Abstract
Key points
Electrical pacemaking in gastrointestinal muscles is generated by specialized interstitial cells of Cajal that produce the patterns of contractions required for peristalsis and segmentation in the gut.
The calcium‐activated chloride conductance anoctamin‐1 (Ano1) has been shown to be responsible for the generation of pacemaker activity in GI muscles, but this conclusion is established from studies of juvenile animals in which effects of reduced Ano1 on gastric emptying and motor patterns could not be evaluated.
Knocking down Ano1 expression using Cre/LoxP technology caused dramatic changes in in gastric motor activity, with disrupted slow waves, abnormal phasic contractions and delayed gastric emptying; modest changes were noted in the small intestine.
Comparison of the effects of Ano1 antagonists on muscles from juvenile and adult small intestinal muscles suggests that conductances in addition to Ano1 may develop with age and contribute to pacemaker activity.
Abstract
Interstitial cells of Cajal (ICC) generate slow waves and transduce neurotransmitter signals in the gastrointestinal (GI) tract, facilitating normal motility patterns. ICC express a Ca2+‐activated Cl− conductance (CaCC), and constitutive knockout of the channel protein anoctamin‐1 leads to loss of slow waves in gastric and intestinal muscles. These knockout experiments were performed on juvenile mice. However, additional experiments demonstrated significant differences in the sensitivity of gastric and intestinal muscles to antagonists of anoctamin‐1 channels. Furthermore, the significance of anoctamin‐1 and the electrical and mechanical behaviours facilitated by this conductance have not been evaluated on the motor behaviours of adult animals. Cre/loxP technology was used to generate cell‐specific knockdowns of anoctamin‐1 in ICC (KitCreERT2/+;Ano1tm2jrr/+) in GI muscles. The recombination efficiency of KitCreERT was evaluated with an eGFP reporter, molecular techniques and immunohistochemistry. Electrical and contractile experiments were used to examine the consequences of anoctamin‐1 knockdown on pacemaker activity, mechanical responses, gastric motility patterns, gastric emptying and GI transit. Reduced anoctamin‐1 caused loss of gastric, but not intestinal slow waves. Irregular spike complexes developed in gastric muscles, leading to uncoordinated antral contractions, delayed gastric emptying and increased total GI transit time. Slow waves in intestinal muscles of juvenile mice were more sensitive to anoctamin‐1 antagonists than slow waves in adult muscles. The low susceptibility to anoctamin‐1 knockdown and weak efficacy of anoctamin‐1 antagonists in inhibiting slow waves in adult small intestinal muscles suggest that a conductance in addition to anoctamin‐1 may develop in small intestinal ICC with ageing and contribute to pacemaker activity.
Keywords: ICC, slow waves, Calcium‐activated chloride conductance (CaCC), Anoctamin‐1 (Ano1), delayed gastric emptying, Cre recombinase/LoxP
Key points
Electrical pacemaking in gastrointestinal muscles is generated by specialized interstitial cells of Cajal that produce the patterns of contractions required for peristalsis and segmentation in the gut.
The calcium‐activated chloride conductance anoctamin‐1 (Ano1) has been shown to be responsible for the generation of pacemaker activity in GI muscles, but this conclusion is established from studies of juvenile animals in which effects of reduced Ano1 on gastric emptying and motor patterns could not be evaluated.
Knocking down Ano1 expression using Cre/LoxP technology caused dramatic changes in in gastric motor activity, with disrupted slow waves, abnormal phasic contractions and delayed gastric emptying; modest changes were noted in the small intestine.
Comparison of the effects of Ano1 antagonists on muscles from juvenile and adult small intestinal muscles suggests that conductances in addition to Ano1 may develop with age and contribute to pacemaker activity.
Introduction
Interstitial cells of Cajal (ICC) are specialized cells in the gastrointestinal (GI) tract that organize and coordinate the contractile behaviours of the tunica muscularis and permit generation and modulation of motor patterns necessary for digestion, nutrient absorption, peristalsis and segmentation (Ward et al. 1994; Torihashi et al. 1995; Huizinga et al. 1995; Sanders, 1996, Sanders et al. 2014). ICC make up a small proportion of the total cellular population of the tunica muscularis and are classified according to their specific locations within the muscle wall and their physiological roles (Chen et al. 2007; Blair et al. 2014). The functions of ICC have been deduced from studies in which loss of these cells is associated with defects in GI motility. A mouse model carrying an inducible Cre‐recombinase allele driven by the endogenous promoter of Kit (KitCreERT2) has enabled investigations to target and delete genes in ICC (Klein et al. 2013; Malysz et al. 2017; Sung et al. 2018). One study disrupted ICC through expression of diphtheria toxin A (DTA), and these mice displayed loss of intestinal slow waves, reduced responses to nerve stimulation, increased intestinal transit time and delayed gastric emptying (Klein et al. 2013).
A Ca2+‐activated Cl− conductance (CaCC) in ICC has been proposed to be involved in the generation of electrical slow waves and responses to neural inputs because pacemaker activity is inhibited by membrane‐permeable Ca2+ buffers and Cl− channel blocking drugs (Hirst et al. 2002; Kito et al. 2002; Kito & Suzuki, 2003). In fact, ICC prominently express anoctamin‐1, a CaCC protein encoded by Tmem16a and more recently known as Ano1 (Gomez‐Pinella et al. 2009; Hwang et al. 2009). This conductance generates spontaneous transient inward currents (STICs) in isolated ICC and slow waves in intact GI smooth muscles (Hwang et al. 2009; Zhu et al. 2009). Juvenile mice with global deactivation of Ano1 do not exhibit slow waves in the stomach or small intestine (Hwang et al. 2009; Singh et al. 2014). However, due to developmental defects in mice with global knockout of Ano1, their life span is reduced to only a few days. Furthermore, treating GI muscles of adult mice with Ano1 antagonists has shown disparate effects on slow waves: intestinal slow waves are quite resistant to Ano1 antagonists but gastric antrum slow waves are readily blocked by these drugs (Hwang et al. 2009, 2016). Therefore, the importance of Ano1 expression in ICC, in terms of regulating gastric motility patterns, gastric emptying and intestinal transit in adult tissues, needs further assessment.
In the present study, we crossed KitCreERT2 mice with Ano1fx mice to ascertain the importance of Ano1 (iAno1−/−) in adult gastric and intestinal muscles. We characterized the recombination efficiency of KitCreERT2 alleles in ICC using an eGFP reporter and examined the efficiency of the knockdown of Ano1 in ICC by assessing the levels of gene transcripts by qPCR and Ano1 protein by immunohistochemistry. We recorded slow waves from iAno1−/− knockdown mice to determine the degree to which this conductance is required for electrical rhythmicity in gastric and small intestinal muscles of adult mice. We also evaluated the effects of Ano1 knockdown on in vivo gastric transit, propagating motility patterns and gastric emptying. Reducing Ano1 expression in ICC in adult tissues using a Cre/LoxP approach caused dramatic motor effects including reduced or loss of gastric slow waves, disrupted contractile patterns, reduced rate of gastric emptying and slow overall GI transit. Electrical and mechanical defects of Ano1 knockdown were far less severe in the small intestine. Responses of juvenile and adult small intestinal muscles to Ano1 antagonists and patch clamp studies suggested that conductances other than Ano1 may develop in adult intestinal ICC and contribute to slow waves.
Methods
Mouse generation
Mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) or specific strains generated in‐house at the University of Nevada, Reno, NV, USA or University California San Francisco, CA, USA. KitCreERT2Ejb1 /+ (KitCreERT2) mice were obtained from Dr Dieter Saur (Technical University Munich, Germany) (Klein et al. 2013) and crossed with the previously described Ano1f/f mice (Faria et al. 2014; Schreiber et al. 2015; Sung et al. 2018) to generate KitCreERT2/+;Ano1f/f and KitCreERT2/+;Ano1f/+ animals. KitCreERT2 /+ mice were also crossed with Gt(ROSA)26Sortm4 ( ACTB‐tdTomato,‐EGFP ) Luo/J reporter mice to produce KitCreERT2/;Ano1tm2jrr/+; Rosamtmg and KitCreERT2/;Ano1tm2jrr/−;Rosamtmg animals (Sung et al. 2018). Age‐matched C57Bl6/6J (The Jackson Laboratory) were also used as wild‐type (+/+) control mice where identified (see Table 1 for complete mice information and abbreviated names used throughout the study). Experiments were also performed on juvenile mice aged between postnatal day (P) 7 and P12 and on animals 4–6 weeks of age.
Table 1.
Mouse strains utilized
| Strain name | Source | Abbreviated name |
|---|---|---|
| C57Bl6/6J wild type | The Jackson Laboratory, Bar Harbor, NE, USA | (+/+) |
| Ano1+/+ ( tm1Bdh )( /tm1Bdh ) * | Jason Rock, Boston University, MA, USA | Ano1+/+ |
| Ano1−/− ( tm1Bdh )( /tm1Bdh )) * | Jason Rock, Boston University, MA, USA | Ano1−/− |
| KitCreERT2Ejb1 /+( Kit‐Cre ) | Deiter Sauer (Technical University Munich, Germany) | KitCreERT2 |
| Ano1tm2jrr | The Jackson Laboratory, Bar Harbor, NE, USA | |
| KitCreERT2/+; Ano1tm2jrr/+ | University of Nevada, Reno, NV, USA | iAno1+/− |
| KitCreERT2/+; Ano1tm2jrr/− | University of Nevada, Reno, NV, USA | iAno1−/− |
| Gt(ROSA)26Sortm4 ( ACTB‐tdTomato,‐EGFP ) Luo/J | The Jackson Laboratory, Bar Harbor, NE, USA | mT/mG |
| KitCreERT2/; Ano1tm2jrr/+; Rosamtmg | Jason Rock, Boston University, MA, USA | iAno1+/−;Rosa |
| KitCreERT2/;Ano1tm2jrr/−; Rosamtmg | Jason Rock, Boston University, MA, USA | iAno1−/−;Rosa |
| KitcopGFP/+ | University of Nevada, Reno, NV, USA | KitcopGFP |
| Pdgfratm11 ( EGFP ) Sor/J | The Jackson Laboratory, Bar Harbor, NE, USA | PdgfraeGFP/+ |
| B6.Cg‐Tg ( Myh11‐cre,eGFP ) | Michel Kotlikoff, Cornel University, Ithaca, NY, USA | Myh11eGFP/+ |
* Ano1+/+ ( tm1Bdh )( /tm1Bdh ) and Ano1−/− ( tm1Bdh )( /tm1Bdh ) mice were previously termed Tmem16a+/+ ( tm1Bdh )( /tm1Bdh ) and Tmem16−/− ( tm1Bdh )( /tm1Bdh ) mice, respectively.
Cre recombinase was induced in 8‐week‐old mice by treating with tamoxifen (Sigma‐Aldrich; St Louis, MO, USA; 2.0 mg intraperitoneal injection made up as a 20 mg ml−1 solution in safflower oil). Each animal received 3 consecutive doses of tamoxifen given every other day and experiments were performed 50 days following the last injection. This time period has been reported to be successful in obtaining maximal gene knockdown in GI muscles (Groneberg et al. 2011).
The Institutional Animal Use and Care Committees at the University of Nevada and University of California, San Francisco approved procedures used on mice. Animals were fed ad libitum and had free access to water. Animals were humanely killed by isoflurane sedation followed by cervical dislocation and exsanguination. The investigators involved in the present study are aware of the ethical principles under which The Journal of Physiology operates and confirm that the use of animals presented here complies with the check list in Grundy (2015).
Genotyping
KitCreERT2Ejb1 /+ (Kit‐Cre) were generated by inserting CreERT2 under control of the mouse proto‐oncogene receptor tyrosine kinase (Kit) promoter/enhancer regions on the BAC transgene. Genomic DNA was isolated from transgenic mice tails using standard procedures. DNA (0.5 μl) was amplified in each PCR reaction to determine the genotypes of the transgenic mice. A 685 bp PCR fragment was amplified from the KitCreERT2 +/+ allele with primers that span the insertion region. The 330 bp PCR fragment was amplified from the KitCreERT2 +/− allele with primers that bind to the CreERT2 insert (Klein et al. 2013). When KitCreERT2+/− mice were bred with Ano1tm2jrr mice containing a loxP‐flanked sequence, tamoxifen‐inducible, Cre‐mediated recombination resulted in deletion of the floxed sequences in Kit‐positive cells of the offspring (Faria et al. 2014; Schreiber et al. 2015; Sung et al. 2018).
Morphological studies
Whole‐mount preparations were made by removing the mucosa from antral and intestinal segments. The tunica muscularis was pinned to the base of a dish filled with Sylgard elastomer (Dow Corning Corp., Midland, MI, USA) with the circular muscle layer facing upwards and fixed in either acetone (4°C; 10 min) or paraformaldehyde (4% w/v in 0.1 M phosphate buffer (PB) for 20 min at 4°C). Following fixation, the muscles were washed for 60 min in phosphate buffered saline (PBS; 0.01 M, pH 7.4). Incubation of tissues in a solution with bovine serum albumin (BSA; 1%) and Triton X‐100 (0.3%) for 1 h at room temperature was performed to reduce non‐specific antibody binding. For double labelling, tissues were incubated sequentially in a combination of primary antibodies (Kit/Ano1; eGFP/Kit combinations); see Table 2. The first incubation was carried out for 48 h at 4°C, tissues were subsequently washed in PBS before being incubated in a second antibody for an additional 48 h at 4°C. The combinations and concentrations of antibodies used were chicken/goat and goat/rabbit (see Table 2 for details). Following incubation in primary antibodies, tissues were washed and incubated separately in secondary antibodies (Alexa Fluor 488, 594 and 647 diluted to 1:1000 in PBS for 1 h at room temperature, Life Technologies, Eugene OR, USA). Control tissues were prepared by either omitting primary or secondary antibodies from the incubation solutions. Tissues were examined with a Zeiss LSM 510 Meta (Zeiss, Germany) or a Nikon A1R confocal microscope with appropriate excitation wavelengths. Confocal micrographs were digital composites of Z‐series scans of 10–15 optical sections through a depth of 2–40 μm. Final images were constructed and montages were assembled using Zeiss LSM 5 Image Examiner or Nikon NIS Elements and converted to Tiff files for final processing in Adobe Photoshop CS5 software (Adobe Co., Mountain View, CA, USA) and Photoshop 7.0 and Corel Draw X4 (Corel Corp. Ontario, Canada).
Table 2.
Details of antibody combinations used for double‐label immunohistochemistry
| Combined antibodies | Primary antibody source | Mono‐ or poly‐clonal antibodies | Host/dilution | 2nd antibody host/source/dilution |
|---|---|---|---|---|
| eGFP/mSCFR | Abcam, Cambridge, MA, USA | Poly‐/poly‐ | Chicken/goat | Rabbit/donkey |
| mSCFR/TMEM16A | R&D Systems Inc., Minneapolis, MN, USA R&D Systems Inc., Minneapolis, MN, USA/Abcam, Cambridge, MA, USA |
Poly‐/poly‐ |
1:1000/1:100 Goat/ rabbit 1:100/1:800 |
Jackson IR West Grove PA, USA/Life Technologies Eugene, OR, USA 1:800/1:1000 Donkey/donkey Life Technologies, Eugene, OR, USA for both 1:1000/1:1000 |
Note: mSCFR: anti‐mouse stem cell factor/Kit receptor; TMEM16A: anti‐anoctamin 1 (Ano1).
RNA isolation and quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR)
Strips of gastric antral and intestinal muscles were isolated as previously described (Ward et al. 1994; Shaylor et al. 2016). Briefly, tissues were opened along the lesser curvature of the stomach and along the mesenteric border for the intestine. The mucosa was rapidly removed by sharp dissection and muscle strips were flash frozen in liquid nitrogen and stored at −80°C until RNA was isolated. Total ribonucleic acid (RNA) was isolated from antrum and intestinal tissues as previously described (Sung et al. 2018). Concentration and purity of RNA were measured using an ND‐1000 Nanodrop Spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Total RNA was reverse transcribed with qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD, USA) in a 5× reaction buffer containing optimized concentrations of MgCl2, dNTPs (dATP, dCTP, dGTP, dTTP), recombinant RNase inhibitor protein, qScript reverse transcriptase, random primers, oligo(dT) primer and stabilizers, followed by heat inactivation. PCR was performed with specific primers to Ano1, Kit, Myh11, Pdgfra and Gapdh from tamoxifen‐treated KitCreERT2;Ano1f/f and KitCreERT2;Ano1f/+ mice (see Table 3). qRT‐PCR was performed with the same primers as PCR using Fast Sybr green chemistry on the 7900HT Real Time PCR System (Applied Biosystems, Foster City, CA, USA). Normalized values and standard deviations were calculated in differences of relative gene expression from four dilutions of technical triplicates of antrum and small intestine from each animal. The data are shown as averages and standard deviations of sextuplet samples (n = 6) and triplicate in (+/+) samples (n = 3). Genes with a fold change P value less than 0.05 between tissues represents a statistically significant difference. Unpaired Student's t test was used to determine P values in the parametric analysis.
Table 3.
Details of primers used
| Gene name | Sequence (sense primer on top) | Accession no. |
|---|---|---|
| Gapdh | GCCGATGCCCCCATGTTTGTGA | NM_008084 |
| GGGTGGCAGTGATGGCATGGAC | ||
| Pdgfra | ATGACAGCAGGCAGGGCTTCAACG | NM_011058 |
| CGGCACAGGTCACCACGATCGTTT | ||
| Ano1 | TAACCCTGCCACCGTCTTCT | NM_178642 |
| ATGATCCTTGACAGCTTCCTCC | ||
| Kit | CGCCTGCCGAAATGTATGACG | NM_021099 |
| GGTTCTCTGGGTTGGGGTTGC | ||
| Myh11 | CCCAAGCAGCTAAAGGACAA | NM_013607 |
| AGGCACTTGCATTGTAGTCC |
Total gastrointestinal transit time
To estimate total GI transit time, mice were administered 100 μl of the carmine dye solution by oral gavage. The elapsed time before excretion of the first red‐stained fecal pellet was recorded for each mouse at baseline and again following tamoxifen treatment. The change in transit time was calculated by subtracting the baseline transit time prior to tamoxifen administration from the transit time after treating animals with tamoxifen and waiting 50 days for Cre‐mediated recombination, deletion of the floxed sequences and reduction in Ano1 to occur.
Small intestine transit
To measure small intestine transit time, mice were administered 100 μl of indocyanine green (ICG) solution by oral gavage. The animals were killed by cervical dislocation either 20 min or 1 h after gavage. The intact GI tracts were then dissected from the mice and imaged with an Odyssey imaging system (LI‐COR Biotechnology, Lincoln, NE, USA). The distance travelled and GI tract area covered by the ICG fluorescent dye was analysed using Fiji image analysis software (Schindelin et al. 2012).
Gastric emptying
To measure gastric emptying, mice were fasted overnight for 12 h and then orally administered 100 μl of 25 mg ml−1 rhodamine‐dextran tracer (Sigma R9379) dissolved in 2% methylcellulose in PBS (De Lisle, 2007). After 20 min, the animals were killed by cervical dislocation. After removing the GI tracts, the stomachs were dissected and the small intestine was cut into 10 segments of equal length. The stomach and small intestine segments were opened and washed with 1 ml PBS in microcentrifuge tubes. The luminal contents were then centrifuged at 2500 g, the supernatants were collected and fluorescence was measured using a FLUOstar OPTIMA microplate reader (BMG LABTECH). The fraction of rhodamine‐dextran remaining in the stomach relative to the total amount recovered from the small intestine segments and stomach was used to calculate gastric emptying. Small intestine transit was analysed by measuring the geometric centre of the fluorescence (GCF) distribution within the small intestine. GCF = Σ((fluorescence fraction in segment) × (segment number)) (Miller et al. 1981; Capasso et al. 2005; De Lisle, 2007; Kondo et al. 2015).
Isometric force measurements
Isometric force recordings were performed as previously described (Shaylor et al. 2016). Briefly, antral and intestinal tissues were prepared as described below for intracellular recordings. Strips of tissues (3 × 8 mm) were attached at one end with suture thread to a fixed‐mount and the opposite end to a Gould isometric strain gauge (Gould UC3; Gould Instruments, OH, USA). A resting force of 5 mN was applied, which has been shown to set the muscles at optimum length. This was followed by an equilibration period of 1 h, during which time the bath was continuously perfused with oxygenated Krebs Ringer buffer (KRB).
Intracellular microelectrode recordings
The GI tracts were removed from animals and placed in KRB for further dissection. Stomachs were opened along the lesser curvature and gastric contents were washed away with KRB. The gastric corpus and antrum were pinned to the base of a Sylgard silicone elastomer (Dow Corning Corp., Midland, MI, USA) dish and the mucosa was removed by sharp dissection. Antrums were subsequently isolated by a surgical incision across the stomach at the level of the incisura angularis. Strips of gastric antrum (2 × 6 mm) were isolated from along the greater curvature and placed in a recording chamber with the serosal aspect of the muscle facing upward for intracellular microelectrode recordings. A similar procedure was performed on intestinal muscles except the tissues were opened along the mesenteric border, luminal contents washed with KRB and the mucosa removed by sharp dissection. Small intestine tissues were mounted in the organ bath with the submucosal aspect of the muscle facing upwards.
Intracellular microelectrode recordings were performed as previously described (Ward et al. 1994). Briefly, impalements of circular muscle cells along the greater curvature of the antrum and anti‐mesenteric border of the small intestine were made with glass microelectrodes having resistances of 80–120 MΩ. Transmembrane potentials were recorded with a high impedance electrometer (Axon Instruments, Union City, CA, USA). Multiple electrical recordings (≤11 for antrum and ≤6 for intestine for periods up to 2 h) were performed in an array‐like arrangement to ensure satisfactory recordings representative of the activity were obtained from each tissue. Data were recorded on a PC running AxoScope 10 data acquisition software (Axon Instruments) and hard copies were made using Clampfit analysis software (Axon Instruments). Some experiments were performed in the presence of nifedipine (1 μM) to reduce contractions and facilitate impalements of cells for extended periods. It has been previously demonstrated that slow waves in the murine antrum and small intestine are not significantly affected by nifedipine (Ward et al. 1994; Suzuki & Hirst, 1999).
Spatiotemporal mapping of whole‐stomach motility patterns
Video analysis was performed on gastric motility patterns from tamoxifen‐treated iAno1−/− and iAno1+/− control stomachs using bright‐field imaging as previously described (Hennig et al. 1999) Briefly, videos were recorded using a Nikon SMZ1000 microscope and an Imagingsource DMK camera recording at 7.5 frames s−1 (FPS) for 15 min. Videos were subsequently analysed using a curved edge‐tracking along the greater curvature of each stomach and analysed using volumetry (GWH v.8a). Greyscale‐coded pixels from each frame of the movie sequence were used to construct a single row and sequential rows, producing spatiotemporal maps (STMaps). STMaps illustrate the gastric motility behaviour (tangential displacement of STMaps) on a time vs. space axis. The y‐axis represents space (i.e. the antrum with oral and anal directionality as indicated) and the x‐axis represents time.
Patch clamp electrophysiological recordings
ICC were isolated from Kit+/copGFP mice, 4—6 weeks of age as previously described (Zhu et al. 2009). Briefly, jejunal muscles were cut into small pieces (1 mm) and then incubated in Ca2+‐free Hanks’ solution for 20 min. Then the muscle fragments were incubated for 35 min in a Ca2+‐free enzyme solution containing: collagenase (Worthington Type II; 4 mg ml−1), bovine serum albumin (Sigma‐Aldrich; 8 mg ml−1), trypsin inhibitor (Sigma‐Aldrich; 8 mg ml−1). Cells were plated onto coverslips coated with murine collagen (2.5 mg ml−1, BD Falcon, Franklin Lakes, NJ, USA) in 35 mm culture dishes. The cells were allowed to stabilize for 2—6 h at 37°C in an incubator (atmosphere 95% O2–5% CO2) in culture media (Clonetics, San Diego, CA, USA) supplemented with 2% antibiotic–antimycotic (Gibco, Grand Island, NY, USA) and stem cell factor (5 ng ml−1, Sigma‐Aldrich).
ICC were identified by the green fluorescent protein reporter using an inverted fluorescence microscope. Standard whole‐cell patch clamp configuration was employed to record membrane currents (voltage clamp mode). Currents were amplified with an Axopatch 200B patch clamp amplifier (Axon Instruments) and digitized with a 16‐bit analog to digital converter (Digidata 1440A, Axon Instruments) and stored using pClamp software (version 10.2, Axon Instruments). All membrane potential data is corrected for 15 mV junction potential. Data were sampled at 4 kHz and filtered at 2 kHz using an eight‐pole Bessel filter for whole‐cell experiments.
Solutions and drugs
Tissues were constantly perfused with oxygenated KRB of the following composition (mM): NaCl 118.5; KCl 4.5; MgCl2 1.2; NaHCO3 23.8; KH2PO4 1.2; dextrose 11.0; CaCl2 2.4. The pH of the KRB was 7.3–7.4 when bubbled with 97% O2–3% CO2 at 37 ± 0.5°C. Muscles were left to equilibrate for at least 1 h before experiments were begun. Nifedipine was purchased from Sigma‐Aldrich, dissolved in ethanol to a concentration of 10 mM and added to the perfusion KRB solution at a final concentration of 1 μM. CaCCinh‐Ano1 was purchased from Tocris (Bristol, UK) and dissolved in DMSO at 100 mM before being added to the perfusion solution at the stated concentration.
Total intestinal transit experiments used a solution containing carmine dye (60 mg ml−1; Sigma C1022) suspended in methylcellulose (0.5%; Sigma M0262) in PBS. A solution containing a 1:500 dilution of indocyanine green (MP Biomedicals) in methylcellulose (0.5%) in PBS was used for small intestinal transit experiments. Gastric emptying was assessed by giving mice a solution containing rhodamine‐dextran tracer (25 mg ml−1; Sigma R9379) dissolved in methylcellulose (2%) in PBS (De Lisle, 2007).
The external solution for whole‐cell patch clamp experiments was Ca2+‐containing physiological salt solution (CaPSS) containing (mM): 5 KCl, 135 NaCl, 2 CaCl2, 10 glucose, 1.2 MgCl2, and 10 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid (HEPES), adjusted to pH 7.4 with tris(hydroxymethyl)aminomethane (Tris). The equilibrium potential for Cl− ions (EC l) was estimated to be −40 mV after junction potential correction using an internal solution containing (mM): 30 CsCl, 110 caesium aspartate, 10 HEPES, 3 MgATP, 0.1 NaGTP, 2.5 creatine phosphate disodium, adjusted to pH 7.2 with Tris. In some experiments, [Na+]o was adjusted to 0 mM by replacing NaCl in the external solution with equimolar NMDG‐Cl.
Data analysis of physiological experiments
Several electrical parameters from juvenile mice were analysed: (i) resting membrane potential (RMP), (ii) amplitude of slow waves (mV), (iii) frequency of slow waves (cycles min−1), (iv) half‐maximal duration of slow waves (s), (v) dV/dt of slow wave upstroke (mV s−1). Figures displayed were made from digitized data using Corel Draw X4. Data are expressed as means ± standard errors of the mean (SEM). Differences between the reported means of mouse groups were evaluated using a Student's unpaired t test. P values < 0.05 were taken as a statistically significant difference. Differences between the means of three or more measured parameters were evaluated using repeated measures of one‐way analysis of variance (ANOVA) where appropriate in conjunction with the Dunnett's multiple comparison test. P values < 0.05 were taken as a statistically significant difference. Statistical tests were performed using Prism 5.03 (GraphPad Software, Inc., La Jolla, CA, USA). The n values reported in the text refer to the number of animals used for each experimental protocol.
Results
Mapping genetic information, determination of recombination efficiency and knockdown of Ano1
To produce a floxed KitCreERT2+ Ano1 mouse line (KitCreERT2+;Ano1f/f) with eGFP expression to determine Cre recombinase (Cre) efficiency we initially crossed KitCreERT2/+ with RosaMtmg mice. Offspring of these mice were subsequently crossed with Ano1f/f to produce KitCreERT2/+;Ano1f/+. Offspring of these mice were back crossed to produce KitCreERT2+;Ano1f/f and KitCreERT2+;Ano1f/+ animals (Faria et al. 2014; Schreiber et al. 2015; Sung et al. 2018).
To determine the efficiency of the inducible Cre and cellular localization of Cre in gastric and intestinal tissues we examined eGFP after its induction following tamoxifen treatment. We utilized KitCreERT2/;Ano1tm2jrr/+;Rosamtmg (iAno1+/−;Rosa) and KitCreERT2/;Ano1tm2jrr/−;Rosamtmg (iAno1−/−;Rosa) mice. td‐Tomato was localized in a variety of cell types, including enteric neurons (Fig. 1 A) and smooth muscle cells (Fig. 1 D, G and J). In the antrum, eGFP expression was localized to populations of cells that had an appearance of ICC (Fig. 1 B, E, H and K). To confirm that eGFP was localized in ICC we performed double labelling of these tissues with antibodies against Kit. Double labelling revealed that eGFP expression was localized to distinct populations of ICC in the gastric antrum. In the antrums of iAno1+/− and iAno1−/−;Rosa animals eGFP expression was limited to ICC at the level of the myenteric plexus (ICC‐MY; Fig. 1 B for iAno1+/−;Rosa controls and Fig. 1 H for iAno1−/−;Rosa animals) and intramuscular ICC (ICC‐IM; Fig. 1 E for iAno1+/−;Rosa controls and Fig. 1 K for iAno1−/−;Rosa animals) within the circular layer. Confirmation that eGFP expression was restricted to ICC‐MY and ICC‐IM was performed by double labelling (633 nm; Fig. 1 C and F for ICC‐MY and ICC‐IM in iAno1+/−;Rosa controls and Fig. 1 I and L for ICC‐MY and ICC‐IM in iAno1−/−;Rosa animals).
Figure 1. Determination of recombination efficiency of Cre by eGFP in the gastric antrum.
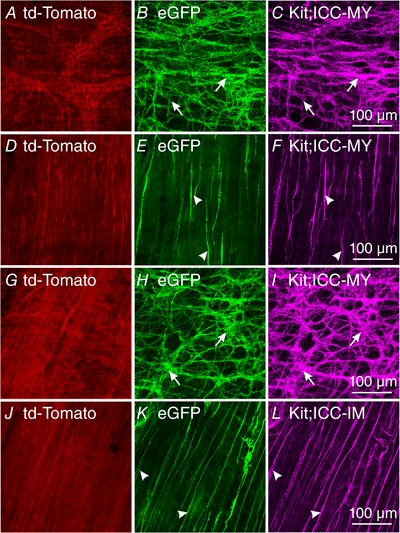
To determine the efficiency of the inducible Cre and cellular localization of Cre in gastric tissues, eGFP was examined after its induction by Cre recombination following tamoxifen treatment. KitCreERT2/;Ano1tm2jrr/+; Rosamtmg (iAno1+/−;Rosa) and KitCreERT2/;Ano1tm2jrr/−;Rosamtmg (iAno1−/−;Rosa) mice were utilized to determine recombination efficiency determined by eGFP expression. A–F, in the gastric antrums of iAno1+/−;Rosa mice, eGFP expression occurred in ICC‐MY (A–C) and ICC‐IM (D–F). A, td‐Tomato was expressed in a variety of cells at the level of the myenteric plexus, including enteric neurons. B, eGFP expression (arrows) in a population of cells with a morphology similar to ICC‐MY. C, confirmation that eGFP expression induced in ICC‐MY following Cre induction was performed by double labelling with Kit (magenta; arrows – same cells labelled in B). D–F, a similar approach showed eGFP expression in Kit+ ICC‐IM (arrowheads) in the gastric antrums within the circular muscle of iAno1+/−;Rosa mice. G–I, eGFP expression in ICC‐MY of iAno1−/−;Rosa mice shows that the majority of Kit+ ICC‐MY (magenta; arrows, I) expressed eGFP (H). J–L, likewise Kit+ ICC‐IM (L, arrowheads) in gastric antrums of iAno1−/−;Rosa mice also revealed eGFP expression (K). Scale bars in panels C, F, I and L = 100 μm, and apply to other panels.
In intestinal muscles of iAno1+/−;Rosa animals, td‐Tomato was localized to a variety of cells including those with a smooth muscle appearance (Fig. 2 A, D, G and J). eGFP expression was limited to ICC‐MY (Fig. 2 B) and ICC at the level of the deep muscular plexus (ICC‐DMP; Fig. 2 E). Likewise, the small intestines of iAno1−/−;Rosa controls also displayed eGFP expression in ICC‐MY (Fig. 2 H) and ICC‐DMP (Fig. 2 K). The eGFP expression in iAno1−/−;Rosa mice did not appear different from iAno1+/−;Rosa animals. As in the intestine, confirmation that eGFP expression was limited to ICC‐MY and ICC‐DMP was performed by double labelling (Fig. 2 C and F for ICC‐MY and ICC‐DMP in iAno1+/−;Rosa controls and Fig. 2 I and L for ICC‐MY and ICC‐DMP in iAno1−/−;Rosa animals). Control animals not treated with tamoxifen did not display eGFP expression. These results demonstrate that tamoxifen caused efficient induction of Cre in a cell‐specific manner (i.e. ICC) in the gastric antrums and intestines and the degree of induction did not appear different between iAno1+/−;Rosa and iAno1−/−;Rosa animals.
Figure 2. Determination of recombination efficiency of Cre by eGFP in the small intestine.
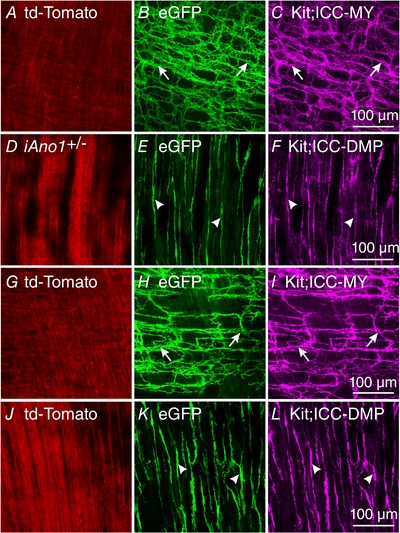
Double labelling with antibodies against eGFP and Kit demonstrated the efficiency and cellular localization of Cre recombination in the small intestines of iAno1+/−;Rosa and iAno1−/−;Rosa mice. A, td‐Tomato expression in a variety of cells at the level of the myenteric plexus. B and C, in iAno1+/−;Rosa mice, eGFP expression (B) was limited to a population of cells with an ICC‐MY phenotype (arrows) and this was confirmed using Kit labelling (majenta; arrows in C). D–F, td‐Tomato, eGFP and Kit expression in cells at the level of the deep muscular plexus in iAno1+/−;Rosa mice. E, eGFP expression in cells with a morphology of ICC‐DMP and this was confirmed by Kit labelling in F (arrowheads). G–I, td‐Tomato (G) and robust eGFP expression in iAno1−/−;Rosa mice at the level of the myenteric plexus was limited to cells that possessed a ICC‐MY morphology (H, arrows) and this was confirmed with Kit double labelling (I; arrows). J–L, td‐Tomato, eGFP and Kit expression in cells at the level of the deep muscular plexus in iAno1−/−;Rosa mice. K, eGFP revealed Cre recombination in cells with an ICC‐DMP morphology (arrowheads) and this was confirmed by Kit double labelling (L, arrowheads). Scale bars in panels C, F, I and L = 100 μm, and apply to other panels.
After tamoxifen, Ano1 transcripts were reduced in antrums and intestines of KitCreERT2/+; Ano1tm2jrr/− (iAno1−/−) and iAno1−/−;Rosa mice, compared to tamoxifen‐treated KitCreERT2/+;Ano1tm2jrr/+ (iAno1+/−) control, iAno1+/−; Rosa and (+/+) mice and revealed by qPCR and Ano1 immunohistochemistry. Antral muscles of iAno1−/− mice showed a −3.7‐fold reduction (0.007 ± 0.0006) in Ano1 transcripts, compared to age‐matched iAno1+/− siblings (0.026 ± 0.002; P = 0.001; n = 6 animals for both). There was no significant difference in Ano1 transcript expression between tamoxifen‐treated iAno1+/− mice and (+/+) mice (0.039 ± 0.003; P > 0.05; n = 3). Intestinal muscles of iAno1−/− mice showed a −4.3‐fold reduction in Ano1 transcripts, 0.014 ± 0.002 compared to 0.06 ± 0.005 for age‐matched iAno1+/− siblings (P = 0.001; n = 6 animals for both groups). Like gastric antrum there was no significant difference in Ano1 transcript expression between tamoxifen‐treated iAno1+/− mice and (+/+) mice (0.07 ± 0.002; P > 0.05; n = 3; Fig. 3).
Figure 3. Ano1 reduction causes changes in gene expression in cells of the SIP syncytium.
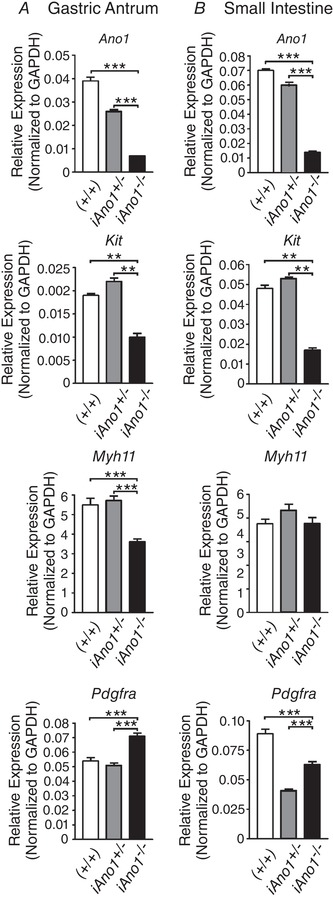
A, Ano1, Kit and Myh11 were significantly reduced in ICC and smooth muscle cells of gastric antrum tissues from iAno1−/− animals compared to tissues from iAno1+/− and (+/+) controls. In comparison, Pdgfra expression was significantly upregulated. B, in the small intestine, Ano1, Kit and Pdgfra were significantly downregulated in tissues from iAno1−/− animals compared to iAno1+/− and (+/+) controls. There were no differences in Myh11 expression. Means ± SD; ** P<0.01, *** P<0.001.
We reported previously that Kit+ ICC were not reduced in the intestines and gastric fundus of global Ano1−/− mice (Hwang et al. 2009; Sung et al. 2018). We sought to determine if Kit transcripts changed in ICC in the gastric antrums of tamoxifen‐treated iAno1−/− or iAno1+/− Rosa mice (Fig. 3). Interestingly, there was a −2.2‐fold reduction in Kit transcripts in antral muscles of iAno1−/− mice (0.01 ± 0.002), compared to iAno1+/− (0.022 ± 0.002) and (+/+) (0.019 ± 0.0008) controls (P = 0.002 and P = 0.003, respectively, n = 6 for iAno1−/− and iAno1+/− mice and n = 3 for (+/+) mice). Similarly, there was also a −3.1‐fold reduction in Kit transcript expression in the small intestines of iAno1−/− mice (0.017 ± 0.003) in comparison to the intestinal muscles of iAno1+/− mice (0.053 ± 0.002; P = 0.004; n = 6 for both groups) and a −2.8‐fold reduction in Kit when compared to (+/+) mice (0.048 ± 0.003; P = 0.002; n = 3; Fig. 3). There was no significant difference in Kit expression between iAno1+/− versus (+/+) mice. Again, there was no obvious difference in the number of ICC in the intestines of tamoxifen‐treated iAno1−/− compared to iAno1+/− mice.
ICC are a cellular element in the smooth muscle cell, ICC and PDGFRα+ cell (SIP) syncytium. Therefore, we also tested whether transcripts of cell identifying genes were also affected by reduction in Ano1. Transcripts of Myh11, a biomarker for SMCs, were slightly decreased in the antrums of iAno1−/− animals in comparison to iAno1+/− and (+/+) controls, but a similar difference was not observed in intestinal muscles. In the gastric antrums there was a −1.58‐fold decrease in Myh11 in iAno1−/− (3.61 ± 0.37), as compared with iAno1+/− (5.72 ± 0.56) animals (P = 0.0001; n = 6; Fig. 3). In (+/+) mice animals Myh11 transcript levels were 5.49 ± 0.59 and not different from iAno1+/− controls but increased compared to iAno1−/− animals. In the small intestines Myh11 was slightly decreased (−1.1 fold, i.e. 3.77 ± 0.62 in iAno−/− mice compared to 4.33 ± 0.62 in iAno1+/− animals (P = 0.15; n = 6), but this did not reach a statistically significant difference and was also unchanged in (+/+) controls (i.e. 3.76 ± 0.32; P = 0.97; n = 3; Fig. 3). Pdgfra transcripts, a biomarker for fibroblast‐like cells, were increased (1.4‐fold) in the antrums of iAno1−/− mice (0.071 ± 0.005) compared to iAno1+/− mice (0.051 ± 0.004; P = 0.001) and increased 1.3‐fold compared to (+/+) mice (0.054 ± 0.004; P = 0.001; Fig. 3). In the small intestine Pdgfra transcripts were increased 1.2‐fold, i.e. an average of 0.063 ± 0.006 in iAno1−/− mice compared to 0.041 ± 0.003 in iAno1+/− mice (P = 0.001) and showed a −1.4‐fold decrease compared to (+/+) controls (0.089 ± 0.007; P = 0.0001). These data demonstrate that reduced Ano1 expression in ICC in the gastric antrum and small intestine led to reduced Kit expression, but reduced Ano1 had differential effects on genes that identified neighbouring cells in the SIP syncytium (i.e. smooth muscle cells and PDGFRα+ cells). There were also significant differences in the gastric antrum versus the intestine, demonstrating organ level variability on the interactions of cells that constitute the SIP syncytium.
Reduced Ano1 transcript expression results in decrease in Ano1 protein translation
The reduction in Ano1 transcripts translated into decreased Ano1 protein immunoreactivity. A comparison of Ano1 protein in the antrums of iAno1+/− controls (Fig. 4 B and C) and iAno1−/− mice (Fig. 4 E and F) by confocal immunohistochemistry showed significantly depressed Ano1 immunoreactivity in ICC‐MY, ICC‐IM and ICC‐DMP of iAno1−/− animals. Although significant reduction in Ano1 was noted in gastric antrums of iAno1−/− mice, weak immunoreactivity persisted. Similarly, robust expression of Ano1 was observed in ICC‐MY and ICC‐DMP of the small intestines of iAno1+/− controls (Fig. 4 H and I), as compared to iAno1−/− animals (Fig. 4 K and L).
Figure 4. Confocal immunohistochemistry verifies reduction in Ano1 protein in gastric antrum and intestine following induction of Cre recombinase by tamoxifen.
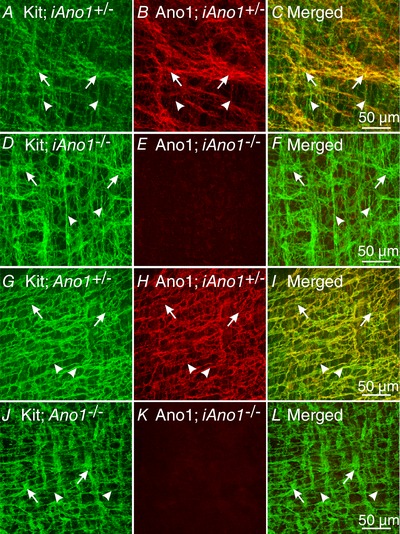
A–C, Kit and Ano1 expression in the gastric antrum of an iAno+/− mouse. A, Kit labelling in ICC‐MY (green, arrows) and ICC‐IM (green, arrowheads). B, Ano1 labelling of ICC (red, arrows for ICC‐MY and arrowheads for ICC‐IM) in the same confocal reconstruction as A. C, merged image of Kit and Ano1 labelling revealing cellular localization of Ano1 in Kit+ ICC after tamoxifen treatment in an iAno1+/− control. D–F, reduction in Ano1 expression in the gastric antrum of a tamoxifen‐treated iAno1−/− mouse. D, Kit labelling (green) revealed the presence of ICC‐MY (arrows) and ICC‐IM (arrowheads) in the gastric antrum of an iAno1−/− mouse. E, although ICC were present, Ano1 labelling was almost non‐detectable. F, merged image showing presence of ICC but absence of Ano1 in the antrum of a tamoxifen‐treated iAno1−/− animal. G–I, Kit and Ano1 expression in the small intestine of a tamoxifen‐treated iAno1+/− mouse. G, Kit labelling in ICC‐MY (green, arrows) and ICC‐DMP (green, arrowheads). H, expression of Ano1 in ICC (red, arrows, ICC‐MY) and ICC‐DMP (arrowheads) in the same confocal reconstruction as G. I, merged image of Kit and Ano1 labelling revealing cellular localization of Ano1 in ICC following tamoxifen treatment in an iAno1+/− control. J–L, reduction in Ano1 expression in the small intestine following tamoxifen treatment of an iAno1−/− mouse. J, Kit labelling of ICC‐MY and ICC‐DMP (green, arrows and arrowheads respectively) in the small intestine of an iAno1−/− mouse. K, Ano1 labelling was almost absent in the small intestine of a tamoxifen‐treated iAno1−/− mouse. L, merged image of J and K showing presence of ICC but absence of Ano1 in the small intestine of a treated iAno1−/− animal. Scale bars in panels C, F, I and L = 50 μm and represent all panels.
Others have reported that mice lacking Ano1 have reduced ICC, raising the possibility that Ano1 is involved in proliferation of these cells (Stanich et al. 2011). This observation was supported by a greater than 2‐fold reduction in Kit transcripts in the antrums and intestines of Ano1−/− compared to Ano1+/− mice. Therefore, we sought to determine whether there was a reduction in ICC populations in iAno1−/− compared to iAno1+/− animals. Double labelling with Ano1 and Kit antibodies was performed to determine whether the loss of Ano1 was associated with reduced Kit+ ICC. Immunohistochemical analysis showed that reduced Ano1 in iAno1−/− animals (Fig. 4 D and E and Fig. 4 J and K for antrum and intestine of iAno1−/− animals, respectively) did not lead to reduced Kit+ ICC. Immunohistochemical analysis of antrums and intestines on Ano1−/− and age‐matched Ano1+/+ controls was also performed to compare expression levels of tamoxifen‐treated animals with global deletion of Ano1 and check the specificity of the Ano1 antibody used for immunohistochemistry. Ano1 immunoreactivity was not detectable in antrums and small intestines of Ano1−/− mice (Fig. 5 E and K), but robust labelling was observed in all Ano1+/+ age‐matched siblings (Fig. 5 B and H), confirming the specificity of the antibody. Further, as in iAno1−/− mutants, there were no significant differences in the distribution and density of Kit+ ICC in the antrums and small intestines of Ano1−/− mutants (Fig. 5 D and J) compared to (+/+) controls (Fig. 5 A and G). These data show that although Ano1 was reduced below levels of immunodetection, ICC were not reduced in the antrum or small intestine of iAno1−/− animals.
Figure 5. Loss of Ano1 but normal Kit+ ICC networks in constitutive Ano1−/− mutants.
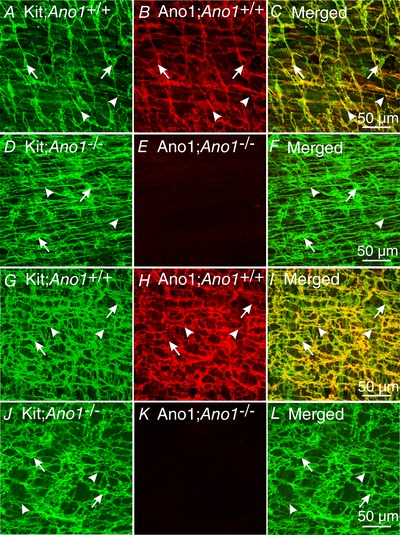
A, Kit+ ICC‐MY (green; arrows) and ICC‐IM (arrowheads) networks in the gastric antrum of a P3 (+/+) control express Ano1 (B; red). C is a merged image of A and B showing cellular co‐localization of Kit and Ano1 (yellow). D–F show normal Kit+ ICC networks (D; green, ICC‐MY‐arrows and ICC‐IM‐arrowheads) but an absence of Ano1 in global Ano1−/− mutants (E). F, merged image shows only Kit+ ICC‐MY and ICC‐IM. G–I, robust Kit+ ICC (G; green, ICC‐MY‐arrows; ICC‐DMP, arrowheads) and Ano1 expression (H; red) in the small intestine of an Ano1+/+ control. I shows merged image and cellular co‐localization of Kit and Ano1 (yellow). J–L, as in the antrum, Ano1−/− mutants showed normal Kit+ ICC networks (J; green, ICC‐MY‐arrows; ICC‐DMP, arrowheads), but a loss of Ano1 expression (K). L, merged image shows only Kit+ ICC. Scale bars in panels C, F, I and L = 50 μm and and apply to other panels.
Reduced Ano1 expression in ICCs delayed gastric and intestinal transit times
Global Ano1−/− mice display 90% lethality within 9 days after birth (Rock et al. 2008). Thus, it has not been possible to perform studies of gastrointestinal transit on these animals. We sought to determine whether adult iAno1−/− mice had reduced GI transit in comparison to iAno1+/− animals. GI transit times were measured for each mouse before and after administration of tamoxifen. Carmine red dye was delivered via oral gavage. iAno1−/− animals demonstrated an average of 112.3 ± 16.8 min increase in total GI transit time after tamoxifen treatment while iAno1+/− animals experienced no change in GI transit following tamoxifen treatment (Fig. 6 A and D; P = 0.001).
Figure 6. Reduced Ano1 expression delayed gastric emptying and total intestinal transit time in vivo .
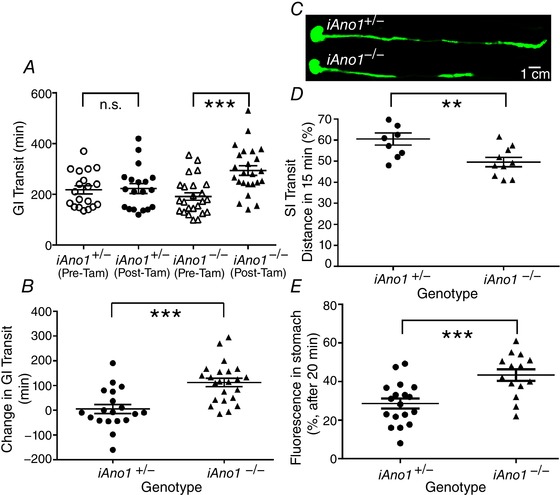
A, total GI transit time was measured using carmine dye solution (100 μl) delivered by oral gavage. The GI transit of carmine red was measured for each mouse before excretion of the first red‐stained fecal pellet was recorded as total GI transit under control conditions and repeated after administration of tamoxifen to induce Cre. No significant (n.s.) difference in total GI transit time was observed in iAno1+/− animals but was observed in iAno1−/− mice. B, the change in GI transit time was calculated by subtracting the baseline transit time prior to tamoxifen administration from the transit time after treating animals with tamoxifen. iAno1−/− mice experienced an increase in total transit time of approximately 120 min after tamoxifen treatment, while the iAno1+/− mice showed no change after tamoxifen. C, gastric emptying and small intestine (SI) transit in iAno1+/− (upper panel) and iAno1−/− (lower panel) mice were measured using indocyanine green fluorescent dye (ICG; 100 μl) delivered via oral gavage. Mice were killed after 15 min or 1 h. The digestive tracts were dissected and imaged using an Odyssey imaging system. Representative images of the dissected GI tracts showing the decrease in relative distance travelled by the fluorescent dye after 15 min in iAno1+/− versus iAno1−/− mice. These data are summarized in D. E, fluorescence remaining in stomach after 20 min. Mice were administered rhodamine‐dextran fluorescent tracer (100 μl) by oral gavage and killed after 20 min. The fraction of fluorescence remaining in the stomach relative to the total fluorescence recovered from the stomach and small intestine is shown. iAno1−/− mice showed an increased percentage of fluorescence remaining in the stomach (43.4 ± 3.0%) relative to iAno1+/− controls (28.7 ± 2.6%; P = 0.008). (n.s. = not significant; ** P<0.01; *** P<0.001). [Color figure can be viewed at wileyonlinelibrary.com]
To determine if the increased transit time in iAno1−/− mice was a consequence of delayed gastric emptying or decreased small intestinal transit we further assessed in vivo gastric emptying and small intestine transit in tamoxifen‐treated mice using indocyanine green fluorescent dye (ICG). ICG was administered by oral gavage and fluorescence in the upper GI tract was imaged 15 min and 1 h after ICG administration. The length of intestine with ICG present after 15 min was reduced significantly in iAno1−/− mice (49.8 ± 2.2%, compared to 60.5 ± 2.8% in iAno1+/− animals; n = 9; P = 0.007). After 1 h, the intestinal length occupied by ICG was still significantly reduced in iAno1−/− mice (43.4 ± 1.62%; n = 9, compared to 49.04 ± 1.3% in iAno1+/− animals; n = 8; P = 0.019). There was also a shift in the geometric centre of the fluorescence distribution toward the more proximal small intestine in iAno1−/− from 3.145 to 2.304 compared to iAno1+/− mice (n = 18, P = 0.0253). These data suggest that decreased GI transit occurring in iAno1−/− was a consequence of delayed gastric emptying or small intestine transit.
Loss of Ano1 causes delayed gastric emptying
A significant number of iAno1−/− mice had distended stomachs. Therefore, to quantify gastric function specifically evaluations of gastric emptying were performed using an indigestible rhodamine B‐conjugated dextran tracer. Overnight‐fasted mice were administered rhodamine‐dextran by oral gavage and killed after 20 min. The percentage of total fluorescence remaining in the stomach was significantly greater in the iAno1−/− (43.4 ± 3.0%) relative to iAno1+/− mice (28.7 ± 2.6%; P = 0.008; Fig. 6 E).
Phasic contractions are disrupted in gastric antrums but not in intestines of iAno1−/− animals
Since there was a significant delay in gastric emptying and intestinal transit times in iAno1−/− animals compared to iAno1+/− siblings, we sought to determine if there was a difference in the phasic contractile activities of muscles from the gastric antrums and intestines. A comparison of the contractile activity in the gastric antrums of iAno1+/− and iAno1−/− animals revealed significant differences in both amplitudes and patterns of phasic contractile activities. Contractions of the circular layer of the gastric antrums of iAno1+/− animals averaged 0.61 ± 0.07 mN in amplitude and occurred at a frequency of 3.6 ± 0.5 cycles min−1 (n = 11; Fig. 7). Contractile force of antral muscles from iAno1−/− animals averaged 0.3 ± 0.08 mN (P = 0.01 compared to iAno1+/− muscles; n = 14). Although the average frequency of contractile activity was not statistically different (3.2 ± 0.6 versus 3.6 ± 0.5 cycles min−1; P = 0.57), there was a marked difference in the regularity of contractions. Gastric muscles from iAno1+/− animals generated regular phasic contractions but muscles from iAno1−/− animals were highly irregular in both frequency and amplitude (Fig. 7). Isometric force measurements of the circular layer of the intestines from iAno1+/− mice displayed regular phasic contractions with an amplitude of 1.15 ± 0.17 mN and frequency of 38.3 ± 1.1 cycles min−1 (n = 11; Fig. 7). Intestines from iAno1−/− animals also generated regular phasic contractions averaging 0.97 ± 0.12 mN and occurred at a frequency of 37.2 ± 0.12 cycles min−1 (n = 14; Fig. 7). There was no statistical difference in amplitude or frequency of intestinal contractions between tamoxifen‐treated iAno1+/− and iAno1−/− animals (P > 0.05 for both parameters).
Figure 7. Phasic contractile activity is disrupted in the antrums of iAno1−/− mice but is normal in the small intestines.
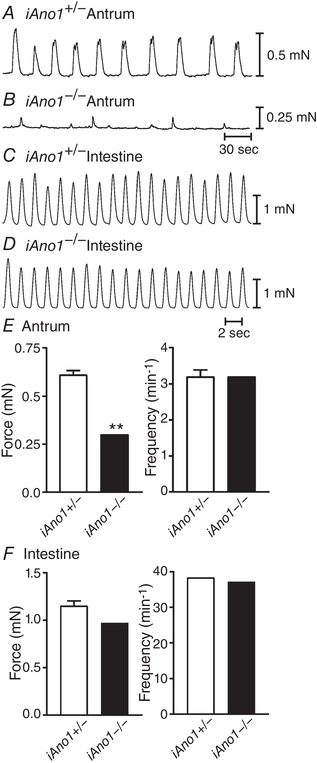
A and B, spontaneous contractile activity of the circular muscle layer from the gastric antrums of iAno1+/− controls and iAno1−/− mutants, respectively. C and D, phasic contractions from the circular muscle layer of the small intestines of iAno1+/− controls and iAno1−/− mutants. Note there was little or no difference in phasic contractions in the intestines but a marked difference in the antrums. E, summarized data of the differences in contractile force and frequency between iAno1+/− mice (white bars) and iAno1−/− mutants (black bars) in the gastric antrum. There was a significant difference in the contractile force that was generated in the gastric antrum. Although there was no statistical difference in the overall frequency of contractile activity, the phasic contractions in iAno1+/− controls were regular, whereas the activity observed in iAno1−/− mutants was highly irregular. F, summarized data of force and frequency from the intestines of iAno1+/− controls (white bars) and iAno1−/− mutants (black bars). There were no statistical differences noted in the small intestines. ** P<0.01 for E.
Electrical activities underlying phasic contractile activity in gastric antrums and intestines of iAno1−/− and iAno1+/− mice
Mice homozygous for the null allele of Ano1 do not generate electrical pacemaker activity in the gastric antrums or small intestines (Hwang et al. 2009; Singh et al. 2014) whereas heterozygous Ano1+/− mice have normal slow waves in both organs (Hwang et al. 2009). We sought to determine whether Cre induction caused a significant reduction of Ano1 to levels where slow wave activity was no longer supported in the stomachs or intestines by performing intracellular microelectrode recordings from intestinal and antral tissues from tamoxifen‐treated iAno1+/− and iAno1−/− mice.
Intestinal muscles of iAno1+/− animals displayed RMPs averaging −65 ± 1.0 mV and electrical slow waves 25.0 ± 2.0 mV in amplitude and 0.54 ± 0.04 s in half‐maximal duration occurred at a frequency of 35.8 ± 0.31 cycles min−1 in nifedipine (n = 6). Intestinal muscles of iAno1−/− mice had RMPs averaging −62 ± 0.3 mV, and slow wave activity was present in 22 of 23 animals examined. There was slight variability in amplitude and frequency in intestinal pacemaker activity of iAno1−/−, compared with iAno1+/− muscles, but this difference did not reach a level of statistical significance (Fig. 8). Intestinal slow waves of iAno1−/− mice averaged 25.0 ± 2.0 mV in amplitude, occurred at a frequency of 35.0 ± 1.7 cycles min−1 and had a half‐maximal duration of 0.5 ± 0.03. RMPs and slow wave parameters were not significantly different from tamoxifen‐treated iAno1−/− and iAno1+/− littermates (Fig. 8; P > 0.05 for all parameters).
Figure 8. Normal electrical slow waves in the small intestines of iAno1−/− animals.
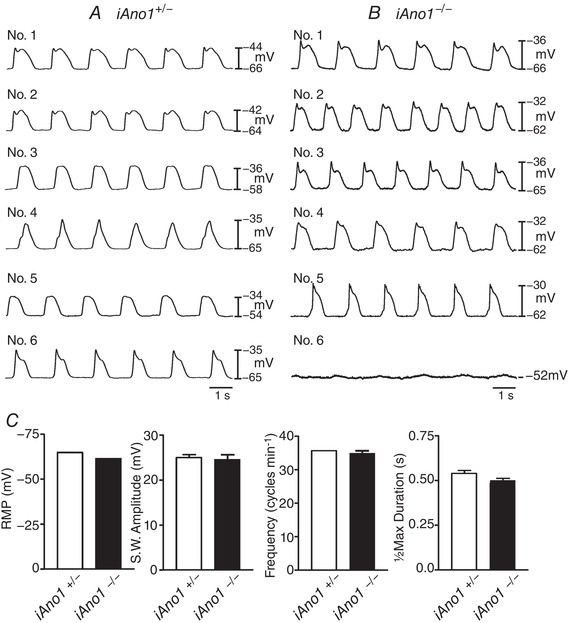
A and B, representative microelectrode recordings from the intestines of 6 different iAno1+/− controls (A; Nos 1–6) and 6 iAno1−/− animals (B; Nos. 1–6). All but one iAno1−/− animal, displayed robust slow waves of similar amplitude, duration and frequency to that recorded from iAno1+/− controls. Only one iAno1−/− intestine (No. 6 of 23 animals) did not display slow waves, typical of that observed in all other Ano1−/− animals. C, summarized data of resting membrane potential (RMP), slow wave amplitude (mV), slow wave frequency (cycles min−1) and half‐maximal duration (s) of slow waves. There was no statistical difference between iAno1+/− controls (white bars) and iAno1−/− animals (black bars) for any electrical parameters.
Since the decrease in GI transit in iAno1−/− animals may likely have been a consequence of delayed gastric emptying (see Fig. 6) we examined pacemaker activity in tamoxifen‐treated iAno1−/− animals and their iAno1+/− littermates. Circular muscles of the gastric antrum of tamoxifen‐treated iAno1+/− mice had resting membrane potentials (RMPs) averaging −64.0 ± 3.0 mV and regularly occurring slow waves 23.0 ± 3.0 mV in amplitude and 3.1 ± 0.23 s in half‐maximal duration occurred at a frequency of 4.3 ± 0.6 cycles min−1 (Fig. 9). In comparison, the gastric antrums of iAno1−/− animals had RMPs averaging −56.0 ± 1.0 mV and slow waves were absent (Fig. 9; n = 23; in presence of nifedipine). RMPs and slow wave parameters were significantly different from tamoxifen‐treated iAno1−/− and iAno1+/− littermates (P = 0.01 for RMP and all slow wave parameters).
Figure 9. Gastric antrum slow waves are absent in iAno1−/− animals.
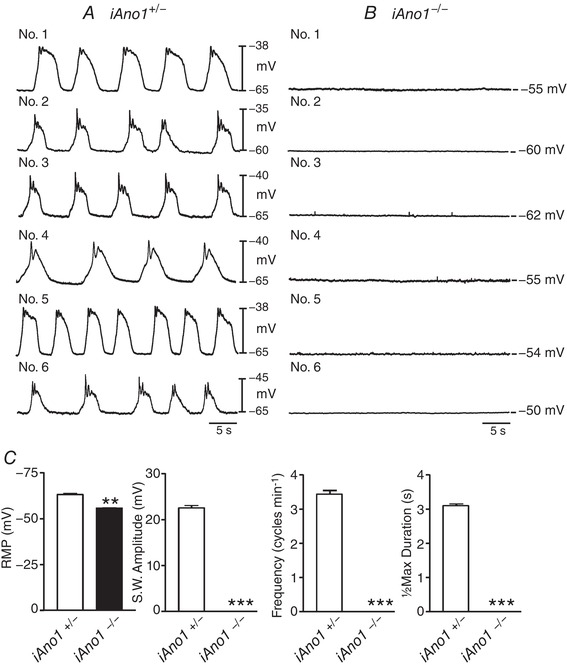
A and B, typical traces of antral slow waves from 6 iAno1+/− controls (A; Nos 1–6) and 6 iAno1−/− animals (B; Nos 1–6). A, regular pacemaker slow waves recorded from the gastric antrums of iAno1+/− controls. Slow waves were present in the gastric antrums of all animals. B, in comparison, the resting membrane potential (RMP) of iAno1−/− animals was more depolarized and slow waves of were absent in all tissues. C, summarized data of RMP, slow wave amplitude (mV), slow wave frequency (cycles min−1) and half‐maximal duration (s) of slow waves from iAno1+/− mice (white bars) and iAno1−/− mutants (black bar). Experiments were performed in the presence of the L‐type calcium channel blocker nifedipine (1 μM). ** P<0.01; *** P<0.001.
There was a marked difference in the regularity of antral phasic contractions in iAno1+/− and iAno1−/− animals, and we sought to determine the mechanism(s) underlying the differences in activity. Slow waves in the small intestine and gastric antrum are not affected significantly by the L‐type calcium channel antagonist nifedipine (Ward et al. 1994; Suzuki & Hirst, 1999), but action potentials generated by smooth muscle cells are inhibited by this compound (Ward et al. 1995). We performed a series of experiments to examine the electrical activities of the gastric antrums of tamoxifen‐treated iAno1−/− and iAno1+/− animals in the absence of nifedipine to unmask electrical events underlying the irregular phasic contractile activity. RMP and slow wave activity of antral muscles from iAno1+/− animals was similar in amplitude and frequency with and without nifedipine (P > 0.05 for all parameters). In the absence of nifedipine, RMP of antral muscles from iAno1−/− was more depolarized than in iAno1+/− animals (−52 ± 1.0 mV; P = 0004), and under these conditions spike complexes, irregular in frequency, occurred in iAno1−/− muscles (Fig. 10). Spike complexes, which are highly irregular for antral muscles, were recorded in 8 of 8 muscles from iAno1−/− animals. These events were variable in amplitude and duration. Nifedipine (1 μM) blocked this activity in all but one antral muscle, suggesting that the spike complexes were generated via activation of L‐type Ca2+ channels in smooth muscle cells. To further address whether spike complexes with superimposed action potentials was a consequence of the irregular contractile patterns, simultaneous intracellular electrical and mechanical recordings were performed and revealed that spike complexes generated by antral muscles of iAno1−/− animals were associated with irregular phasic contractions that varied in amplitude, frequency and duration (Fig. 11). In comparison, slow waves of antral muscles from iAno1+/− animals were associated with regular phasic contractions of similar amplitudes of the circular layer as previously reported (Fig. 11).
Figure 10. Irregular spike complexes replace electrical slow waves in the gastric antrums of iAno1−/− animals.
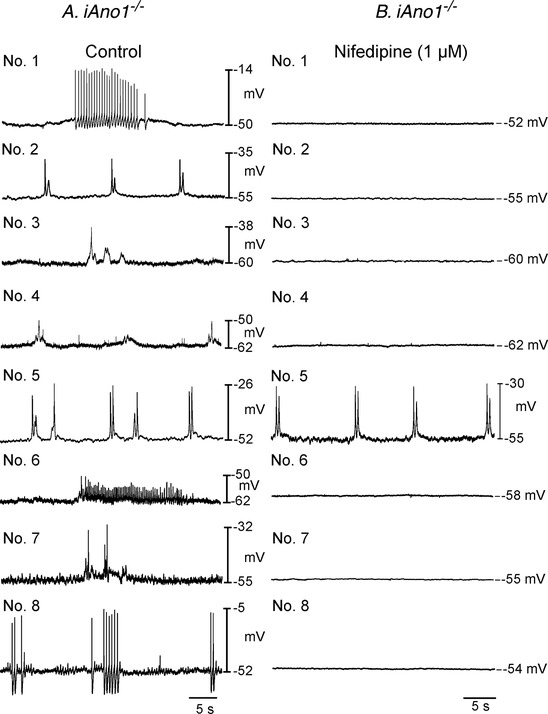
Electrical recordings from eight different iAno1−/− animals before (Control; lefthand panels; Nos 1–8) and after the L‐type calcium channel antagonist nifedipine (1 μM; righthand panels; Nos 1–8). Irregular spike complexes of varying amplitudes, frequencies and durations were recorded from the antrums of iAno1−/− animals under control conditions. All but one of these complexes (animal No. 5) were inhibited by the L‐type calcium channel antagonist nifedipine (1 μM).
Figure 11. Uncoordinated spike complexes in the antrums of iAno1−/− animals lead to irregular phasic contractions of the circular muscle layer compared to iAno1+/− controls.

A, simultaneous intracellular microelectrode recordings and isometric force measurements from tamoxifen‐treated iAno1+/− controls display regular slow waves with associated phasic contractions. Each slow wave (upper trace) generated large amplitude regular phasic contractions (lower trace). B, antrums of treated iAno1−/− animals generated irregular spike complexes (upper trace) that led to dis‐coordinated contractile activity (lower trace). Irregular spike complexes are associated with small irregular phasic contractions of the circular layer. C, slow wave and associated contraction identified in A by dashed box and shown at a faster sweep speed. D, spike complex and associated contraction identified in B by dashed box, shown at a higher resolution.
Spatiotemporal mapping of gastric motility patterns
The consequences of reduction in generation and/or propagation of slow waves in antral muscles of iAno1−/− mice were also examined in intact stomachs by analysis of video images and generation of spatiotemporal maps (STMaps), as previously described (Hennig et al. 1999). Stomachs of iAno1+/− mice generated normal slow wave activity (as in Fig. 9) and exhibited propagating antral contractions of similar amplitudes and velocities from event to event. In comparison, stomachs of iAno1−/− animals exhibited contractions of varying amplitudes and propagation velocities, as shown in STMaps (Fig. 12). Analysis of STMaps showed that the gastric antrums of iAno1−/− mice exhibited greater periods between propagating contractions than was observed in iAno1+/− stomachs. Differences between iAno1−/− and iAno1+/− gastric motor activities are tabulated in Fig. 12 H–J. These observations suggest that Ano1 currents and slow waves generated by ICC provide a critical level of coordination in gastric muscles that permits oral to aboral propagation of peristaltic contractions.
Figure 12. Disrupted propagating gastric contractions in iAno1−/− mutants.
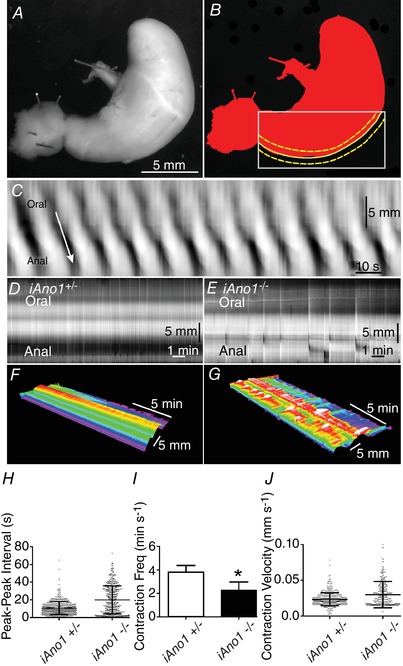
Spatiotemporal maps (STMaps) used to quantify gastric motility patterns. A, video analysis was performed on whole stomachs of tamoxifen‐treated iAno1+/− and iAno1−/− animals using bright‐field imaging at 7.5 frames s−1 (FPS) for 15 min. B, propagating gastric contractions (between dashed yellow lines) were evaluated from videos using a curved edge‐tracking along the greater curvature of each stomach (dashed yellow lines within grey box) and analysed using volumetry (GWH v.8a). C, grey scale‐coded pixels from each frame of the movie sequence were used to construct a single row and sequential rows, producing STMaps. Time is on the x‐axis and distance along the greater curvature on the y‐axis. Propagating contractile waves (dark bands), from a iAno1+/− mice, displaying regular orally to anally propagating contractions (white arrow). D and E, STMaps illustrate the differences in gastric motility behaviour between iAno1+/− controls and iAno1−/− animals. D, regular propagating gastric contractions of an iAno1+/− control. E, irregular, dis‐coordinated gastric contractions of an iAno1−/− animal. F and G, colour 3D STMaps show the coordinated contractile activity of an iAno1+/− control (F) in comparison to the dysrhythmic uncoordinated contractility of an iAno1−/− animal (G). H–J, summary of gastric STMaps reveal coordinated propagating gastric contractions of iAno1+/− controls compared to disrupted motor activity of iAno1−/− animals. H, shows that iAno1−/− animals had greater degree of variability in contractile activity. I shows there was a reduction in frequency of antral contractions in iAno1−/− animals. J shows that there was an increase in the velocity of contractions in iAno1−/− animals compared to iAno1+/− controls. * P<0.05.
Resistance of pacemaker activity in iAno1−/− small intestines versus gastric antrums
As stated above, juvenile mice homozygous null for Ano1 do not generate slow waves in the gastric antrum or small intestine (Hwang et al. 2009; Singh et al. 2014). However, these animals die early in life and the present study suggests that there may developmental changes in the reliance on Ano1 in the small intestine as the animals age. Such a change might account for the persistence of slow waves in the small intestines of iAno1−/− mice. Therefore, we examined the effects of CaCCinh‐Ano1, an Ano1 antagonist, on slow waves in the gastric antrum and small intestine of juvenile animals between postnatal day (P) 9 and P11. RMP of intestinal circular muscle cells of these animals averaged −66 ± 1.8 mV and slow waves 23.3 ± 0.7 mV in amplitude, occurred at a frequency of 26 ± 1.0 cycles min−1. The half‐maximal duration of slow waves was 0.96 ± 0.04 s and the maximal rate of rise averaged 141 ± 21 mV s−1 (Fig. 13; n = 6). Slow wave parameters from juvenile animals were all significantly smaller than recorded from small intestinal muscles of adult animals (Hwang et al. 2016). CaCCinh‐Ano1 (1‐30 μM), dose dependently inhibited slow waves. Amplitude, half‐maximal duration and maximal rate of rise were the most sensitive, and significant inhibition was noted at 1–3 μM. Significant differences for the other parameters tabulated was observed at 5–10 μM (Fig. 13). In comparison, intestinal slow waves in adult muscles were resistant to CaCCinh‐Ano1 up to approximately 30 μM (cf. Fig. 13 with Fig. 7 in Hwang et al. 2016).
Figure 13. Effects of CaCCinh‐A01 on intestinal slow waves from juvenile mice.

A, concentration‐dependent effects of CaCCinh‐A01 on intestinal slow waves from P9–P11animals. B–E, effects of CaCCinh‐A01 on slow wave amplitude, frequency, half‐maximal duration and maximal rate of rise. B, reduction in slow wave amplitude (mV) reached statistical significance at 1 μM. C, reduction in slow wave frequency (cycles min−1) reached statistical significance at 5 μM. D, half‐maximal duration of slow wave duration (s) was reduced at 5 μM. E, statistically significant reduction in the maximal rate of rise of the slow wave upstroke (dV/dt; mV s−1) was reached at 3 μM CaCCinh‐A01. Data shown in B–E are means ± SEM; n = 6. * P<0.05; ** P<0.01; *** P<0.001; one‐way ANOVA. Juvenile small intestinal muscles are much more sensitive to the Ano1 antagonist than adult muscles (Hwang et al. 2016).
We also sought to determine if there is a difference in the sensitivity to the Ano1 antagonist in gastric antrums. In the gastric antrum of juvenile animals RMP averaged −71.6 ± 3.0 mV and slow waves, 32 ± 2 mV in amplitude, occurred at a frequency of 2.5 ± 0.2 cycles min−1. The half‐maximal duration of gastric slow waves was 6.1 ± 0.2 s and the maximal rate of rise averaged 38 ± 11 mV s−1 (n = 5). Slow waves in adult and juvenile muscles were essentially similar, but slow wave frequency was slower (Fig. 14; cf. Fig. 1 in Hwang et al. 2016). Gastric slow waves of juvenile animals were slightly more sensitive to CaCCinh‐Ano1 than in adult muscles, but the differences were far less than observed in the small intestine. For example, slow wave frequency was significantly reduced at 1 μM, amplitude and maximal rate of rise at 3 μM, whereas these parameters required 3 μM, 5 μM and 5 μM respectively in adult tissues (n = 5; Fig. 14; cf. Fig. 1 in Hwang et al. 2016). RMP of juvenile gastric tissues were significantly more depolarized by CaCCinh‐Ano1 at 3 μM (i.e. from −71.6 ± 3.0 mV to −67.6 ± 2.7 mV; P = 0.003), this depolarization was similar to that observed in adult tissues at the same concentration.
Figure 14. Reduction in gastric antrum slow waves from juvenile mice by CaCCinh‐A01.

A, dose‐dependent effects of CaCCinh‐A01 on gastric slow waves from juvenile animals (P9–P11). B, reduction in slow wave amplitude (mV) reached statistical significance at 3 μM. C, reduction in slow wave frequency (cycles min−1) reached statistical significance at 1 μM. D, half‐maximal duration of slow wave duration (s) was reduced at 1 μM. E, reduction in the maximal rate of rise of the slow wave upstroke (dV/dt; mV s−1) reached statistical significance at 3 μM CaCCinh‐A01. Data shown in B–E are means ± SEM; n = 5. * P<0.05; ** P<0.01; *** P<0.001; one‐way ANOVA.
Non‐selective cation conductance (NSCC) contributes to pacemaker currents in adult intestinal ICC
ICC from juvenile mouse intestines (P7–P12) generated inward currents that reversed in accordance with E Cl (Zhu et al. 2009). In this study, we isolated jejunal ICC from adult Kit+/copGFP mice (P30–P40), as previously described (Zhu et al. 2009), to determine whether a conductance other than a Cl− contributes to pacemaker activity in cells from adult animals. ICC were voltage clamped using the whole‐cell configuration with cell dialysis, and E Cl was set at −40 mV (see Methods). When ICC were held at −95 mV large spontaneous transient inward currents (STICs) averaging −151 ± 22 pA and 1.0 ± 0.1 ms in duration occurred (Fig. 15 Aa and Ba; n = 9). When cells were stepped, and held at −55 mV, the amplitude and frequency of the STICs was reduced (Fig. 15Ab). Changing the holding potential to −35 mV, the amplitude and frequency of STICs was further reduced, and the currents were biphasic, consisting of small outward currents followed by larger inward currents (Fig. 15 Ac). Replacement of extracellular Na+ ([Na+]o) with equimolar NMDG, abolished the inward currents at −35 mV (Fig. 15 Bb), suggesting the inward current component of the spontaneous currents at −35 mV was carried by a non‐selective cation conductance (NSCC). The effects of replacing [Na+]o with an impermeant cation are summarized in Fig. 15 C and D).
Figure 15. Cation conductance participates in spontaneous inward currents (STICs) in adult small intestine ICC.
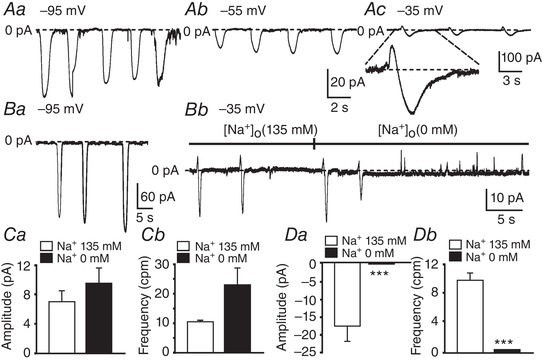
A, representative trace showing STICs at various holding potentials (Aa, −95 mV; Ab, −55 mV; Ac, −35 mV). EC l was adjusted to −40 mV in these experiments. At −95 mV and −55 mV, spontaneous inward currents were observed, but at −35 mV (Ac), two phase currents were observed consisting of a transient outward current that reversed to an inward current (dashed lines). One of these events is shown at higher resolution below Ac. B shows the effect of replacing [Na+]o with an impermeant cation (NMDG). External solution was CaPSS and EC l was set at −40 mV. Representative traces show spontaneous currents at −95 mV(Ba) and −35 mV (Bb). At −35 mV, inward currents and outward currents were observed (dashed line), replacement of [Na+]o (135 mM) with equimolar NMDG (i.e. [Na+]o = 0 mM) abolished the inward currents, suggesting a non‐selective cation conductance was involved in the generation of STICs (dashed line). Ca and Cb show summaries of the amplitude (pA) and frequency (cycles min−1, cpm) of outward currents in the presence of 135 mM (white bars) and 0 mM [Na+]o (black bars; n = 4. Da and Db show summaries of the amplitude (pA) and frequency (cycles min−1, cpm) of the inward current phase in the presence of 135 mM (white bars) and 0 mM [Na+]o (black bars; n = 4.
Discussion
In this study, mice with a KitCreERT2 allele (Klein et al. 2013) were crossed with Ano1f/f (Faria et al. 2014; Schreiber et al. 2015) to knockdown Ano1 (iAno1−/−) in ICC of adult mice and investigate the consequences of reduced Ano1 expression on the electrophysiological and motor behaviours of gastric and intestinal smooth muscles. The efficiency of recombination was tested with a Rosamtmg reporter (Sung et al. 2018), and found to be significant in gastric antral and intestinal ICC after induction of iCre by tamoxifen treatments. Analysis of Ano1 expression by qPCR demonstrated significantly reduced Ano1 transcripts in antral and small intestinal muscles of iAno1−/− animals (approx. −4‐fold) compared to iAno1+/− and wild‐type (+/+) muscles. Reduction in Ano1 transcripts was mirrored by reduced Ano1 protein, as evaluated by confocal immunohistochemistry. Ano1‐like immunoreactivity (Ano1‐LI) was reduced to a point where it was not resolved in ICC‐MY or ICC‐IM in the antrum and in ICC‐MY or ICC‐DMP in the small intestine. Reduced Ano1 expression in adult iAno1−/− animals was associated with delayed GI transit. However, the delay in total GI transit probably resulted from prolonged gastric emptying rather than a deficit in intestinal transit, as pacemaker activity and phasic contractions were markedly disrupted in the gastric antrum and much less so in intestinal muscles.
All classes of ICC in the stomach and small intestine (ICC‐MY and IC‐IM in the stomach and ICC‐MY and ICC‐DMP in the intestine) express Ano1; however, Ano1 is not resolved by immunohistochemistry in SMCs or enteric neurons in laboratory animals and humans (Gomez‐Pinilla et al. 2009; Hwang et al. 2009; Blair et al. 2012; Cobine et al. 2017). A role for Ano1 in slow waves was demonstrated using global Ano1−/− mice and recording electrical activity in juvenile animals (up to 23 days; this strain does not survive long after birth) (Rock et al. 2008; Hwang et al. 2009). The present study using iAno1−/− mice supported findings from global knockouts in gastric muscles, but only 1 of 23 animals displayed loss of slow waves in the small intestine. This difference was in spite of the observation that antral and small intestine exhibited similar reductions in Ano1 transcripts, as assessed by qPCR (−3.7 and −4.3‐fold reduction, respectively), and in protein by immunohistochemistry. The relatively lower sensitivity of intestinal muscles to Ano1 knockdown is mirrored by results obtained using pharmacological antagonists of Ano1 (Hwang et al. 2009; 2016) and by a previous study that also reported incomplete loss of slow waves in intestinal muscles of mice with inducible knockdown of Ano1−/− (Malysz et al. 2017). It should also be noted that gastric muscles of non‐human primates are also significantly more sensitive to CaCC blockers, such as niflumic acid, DIDS and NPPB, than intestinal muscles (Hwang et al. 2009; 2016). In an earlier study, we suggested that the difference in sensitivity to Ano1 antagonists in gastric and small intestinal muscles might be due to differences in Ano1 splice variants or differences in channel sensitivity to antagonists resulting from differences in intracellular Ca2+ (Sung et al. 2016). However, the current study showed that knockdown of Ano1 was equivalent in intestinal and gastric muscles, yet slow waves were retained in small intestinal muscles but largely absent in the stomach. Comparison of the sensitivities of slow waves in juvenile (this study) and adult (Hwang et al. 2016) small intestinal muscles to an Ano1 antagonist suggested that the dominance of Ano1, as the pacemaker conductance, decreases with age.
Another explanation for the lower sensitivity of small intestinal muscles to Ano1 deactivation is that ICC in this organ have a high safety factor for maintenance of slow wave activity even when Ano1 availability is low. Our current concept of the slow wave mechanism is that Ano1 channels are both a primary and a secondary conductance in the generation and propagation of slow waves. These channels have a primary function in that spontaneous release of Ca2+ in ICC causes STICs due to activation of Ano1 channels (Zhu et al. 2015). These inward currents and subsequent depolarization activate voltage‐dependent Ca2+ channels (T‐type channels; Zheng et al. 2014; Drumm et al. 2017), and this conductance is responsible for active propagation within ICC networks (Drumm et al. 2017). Ca2+ entry via T‐type Ca2+ channels initiates Ca2+‐induced Ca2+ release that further activates Ano1 channels in ICC and generates the sustained, plateau phase of slow waves (Drumm et al. 2017). The plateau potential of slow waves is the secondary role of Ano1 channels. Differences in the safety factor for slow waves could result from structural and functional differences between gastric and small intestinal ICC, such as differences in: (i) Ca2+ release mechanisms and extent of plasma membrane‐endoplasmic reticulum (PM‐ER) junctions; (ii) density of Ano1 channel localization in PM‐ER junctions; (iii) density and localization of Ca2+ entry mechanisms within PM‐ER junctions and (iv) density of ICC in pacemaker networks. Differences in safety factor and potential differences in the pacemaker and Ca2+ handling mechanisms in different types of ICC will require further investigation.
The reversal potentials of whole‐cell spontaneous inward currents in juvenile small intestinal ICC and blockade of CaCC antagonists suggested that the primary pacemaker conductance in ICC was due to a Cl− current (Zhu et al. 2009). Intestinal ICC also displayed channel activity consistent with the properties of Ano1 channels expressed in HEK293 cells (Yang et al. 2008; Zhu et al. 2009). However, in the current study spontaneous inward currents recorded from adult small intestinal ICC contained a current component that reversed at E Cl and another that remained inward and was blocked by replacement of [Na+]o with an impermeant cation. Taken together, previous and current data are consistent with the idea that juvenile ICC utilize Ano1 as the primary pacemaker conductance. The central importance of this conductance for pacemaker activity is retained in the adult gastric antrum, but an additional cation conductance, possibly an NSCC, develops in small intestinal ICC and can sustain slow wave activity when Ano1 is blocked or genetically deactivated.
Involvement of two conductances in slow waves in adult mice might explain previous confounding reports in the literature in which the autonomous current in freshly dispersed intestinal ICC from adult mice (Goto et al. 2004) and the conductance responsible for rhythmic inward currents and slow wave‐like activity in cultured ICC from the mouse small intestine (Koh et al. 1998) displayed properties of an NSCC. A recent study of cultured mouse small intestinal ICC also reported a lack of sensitivity of slow waves to Ano1 antagonists (Choi et al. 2018). Thus, it appears that development of a cation conductance may occur as a function of age in small intestinal pacemaker ICC, and this process may be accelerated or possibly replace Ano1 in cell culture conditions. The identity of the cation conductance is unknown, but deep sequencing of transcripts from adult cells identified expression of TRPM and TRPC family genes in small intestinal ICC (Lee et al. 2017). Additional experiments will be required to characterize the properties of the cation current in adult ICC, to learn how channels are gated during slow waves and to test the effects of knockouts of channel candidates on slow waves and small bowel motility behaviours.
To ensure maximal knockdown of Ano1, experiments in the current study were performed 50 days after the last tamoxifen treatment (Groneberg et al. 2011; Sung et al. 2018). Slow waves were disrupted in gastric antral muscles after tamoxifen, and this exposed atypical behaviour in smooth muscle cells. Tamoxifen‐treated iAno1−/− muscles generated irregular spike complexes of differing durations with action potentials of variable amplitudes. These spike complexes were associated with irregular contractions and were inhibited by the L‐type Ca2+ channel antagonist nifedipine. This type of activity was never observed in antral muscles of iAno1+/− mice. It is likely that the spike complexes and irregular contractions in iAno1−/− muscles were due to spontaneous activity of smooth muscle cells, and similar activity was also observed in small intestinal muscles of Sl/Sld mutant mice that suffer developmental defects in pacemaker ICC (Ward et al. 1995). Use of the Cre/LoxP approach can rule out developmental compensatory effects, but compensatory mechanisms may also develop in adult animals, and in the present study the excitability of SMCs appears to have increased when Ano1 expression decreased. The spike complex behaviour of muscles after tamoxifen treatment, however, was not capable of generating normal motility patterns, and gastric arrhythmias and delayed gastric emptying occurred in iAno1−/− animals.
Following induction of Cre and reduction in Ano1 transcripts we sought to determine if there was a reduction in Kit expression or disruption in ICC networks in the gastric antrums and small intestine of adults. Since ICC are a component of the SIP syncytium, that includes smooth muscle cells (SMCs), ICC and PDGFRα+ cells and regulates motility patterns of the GI tract (Sanders et al. 2012, 2014), we also sought to determine if SMCs and PDGFRα+ cells were also affected by reduction in Ano1 in ICC. Although Kit transcripts were reduced by 2‐fold in the antrums following tamoxifen in iAno1−/− animals in comparison to tamoxifen‐treated iAno1+/− and (+/+) controls, the number of Kit+ ICC was similar in these animals. A greater than 3‐fold reduction in Kit was observed in the small intestines of tamoxifen‐treated iAno1−/− mice in comparison to iAno1+/− animals and a 2.8‐fold reduction was observed in comparison to (+/+) animals. Since the numbers and labelling of immunoreactive ICC were not significantly affected in iAno1−/− mice this would suggest that translation from Kit transcripts to Kit protein is non‐linear. Transcripts for Myh11, a biomarker for smooth muscle cells, were only slightly decreased in the gastric antrums of iAno1−/− mice in comparison to iAno1+/− controls and unchanged in the small intestine. Pdgfra transcripts slightly increased in iAno1−/− mice, compared to iAno1+/− mice in both antrum and intestine. These findings differ from observations made in studies of the murine gastric fundus. Reduction of Ano1 transcripts was associated with reduction in transcripts representing the cells of the SIP syncytium, i.e. Kit, Myh11 and Pdgfra (Sung et al. 2018). These data suggest that the interdependence between cells within the SIP syncytium, as found in gastric fundus, is not equivalent in other regions of the GI tract. The differences may be a consequence of the different classes of ICC in the different organs. The mouse gastric fundus contains only one class of ICC, i.e. ICC‐IM, where the antrum and small intestine possess at least two classes, ICC‐MY and ICC‐IM in the antrum and ICC‐MY and ICC‐DMP in the small intestine. It is possible that loss of Ano1 in either of these classes of ICC is not as detrimental to expression profiles of other cells in the SIP syncytium.
Constitutive Ano1−/− mice die within the first week of life (Rock et al. 2008), so experiments to test the function of Ano1 and slow waves in GI motility could not be performed in vivo. In the present study, reducing Ano1 expression in ICC of adult mice and small intestine increased GI transit time approximately 2‐fold, as measured using carmine red dye suspended in methylcellulose. The observation that most iAno1 −/ − mice still generated slow waves in the small intestine and had regular phasic contractions of similar amplitude and frequency suggested that defects in total transit and small intestine transit were a result of delayed gastric emptying. The assays used to test small intestine transit efficiency and total transit time could not differentiate between effects associated specifically with gastric emptying or small intestine transit. Despite these complications, the experiments collectively demonstrated that slow waves generated by Ano1 regulate gastrointestinal motility in vivo and their loss leads to significant delay in gastric emptying and impairment in the transit of food along the gastrointestinal tract.
The effects of Ano1 reduction on gastric motor activity were evaluated by video analysis of whole‐stomach motor patterns. Spatiotemporal analysis of iAno1 +/ − animals revealed coordinated motor patterns in the proximal antrum and these contractions propagated toward the pyloric sphincter. iAno1 −/ − animals displayed uncoordinated contractile behaviour and propagating contractile waves that were intermittent and often collided with one another to produce a wide variability in waveforms and disrupted motor activity. These data show for the first time the importance of Ano1 in ICC for generating coordinating gastric motor patterns and efficacious gastric emptying.
Delayed gastric emptying is associated with nausea and vomiting in patients with both idiopathic and diabetic gastroparesis (Webb & Fogel, 1995; Camilleri, 1996). Both forms of gastroparesis have been associated with a loss of gastric and pyloric ICC (Grover et al. 2011; Bashashati & McCallum, 2015; Farrugia, 2015; Moraveji et al. 2016). It has also been shown that different splice variants of Ano1 exist in patients with diabetic gastroparesis and that these variants alter the electrophysiological properties of this ion channel, including sensitivity to intracellular Ca2+ and CaCC inhibitors (Mazzone et al. 2011; Strege et al. 2017). The data presented in the present paper show a direct correlation between Ano1 reduction in ICC and defects in propagating gastric contractions and delayed gastric emptying. The study also shows that delayed gastric emptying does not require loss of gastric ICC, and many loss‐of‐function mutations in key molecules involved in generation and propagation of slow waves might have a similar clinical signature to ICC loss.
In summary cell‐specific reduction of Ano1 in ICC using the Cre/LoxP approach, caused dramatic effects on gastric antral electrical and mechanical activities in adult mice. Defects included loss of rhythmic slow waves in most animals, abnormal clusters of nifedipine‐sensitive, action potential‐like electrical transients, irregular contractile activity, aberrant spread of contractions and delayed gastric emptying. A far less dramatic phenotype was observed in the small intestine, as previously reported (Malysz et al. 2017). Differences in sensitivity to reduced Ano1 in the stomach and small intestine mirror the differences observed in responsiveness to Ano1 antagonists (Hwang et al. 2009, 2016). These findings raise important questions about the generalized role of Ano1 in pacemaker activity in all organs of the GI tract. Our results suggest that an additional conductance may develop in ICC as a function of age and contribute inward currents that sustain slow waves when Ano1 channels are genetically deactivated or blocked by antagonists. The identity and gating mechanism of the age‐dependent conductance are unknown, so how this conductance contributes to pacemaker activity in small intestinal muscles is a topic for further investigation.
Additional information
Competing interests
None of the authors has any conflicts of interests.
Author contributions
S.J.H. performed sharp electrode experiments on whole muscles, analysed data and wrote drafts of the manuscipt. D.M.P and S.D.V. performed stomach and intestinal transit experiments, analysed data and wrote drafts of manuscript for that portion of the study. H.Z. performed patch clamp experiments, analysed data and wrote drafts of manuscript. Y.B. performed immunohistochemical studies. P.J.B., R.F.‐G., R.S.C., M.W. and M.B. planned and performed intracellular recordings and isometric force measurements. M.C.S. and G.W.H. performed whole‐stomach video recordings and spatio‐temporal analysis. J.R. provided strains of mice and helped in the analysis of data. K.M.S. planned and coordinated experiments and wrote and revised the manuscipt. S.M.W. planned genetic crosses, immunofluorescence and microelectrode experiments, analysed data, and wrote and revised the manuscipt. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by DK57236 to S.M.W. and by P01 DK41315 to K.M.S. and S.M.W. H.Z. was supported by COBRE P20GM130459. Confocal imaging was supported by an equipment grant from the NCRR for the Zeiss LSM510 confocal microscope (1 S10 RR16871). FACS sorting was supported by a COBRE P30 GM110767 Phase III grant.
Acknowledgements
The authors are grateful to: Matthew Wharton and Mathew Battersby for technical assistance, Drs Deiter Sauer (Technical University Munich, Germany) and Dr Mike Kotlikoff (Cornell University, Ithaca, NY, USA) for providing mice strains KitCreERT2Ejb1 /+ ( Kit‐Cre ) and B6.Cg‐Tg ( Myh11‐cre,eGFP ), respectively. Nancy Horowitz for the help in breeding animals, tamoxifen treatments and genotyping and Byoung H. Koh and David M. White for FACS sorting.
Biography
Sung Jin Hwang received his PhD from the Department of Biology in the Wonkwang University of South Korea in 2003. He was a post‐doctoral fellow at the University of Nevada, Reno School of Medicine from 2003 to 2008 and is currently a Research Assistant Professor of Physiology and Cell Biology. He has studied interstitial cells in pacemaking and their role in neuroeffector transmission in the gastrointestinal tract.

D. M. Pardo contributed equally to this study and should be considered co‐first author.
Edited by: Kim Barrett & David Grundy
References
- Bashashati M & McCallum RW (2015). Motility: Is ‘ICC‐opathy’ present in gastroparesis‐like syndrome? Nat Rev Gastroenterol Hepatol 12, 375–376. [DOI] [PubMed] [Google Scholar]
- Blair PJ, Bayguinov Y, Sanders KM & Ward SM (2012). Interstitial cells in the primate gastrointestinal tract. Cell Tissue Res 350, 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair PJ, Rhee PL, Sanders KM & Ward SM (2014). The significance of interstitial cells in neurogastroenterology. J Neurogastroenterol Motil 20, 294–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M (1996). Gastrointestinal problems in diabetes. Endocrinol Metab Clin North Am 25, 361–378. [DOI] [PubMed] [Google Scholar]
- Capasso R, Matias I, Lutz B, Borrelli F, Capasso F, Marsicano G, Mascolo N, Petrosino S, Monory K, Valenti M, Di Marzo V & Izzo AA (2005). Fatty acid amide hydrolase controls mouse intestinal motility in vivo. Gastroenterology 129, 941–951. [DOI] [PubMed] [Google Scholar]
- Chen H, Ordög T, Chen J, Young DL, Bardsley MR, Redelman D, Ward SM & Sanders KM (2007). Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine. Physiol Genomics 31, 492–509. [DOI] [PubMed] [Google Scholar]
- Choi S, Kang HG, Wu MJ, Jiao HY, Shin DH, Hong C & Jun JY (2018). Effects of Ca2+‐activated Cl− channel ANO1inhibitors on pacemaker activity in interstitial cells of Cajal. Cell Physiol Biochem 51, 2887–2899. [DOI] [PubMed] [Google Scholar]
- Cobine CA, Hannah EE, Zhu MH, Lyle HE, Rock JR, Sanders KM, Ward SM & Keef KD (2017). ANO1 in intramuscular interstitial cells of Cajal plays a key role in the generation of slow waves and tone in the internal anal sphincter. J Physiol 595, 2021–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lisle RC (2007). Altered transit and bacterial overgrowth in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol 293, G104–G111 [DOI] [PubMed] [Google Scholar]
- Drumm BT, Hennig GW, Battersby MJ, Cunningham EK, Sung TS, Ward SM, Sanders KM & Baker SA (2017). Clustering of Ca2+ transients in interstitial cells of Cajal defines slow wave duration. J Gen Physiol 149, 703–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria D, Rock JR, Romao AM, Schweda F, Bandulik S, Witzgall R, Schlatter E, Heitzmann D, Pavenstädt H, Herrmann E, Kunzelmann K & Schreiber R (2014). The calcium‐activated chloride channel Anoctamin 1 contributes to the regulation of renal function. Kidney Int 85, 1369–1381. [DOI] [PubMed] [Google Scholar]
- Farrugia G (2015). Histologic changes in diabetic gastroparesis. Gastroenterol Clin North Am 44, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Pinilla PJ, Gibbons SJ, Bardsley MR, Lorincz A, Pozo MJ, Pasricha PJ, Van de Rijn M, West RB, Sarr MG, Kendrick ML, Cima RR, Dozois EJ, Larson DW, Ordog T & Farrugia G (2009). Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol 296, G1370–G1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K, Matsuoka S & Noma A (2004). Two types of spontaneous depolarizations in the interstitial cells freshly prepared from the murine small intestine. J Physiol 559, 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneberg D, Konig P, Koesling D & Friebe A (2011). Nitric oxide‐sensitive guanylyl cyclase is dispensable for nitrergic signaling and gut motility in mouse intestinal smooth muscle. Gastroenterology 140, 1608–1617. [DOI] [PubMed] [Google Scholar]
- Grover M, Farrugia G, Lurken MS, Bernard CE, Faussone‐Pellegrini MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL, Ünalp‐Arida A, Nguyen L, Koch KL, Calles J, Lee L, Tonascia J, Hamilton FA & Pasricha PJ & NIDDK Gastroparesis Clinical Research Consortium (2011). Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology 140, 1575–1585.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig GW, Costa M, Chen BN & Brookes SJ (1999). Quantitative analysis of peristalsis in the guinea‐pig small intestine using spatio‐temporal maps. J Physiol 517, 575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Bramich NJ, Teramoto N, Suzuki h & Edwards FR (2002). Regenerative component of slow waves in the guinea‐pig gastric antrum involves a delayed increase in [Ca2+]i and Cl− channels. J Physiol 540, 907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Klüppel M, Malysz J, Mikkelsen HB & Bernstein A (1995). W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 373, 347–349. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Basma N, Sanders KM & Ward SM (2016). Effects of new‐generation inhibitors of the calcium‐activated chloride channel anoctamin 1 on slow waves in the gastrointestinal tract. Br J Pharmacol 173, 1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Blair PJ, Britton FC, O'Driscoll KE, Hennig G, Bayguinov YR, Rock JR, Harfe BD, Sanders KM & Ward SM (2009). Expression of anoctamin 1/TMEM16A by interstitial cells of Cajal is fundamental for slow wave activity in gastrointestinal muscles. J Physiol 587, 4887–4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Fukuta h & Suzuki h (2002). Components of pacemaker potentials recorded from the guinea pig stomach antrum. Pflugers Arch 445, 202–217. [DOI] [PubMed] [Google Scholar]
- Kito Y & Suzuki h (2003). Properties of pacemaker potentials recorded from myenteric interstitial cells of Cajal distributed in the mouse small intestine. J Physiol 553, 803–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Seidler B, Kettenberger A, Sibaev A, Rohn M, Feil R, Allescher HD, Vanderwinden JM, Hofmann F, Schemann M, Rad R, Storr MA, Schmid RM, Schneider G & Saur D (2013). Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow‐wave activity. Nat Commun 4, 1630. [DOI] [PubMed] [Google Scholar]
- Koh SD, Sanders KM & Ward SM (1998). Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol 513, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo J, Powell AE, Wang Y, Musser MA, Southard‐Smith EM, Franklin JL & Coffey RJ (2015). LRIG1 regulates ontogeny of smooth muscle‐derived subsets of interstitial cells of Cajal in mice. Gastroenterology 149, 407–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MY, Ha SE, Park C, Park PJ, Fuchs R, Wei L, Jorgensen BG, Redelman D, Ward SM, Sanders KM & Ro S (2017). Transcriptome of interstitial cells of Cajal reveals unique and selective gene signatures. PLoS One 12, e0176031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malysz J, Gibbons SJ, Saravanaperumal SA, Du P, Eisenman ST, Cao C, Oh U, Saur D, Klein S, Ordog T & Farrugia G (2017). Conditional genetic deletion of Ano1 in interstitial cells of Cajal impairs Ca2+ transients and slow waves in adult mouse small intestine. Am J Physiol Gastrointest Liver Physiol 312, G228–G245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone A, Bernard CE, Strege PR, Beyder A, Galietta LJ, Pasricha PJ, Rae JL, Parkman HP, Linden DR, Szurszewski JH, Ördög T, Gibbons SJ & Farrugia G (2011). Altered expression of Ano1 variants in human diabetic gastroparesis. J Biol Chem 286, 13393–13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Galligan JJ & Burks TF (1981). Accurate measurement of intestinal transit in the rat. J Pharmacol Methods 1981 6, 211–217. [DOI] [PubMed] [Google Scholar]
- Moraveji S, Bashashati M, Elhanafi S, Sunny J, Sarosiek I, Davis B, Torabi A & McCallum RW (2016). Depleted interstitial cells of Cajal and fibrosis in the pylorus: Novel features of gastroparesis. Neurogastroenterol Motil 28, 1048–1054. [DOI] [PubMed] [Google Scholar]
- Rock JR, Futtner CR & Harfe BD (2008). The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol 321, 141–149. [DOI] [PubMed] [Google Scholar]
- Sanders KM (1996). A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 111, 492–515. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Koh SD, Ro S & Ward SM (2012). Regulation of gastrointestinal motility–insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol 9, 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Ward SM & Koh SD (2014). Interstitial cells of Cajal: Regulators of smooth muscle function. Physiol Rev 94, 859–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda‐Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P & Cardona A (2012). Fiji: an open‐source platform for biological‐image analysis. Nat Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Faria D, Skryabin BV, Wanitchakool P, Rock JR & Kunzelmann K (2015). Anoctamins support calcium‐dependent chloride secretion by facilitating calcium signaling in adult mouse intestine. Pflugers Arch 467, 1203–1213. [DOI] [PubMed] [Google Scholar]
- Shaylor LA, Hwang SJ, Sanders KM & Ward SM (2016). Convergence of inhibitory neural inputs regulate motor activity in the murine and monkey stomach. Am J Physiol Gastrointest Liver Physiol 311, G838–G851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RD, Gibbons SJ, Saravanaperumal SA, Du P, Hennig GW, Eisenman ST, Mazzone A, Hayashi Y, Cao C, Stoltz GJ, Ordog T, Rock JR, Harfe BD, Szurszewski JH & Farrugia G (2014). Ano1, a Ca2+‐activated Cl− channel, coordinates contractility in mouse intestine by Ca2+ transient coordination between interstitial cells of Cajal. J Physiol 592, 4051–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanich JE, Gibbons SJ, Eisenman ST, Bardsley MR, Rock JR, Harfe BD, Ordog T & Farrugia G (2011). Ano1 as a regulator of proliferation. Am J Physiol Gastrointest Liver Physiol 301, G1044–G1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strege PR, Gibbons SJ, Mazzone A, Bernard CE, Beyder A & Farrugia G (2017). EAVK segment “c” sequence confers Ca2+‐dependent changes to the kinetics of full‐length human Ano1. Am J Physiol Gastrointest Liver Physiol 312, G572–G579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung TS, Hwang SJ, Koh SD, Bayguinov Y, Peri LE, Blair PJ, Webb TI, Pardo DM, Rock JR, Sanders KM & Ward SM (2018). The cells and conductance mediating cholinergic neurotransmission in the murine proximal stomach. J Physiol 596, 1549–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung TS, O'Driscoll K, Zheng H, Yapp NJ, Leblanc N, Koh SD & Sanders KM (2016). Influence of intracellular Ca2+ and alternative splicing on the pharmacological profile of ANO1 channels. Am J Physiol Cell Physiol 311, C437–C451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki h & Hirst GDS (1999). Regenerative potentials evoked in circular smooth muscle of the antral region of guinea‐pig stomach. J Physiol 517, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torihashi S, Ward SM, Nishikawa S, Nishi K, Kobayashi S & Sanders KM (1995). c‐kit‐dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res 280, 97–111. [DOI] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Harney SC & Sanders KM (1995). Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol Cell Physiol 269, C1577–C1585. [DOI] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S & Sanders KM (1994). Mutation of the proto‐oncogene c‐kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol 480, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb WW & Fogel RP (1995). Gastroparesis: current management. Compr Ther 21, 741–45. [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK & Oh U (2008). TMEM16A confers receptor‐activated calcium‐dependent chloride conductance. Nature 455, 1210–1215. [DOI] [PubMed] [Google Scholar]
- Zheng H, Park KS, Koh SD & Sanders KM (2014). Expression and function of a T‐type Ca2+ conductance in interstitial cells of Cajal of the murine small intestine. Am J Physiol Cell Physiol 306, C705–C713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MH, Kim TW, Ro S, Yan W, Ward SM, Koh SD & Sanders KM (2009). A Ca2+‐activated Cl− conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol 587, 4905–4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MH, Sung TS, O'Driscoll K, Koh SD & Sanders KM (2015). Intracellular Ca2+ release from endoplasmic reticulum regulates slow wave currents and pacemaker activity of interstitial cells of Cajal. Am J Physiol Cell Physiol 308, C608–C620. [DOI] [PMC free article] [PubMed] [Google Scholar]


