure 1. Association of LPA Variants With Lipoprotein(a) (Lp[a]) Concentration and Coronary Heart Disease (CHD) Risk.
Marginal genetic associations for each of the 27 evaluated single-nucleotide polymorphisms (SNPs), with Lp(a) concentrations on the x-axis (denoting absolute change in milligrams per deciliter) and CHD risk on the y-axis (log odds ratio). For direct comparison, the data from Burgess et al9 are illustrated in orange color and our data are given in blue color. Effect sizes refer to the minor allele and error bars give 95% confidence intervals. The lines indicate the mendelian randomization estimates from Lp(a) on CHD risk. Black arrows show the reduction of Lp(a) estimates exemplarily for 2 SNPs when taking our estimates instead of the estimates from Burgess at al.9 These data underline that the higher estimates of the SNPs on Lp(a) concentrations from Burgess et al9 result in markedly lower estimates for CHD risk per 10-mg/dL lower genetically predicted Lp(a) concentrations compared with our data.
SI conversion factor: To convert cholesterol levels to millimoles per liter, multiply by 0.0259.
Figure 2. Estimates of Coronary Heart Disease (CHD) Risk Reduction With Lowering of Lipoprotein(a) (Lp[a]) Concentration.
The solid lines provide the genetically predicted lifelong reduction of CHD risk by varying amount of genetically caused Lp(a) reductions. The blue lines provide data from Burgess et al9 and the orange lines are based on the estimates from our study. The dashed lines give the predicted short-term trial estimates from both studies. The intersections of the horizontal and vertical dotted lines illustrate the required amount of Lp(a) lowering to reach the same effect on clinical outcomes as a 38.67 mg/dL genetically caused reduction of LDL-C (corresponds to 45% risk reduction) or therapeutic reduction of LDL-C derived from the CTT trial13 (corresponds to 22% risk reduction).
SI conversion factor: To convert cholesterol levels to millimoles per liter, multiply by 0.0259.
Discussion
This mendelian randomization analysis revealed that a 65.7-mg/dL Lp(a) lowering would be required by a specific therapy targeting Lp(a) to reach the same potential effect on clinical outcomes as a 38.67-mg/dL therapeutic reduction of LDL-C levels. This estimate is similar to calculations from a 2018 substudy of Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), from which a required 50 to 60 mg/dL reduction of Lp(a) can be deducted.8 However, it is less pronounced compared with a 2018 study by Burgess et al,9 who used the same approach as our study. Because we also used the same effect estimates on CHD risk (based on a subsample of the CHD Exome+ consortium, as provided in Burgess et al9), the only difference is the data basis for effect estimates for each of the SNPs with Lp(a) concentrations. Burgess et al9 used studies with a heterogeneous distribution in Lp(a) concentrations and much higher median values for Lp(a) than one would expect: the range of medians was 13.6 to 43.3 mg/dL, and the study with the highest median contributed 44% to the entire sample. To our knowledge, such high concentrations have never been observed before in a large white population except for patients with nephrotic syndrome, who have severe disturbances in Lp(a) metabolism, with an overproduction of Lp(a) and other lipoproteins.14
Because the calculations for Lp(a) were associated with the observed effect estimates of SNPs on Lp(a) concentrations, it is likely that the estimated Lp(a)-lowering effect size is overestimated, possibly owing to the winner’s curse or any consequence of Lp(a) measurement. Using our effect estimates from a large meta-analysis of almost 14 000 individuals with all measurements from 1 laboratory with markedly lower Lp(a) concentrations10 argues for a smaller reduction of Lp(a) to be successful in terms of clinical outcomes. It might be speculated that even lower required Lp(a)-lowering effect estimates could be calculated if use estimates from a 2017 large population study15 from several European countries with even lower median Lp(a) concentrations (between 4.9-10.9 mg/dL) were used. There is no reason to believe that the absolute effects of singular SNPs on Lp(a) concentrations are always the same independent of whether an assay measures 2 to 3 times higher absolute values as another assay. Because the standardization of Lp(a) assays is not yet solved sufficiently, this matter becomes even more important.
These results have important implications for the planning of randomized clinical trials of drugs that target Lp(a) concentrations. First, these drugs need to have a pronounced Lp(a)-lowering potential, which we estimated to be 65.7 mg/dL, but more than 100 mg/dL might be an overestimate. This is supported by a 2018 analysis from the FOURIER Trial,16 which revealed that the PCSK9-inhibitor evolocumab was associated with reduced risk of CVD outcomes by 23% in patients with a baseline Lp(a) level greater than the median, and by 7% in those at the median or less (P value interaction = .07), although the overall median Lp(a) reduction was only 26.9%. Second, the upcoming first trials should include patients with Lp(a) greater than 100 mg/dL at baseline to achieve an Lp(a) concentration less than 30 mg/dL by treatment. Third, the Lp(a) assay used for identification of patients for treatment have to be well standardized to avoid an overestimation or underestimation of Lp(a) concentrations and thereby an inappropriate recruitment of patients.
One further central question is how a mendelian randomization estimate can be transferred to what might be finally observed in interventional trials. The aim of Burgess et al9 and our study was “to estimate how much Lp(a) concentration must be lowered pharmacologically to produce the same change as lowering LDL-C by 38.67 mg/dL with a statin.” However, this is based on the important assumption that the lifetime risk reduction based on genetic effects for 38.67 mg/dL of LDL-C (approximately 45% according to Burgess et al9) compared with the short-time risk reduction based on therapeutic lowering for LDL-C by 38.67 mg/dL (approximately 22% derived from LDL-C trials13) shows a similar proportion for Lp(a) as observed for LDL-C. Whether this is indeed the case needs to be evaluated in future outcome trials. Finally, because an LDL-C lowering of less than 38.67 mg/dL has beneficial effects on outcomes, even a lowering of Lp(a) less than 65.7 mg/dL might be beneficial.
Limitations
Our study has limitations. First, it is based on the assumption that the pathophysiological effects and mechanisms of LDL-C and Lp(a) for atherosclerosis development are similar, which is not necessarily the case. There is some evidence that Lp(a) has beside the atherogenic also thrombogenic properties.1 Second, the standardization of Lp(a) assays in general is not resolved sufficiently which is caused by the repetitive kringle-IV structure of apolipoprotein(a) and the antibodies used in the various assay. Therefore, the estimates of the SNP effects on Lp(a) concentrations used in a mendelian randomization analysis will depend on the assay used and can result in an overestimation or underestimation of the required therapeutical Lp(a) lowering. Both limitations are valid for this and other studies.8,9
Conclusions
By using a mendelian randomization analysis, we estimated that the required reduction in Lp(a) effect would be 65.7 mg/dL to reach the same potential effect on clinical outcomes as a 38.67 mg/dL lowering of LDL-C.
References
- 1.Kronenberg F, Utermann G. Lipoprotein(a): resurrected by genetics. J Intern Med. 2013;273(1):6-30. doi: 10.1111/j.1365-2796.2012.02592.x [DOI] [PubMed] [Google Scholar]
- 2.Sandholzer C, Saha N, Kark JD, et al. Apo(a) isoforms predict risk for coronary heart disease: a study in six populations. Arterioscler Thromb. 1992;12(10):1214-1226. doi: 10.1161/01.ATV.12.10.1214 [DOI] [PubMed] [Google Scholar]
- 3.Erqou S, Thompson A, Di Angelantonio E, et al. Apolipoprotein(a) isoforms and the risk of vascular disease: systematic review of 40 studies involving 58,000 participants. J Am Coll Cardiol. 2010;55(19):2160-2167. doi: 10.1016/j.jacc.2009.10.080 [DOI] [PubMed] [Google Scholar]
- 4.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331-2339. doi: 10.1001/jama.2009.801 [DOI] [PubMed] [Google Scholar]
- 5.Clarke R, Peden JF, Hopewell JC, et al. ; PROCARDIS Consortium . Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518-2528. doi: 10.1056/NEJMoa0902604 [DOI] [PubMed] [Google Scholar]
- 6.Wei WQ, Li X, Feng Q, et al. LPA variants are associated with residual cardiovascular risk in patients receiving statins. Circulation. 2018;138(17):1839-1849. doi: 10.1161/CIRCULATIONAHA.117.031356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388(10057):2239-2253. doi: 10.1016/S0140-6736(16)31009-1 [DOI] [PubMed] [Google Scholar]
- 8.Parish S, Hopewell JC, Hill MR, et al. ; HPS2-THRIVE Collaborative Group . Impact of apolipoprotein(a) isoform size on lipoprotein(a) lowering in the HPS2-THRIVE study. Circ Genom Precis Med. 2018;11(2):e001696. doi: 10.1161/CIRCGEN.117.001696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess S, Ference BA, Staley JR, et al. ; European Prospective Investigation Into Cancer and Nutrition–Cardiovascular Disease (EPIC-CVD) Consortium . Association of LPA variants with risk of coronary disease and the implications for lipoprotein(a)-lowering therapies: a mendelian randomization analysis. JAMA Cardiol. 2018;3(7):619-627. doi: 10.1001/jamacardio.2018.1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mack S, Coassin S, Rueedi R, et al. ; KORA-Study Group . A genome-wide association meta-analysis on lipoprotein (a) concentrations adjusted for apolipoprotein (a) isoforms. J Lipid Res. 2017;58(9):1834-1844. doi: 10.1194/jlr.M076232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880-1906. doi: 10.1002/sim.6835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yavorska OO, Burgess S. MendelianRandomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734-1739. doi: 10.1093/ije/dyx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baigent C, Blackwell L, Emberson J, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681. doi: 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kronenberg F, Lingenhel A, Lhotta K, et al. The apolipoprotein(a) size polymorphism is associated with nephrotic syndrome. Kidney Int. 2004;65(2):606-612. doi: 10.1111/j.1523-1755.2004.00418.x [DOI] [PubMed] [Google Scholar]
- 15.Waldeyer C, Makarova N, Zeller T, et al. Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium. Eur Heart J. 2017;38(32):2490-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Donoghue ML, Fazio S, Giugliano RP, et al. Lipoprotein(a), PCSK9 inhibition and cardiovascular risk: insights from the FOURIER trial. Circulation. 2018. doi: 10.1161/CIRCULATIONAHA.118.037184 [DOI] [PubMed] [Google Scholar]