Key Points
Question
Does single-fraction stereotactic body radiotherapy (SBRT) for bone metastases lead to better pain response rates than standard multifraction radiotherapy (MFRT)?
Findings
In this prospective randomized phase 2 noninferiority trial, 160 patients with mostly nonspine bone lesions were randomly assigned to receive single-fraction SBRT (12 Gy for ≥4-cm lesions or 16 Gy for <4-cm lesions) or MFRT to 30 Gy in 10 fractions. Single-fraction radiation led to more patients experiencing complete or partial pain response at 2 weeks, 3 months, and 9 months compared with standard MFRT.
Meaning
Pain response rates were higher for high-dose, single-fraction SBRT, which should be considered for patients with bone metastases and long estimated survival times.
This randomized phase 2 noninferiority trial assesses the efficacy of high-dose, single-fraction stereotactic radiation therapy vs standard multifraction radiation therapy for pain relief in patients with mostly nonspine bone metastases.
Abstract
Importance
Consensus is lacking as to the optimal radiotherapy dose and fractionation schedule for treating bone metastases.
Objective
To assess the relative efficacy of high-dose, single-fraction stereotactic body radiotherapy (SBRT) vs standard multifraction radiotherapy (MFRT) for alleviation of pain in patients with mostly nonspine bone metastases.
Design, Setting, and Participants
This prospective, randomized, single-institution phase 2 noninferiority trial conducted at a tertiary cancer care center enrolled 160 patients with radiologically confirmed painful bone metastases from September 19, 2014, through June 19, 2018. Patients were randomly assigned in a 1:1 ratio to receive either single-fraction SBRT (12 Gy for ≥4-cm lesions or 16 Gy for <4-cm lesions) or MFRT to 30 Gy in 10 fractions.
Main Outcomes and Measures
The primary end point was pain response, defined by international consensus criteria as a combination of pain score and analgesic use (daily morphine-equivalent dose). Pain failure (ie, lack of response) was defined as worsening pain score (≥2 points on a 0-to-10 scale), an increase in morphine-equivalent opioid dose of 50% or more, reirradiation, or pathologic fracture. We hypothesized that SBRT was noninferior to MFRT.
Results
In this phase 2 noninferiority trial of 96 men and 64 women (mean [SD] age, 62.4 [10.4] years), 81 patients received SBRT and 79 received MFRT. Among evaluable patients who received treatment per protocol, the single-fraction group had more pain responders than the MFRT group (complete response + partial response) at 2 weeks (34 of 55 [62%] vs 19 of 52 [36%]) (P = .01), 3 months (31 of 43 [72%] vs 17 of 35 [49%]) (P = .03), and 9 months (17 of 22 [77%] vs 12 of 26 [46%]) (P = .03). No differences were found in treatment-related toxic effects or quality-of-life scores after SBRT vs MFRT; local control rates at 1 and 2 years were higher in patients receiving single-fraction SBRT.
Conclusions and Relevance
Delivering high-dose, single-fraction SBRT seems to be an effective treatment option for patients with painful bone metastases. Among evaluable patients, SBRT had higher rates of pain response (complete response + partial response) than did MFRT and thus should be considered for patients expected to have relatively long survival.
Trial Registration
ClinicalTrials.gov identifier: NCT02163226
Introduction
Bone is a common site of metastasis in advanced cancer, and bone metastases often result in debilitating cancer-related pain. For decades, radiation therapy has been the gold standard for palliation of pain from osseous metastases.1,2 However, the treatment paradigm for metastatic cancer has changed substantially with the advent of newer systemic therapies, targeted agents, and immunotherapy. Because patients with metastatic disease can have improved outcomes and longer survival times, providing durable pain control is important for preserving their quality of life.
Despite numerous prospective randomized clinical trials (RCTs), consensus has not been reached regarding the optimal radiation dose and fractionation for palliation of painful bone metastases. Since the early 1980s, several trials have shown that palliative radiation, delivered in single or multiple fractions, can produce equivalent pain relief, but the single-fraction regimens generally led to higher retreatment rates.1,2,3,4 In attempts to analyze these and other less consistent results, 2 meta-analyses by Wu et al5 and Sze et al6 concluded that neither complete nor overall pain relief rates were different for patients given single- vs multiple-fraction radiation regimens.
However, surveys of practice patterns7,8,9 worldwide demonstrate that many clinicians remain reluctant to use single-fraction radiation, perhaps because of the reported high retreatment rates or perception of a lack of durable pain control, especially for patients with long life expectancy. Herein we report the findings from the first (to our knowledge) prospective RCT of single- vs multiple-fraction radiation for mostly nonspine bone metastases. The trial objective was to compare pain relief from high-dose single-fraction stereotactic body radiotherapy (SBRT) with that from conventional multifraction radiotherapy (MFRT).
Methods
Study Design and Participants
This phase 2, nonblinded, prospective, randomized clinical noninferiority trial (Supplement 1) was conducted at The University of Texas MD Anderson Cancer Center after approval by the institutional review board. Each patient provided written informed consent before enrollment. The primary end point in this trial was pain response; failure (that is, lack of pain control) was defined according to international consensus criteria10,11 as a combination of worsening of patient-reported pain score and increased consumption of narcotics. We also considered the need for reirradiation or radiographic evidence of disease progression after treatment to be pain failures. Secondary end points were (1) quality of life and symptom burden, as assessed by the 13 core items of the MD Anderson Symptom Inventory (MDASI)12; (2) clinician-rated toxic effects per the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0; (3) local control of irradiated metastases; and (4) overall survival (OS).
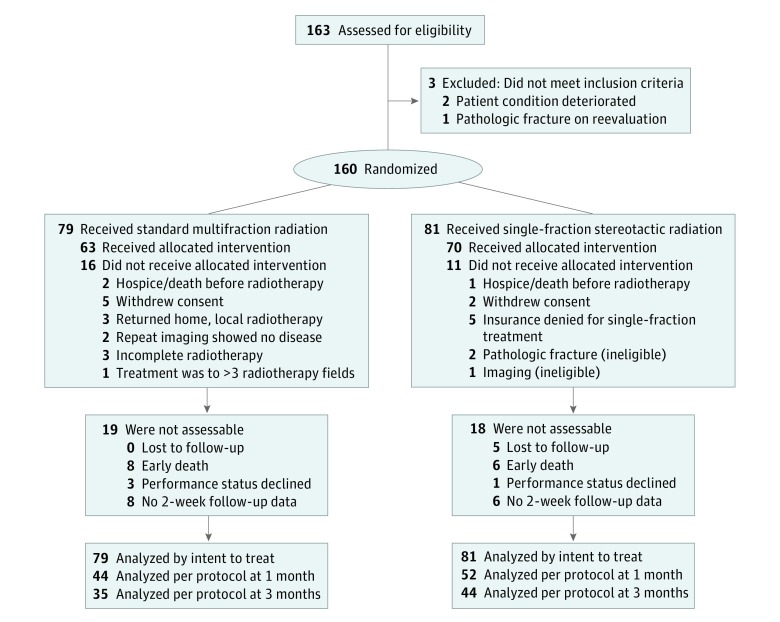
Eligible patients met the following criteria: (1) pathologic diagnosis of cancer, (2) painful bone metastases (ie, a score of at least 2 on a 0-to-10 scale), and (3) age 18 years or older with a life expectancy of more than 3 months. Concurrent treatment of up to 3 radiation fields was allowed. Exclusion criteria included prior radiation to the site being evaluated, untreated spinal cord compression, pathologic fracture at the evaluated site, and previous receipt of radioactive isotope therapy (eg, strontium 89) within 30 days of randomization. Study inclusions and exclusions are shown in Figure 1.
Figure 1. Flow Diagram of Patient Inclusions and Exclusions.
Randomization and Masking
Eligible patients were randomly assigned (ratio, 1:1) to 1 of 2 treatment groups: single-fraction SBRT (12 Gy for lesions >4 cm or 16 Gy for lesions ≤4 cm) or standard MFRT (30 Gy delivered in 10 3-Gy fractions). After a research nurse enrolled each patient, the randomization was done through MD Anderson’s Clinical Oncology Research information management system. Patients were stratified by the following variables: (1) tumor size (≤4 cm vs >4 cm), (2) site of bone metastases (extremities, pelvis, abdomen, head/neck, or thorax), and (3) number of sites irradiated (1 vs >1).
Treatment Procedures and Follow-up Evaluation
Standard concurrent chemotherapy, immunotherapy, or targeted therapy was allowed. Radiation was delivered as 2-dimensional or 3-dimensional conformal radiation therapy, intensity-modulated radiation therapy, or volumetric arc therapy. Patients in the SBRT group were immobilized with stereotactic devices to ensure reproducible positioning. The planning target volumes were standardized in both treatment groups. The gross tumor volume was delineated on computed tomographic (CT) scans obtained at treatment simulation. The planning target volume was defined as the gross tumor volume plus a 5-mm circumferential margin. No clinical target volumes were used. A prophylactic 7-day course of low-dose dexamethasone was recommended for patients receiving SBRT to reduce the risk of painful bone flares; if the patients in the standard MFRT group experienced bone flares, they could also receive low-dose dexamethasone at the treating physician’s discretion.
At baseline and every follow-up visit, patients were asked to rate their pain at the irradiated site at its worst on a scale from 0 to 10; analgesic intake was also recorded and converted to morphine-equivalent doses (MEDs). The pain response end point was defined as a combination of pain score and analgesic consumption, as recommended in international consensus guidelines.10,11 According to those guidelines, 4 types of response are possible: complete response (CR), partial response (PR), pain progression (PP), or indeterminate response (IR). Complete response is a pain score of 0 at the treated site and no increase in MED, whereas PR is a reduction in pain score of 2 or more points above baseline with no increase in MED. Pain progression is an increase in pain score of 2 or more points above baseline with no change in MED, or an increase in pain score of 1 point above baseline with an increase in MED of 25% or more. Indeterminate responses were all other responses. Both PP and IR indicated pain failure. Follow-up visits for patients in both treatment groups were scheduled at 1 to 3 months after treatment and then at 6 months, 9 months, and 12 months after treatment for the first year, and at the physician’s discretion thereafter. For the secondary end points, the extent to which pain interfered with patients’ quality of life and daily living was rated with the 13-core-item MDASI. Response to radiotherapy (as opposed to pain response) at the irradiated sites was assessed with X-ray, CT, positron emission tomography/CT, magnetic resonance imaging, or bone scan. Toxic effects were also assessed by clinicians using the CTCAE version 4.0 during treatment and at every follow-up visit.
Statistical Analyses
This study was registered as a phase 2, nonblinded RCT with ClinicalTrials.gov (NCT02163226). We estimated that a sample size of 150 patients (75 patients randomized to each treatment arm) would yield 90% power with a 1-sided significance level of 0.20, as recommended by Rubenstein,13 to reject the null hypothesis and conclude that single-fraction SBRT was not inferior to standard MFRT in terms of pain response (that is, CR/PR vs PP/IR).
We analyzed the proportions of patients in each group experiencing CR or PR by using a Cochran-Mantel-Haenszel test14 and calculated the 1-sided upper 95% confidence limit of the difference between the groups. The noninferiority margin was defined as 10%, so if the upper boundary of the 95% confidence limit for the difference in overall response among patients assigned to single-fraction SBRT was no more than 10% less than the overall response among patients assigned to MFRT, we would conclude that the single-fraction SBRT regimen was not inferior to the MFRT regimen. The primary analysis was by intent to treat. Because death and dropout rates are high for patients with stage IV cancer, we also conducted a per-protocol analysis that excluded patients who were found to be ineligible, failed to receive the allocated treatment after randomization, or whose response could not be assessed at 3 months.
We used the Wilcoxon rank sum test to compare changes in pain scores and analgesic use at each assessment point relative to baseline by treatment group. Increases or decreases of 2 or more points on a scale of 0 to 10 indicated improving or worsening pain. Fisher exact tests were used to compare the distribution of pain responders (CR + PR) and nonresponders (PP + IR) between the 2 treatment groups.
For the secondary end points, Kaplan-Meier estimates of local failure and OS over time were provided for each treatment group, and log-rank and Wilcoxon tests were used to compare distributions between groups. The cumulative incidence function of local failure was estimated and plotted by treatment group according to Fine and Gray,15 with death considered a competing risk. Hazard ratios (HRs) with 95% CIs were calculated with a Cox proportional hazards model to compare pain response rates between the SBRT and MFRT groups. All analyses were conducted with Statistical Analysis Software version 9.4 for Windows (SAS Institute) and R version 3.5.0.
Results
The study was opened for enrollment in September 19, 2014, and closed on June 19, 2018. A total of 160 patients (96 men, 64 women; mean [SD] age, 62.4 [10.4] years) were randomly assigned to either single-fraction SBRT or standard MFRT. Twenty-seven patients (16 in the MFRT group and 11 in the SBRT group) were found to be ineligible and therefore did not receive treatment per randomization (Figure 1). Treatment groups were well balanced in terms of sex, age, ethnicity, tumor histology, sites of bony metastases, baseline pain scores, number of sites irradiated, and Karnofsky performance status scores (eTable in Supplement 2).
Pain Response by Treatment
In the intent-to-treat analysis, 81 patients were randomized to receive single-fraction SBRT (12 Gy or 16 Gy depending on lesion size) and 79 were randomized to receive standard MFRT (30 Gy in 10 fractions). The response rates (CR + PR) at 1 month were 44% (n = 36) for the SBRT group vs 30% (n = 24) for the MFRT group (P = .18), and the corresponding rates at 3 months were 38% (n = 31) vs 21% (n = 17) (P = .05) (Table). Among evaluable patients who received treatment per protocol (ie, the per-protocol analysis), the SBRT group had more pain responders (CR+PR) than the MFRT group at 2 weeks (34 of 55 [62%] vs 19 of 52 [36%]) (P = .01), 3 months (31 of 43 [72%] vs 17 of 35 [49%]) (P = .03), and 9 months (17 of 22 [77%] vs 12 of 26 [46%]) (P = .04) (Table).
Table. Pain Response Over Time by Treatment Group.
| Group | Patients, No. (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 Weeks | 1 Month | 3 Months | 6 Months | 9 Months | |||||||||||
| Single Fx | Multifx | P Value | Single Fx | Multifx | P Value | Single Fx | Multifx | P Value | Single Fx | Multifx | P Value | Single Fx | Multifx | P Value | |
| Intent-to-Treat Population | |||||||||||||||
| Responders (CR + PR) | 34 of 81 (42) | 19 of 79 (24) | .03 | 36 of 81 (44) | 24 of 79 (30) | .18 | 31 of 81 (38) | 17 of 79 (21) | .05 | 19 of 81 (23) | 17 of 79 (21) | .89 | 17 of 81 (21) | 12 of 79 (15) | .06 |
| Nonresponders (PP + IR) | 21 of 81 (26) | 33 of 79 (42) | 17 of 81 (21) | 20 of 79 (25) | 12 of 81 (15) | 18 of 79 (23) | 9 of 81 (11) | 11 of 79 (14) | 5 of 81 (6) | 14 of 79 (18) | |||||
| Per-Protocol Treated Population | |||||||||||||||
| Responders (CR + PR) | 34 of 55 (62) | 19 of 52 (36) | .01 | 36 of 44 (68) | 24 of 44 (45) | .21 | 31 of 43 (72) | 17 of 35 (49) | .03 | 19 of 28 (68) | 17 of 28 (61) | .78 | 17 of 22 (77) | 12 of 26 (46) | .04 |
| Nonresponders (PP + IR) | 21 of 55 (38) | 33 of 52 (64) | 36 of 44 (68) | 20 of 44 (45) | 12 of 43 (28) | 18 of 35 (51) | 9 of 28 (32) | 11 of 28 (39) | 5 of 22 (23) | 14 of 26 (54) | |||||
In a subset analysis of radiation dose in the SBRT intent-to-treat group, rates of pain response (CR + PR) were higher for those treated with 16 Gy (62% [n = 13 of 21]) than for those treated with 12 Gy (30% [n = 18 of 60]) or 30 Gy in 10 fractions (21% [n = 17 of 79]; P = .003) at 3 months. At longer follow-up times, 16-Gy SBRT produced the most durable pain control; at 9 months, the pain response rates were 42.9% (n = 9 of 21) for the 16-Gy SBRT group vs 13.3% (n = 8 of 60) for the 12-Gy SBRT group and 15.2% (n = 12 of 79) for the 30-Gy MFRT group (P = .005).
Local Progression-Free Survival and Overall Survival by Treatment
Local progression-free survival rates were higher in the SBRT group than MFRT group at 1 year (100% [n = 17 of 17] vs 90.5% [n = 2 of 17]) and at 2 years (100% [n = 4 of 4] vs 75.6% [n = 1 of 6]) (P = .01) (Figure 2). Among the 81 patients in the intent-to-treat group given SBRT, none had local failures, 42 patients had competing events of death, and 39 patients were censored, for a cumulative incidence of local failure of 0% at 6 months and up to 24 months. Among the 79 patients in the intent-to-treat group given MFRT, 6 experienced local failure, 49 patients had competing events of death, and 24 patients were censored, for a 4.2% cumulative incidence of local failure at 6 months, 5.9% at 12 months, and 9.7% at 24 months (P = .02). Interestingly, the reirradiation rates seemed to be lower in the SBRT group (0% at both 1 and 2 years) than in the MFRT group (3.3% [n = 3 of 24] at 1 year and 5.3% [n = 1 of 10] at 2 years), although this difference was not statistically significant (P = .10).
Figure 2. Local Progression-Free Survival According to Treatment.
MFRT indicates standard-dose multifraction radiation therapy (10 fractions of 3 Gy each, for a total of 30 Gy); SBRT, high-dose, single-fraction stereotactic radiation therapy with a dose of 12 Gy or 16 Gy (solid line).
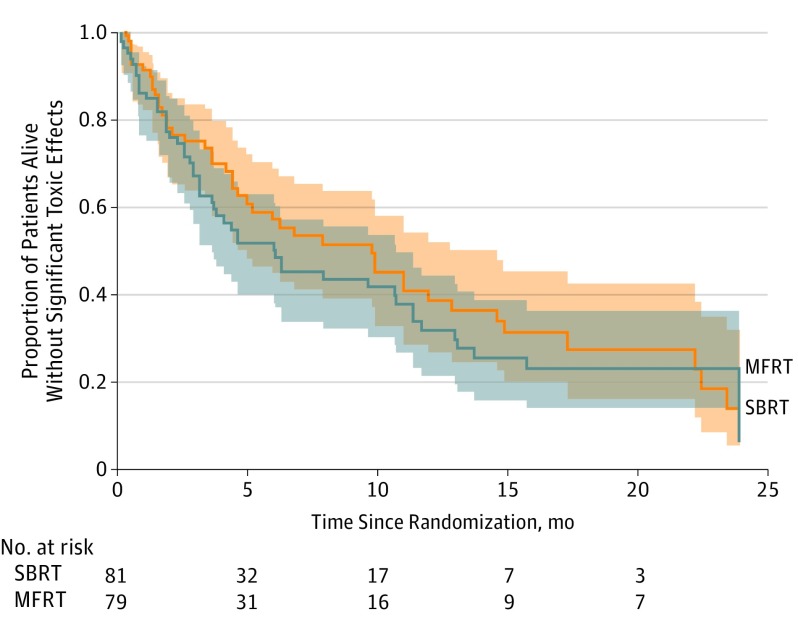
No difference was found in OS between the 2 treatment groups in the intent-to-treat analysis (eFigure 1 in Supplement 2); the median survival times were the same in both groups at 6.7 months (range, 0.1-36.3 months; 95% CI, 4.6-10.9 months). However, when we applied a quality-life-adjusted survival analysis using the Q-TWiST method,16 the OS was found to be significantly higher in the SBRT group than in the MFRT group (Figure 3).
Figure 3. Overall Quality-Adjusted Product-Limit Survival According to Treatment.
The Q-TWiST method was used to analyze quality-adjusted overall survival time, that is, the proportion of patients alive without significant toxic effects (all MD Anderson Symptom Inventory [MDASI] scores <5) over the interval since randomization, in months. The shaded areas represent interval confidence limits. MFRT indicates standard-dose multifraction radiation therapy (10 fractions of 3 Gy each, for a total of 30 Gy); SBRT, high-dose, single-fraction stereotactic radiation therapy with a dose of 12 Gy or 16 Gy (solid line).
Among the intent-to-treat patients, the calculated hazard ratio (HR) for the composite measure worsening of pain and change in MED (ie, PP and IR) at 3 months for the SBRT vs MFRT groups was 0.73 (95% CI, 0.16-3.27). The corresponding HR for an increase in pain score of 2 points or more from baseline to 3 months was 1.22 (95% CI, 0.82-1.81), and the HR for an increase of 50% or more in narcotic usage (by MED) from baseline to 3 months was 0.26 (95% CI, 0.07-0.95). The HR for local failure was 0.18 (95 CI, 0.02-1.47), and the HR for reirradiation was 0.13 (95% CI, 0.004–4.010). Collectively, these results show that SBRT was noninferior to MFRT.
Toxic Effects According to Treatment
No differences in toxic effects were found between the 2 treatment groups. Rates of grade 2 and 3 nausea were 21% (n = 20 of 79) and 1.2% (n = 1 of 81), respectively, for the SBRT group and 25.3% (n = 10 of 79) and 5.0% (n = 4 of 79), respectively, for the MFRT group (P = .58 for grade 2 and P = .21 for grade 3). Rates of grade 2 and 3 vomiting were 8.6% (n = 7 of 81) and 0% (n = 0 of 81), respectively, for the SBRT group and 13.9% (n = 11 of 79) and 2.5% (n = 2 of 79), respectively, in the MFRT group (P = .33 for grade 2 and P = .24 for grade 3). Rates of grade 3 fatigue were 9.9% (n = 8 of 81) in the SBRT group vs 5.1% (n = 4 of 49) in the MFRT group (P = .37). Rates of radiation dermatitis were quite low at 1.2% (n = 1 of 81 patients) for the SBRT group and 2.5% (n = 2 of 79) for the MFRT group (P = .62), and rates of fracture were 1.2% (n = 1 of 81) after SBRT and 0% (n = 0 of 79) after MFRT (P = .99). Both SBRT and MFRT led to improved MDASI quality-of-life symptom scores, but no significant difference in scores was found between the treatments (eFigure 2 in Supplement 2).
Discussion
To our knowledge, this is the first RCT to compare high-dose, single-fraction SBRT with standard-dose MFRT for patients with painful, predominantly nonspine bone metastases. Our intent-to-treat analysis revealed that single-fraction SBRT with either 12 Gy or 16 Gy was not inferior to standard MFRT with regard to pain control and time to local progression. When we assessed overall pain response as recommended by international consensus criteria, the HRs were less than 1.5 at 3 months, 6 months, and 12 months for both groups, further indicating that stereotactic doses were noninferior to conventionally fractionated radiation. Among evaluable patients, rates of overall pain response (CR+PR) were significantly higher in the SBRT group than in the MFRT group at 2 weeks, 3 months, and 9 months. Collectively, these findings support that delivery of the higher dose via SBRT could produce better short-term and long-term pain response. These findings may well change the standard of care to allow higher single-fraction doses (12-16 Gy) instead of the traditional 8-Gy dose for more effective and durable palliation of pain.
The debate continues over which fractionation scheme produces the best results for palliation of painful bone metastases, despite numerous published RCTs.3,4,17,18,19 Findings from these trials showed no significant difference in pain control between a short single-fraction course or longer multifraction courses, although the 8-Gy single-fraction regimen led to higher retreatment rates, suggesting that that regimen did not produce sufficiently durable responses. In contrast, the findings of the present study showed that delivering a single fraction of 12 to 16 Gy, with the correspondingly higher biologically effective doses (BEDs), was not associated with higher retreatment rates relative to patients given 30 Gy in 10 fractions. Indeed, this study is the first RCT to show that higher-dose SBRT resulted in more durable pain response and improved local control compared with the standard-dose MFRT regimen. Moreover, the findings for the secondary end point of local failure showed that local progression-free survival rates were higher in the single-fraction group than in the multifraction group at 1 year and at 2 years (Figure 2). Thus, higher doses of 12 to 16 Gy delivered stereotactically led to better pain response and better local control. These improved outcomes, in addition to the convenience of shorter-term radiation for patients, seem to indicate that high-dose, single-fraction SBRT is the more desirable treatment option.
Stereotactic body radiotherapy has the advantage of delivering significantly higher BEDs than are possible with conventionally fractionated radiotherapy, which as we found can result in both improved tumor control and durable pain control. The subset analysis comparing the 3 different doses delivered suggested that the higher BED provided superior pain response at 9 months; receipt of SBRT with 16 Gy led to the highest durable pain response rate (42.8% [9 of 21] vs 13.3% [8 of 60] for 12-Gy single fraction or 15.5% [12 of 79] 30-Gy multifraction radiation, P < .01). Therefore, for patients with a relatively long life expectancy and good performance status, use of an SBRT dose of at least 16 Gy may be most effective. In terms of local control, to date, 15 reviews of stereotactic radiotherapy for nonspine bone metastases and found that local control rates were all higher than 85%.20,21
For the survival analysis, we used the Q-TWiST (Quality adjusted Time without Symptoms and Toxicity) method, which computes survival analysis and quality-of-life-adjusted survival, taking into account treatment-related adverse effects over time.16 We observed an improvement in Q-TWiST survival for patients receiving SBRT vs MFRT probably related to the significant reduction in pain. A few studies have shown that physical health quality-of-life measures as well as pain, eating, and speech were highly associated with survival after head and neck cancer.22,23,24 We recommend that this Q-TWiST analysis be validated with a larger group of patients.
Limitations
Several factors complicate the interpretation of our results, most of which reflect the natural progression of metastatic disease. Patients in the present study had solid primary cancer with multiple metastases to the bone, which undoubtedly contributed to the poor prognosis and limited survival. Having metastatic cancer, with the correspondingly high risks of death, could influence the findings, although some degree of attrition could be expected. Indeed, the attrition rate in this study rose with increasing follow-up time. However, among patients who survived 3 months and 9 months after treatment, more experienced overall pain response and durable local control in the single-fraction group, which is consistent with previous studies reporting pain relief rates of 80% from SBRT for spinal metastases.18,25 Another potential shortcoming of the study is the inclusion of patients with metastases from several types of primary cancer. Differences in tumor histology and molecular subtype may have influenced the response to either SBRT or MFRT and, in turn, pain response and local control. We plan to report responses according to histology separately. Finally, the findings from the subset analysis of dose could have been influenced by lesion size because the dose in this study was based on lesion size. This possibility needs further testing in future prospective trials.
Conclusions
In conclusion, we found that single-fraction SBRT was noninferior to standard-dose MFRT in terms of pain control and local disease control. These findings represent the first prospective randomized evidence to suggest that SBRT should be the standard of care for patients with excellent performance status, longer life expectancy, and limited bone metastases. We recommend that higher single-fraction SBRT doses be further tested in larger phase 3 studies to validate our findings.
Trial Protocol
eTable. Patient Characteristics
eFigure 1. Overall survival estimates by treatment group in the intent-to-treat analysis
eFigure 2. Proportions of patients with or without severe symptoms on the 13-core-item MD Anderson Symptom Index (MDASI) after irradiation of bone metastases
Data Sharing Statement
References
- 1.Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423-1436. doi: 10.1200/JCO.2006.09.5281 [DOI] [PubMed] [Google Scholar]
- 2.Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol). 2012;24(2):112-124. doi: 10.1016/j.clon.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 3.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804. doi: 10.1093/jnci/dji139 [DOI] [PubMed] [Google Scholar]
- 4.Bone Pain Trial Working Party 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Radiother Oncol. 1999;52(2):111-121. doi: 10.1016/S0167-8140(99)00097-3 [DOI] [PubMed] [Google Scholar]
- 5.Wu JS, Wong R, Johnston M, Bezjak A, Whelan T; Cancer Care Ontario Practice Guidelines Initiative Supportive Care Group . Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys. 2003;55(3):594-605. doi: 10.1016/S0360-3016(02)04147-0 [DOI] [PubMed] [Google Scholar]
- 6.Sze WM, Shelley MD, Held I, Wilt TJ, Mason MD. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy—a systematic review of randomised trials. Clin Oncol (R Coll Radiol). 2003;15(6):345-352. doi: 10.1016/S0936-6555(03)00113-4 [DOI] [PubMed] [Google Scholar]
- 7.Ganesh V, Chan S, Raman S, et al. A review of patterns of practice and clinical guidelines in the palliative radiation treatment of uncomplicated bone metastases. Radiother Oncol. 2017;124(1):38-44. doi: 10.1016/j.radonc.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 8.McDonald R, Chow E, Lam H, Rowbottom L, Soliman H. International patterns of practice in radiotherapy for bone metastases: A review of the literature. J Bone Oncol. 2014;3(3-4):96-102. doi: 10.1016/j.jbo.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura N, Shikama N, Wada H, et al. ; Japanese Radiation Oncology Study Group Working Subgroup of Palliative Radiotherapy . Patterns of practice in palliative radiotherapy for painful bone metastases: a survey in Japan. Int J Radiat Oncol Biol Phys. 2012;83(1):e117-e120. doi: 10.1016/j.ijrobp.2011.11.075 [DOI] [PubMed] [Google Scholar]
- 10.Chow E, Wu JS, Hoskin P, Coia LR, Bentzen SM, Blitzer PH. International consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Radiother Oncol. 2002;64(3):275-280. doi: 10.1016/S0167-8140(02)00170-6 [DOI] [PubMed] [Google Scholar]
- 11.Chow E, Hoskin P, Mitera G, et al. ; International Bone Metastases Consensus Working Party . Update of the international consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):1730-1737. doi: 10.1016/j.ijrobp.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 12.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634-1646. doi: [DOI] [PubMed] [Google Scholar]
- 13.Rubinstein LV, Korn EL, Freidlin B, Hunsberger S, Ivy SP, Smith MA. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23(28):7199-7206. doi: 10.1200/JCO.2005.01.149 [DOI] [PubMed] [Google Scholar]
- 14.O’Gorman TW, Woolson RF, Jones MP. A comparison of two methods of estimating a common risk difference in a stratified analysis of a multicenter clinical trial. Control Clin Trials. 1994;15(2):135-153. doi: 10.1016/0197-2456(94)90017-5 [DOI] [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 16.Bogart E, Jouin A, Béhal H, Duhamel A, Filleron T, Kramar A. Analysis of survival adjusted for quality of life using the Q-TWiST function: interface in R. Comput Methods Programs Biomed. 2016;125:79-87. doi: 10.1016/j.cmpb.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 17.Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study [published correction appears in Radiother Oncol 1999;53(2):167]. Radiother Oncol. 1999;52(2):101-109. doi: 10.1016/S0167-8140(99)00110-3 [DOI] [PubMed] [Google Scholar]
- 18.Sande TA, Ruenes R, Lund JA, et al. Long-term follow-up of cancer patients receiving radiotherapy for bone metastases: results from a randomised multicentre trial. Radiother Oncol. 2009;91(2):261-266. doi: 10.1016/j.radonc.2009.02.014 [DOI] [PubMed] [Google Scholar]
- 19.Kaasa S, Brenne E, Lund JA, et al. Prospective randomised multicenter trial on single fraction radiotherapy (8 Gy x 1) versus multiple fractions (3 Gy x 10) in the treatment of painful bone metastases. Radiother Oncol. 2006;79(3):278-284. doi: 10.1016/j.radonc.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 20.Erler D, Brotherston D, Sahgal A, et al. Local control and fracture risk following stereotactic body radiation therapy for non-spine bone metastases. Radiother Oncol. 2018;127(2):304-309. doi: 10.1016/j.radonc.2018.03.030 [DOI] [PubMed] [Google Scholar]
- 21.Bedard G, McDonald R, Poon I, et al. Stereotactic body radiation therapy for non-spine bone metastases—a review of the literature. Ann Palliat Med. 2016;5(1):58-66. [DOI] [PubMed] [Google Scholar]
- 22.Karvonen-Gutierrez CA, Ronis DL, Fowler KE, Terrell JE, Gruber SB, Duffy SA. Quality of life scores predict survival among patients with head and neck cancer. J Clin Oncol. 2008;26(16):2754-2760. doi: 10.1200/JCO.2007.12.9510 [DOI] [PubMed] [Google Scholar]
- 23.Goldstein DP, Hynds Karnell L, Christensen AJ, Funk GF. Health-related quality of life profiles based on survivorship status for head and neck cancer patients. Head Neck. 2007;29(3):221-229. doi: 10.1002/hed.20507 [DOI] [PubMed] [Google Scholar]
- 24.Grignon LM, Jameson MJ, Karnell LH, Christensen AJ, Funk GF. General health measures and long-term survival in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133(5):471-476. doi: 10.1001/archotol.133.5.471 [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharya IS, Hoskin PJ. Stereotactic body radiotherapy for spinal and bone metastases. Clin Oncol (R Coll Radiol). 2015;27(5):298-306. doi: 10.1016/j.clon.2015.01.030 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Patient Characteristics
eFigure 1. Overall survival estimates by treatment group in the intent-to-treat analysis
eFigure 2. Proportions of patients with or without severe symptoms on the 13-core-item MD Anderson Symptom Index (MDASI) after irradiation of bone metastases
Data Sharing Statement