This systematic review and meta-analysis describes the various adverse events associated with PD-1 and PD-L1 inhibitor drugs reported in clinical trials indexed in PubMed, Web of Science, Embase, and Scopus.
Key Points
Question
What are the incidences of treatment-related adverse events of PD-1 and PD-L1 inhibitors, and do they differ between different drugs and cancer types?
Findings
In this systematic review and meta-analysis of 125 clinical trials involving 20 128 patients, the overall incidences of all-grade adverse events were 66.0% and of grade 3 or higher adverse events were 14.0%. The overall mean adverse event incidences were similar across different cancer types but varied between different drugs.
Meaning
A comprehensive summary of treatment-related adverse events for PD-1 and PD-L1 inhibitors in clinical trials may be an important guide for clinical practice.
Abstract
Importance
Programmed cell death (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors have been increasingly used in cancer therapy. Understanding the treatment-related adverse events of these drugs is critical for clinical practice.
Objective
To evaluate the incidences of treatment-related adverse events of PD-1 and PD-L1 inhibitors and the differences between different drugs and cancer types.
Data Sources
PubMed, Web of Science, Embase, and Scopus were searched from October 1, 2017, through December 15, 2018.
Study Selection
Published clinical trials on single-agent PD-1 and PD-L1 inhibitors with tabulated data on treatment-related adverse events were included.
Data Extraction and Synthesis
Trial name, phase, cancer type, PD-1 and PD-L1 inhibitor used, dose escalation, dosing schedule, number of patients, number of all adverse events, and criteria for adverse event reporting data were extracted from each included study, and bayesian multilevel regression models were applied for data analysis.
Main Outcomes and Measures
Incidences of treatment-related adverse events and differences between different drugs and cancer types.
Results
This systematic review and meta-analysis included 125 clinical trials involving 20 128 patients; 12 277 (66.0%) of 18 610 patients from 106 studies developed at least 1 adverse event of any grade (severity), and 2627 (14.0%) of 18 715 patients from 110 studies developed at least 1 adverse event of grade 3 or higher severity. The most common all-grade adverse events were fatigue (18.26%; 95% CI, 16.49%-20.11%), pruritus (10.61%; 95% CI, 9.46%-11.83%), and diarrhea (9.47%; 95% CI, 8.43%-10.58%). The most common grade 3 or higher adverse events were fatigue (0.89%; 95% CI, 0.69%-1.14%), anemia (0.78%; 95% CI, 0.59%-1.02%), and aspartate aminotransferase increase (0.75%; 95% CI, 0.56%-0.99%). Hypothyroidism (6.07%; 95% CI, 5.35%-6.85%) and hyperthyroidism (2.82%; 95% CI, 2.40%-3.29%) were the most frequent all-grade endocrine immune-related adverse events. Nivolumab was associated with higher mean incidences of all-grade adverse events compared with pembrolizumab (odds ratio [OR], 1.28; 95% CI, 0.97-1.79) and grade 3 or higher adverse events (OR, 1.30; 95% CI, 0.89-2.00). PD-1 inhibitors were associated with a higher mean incidence of grade 3 or higher adverse events compared with PD-L1 inhibitors (OR, 1.58; 95% CI, 1.00-2.54).
Conclusions and Relevance
Different PD-1 and PD-L1 inhibitors appear to have varying treatment-related adverse events; a comprehensive summary of the incidences of treatment-related adverse events in clinical trials provides an important guide for clinicians.
Introduction
Programmed cell death (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors have revolutionized cancer therapy.1,2 To date, 2 PD-1 inhibitors (nivolumab and pembrolizumab) and 3 PD-L1 inhibitors (atezolizumab, avelumab, and durvalumab) have been approved by the US Food and Drug Administration for various indications. These drugs work by blocking the PD-1 or PD-L1 immune checkpoint pathway to reactivate T cell–mediated antitumor immunity.2 With reactivation of cellular immunity, these checkpoint inhibitors have been reported to cause autoimmune-like disorders.2,3 Given the increasing use of PD-1 and PD-L1 inhibitors, understanding their toxicologic profile is crucial.
Clinical trials of PD-1 and PD-L1 inhibitors report treatment-related adverse events according to standard guidelines, such as the National Cancer Institute Common Terminology Criteria for Adverse Events, and represent an ideal resource for comprehensive analysis of incidences of treatment-related adverse events. However, substantial variations exist in cancer type, drug and dosing schedule, and adverse event reporting criteria in the publication. Ignoring these variations and missing data patterns in adverse event reporting can lead to inaccurate estimation of the true incidences of treatment-related adverse events associated with PD-1 and PD-L1 inhibitors.
We performed a systematic review and meta-analysis of treatment-related adverse events of the Food and Drug Administration–approved PD-1 and PD-L1 inhibitors in published clinical trials. Using a novel bayesian approach to derive exact inferences based on patient-level data, we investigated the incidences of different treatment-related adverse events associated with these drugs, and we quantified the potential differences in adverse event incidences among a variety of cancer types, drugs, and dosing schedules.
Methods
Search Methods and Study Selection
A systematic search of the literature was conducted to identify published clinical trials of PD-1 and PD-L1 inhibitors that reported treatment-related adverse events. The search was done in PubMed, Web of Science, Embase, and Scopus using the terms nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab, PD-1 inhibitor, and PD-L1 inhibitor. The search was conducted from October 1, 2017, through final search for updates on December 15, 2018. The references of relevant published trials and review articles were also searched for additional eligible studies. Studies eligible for inclusion met all of the following criteria: (1) cancer therapy clinical trial, (2) participants were treated with a single-agent PD-1 or PD-L1 inhibitor, (3) reported tabulated data on treatment-related adverse events, and (4) published in English. Studies published online ahead of print were eligible, but meeting abstracts were excluded. When multiple publications reporting on the same study population were identified, the one with the most updated and/or comprehensive adverse event data was selected. The literature search, study selection, and data extraction were performed independently by 2 of us (F.Y. and X.W.), and discrepancies were reviewed by another investigator on the team (Y.W.) and resolved by consensus. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline.
Data Extraction
The trial name, phase, cancer type, PD-1 and PD-L1 inhibitor used, dose escalation, dosing schedule, number of patients, number of all adverse events, and criteria for adverse event reporting in the publication were obtained from each included study. All-grade (severity) adverse event and grade 3 or higher (severity) adverse event data were both extracted.
Statistical Analysis
The response variable is the number of reported all-grade or grade 3 or higher adverse events, assumed to follow a binomial distribution. To explain the between-study variation in the meta-analysis, we adjusted the incidence probability of adverse events by study-level moderators, including the therapeutic regimen and dosing schedule, cancer type, and adverse event.
We applied bayesian multilevel regression models for data analysis. Because many less frequently observed adverse events were not reported given a predetermined study-specific cutoff value, binomial distribution was proposed for fully reported adverse events and cumulative binomial probabilities were proposed for left-censored adverse events, in the likelihood of coherent parameter estimation. With a logit transformation (logit(z) = log(z)-log(1-z)) on the incidence probability, we assumed normal distributions for the additive effects of study-level moderators to adjust for study-specific effects. A noninformative prior distribution was proposed for the mean parameters of normal distributions, and weakly informative Cauchy prior distributions with mode at 0 and scale at 25 was proposed for the SD parameters.4,5 The same statistical model was separately applied to all-grade and grade 3 or higher adverse events.
For all bayesian analyses, we found the joint posterior distributions of the model parameters using a Markov chain Monte Carlo algorithm. Because closed forms of the full-conditional distributions are not available, we generated these distributions using Gibbs sampling and the Metropolis-Hastings algorithm. For data analysis, we used statistical software R, version 3.4.3 (with packages rjags_v4-6, coda_v0.19-1 and ggplot2_v2.2.1; R Foundation for Statistical Computing), and JAGS, version 4.3.0 (GNU General Public License). For both all-grade and grade 3 or higher adverse events, we plotted the incidences and their 95% probability intervals (bayesian credible intervals [CrIs]) by study, therapeutic regimen, cancer type, and adverse event using forest plots. Odds ratios (ORs), risk ratios (RRs) of grade 3 or higher adverse events to all-grade adverse events, and their CrIs were estimated from the medians and the 2.5 percentile and 97.5 percentile of the posterior distributions. For bayesian inferences, values of the posterior probabilities greater than .90 may be considered to show weakly significant positive association; greater than .95, significant positive association; and greater than .99, highly significant positive association of regimen with incidence. Values less than .10 correspond to weakly significant negative association; less than .05, significant negative association; or less than .01, highly significant negative association. Values near .50 correspond to no association.
Results
Eligible Studies and Characteristics
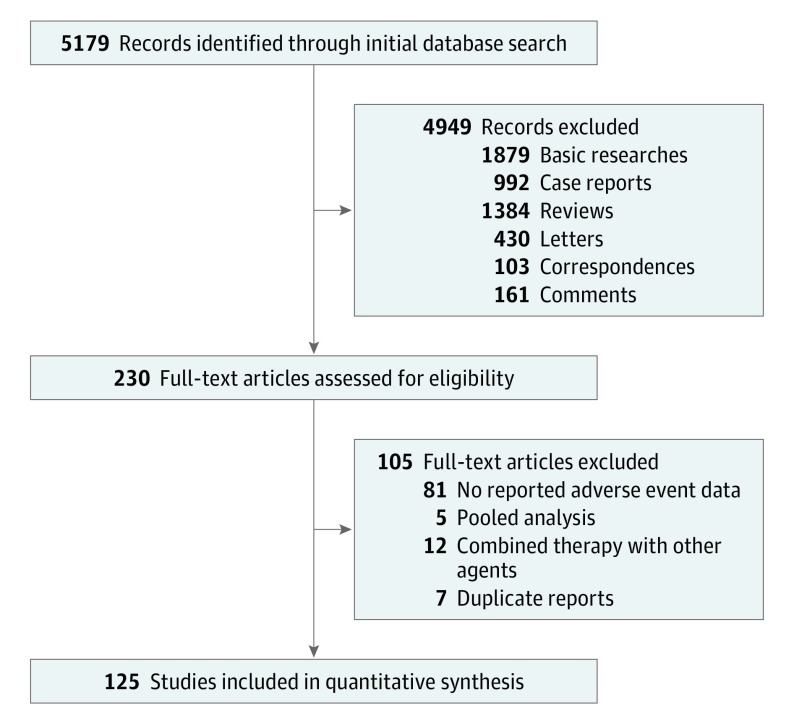
Literature search and review of reference lists identified 5179 relevant publications. After screening and eligibility assessment, we included in the meta-analysis a total of 125 clinical trials involving 20 128 patients (Figure 1; eTable 1 in the Supplement).6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130 The PD-1 and PD-L1 inhibitors used included nivolumab (n = 46), pembrolizumab (n = 49), atezolizumab (n = 15), avelumab (n = 9), and durvalumab (n = 6). The trials involved the treatment of melanoma (n = 16), lung cancer (n = 26), gastrointestinal cancer (n = 14), genitourinary cancer (n = 22), hematologic malignant neoplasm (n = 8), other cancers (n = 31), and mixed cancer types (n = 10). One study had both melanoma and lung cancer arms,60 and another study included all cancer types but reported genitourinary cancer data separately.127
Figure 1. Flow Diagram of the Study Selection Process.
Overall Incidence of Adverse Events
Collectively, the 125 studies reported more than 300 different types of adverse events. Overall, 12 277 (66.0%) of 18 610 patients from 106 studies developed at least 1 adverse event of any grade, and 2627 (14.0%) of 18 715 patients from 110 studies developed at least 1 grade 3 or higher adverse event.
For the meta-analysis, we focused on adverse events that either were reported by at least 10% of the studies or were likely immune-related adverse events (irAEs). Using these criteria, we narrowed down to 75 adverse events, which included most clinically relevant adverse events that are commonly seen in practice. A comprehensive list of the incidences of each adverse event is provided in eFigure 1 in the Supplement. The overall mean incidence of all-grade adverse events was 1.66% (95% CI, 1.47%-1.86%), and the mean incidence of grade 3 or higher adverse events was 0.11% (95% CI, 0.08%-0.14%). The mean incidences of all-grade and grade 3 or higher adverse events across different studies are shown in eFigure 2 in the Supplement.
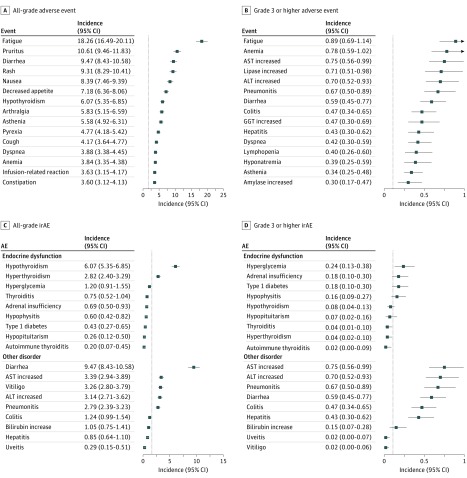
As shown in Figure 2A, the most common all-grade adverse events were fatigue (18.26%; 95% CI, 16.49%-20.11%), pruritus (10.61%; 95% CI, 9.46%-11.83%), and diarrhea (9.47%; 95% CI, 8.43%-10.58%). The most common grade 3 or higher adverse events were fatigue (0.89%; 95% CI, 0.69%-1.14%), anemia (0.78%; 95% CI, 0.59%-1.02%), and aspartate aminotransferase (AST) increase (0.75%; 95% CI, 0.56%-0.99%) (Figure 2B).
Figure 2. Incidences of the Most Common Adverse Events and Immune-Related Adverse Events (irAEs).
A, Incidences of the most common all-grade adverse events. B, Incidences of the most common grade 3 or higher adverse events. C, Incidences of the most common all-grade irAEs. D, Incidences of the most common grade 3 or higher irAEs. Vertical lines in A and C indicate the overall mean incidence of all-grade adverse events (1.66%). Vertical lines in B and D indicate the overall mean incidence of grade 3 or higher adverse events (0.11%). Values to the left of the line are lower than the mean, to the right, higher. ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase.
Incidence of Immune-Related Adverse Events
PD-1 and PD-L1 inhibitors block the immune checkpoint pathway and reactivate cellular immunity and can cause autoimmune-mediated adverse events. These irAEs are of particular clinical interest and importance. Commonly recognized irAEs include various endocrine dysfunctions and other autoimmune-like disorders. We analyzed the incidences of adverse events that are likely immune-related.
Among the endocrine dysfunctions, the most frequent all-grade adverse events were hypothyroidism (6.07%; 95% CI, 5.35%-6.85%) and hyperthyroidism (2.82%; 95% CI, 2.40%-3.29%), followed by hyperglycemia (1.20%; 95% CI, 0.91%-1.55%), thyroiditis (0.75%; 95% CI, 0.52%-1.04%), and adrenal insufficiency (0.69%; 95% CI, 0.50%-0.93%) (Figure 2C). The most common grade 3 or higher adverse events were hyperglycemia (0.24%; 95% CI, 0.13%-0.38%), adrenal insufficiency (0.18%; 95% CI, 0.10%-0.30%), type 1 diabetes (0.18%; 95% CI, 0.10%-0.30%), hypophysitis (0.16%; 95% CI, 0.09%-0.27%), and hypothyroidism (0.08%; 95% CI, 0.04%-0.13%) (Figure 2D).
The most common other all-grade irAEs were diarrhea (9.47%; 95% CI, 8.43%-10.58%), AST increase (3.39%; 95% CI, 2.94%-3.89%), vitiligo (3.26%; 95% CI, 2.80%-3.79%), alanine aminotransferase (ALT) increase (3.14%; 95% CI, 2.71%-3.62%), pneumonitis (2.79%; 95% CI, 2.39%-3.23%), and colitis (1.24%; 95% CI, 0.99%-1.54%) (Figure 2C). For grade 3 or higher irAEs, AST increase (0.75%; 95% CI, 0.56%-0.99%) was most common, followed by ALT increase (0.70%; 95% CI, 0.52%-0.93%), pneumonitis (0.67%; 95% CI, 0.50%-0.89%), diarrhea (0.59%; 95% CI, 0.45%-0.77%), and colitis (0.47%; 95% CI, 0.34%-0.65%) (Figure 2D). Because diarrhea is likely a sign of colitis and ALT or AST increase a sign of hepatitis, incidences of autoimmune pneumonitis, colitis, and hepatitis are clinically significant.
Risk Ratio of Grade 3 or Higher Adverse Events
Some adverse events were more likely to be severe if observed in a patient, as reflected by a high RR of grade 3 or higher adverse event incidence to the respective all-grade adverse event incidence. Notable among these adverse events were hepatitis (RR = 50.59%), lipase increase (RR = 42.01%), γ-glutamyltransferase increase (RR = 41.96%), type 1 diabetes (RR = 41.86%), and colitis (RR = 37.90%) (eTable 2 in the Supplement). The most common all-grade adverse events, such as fatigue, pruritus, diarrhea, rash, and nausea, had relatively lower RRs. The RRs of some irAEs of clinical interest were pneumonitis (RR = 24.01%), ALT increase (RR = 22.29%), AST increase (RR = 22.12%), hypothyroidism (RR = 1.32%), hyperthyroidism (RR = 1.42%), adrenal insufficiency (RR = 26.09%), hypophysitis (RR = 26.67%), and hypopituitarism (RR = 26.92%). These data suggest that, although hypothyroidism and hyperthyroidism tended to be mild, other irAEs, including pneumonitis, hepatitis, colitis, and other endocrine dysfunctions, were more likely to be severe.
Incidence of Treatment-Related Deaths
Among the 125 studies, 112 (89.6%) reported whether any treatment-related deaths occurred. Among these, 40 studies reported at least 1 treatment-related death, with a total of 82 such deaths reported (eTable 3 in the Supplement). The overall incidence of treatment-related death was 0.45% (82 of 18 353).
As shown in the Table, the most common cause of treatment-related death (n = 82) was pneumonitis (23 [28.0%]). Other common causes were pneumonia (5 [6.1%]), sepsis (7 [8.5%]), respiratory failure (5 [6.1%]), and cardiovascular failure (3 [3.7%]). Respiratory causes (39 [48.0%]) accounted for almost half of the treatment-related deaths. Cardiovascular (8 [9.8%]), infectious (7 [8.5%]), hematologic (5 [6.1%]), and hepatic (3 [3.7%]) diseases were other common causes.
Table. Causes of 82 Treatment-Related Deaths in Clinical Trials of PD-1 and PD-L1 Inhibitors.
| Cause of Death | No. (%)a |
|---|---|
| Respiratory (n = 39) | |
| Pneumonitis | 23 (28.0) |
| Radiation pneumonitis | 2 (2.4) |
| Pneumonia | 5 (6.1) |
| Respiratory failure | 5 (6.1) |
| Respiratory distress | 2 (2.4) |
| Exertional dyspnea | 1 (1.2) |
| Pulmonary hypertension | 1 (1.2) |
| Cardiovascular (n = 8) | |
| Cardiovascular failure | 3 (3.7) |
| Cardiac arrest | 1 (1.2) |
| Myocardial infarction | 1 (1.2) |
| Cardiomyopathy | 1 (1.2) |
| Brain natriuretic peptide increase | 1 (1.2) |
| Acute coronary syndrome | 1 (1.2) |
| Infectious (n = 7) | |
| Sepsis | 7 (8.5) |
| Hematologic (n = 5) | |
| Neutropenia | 2 (2.4) |
| Thrombocytopenia | 1 (1.2) |
| Immune thrombocytopenic purpura | 1 (1.2) |
| Disseminated intravascular coagulation | 1 (1.2) |
| Hepatic (n = 3) | |
| Hepatitis | 1 (1.2) |
| Autoimmune hepatitis | 1 (1.2) |
| Acute hepatic failure | 1 (1.2) |
| Cerebrovascular (n = 2) | |
| Ischemic stroke | 1 (1.2) |
| Cerebral hemorrhage | 1 (1.2) |
| Other (n = 11) | |
| Hypercalcemia/pulmonary embolism | 1 (1.2) |
| Urinary tract obstruction | 1 (1.2) |
| Myositis | 2 (2.4) |
| Multiorgan failure | 1 (1.2) |
| Colitis | 1 (1.2) |
| Ulcerative esophagitis | 1 (1.2) |
| Intestinal perforation | 1 (1.2) |
| Toxic epidermal necrolysis | 1 (1.2) |
| General physical health deterioration | 1 (1.2) |
| Severe skin reaction | 1 (1.2) |
| Unspecified (n = 10) | |
| Respiratory, thoracic, and mediastinal disorders | 1 (1.2) |
| Neoplasms | 2 (2.4) |
| Malignant neoplasm progression | 1 (1.2) |
| Unknown | 6 (7.3) |
Abbreviations: PD-1, programmed cell death; PD-L1, programmed cell death ligand 1.
Total number (n = 85) in this table is slightly higher than the total number of deaths (n = 82); percentage values are calculated from 82. One study126 reported 10 treatment-related deaths that occurred in 7 patients (4 patients had pneumonitis, and 1 patient had each of the following: cardiomyopathy, right ventricular failure, respiratory distress, respiratory failure, increased brain natriuretic peptide, and radiation pneumonitis).
Subgroup Analysis of Mean Adverse Event Incidence by Cancer Type
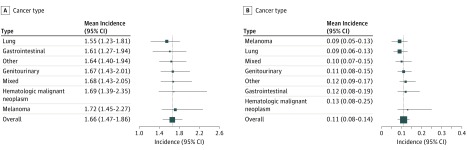
Based on the type of cancer treated in the clinical trials, we classified the 125 studies into 7 different categories: melanoma, lung cancer, gastrointestinal cancer, genitourinary cancer, hematologic malignant neoplasm, other cancers, and mixed cancer types. As shown in Figure 3A, the highest mean all-grade adverse events incidence was observed in melanoma (1.72%; 95% CI, 1.45%-2.27%), which was not much different from the lowest that was observed in lung cancer (1.55%; 95% CI, 1.23%-1.81%). Similarly, no statistically significant difference was found in the mean incidence of grade 3 or higher adverse events between any 2 categories (Figure 3B). These data suggest that the mean incidences of all-grade and grade 3 or higher adverse events were similar across different cancer types.
Figure 3. Mean Incidences of Adverse Events by Cancer Type.
A, Mean incidences of all grade adverse events by cancer type; vertical line indicates the overall mean incidence of all-grade adverse events (1.66%). B, Mean incidences of grade 3 or higher adverse events by cancer type. The vertical line indicates the overall mean incidence of grade 3 or higher adverse events (0.11%). For both panels, values to the left of the line are lower than the mean, to the right, higher.
Subgroup Analysis of Mean Adverse Event Incidence by Drug and Dose
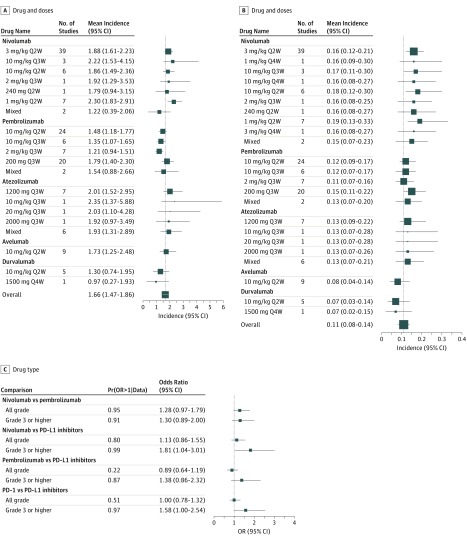
We compared the mean incidences of adverse events between different dosing schedules of the same drug as well as between different drugs. As shown in Figure 4A and B, no statistically significant differences were found in the mean incidences of all-grade or grade 3 or higher adverse events between different dosing schedules for nivolumab. The same was true for pembrolizumab and atezolizumab.
Figure 4. Mean Incidences of Adverse Events by Drug Type.
A, Mean incidences of all-grade adverse events by drug and dose; vertical line indicates the overall mean incidence of all-grade adverse events (1.66%). B, Mean incidences of grade 3 or higher adverse events by drug and dose. C, Comparisons of mean incidences of adverse events between different drugs. The vertical line indicates the overall mean incidence of grade 3 or higher adverse events (0.11%). For A and B, values to the left of the line are lower than mean, to the right, higher. For C, values to the left of the line are higher for second drug in the comparison, to the right, for first drug in comparison. PD-1 indicates programmed cell death; PD-L1, programmed cell death ligand 1.
Nivolumab (3 mg/kg every 2 weeks [Q2W] dose) had higher mean incidences of all-grade adverse events (OR, 1.28; 95% CI, 0.97-1.79) and grade 3 or higher adverse events (OR, 1.30; 95% CI, 0.89-2.00) compared with pembrolizumab (10 mg/kg Q2W dose). Nivolumab also had a higher mean incidence of grade 3 or higher adverse events (OR, 1.81; 95% CI, 1.04-3.01) compared with PD-L1 inhibitors. The overall mean incidence of grade 3 or higher adverse events for PD-1 inhibitors was higher compared with PD-L1 inhibitors (OR, 1.58; 95% CI, 1.00-2.54) (Figure 4C).
Study Heterogeneity
The heterogeneity among studies was statistically quantified using a bayesian multilevel regression model and decomposed into several additive components attributed to various factors, including cancer type, adverse event, regimen and dosing schedule, and the residual heterogeneity owing to underlying clinical baseline variations between the patients enrolled in each study. Because of the nonlinearity of the logit transformation and a much higher mean incidence, all-grade adverse events actually had a larger variation in incidence compared with grade 3 or higher adverse events, even though in the models, grade 3 or higher adverse events showed larger SDs compared with all-grade adverse events for all study moderators. Therefore, we only compared the SDs across various factors within each model. Because between-dose variation by drug was very similar, the estimates were pooled together rather than estimated separately in the models.
eFigure 3 in the Supplement illustrates the posterior median and CIs of the SDs regarding either all-grade or grade 3 or higher adverse events. For both all-grade and grade 3 or higher adverse events, the largest variation came from the heterogeneity between different adverse event categories, for which the SD was statistically significantly larger than that of the residual effect (SD ratio for all-grade adverse events, 1.51; 95% CI, 1.37-1.66; for grade 3 or higher adverse events, 1.52; 95% CI, 1.26-1.83). Here, we consider the heterogeneity of the residual effect, denoting the information that cannot be explained by any of the study moderators, as a benchmark in comparison. The other factors, including drug, dose, and cancer type, all showed a small variation compared with the residual effect, with median SD ratios between 0.11 and 0.51.
Discussion
We performed a systematic review of PD-1 and PD-L1 inhibitor–associated adverse events using a collection of sparse binomial data from published studies. Unlike meta-analyses using continuous summary statistics based on the large-sample theory, this meta-analysis used the number of each treatment-related adverse event to derive exact statistical inferences that were close or even identical to results from individual-level data (if available). Obtaining and merging original individual-level patient data are difficult, but this meta-analysis provides an alternative for estimating the study moderator effects without loss of relative efficiency.131,132 To our knowledge, this is the largest and most comprehensive meta-analysis of treatment-related adverse events for immune checkpoint inhibitors. Previous meta-analyses included fewer studies and primarily focused on certain adverse events, such as pneumonitis, endocrine dysfunction, or selected irAEs.133,134,135,136 A comprehensive analysis of all common treatment-related adverse events reported in clinical trials is critical, as the results constitute an important reference for clinicians. Such a global overview of immune checkpoint inhibitor adverse event incidences is complementary to American Society of Clinical Oncology and National Comprehensive Cancer Network guidelines on management of irAEs137 and informs clinical practice guidelines.
From the standpoint of patient counseling, several results from this meta-analysis are important. Approximately 2 in 3 patients treated with PD-1 or PD-L1 inhibitors in clinical trials had at least 1 adverse event, and 1 in 7 patients experienced at least 1 grade 3 or higher adverse event. These numbers can be important to share with patients before they begin treatment with a PD-1 or PD-L1 inhibitor. Fatigue was the most common all-grade adverse event (18.26%) and the most common grade 3 or higher adverse event (0.89%). Although less likely to be severe at presentation (about 5% chance), fatigue has a relatively high incidence (approximately 1 in 5) that is worth disclosing to patients. Pruritus, diarrhea, and rash are the next most common all-grade adverse events (approximately 1 in 10), but the likelihood of patients experiencing serious manifestations of these adverse events is low.
This meta-analysis showed that most of the most common grade 3 or higher adverse events were likely immune-related, including pneumonitis and dyspnea, diarrhea and colitis, ALT or AST increase and hepatitis, and lipase increase (suggestive of pancreatitis). Close monitoring and early recognition of pertinent symptoms and signs may help enable their proper management, such as prompt initiation of steroids. Dyspnea can be early signs of pneumonitis, diarrhea a sign of colitis, and ALT or AST increase a sign of hepatitis. If not detected early, these autoimmune-mediated disorders tend to present with higher severity and may even be fatal. Our results indicated that 24.01% of pneumonitis cases were grade 3 or higher in severity, and pneumonitis was the most common cause of treatment-related death in patients treated with PD-1 and PD-L1 inhibitors. In addition, hepatitis was the adverse event found most likely to be serious if it occurred, with 50.59% of hepatitis being grade 3 or higher. Diarrhea was the third most common all-grade adverse event, and clinical vigilance is necessary for early recognition and intervention to prevent severe colitis.
Among the irAEs manifesting as endocrine dysfunctions, hypothyroidism (6.07%) and hyperthyroidism (2.82%) were most common. Hyperglycemia, thyroiditis, adrenal insufficiency, hypophysitis, type 1 diabetes, and hypopituitarism were less common. However, these adverse events were more likely to be severe, with approximately 20% to 35% likelihood of being grade 3 or higher, as opposed to about 2% for hypothyroidism and hyperthyroidism. This difference may be partly attributed to frequent monitoring of thyroid-stimulating hormone in clinical trials, which allows for detection of thyroid dysfunction at an earlier stage. Hyperglycemia can be detected easily through routine laboratory work, but interpretation requires vigilance for possible pancreatic dysfunction. Routine monitoring of adrenal and pituitary function is not yet prevalent in clinical practice, likely owing to relatively low incidences of dysfunction. In this setting, a careful interview is important for early detection of pertinent symptoms.
The results of this meta-analysis indicated that the overall mean incidence of all-grade and grade 3 or higher adverse events did not differ between different cancer types. We did not further investigate whether specific adverse events were more common in particular cancer types (for example, pneumonitis in lung cancer or colitis in gastrointestinal cancer), which is a potential focus for future analyses. In addition, we found that nivolumab appeared to have a higher mean incidence of all-grade and grade 3 or higher adverse events, compared with pembrolizumab, but the mechanism and clinical significance are unclear. PD-L1 inhibitors appeared to be associated with lower mean incidence of grade 3 or higher adverse events, compared with nivolumab and pembrolizumab, possibly owing to the presence of the other PD-1 ligand, PD-L2, which may maintain some level of checkpoint signaling. No head-to-head comparison trials have been conducted, and interpretation of these results should be made with caution.
Strengths and Limitations
A major strength of this meta-analysis is the coherent estimation of adverse event incidences with accommodation of both fully reported and censored data. The missing data problem is pivotal in meta-analysis, because published studies do not always provide all of the necessary information, which is especially true for treatment-related adverse events as the primary outcome. Usually, only the prevalent adverse events were reported for each study, and most information regarding less frequently observed adverse events was censored using a predetermined study-specific cutoff value. Furthermore, the larger the scope of the study, the higher the cutoff value (usually a percentage of the total sample size), which brought more uncertainty regarding the censored information. This type of missing information, if treated as missing completely at random, will result in overestimation of the incidence probability of the corresponding adverse events. Therefore, we took an innovative approach by introducing additional cumulative binomial probabilities in the likelihood function to accommodate the censored data. The between-study heterogeneity was simultaneously quantified by study-level moderators using bayesian multilevel modeling, with exact inference avoiding continuity correction for sparse binomial data.138
This meta-analysis has limitations. Small-study effects were observed when studies with smaller sample sizes had different incidences and wider CIs for both all-grade and grade 3 or higher adverse events. Although the forest plots showed no asymmetry favoring low adverse events incidence studies, this meta-analysis is subject to publication bias given that all of our analyses were based on publications. In addition, this study is subject to any biases or errors of the original investigators, and the results are generalizable only to patient groups eligible for these trials.
Conclusions
This meta-analysis, which used an innovative bayesian multilevel regression model, has defined the incidences of all common treatment-related adverse events of PD-1 and PD-L1 inhibitors. The incidences of adverse events are independent of cancer types, but different PD-1 and PD-L1 inhibitors may be associated with different incidences of adverse events. This global overview of the adverse events of PD-1 and PD-L1 inhibitors can be used as a reference by clinicians and may guide clinical practice.
eTable 1. List of the Studies Included in This Meta-Analysis
eTable 2. Relative Ratios of Grade 3 or Higher Adverse Event Incidences to Respective All-grade Adverse Event Incidences
eTable 3. List of Studies That Reported At Least One Treatment-related Death
eFigure 1. Incidences of All Adverse Events, (A) Incidences of All-grade Adverse Events and (B) Incidences of Grade 3 or Higher Adverse Events
eFigure 2. Mean Incidences of Adverse Events by Study, (A) Mean Incidences of All-grade Adverse Events by Study and (B) Mean Incidences of Grade 3 or Higher Adverse Events by Study
eFigure 3. Heterogeneity of Adverse Event Incidence in This Meta-Analysis
eFigure 4. Mean Incidences of All-grade Adverse Events by Study if Ignoring Missing Data
References
- 1.Li X, Shao C, Shi Y, Han W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J Hematol Oncol. 2018;11(1):31. doi: 10.1186/s13045-018-0578-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158-168. doi: 10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 4.Gelman A. Prior distributions for variance parameters in hierarchical models (comment on article by Browne and Draper). Bayesian Anal. 2006;1(3):515-534. doi: 10.1214/06-BA117A [DOI] [Google Scholar]
- 5.Gelman A, Jakulin A, Pittau MG, Su Y-S. A weakly informative default prior distribution for logistic and other regression models. Ann Appl Stat. 2008;2(4):1360-1383. doi: 10.1214/08-AOAS191 [DOI] [Google Scholar]
- 6.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167-3175. doi: 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020-1030. doi: 10.1200/JCO.2013.53.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. doi: 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004-2012. doi: 10.1200/JCO.2014.58.3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015-4022. doi: 10.1200/JCO.2015.62.3397 [DOI] [PubMed] [Google Scholar]
- 14.McDermott DF, Drake CG, Sznol M, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol. 2015;33(18):2013-2020. doi: 10.1200/JCO.2014.58.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motzer RJ, Escudier B, McDermott DF, et al. ; CheckMate 025 Investigators . Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803-1813. doi: 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol. 2015;33(13):1430-1437. doi: 10.1200/JCO.2014.59.0703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257-265. doi: 10.1016/S1470-2045(15)70054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320-330. doi: 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 19.Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883-895. doi: 10.1016/S1470-2045(16)30098-5 [DOI] [PubMed] [Google Scholar]
- 20.Choueiri TK, Fishman MN, Escudier B, et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin Cancer Res. 2016;22(22):5461-5471. doi: 10.1158/1078-0432.CCR-15-2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856-1867. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34(25):2980-2987. doi: 10.1200/JCO.2016.66.9929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(23):2698-2704. doi: 10.1200/JCO.2015.65.9789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol. 2016;17(11):1590-1598. doi: 10.1016/S1470-2045(16)30496-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber J, Gibney G, Kudchadkar R, et al. Phase I/II study of metastatic melanoma patients treated with nivolumab who had progressed after ipilimumab. Cancer Immunol Res. 2016;4(4):345-353. doi: 10.1158/2326-6066.CIR-15-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Ami E, Barysauskas CM, Solomon S, et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: results of a phase 2 study. Cancer. 2017;123(17):3285-3290. doi: 10.1002/cncr.30738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carbone DP, Reck M, Paz-Ares L, et al. ; CheckMate 026 Investigators . First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415-2426. doi: 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502. doi: 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461-2471. doi: 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 30.Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol. 2017;18(5):631-639. doi: 10.1016/S1470-2045(17)30181-X [DOI] [PubMed] [Google Scholar]
- 31.Larkin J, Minor D, D’Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol. 2018;36(4):383-390. doi: 10.1200/JCO.2016.71.8023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maruyama D, Hatake K, Kinoshita T, et al. Multicenter phase II study of nivolumab in Japanese patients with relapsed or refractory classical Hodgkin lymphoma. Cancer Sci. 2017;108(5):1007-1012. doi: 10.1111/cas.13230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris VK, Salem ME, Nimeiri H, et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(4):446-453. doi: 10.1016/S1470-2045(17)30104-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182-1191. doi: 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312-322. doi: 10.1016/S1470-2045(17)30065-7 [DOI] [PubMed] [Google Scholar]
- 36.Weber J, Mandala M, Del Vecchio M, et al. ; CheckMate 238 Collaborators . Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824-1835. doi: 10.1056/NEJMoa1709030 [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto N, Nokihara H, Yamada Y, et al. Phase I study of nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Invest New Drugs. 2017;35(2):207-216. doi: 10.1007/s10637-016-0411-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamazaki N, Kiyohara Y, Uhara H, et al. Efficacy and safety of nivolumab in Japanese patients with previously untreated advanced melanoma: a phase II study. Cancer Sci. 2017;108(6):1223-1230. doi: 10.1111/cas.13241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36(14):1428-1439. doi: 10.1200/JCO.2017.76.0793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Angelo SP, Mahoney MR, Van Tine BA, et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19(3):416-426. doi: 10.1016/S1470-2045(18)30006-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976-1986. doi: 10.1056/NEJMoa1716078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gettinger S, Horn L, Jackman D, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J Clin Oncol. 2018;36(17):1675-1684. doi: 10.1200/JCO.2017.77.0412 [DOI] [PubMed] [Google Scholar]
- 43.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi: 10.1056/NEJMoa1801946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480-1492. doi: 10.1016/S1470-2045(18)30700-9 [DOI] [PubMed] [Google Scholar]
- 45.Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 2018;36(28):2836-2844. doi: 10.1200/JCO.2017.76.6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JS, Lee KH, Cho EK, et al. Nivolumab in advanced non-small-cell lung cancer patients who failed prior platinum-based chemotherapy. Lung Cancer. 2018;122:234-242. doi: 10.1016/j.lungcan.2018.05.023 [DOI] [PubMed] [Google Scholar]
- 47.Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672-681. doi: 10.1016/S1470-2045(18)30139-6 [DOI] [PubMed] [Google Scholar]
- 48.Ma BBY, Lim WT, Goh BC, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the Mayo Clinic phase 2 consortium (NCI-9742). J Clin Oncol. 2018;36(14):1412-1418. doi: 10.1200/JCO.2017.77.0388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018;20(5):674-686. doi: 10.1093/neuonc/nox208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quispel-Janssen J, van der Noort V, de Vries JF, et al. Programmed death 1 blockade with nivolumab in patients with recurrent malignant pleural mesothelioma. J Thorac Oncol. 2018;13(10):1569-1576. doi: 10.1016/j.jtho.2018.05.038 [DOI] [PubMed] [Google Scholar]
- 51.Ready N, Farago AF, de Braud F, et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol. 2019;14(2):237-244. doi: 10.1016/j.jtho.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garon EB, Rizvi NA, Hui R, et al. ; KEYNOTE-001 Investigators . Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018-2028. doi: 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 53.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21(19):4286-4293. doi: 10.1158/1078-0432.CCR-14-2607 [DOI] [PubMed] [Google Scholar]
- 55.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908-918. doi: 10.1016/S1470-2045(15)00083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robert C, Schachter J, Long GV, et al. ; KEYNOTE-006 investigators . Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521-2532. doi: 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 57.Armand P, Shipp MA, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol. 2016;34(31):3733-3739. doi: 10.1200/JCO.2016.67.3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34(32):3838-3845. doi: 10.1200/JCO.2016.68.1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. doi: 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 60.Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976-983. doi: 10.1016/S1470-2045(16)30053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17(6):717-726. doi: 10.1016/S1470-2045(16)00175-3 [DOI] [PubMed] [Google Scholar]
- 62.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460-2467. doi: 10.1200/JCO.2015.64.8931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374(26):2542-2552. doi: 10.1056/NEJMoa1603702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 65.Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315(15):1600-1609. doi: 10.1001/jama.2016.4059 [DOI] [PubMed] [Google Scholar]
- 66.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17(7):956-965. doi: 10.1016/S1470-2045(16)30066-3 [DOI] [PubMed] [Google Scholar]
- 67.Shimizu T, Seto T, Hirai F, et al. Phase 1 study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced solid tumors. Invest New Drugs. 2016;34(3):347-354. doi: 10.1007/s10637-016-0347-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017;18(5):623-630. doi: 10.1016/S1470-2045(17)30169-9 [DOI] [PubMed] [Google Scholar]
- 69.Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483-1492. doi: 10.1016/S1470-2045(17)30616-2 [DOI] [PubMed] [Google Scholar]
- 70.Bellmunt J, de Wit R, Vaughn DJ, et al. ; KEYNOTE-045 Investigators . Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-1026. doi: 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35(14):1542-1549. doi: 10.1200/JCO.2016.70.1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen R, Zinzani PL, Fanale MA, et al. ; KEYNOTE-087 . Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 2017;35(19):2125-2132. doi: 10.1200/JCO.2016.72.1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ding W, LaPlant BR, Call TG, et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129(26):3419-3427. doi: 10.1182/blood-2017-02-765685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Doi T, Piha-Paul SA, Jalal SI, et al. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol. 2018;36(1):61-67. doi: 10.1200/JCO.2017.74.9846 [DOI] [PubMed] [Google Scholar]
- 75.Frenel JS, Le Tourneau C, O’Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1-positive cervical cancer: results from the phase Ib KEYNOTE-028 trial. J Clin Oncol. 2017;35(36):4035-4041. doi: 10.1200/JCO.2017.74.5471 [DOI] [PubMed] [Google Scholar]
- 76.Gangadhar TC, Hwu WJ, Postow MA, et al. Efficacy and safety of pembrolizumab in patients enrolled in KEYNOTE-030 in the United States: an expanded access program. J Immunother. 2017;40(9):334-340. doi: 10.1097/CJI.0000000000000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsu C, Lee SH, Ejadi S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol. 2017;35(36):4050-4056. doi: 10.1200/JCO.2017.73.3675 [DOI] [PubMed] [Google Scholar]
- 78.Ott PA, Bang YJ, Berton-Rigaud D, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol. 2017;35(22):2535-2541. doi: 10.1200/JCO.2017.72.5952 [DOI] [PubMed] [Google Scholar]
- 79.Ott PA, Piha-Paul SA, Munster P, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with recurrent carcinoma of the anal canal. Ann Oncol. 2017;28(5):1036-1041. doi: 10.1093/annonc/mdx029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ott PA, Elez E, Hiret S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2017;35(34):3823-3829. doi: 10.1200/JCO.2017.72.5069 [DOI] [PubMed] [Google Scholar]
- 81.O’Neil BH, Wallmark JM, Lorente D, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12(12):e0189848. doi: 10.1371/journal.pone.0189848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 2017;18(2):212-220. doi: 10.1016/S1470-2045(17)30007-4 [DOI] [PubMed] [Google Scholar]
- 83.Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390(10105):1853-1862. doi: 10.1016/S0140-6736(17)31601-X [DOI] [PubMed] [Google Scholar]
- 84.Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18(11):1493-1501. doi: 10.1016/S1470-2045(17)30624-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamazaki N, Takenouchi T, Fujimoto M, et al. Phase 1b study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced melanoma (KEYNOTE-041). Cancer Chemother Pharmacol. 2017;79(4):651-660. doi: 10.1007/s00280-016-3237-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood. 2017;130(3):267-270. doi: 10.1182/blood-2016-12-758383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase 2 KEYNOTE-086 study [published online November 26, 2018]. Ann Oncol. 2018. doi: 10.1093/annonc/mdy517 [DOI] [PubMed] [Google Scholar]
- 88.Adra N, Einhorn LH, Althouse SK, et al. Phase II trial of pembrolizumab in patients with platinum refractory germ-cell tumors: a Hoosier Cancer Research Network Study GU14-206. Ann Oncol. 2018;29(1):209-214. doi: 10.1093/annonc/mdx680 [DOI] [PubMed] [Google Scholar]
- 89.Cho J, Kim HS, Ku BM, et al. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: an open-label phase II trial [published online June 15, 2018]. J Clin Oncol. 2018;JCO2017773184. [DOI] [PubMed] [Google Scholar]
- 90.Cohen RB, Delord JP, Doi T, et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: findings of the phase 1b KEYNOTE-028 study [published online February 21, 2018]. Am J Clin Oncol. 2018. doi: 10.1097/COC.0000000000000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cohen EEW, Soulières D, Le Tourneau C, et al. ; KEYNOTE-040 investigators . Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156-167. doi: 10.1016/S0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- 92.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789-1801. doi: 10.1056/NEJMoa1802357 [DOI] [PubMed] [Google Scholar]
- 93.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. doi: 10.1001/jamaoncol.2018.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gadgeel SM, Pennell NA, Fidler MJ, et al. Phase II study of maintenance pembrolizumab in patients with extensive-stage small cell lung cancer (SCLC). J Thorac Oncol. 2018;13(9):1393-1399. doi: 10.1016/j.jtho.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. 2018;19(3):347-355. doi: 10.1016/S1470-2045(18)30062-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hansen AR, Massard C, Ott PA, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol. 2018;29(8):1807-1813. doi: 10.1093/annonc/mdy232 [DOI] [PubMed] [Google Scholar]
- 97.Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study [published online October 20, 2018]. J Clin Oncol. 2018;JCO1801148. doi: 10.1200/JCO.18.01148 [DOI] [PubMed] [Google Scholar]
- 98.Rugo HS, Delord JP, Im SA, et al. Safety and antitumor activity of pembrolizumab in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer. Clin Cancer Res. 2018;24(12):2804-2811. doi: 10.1158/1078-0432.CCR-17-3452 [DOI] [PubMed] [Google Scholar]
- 99.Shitara K, Özgüroğlu M, Bang YJ, et al. ; KEYNOTE-061 investigators . Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123-133. doi: 10.1016/S0140-6736(18)31257-1 [DOI] [PubMed] [Google Scholar]
- 100.Zhu AX, Finn RS, Edeline J, et al. ; KEYNOTE-224 investigators . Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940-952. doi: 10.1016/S1470-2045(18)30351-6 [DOI] [PubMed] [Google Scholar]
- 101.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563-567. doi: 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fehrenbacher L, Spira A, Ballinger M, et al. ; POPLAR Study Group . Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-1846. doi: 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 103.McDermott DF, Sosman JA, Sznol M, et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol. 2016;34(8):833-842. doi: 10.1200/JCO.2015.63.7421 [DOI] [PubMed] [Google Scholar]
- 104.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909-1920. doi: 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Balar AV, Galsky MD, Rosenberg JE, et al. ; IMvigor210 Study Group . Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67-76. doi: 10.1016/S0140-6736(16)32455-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mizugaki H, Yamamoto N, Murakami H, et al. Phase I dose-finding study of monotherapy with atezolizumab, an engineered immunoglobulin monoclonal antibody targeting PD-L1, in Japanese patients with advanced solid tumors. Invest New Drugs. 2016;34(5):596-603. doi: 10.1007/s10637-016-0371-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peters S, Gettinger S, Johnson ML, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol. 2017;35(24):2781-2789. doi: 10.1200/JCO.2016.71.9476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi: 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Colevas AD, Bahleda R, Braiteh F, et al. Safety and clinical activity of atezolizumab in head and neck cancer: results from a phase I trial. Ann Oncol. 2018;29(11):2247-2253. doi: 10.1093/annonc/mdy411 [DOI] [PubMed] [Google Scholar]
- 110.Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5(1):74-82. doi: 10.1001/jamaoncol.2018.4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Horn L, Gettinger SN, Gordon MS, et al. Safety and clinical activity of atezolizumab monotherapy in metastatic non-small-cell lung cancer: final results from a phase I study. Eur J Cancer. 2018;101:201-209. doi: 10.1016/j.ejca.2018.06.031 [DOI] [PubMed] [Google Scholar]
- 112.Lukas RV, Rodon J, Becker K, et al. Clinical activity and safety of atezolizumab in patients with recurrent glioblastoma. J Neurooncol. 2018;140(2):317-328. doi: 10.1007/s11060-018-2955-9 [DOI] [PubMed] [Google Scholar]
- 113.McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018;24(6):749-757. doi: 10.1038/s41591-018-0053-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Petrylak DP, Powles T, Bellmunt J, et al. Atezolizumab (MPDL3280A) monotherapy for patients with metastatic urothelial cancer: long-term outcomes from a phase 1 study. JAMA Oncol. 2018;4(4):537-544. doi: 10.1001/jamaoncol.2017.5440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391(10122):748-757. doi: 10.1016/S0140-6736(17)33297-X [DOI] [PubMed] [Google Scholar]
- 116.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374-1385. doi: 10.1016/S1470-2045(16)30364-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase Ib study. J Clin Oncol. 2017;35(19):2117-2124. doi: 10.1200/JCO.2016.71.6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gulley JL, Rajan A, Spigel DR, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol. 2017;18(5):599-610. doi: 10.1016/S1470-2045(17)30240-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Heery CR, O’Sullivan-Coyne G, Madan RA, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017;18(5):587-598. doi: 10.1016/S1470-2045(17)30239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bang YJ, Ruiz EY, Van Cutsem E, et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29(10):2052-2060. doi: 10.1093/annonc/mdy264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barlesi F, Vansteenkiste J, Spigel D, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19(11):1468-1479. doi: 10.1016/S1470-2045(18)30673-9 [DOI] [PubMed] [Google Scholar]
- 122.D’Angelo SP, Russell J, Lebbé C, et al. Efficacy and safety of first-line avelumab treatment in patients with stage IV metastatic Merkel cell carcinoma: a preplanned interim analysis of a clinical trial. JAMA Oncol. 2018;4(9):e180077. doi: 10.1001/jamaoncol.2018.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167(3):671-686. doi: 10.1007/s10549-017-4537-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Le Tourneau C, Hoimes C, Zarwan C, et al. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: phase 1b results from the JAVELIN solid tumor trial. J Immunother Cancer. 2018;6(1):111. doi: 10.1186/s40425-018-0424-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34(26):3119-3125. doi: 10.1200/JCO.2016.67.9761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 127.Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9):e172411. doi: 10.1001/jamaoncol.2017.2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Garassino MC, Cho BC, Kim JH, et al. ; ATLANTIC Investigators . Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521-536. doi: 10.1016/S1470-2045(18)30144-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Necchi A, Giannatempo P, Raggi D, et al. An open-label randomized phase 2 study of durvalumab alone or in combination with tremelimumab in patients with advanced germ cell tumors (APACHE): results from the first planned interim analysis. Eur Urol. 2019;75(1):201-203. doi: 10.1016/j.eururo.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 130.Siu LL, Even C, Mesía R, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol. 2019;5(2):195-203. doi: 10.1001/jamaoncol.2018.4628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Olkin I, Sampson A. Comparison of meta-analysis versus analysis of variance of individual patient data. Biometrics. 1998;54(1):317-322. doi: 10.2307/2534018 [DOI] [PubMed] [Google Scholar]
- 132.Mathew T, Nordström K. On the equivalence of meta-analysis using literature and using individual patient data. Biometrics. 1999;55(4):1221-1223. doi: 10.1111/j.0006-341X.1999.01221.x [DOI] [PubMed] [Google Scholar]
- 133.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(12):1607-1616. doi: 10.1001/jamaoncol.2016.2453 [DOI] [PubMed] [Google Scholar]
- 134.Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):173-182. doi: 10.1001/jamaoncol.2017.3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:k793. doi: 10.1136/bmj.k793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.De Velasco G, Je Y, Bossé D, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5(4):312-318. doi: 10.1158/2326-6066.CIR-16-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brahmer JR, Lacchetti C, Schneider BJ, et al. ; National Comprehensive Cancer Network . Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714-1768. doi: 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351-1375. doi: 10.1002/sim.1761 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. List of the Studies Included in This Meta-Analysis
eTable 2. Relative Ratios of Grade 3 or Higher Adverse Event Incidences to Respective All-grade Adverse Event Incidences
eTable 3. List of Studies That Reported At Least One Treatment-related Death
eFigure 1. Incidences of All Adverse Events, (A) Incidences of All-grade Adverse Events and (B) Incidences of Grade 3 or Higher Adverse Events
eFigure 2. Mean Incidences of Adverse Events by Study, (A) Mean Incidences of All-grade Adverse Events by Study and (B) Mean Incidences of Grade 3 or Higher Adverse Events by Study
eFigure 3. Heterogeneity of Adverse Event Incidence in This Meta-Analysis
eFigure 4. Mean Incidences of All-grade Adverse Events by Study if Ignoring Missing Data