Abstract
Key points
Hypoxia, a potent activator of the sympathetic nervous system, is known to increase muscle sympathetic nerve activity (MSNA) to the peripheral vasculature of native Lowlanders during sustained high altitude (HA) exposure.
We show that the arterial baroreflex control of MSNA functions normally in healthy Lowlanders at HA, and that upward baroreflex resetting permits chronic activation of basal sympathetic vasomotor activity under this condition.
The baroreflex MSNA operating point and resting sympathetic vasomotor outflow both are lower for highland Sherpa compared to acclimatizing Lowlanders; these lower levels may represent beneficial hypoxic adaptation in Sherpa.
Acute hyperoxia at HA had minimal effect on baroreflex control of MSNA in Lowlanders and Sherpa, raising the possibility that mechanisms other than peripheral chemoreflex activation contribute to vascular sympathetic baroreflex resetting and sympathoexcitation.
These findings provide a better understanding of sympathetic nervous system activation and the control of blood pressure during the physiological stress of sustained HA hypoxia.
Abstract
Exposure to high altitude (HA) is characterized by heightened muscle sympathetic neural activity (MSNA); however, the effect on arterial baroreflex control of MSNA is unknown. Furthermore, arterial baroreflex control at HA may be influenced by genotypic and phenotypic differences between lowland and highland natives. Fourteen Lowlanders (12 male) and nine male Sherpa underwent haemodynamic and sympathetic neural assessment at low altitude (Lowlanders, low altitude; 344 m, Sherpa, Kathmandu; 1400 m) and following gradual ascent to 5050 m. Beat‐by‐beat haemodynamics (photoplethysmography) and MSNA (microneurography) were recorded lying supine. Indices of vascular sympathetic baroreflex function were determined from the relationship of diastolic blood pressure (DBP) and corresponding MSNA at rest (i.e. DBP ‘operating pressure’ and MSNA ‘operating point’), as well as during a modified Oxford baroreflex test (i.e. ‘gain’). Operating pressure and gain were unchanged for Lowlanders during HA exposure; however, the operating point was reset upwards (48 ± 16 vs. 22 ± 12 bursts 100 HB−1, P = 0.001). Compared to Lowlanders at 5050 m, Sherpa had similar gain and operating pressure, although the operating point was lower (30 ± 13 bursts 100 HB−1, P = 0.02); MSNA burst frequency was lower for Sherpa (22 ± 11 vs. 30 ± 9 bursts min−1 P = 0.03). Breathing 100% oxygen did not alter vascular sympathetic baroreflex function for either group at HA. For Lowlanders, upward baroreflex resetting promotes heightened sympathetic vasoconstrictor activity and maintains blood pressure stability, at least during early HA exposure; mechanisms other than peripheral chemoreflex activation could be involved. Sherpa adaptation appears to favour a lower sympathetic vasoconstrictor activity compared to Lowlanders for blood pressure homeostasis.
Keywords: Autonomic nervous system, Arterial baroreflex, blood pressure, High Altitude, Hypoxia, Sympathetic nerve activity
Key points
Hypoxia, a potent activator of the sympathetic nervous system, is known to increase muscle sympathetic nerve activity (MSNA) to the peripheral vasculature of native Lowlanders during sustained high altitude (HA) exposure.
We show that the arterial baroreflex control of MSNA functions normally in healthy Lowlanders at HA, and that upward baroreflex resetting permits chronic activation of basal sympathetic vasomotor activity under this condition.
The baroreflex MSNA operating point and resting sympathetic vasomotor outflow both are lower for highland Sherpa compared to acclimatizing Lowlanders; these lower levels may represent beneficial hypoxic adaptation in Sherpa.
Acute hyperoxia at HA had minimal effect on baroreflex control of MSNA in Lowlanders and Sherpa, raising the possibility that mechanisms other than peripheral chemoreflex activation contribute to vascular sympathetic baroreflex resetting and sympathoexcitation.
These findings provide a better understanding of sympathetic nervous system activation and the control of blood pressure during the physiological stress of sustained HA hypoxia.
Introduction
The sympathetic nervous system is the ubiquitous controller of the cardiovascular system in humans, and thus plays a pivotal role in arterial pressure homeostasis (Guyenet, 2006). High altitude (HA) hypoxia is a major physiological stressor that is accompanied by a profound activation of muscle sympathetic nerve activity (MSNA), which is markedly greater than that observed during acute exposure to a similar hypoxic stimulus (Duplain et al. 1999; Lundby et al. 2017). Notably, sympathoexcitation is maintained for the duration of HA exposure, despite normalization of resting arterial oxygen content to near sea‐level values (Hansen & Sander, 2003; Lundby et al. 2017). Furthermore, sympathetic activation is not reversed when breathing 100% oxygen, and persists for up to 3 days following descent to low altitude (LA) (Hansen & Sander, 2003; Mitchell et al. 2018). These data suggest a form of neural ‘remodelling’ associated with prolonged hypoxia.
Several studies have characterized MSNA and arterial pressure responses to sustained HA exposure in healthy Lowlanders (Hansen & Sander, 2003; Lundby et al. 2017; Fisher et al. 2018). Despite a greater probability of a burst of sympathetic vasoconstrictor activity at rest (i.e. 50% as opposed to 25%), any accompanying change in arterial pressure is relatively modest, at least for exposures lasting to 10–50 days. These observations imply a chronic resetting of the neural vasoconstrictor reflexes, which attempt to maintain blood pressure (BP), presumably to balance local vasodilator mechanisms and secure haemodynamic stability. However, the arterial baroreflex control of sympathetic vasoconstrictor activity has never been investigated at HA.
Relatively little is known regarding the consequences of lifelong HA hypoxia on sympathetic activity and BP regulation. A single microneurographic study found similar basal MSNA but lower arterial pressure for Bolivian highlanders compared to well‐acclimatized Lowlanders (Lundby et al. 2017); arterial baroreflex function was not tested. This suggests that sustained sympathetic activation may be an evolutionary adaptation for those living permanently under HA hypoxia. However, distinct differences in physiological adaptation are known to exist between natives of the South American Andes, Himalaya plateau and Ethiopian highlands (Beall, 2006, 2007; Erzurum et al. 2007), with the suggestion that the Sherpa (Himalayan) adaptation represents the most effective phenotype for chronic hypoxia (Gilbert‐Kawai et al. 2014; Horscroft et al. 2017). However, as a result of a lack of microneurographic data for highlanders, other than Bolivians, it is unclear whether differences in the patterns of adaptation extends to sympathetic nervous system control of the cardiovascular system and arterial pressure homeostasis.
Therefore, the present study aimed (i) to examine baroreflex regulation (resetting and gain) in healthy Lowlanders at 5050 m, following 10–20 days of acclimatization and (ii) to compare arterial baroreflex function between acclimatizing Lowlanders and HA native Sherpa. Based upon previous reports for acute hypoxia (Halliwill & Minson, 2002; Halliwill et al. 2003; Steinback et al. 2009; Querido et al. 2011), we hypothesized that the ‘operating pressure’ [i.e. diastolic BP (DBP)] and ‘operating point’ (i.e. MSNA burst incidence) of the vascular sympathetic baroreflex would shift to higher values, during HA acclimatization, with no change in reflex ‘gain’ (i.e. slope). We further hypothesized that Sherpa, who cope extremely well with chronic hypoxia, would have lower arterial pressure and lower MSNA operating point, compared to Lowlanders.
Methods
Ethical approval
All testing procedures had Institutional Review Board approval from the University of British Colombia (H16‐01297/H16‐01028), University of Alberta, and Nepal Health Research Council. All participants were informed, using the native language, of the purpose and the risks involved wih each procedure, and provided their oral and written informed consent, in compliance with the latest revision of the Declaration of Helsinki, except for registration in a database.
Participants
Fourteen Lowlanders (12 males; mean ± SD: aged 27 ± 6 years; height 1.77 ± 0.8 m; weight 72.2 ± 10.1 kg) and nine male Sherpa (aged 33 ± 12 years; height 1.68 ± 0.07 m; weight 65.3 ± 10.3 kg) participated in the study. All Sherpa were natives of the Khumbu valley (>3440 m). None of the participants had any history or symptoms of cardiovascular, respiratory, metabolic and neurological disease, and they were not taking any prescription or over‐the‐counter‐medication during the time of participation. Five Sherpa were self‐reported smokers (1–5 cigarettes per day). None of the Lowlanders experienced clinical acute mountain sickness at the time of testing, as assessed by the Lake Louise questionnaire (LLQ score ≤3); however, one was tested 2 days following an i.m. injection of dexamethasone (half‐life, 3 h). All participants abstained from caffeine and vigorous exercise for 12 h prior to all testing sessions and arrived at the laboratory a minimum of 2 h after a light meal.
Experimental design
The experiment was carried out within the framework of the 2016 UBC Nepal Expedition to the Ev‐K2‐CNR Research Facility (Willie et al. 2018). Participants for the present study were also recruited for a number of other investigations. Therefore, care was taken to ensure that no overlap existed between any of the studies, and the present study addressed its own distinct a priori research question. Data collected during the testing sessions described below, although with separate a priori analyses, are reported elsewhere (Busch et al. 2017).
All participants underwent two testing sessions. Pre‐expedition, LA, testing of Lowlanders was conducted at 344 m (Kelowna, Canada, barometric pressure, 758 ± 8 mmHg). Pre‐expedition testing of Sherpa was conducted at 1400 m [Kathmandu (KT), Nepal; barometric pressure, 652 ± 3 mmHg]; this was performed a minimum of 4 days following descent from their resident altitude. All of the HA testing was performed at 5050 m (barometric pressure: 431 ± 44 mmHg). These tests followed a gradual trek (i.e. 9 or 10 days), starting at 2860 m with rest days at both 3400 and 4240 m. Sherpa were studied on days 1–4 at 5050 m (i.e. 10–14 days above 2860 m) and Lowlanders were studied on days 1–10 (i.e. 10–20 days).
Measurements
Haemodynamics
Heart rate (HR) and beat‐by‐beat BP were continuously recorded using Lead II electrocardiogram and finger photoplethysmography (Finometer Pro; Finapres Medical Systems BV, Amsterdam, The Netherlands). Mean arterial pressure (MAP), as well as systolic BP (SBP) and DBP, were calculated from the arterial pressure waveform, which was calibrated against manual brachial artery pressure measurements. Cardiac output (CO) was estimated using the Model Flow algorithm and used to estimate total peripheral resistance (TPR = MAP/CO). Peripheral capillary oxygen saturation (S pO2) was determined using finger pulse oximetry (Nellcor; Medtronics, Minneapolis, MN, USA).
MSNA
Multi‐unit MSNA was recorded from the peroneal (common fibular) nerve via microneurography (by JPM and CDS) as described previously (Steinback & Shoemaker, 2012; Usselman et al. 2015). MSNA signal was confirmed by pulse‐synchronous activity that responded to end‐expiratory apnea but not to startle stimuli or skin stroking (Delius et al. 1972b, 1972ab). Nerve signals were amplified (1000x pre‐amplifier and 100x variable gain isolated amplifier), bandpass filtered (700–2000 Hz) rectified (model 662C‐3; Iowa University Bioengineering, Iowa City, IA, USA) and integrated (decay constant 0.1 s).
Experimental protocol
Basal sympathetic neural activity
Following arrival at the laboratory, subjects rested in the supine position and an antecubital venous cannula was inserted for subsequent drug administration. Following instrumentation, acquisition of an acceptable MSNA signal and a period of stabilization, 10 min of baseline data were recorded.
Arterial baroreflex function
Vascular sympathetic and cardiovagal baroreflex function was determined from the MSNA and R–R interval (RRI) responses during arterial pressure perturbations induced by a single modified Oxford baroreflex test (Rudas et al. 1999) during ambient air breathing. Briefly, the modified Oxford test involved bolus injection of sodium nitroprusside (SNP), followed 90 seconds later by phenylephrine (PE). Prior to experimental testing, bolus doses of SNP and PE that evoked ∼15 mmHg perturbations above and below resting BP were determined for each individual. The same relative dose (μg kg−1) was administered at LA and HA for a given individual. Doses of vasoactive drugs administered and resultant BP changes are shown in Table 1.
Table 1.
Doses of SNP and PE administered in Lowlanders at 344 m (LA) and 5050 m (HA) and Sherpa at HA and the resultant BP changes
| Lowlanders | Sherpa | ||||
|---|---|---|---|---|---|
| LA (n = 14) | HA (n = 14) | P value | HA (n = 8) | P value | |
| SNP dose (μg kg−1) | 1.71 ± 0.53 | 1.72 ± 0.39 | – | 1.60 ± 0.40 | – |
| PE dose (μg kg−1) | 2.27 ± 0.21 | 2.29 ± 0.24 | – | 2.10 ± 0.20 | – |
| SNP BP decrease (mmHg) | 15 ± 6 | 15 ± 6 | 0.78 | 14 ± 4 | 0.9 |
| PE BP increase (mmHg) | 19 ± 9 | 11 ± 3 | 0.02 | 15 ± 4 | 0.15 |
| Total BP changes (mmHg) | 35 ± 11 | 26 ± 8 | 0.02 | 28 ± 6 | 0.15 |
Arterial baroreflex–peripheral chemoreflex interaction
At LA, a modified Oxford test was also performed when breathing a gas mixture containing 11% oxygen (equivalent to 5050 m) to increase peripheral chemoreceptor drive (acute hypoxia, AH). At both LA and HA, a single modified Oxford test was also performed when participants breathed 100% oxygen (LA + 100% O2, HA + 100% O2) to acutely eliminate peripheral chemoreceptor drive. Participants breathed each of the gas mixtures for a minimum of 5 min; as soon as a new steady state S pO2 was achieved, the modified Oxford test was performed. No attempt was made to control ventilation or end‐tidal CO2 during manipulation of peripheral chemoreceptor drive. At least 20 min separated the modified Oxford tests. The order of trials at LA were not randomized because persistent alterations in MSNA and vascular sympathetic baroreflex function have been shown following acute hypoxia stimulus (Querido et al. 2011).
Data analysis
All haemodynamic data were sampled at 1 kHz using a commercial data acquisition software (LabChart Pro, version 8.3.1; ADInstruments, Sydney, NSW, Australia) and stored for offline analysis. The raw MSNA signal was sampled at 10 kHz. Multi‐unit bursts of MSNA were identified using a semi‐automated detection algorithm (LabChart Pro, version 8.3.1) and confirmed by a trained observer (SAB and CDS). To account for vascular sympathetic baroreflex latency, MSNA data were shifted backwards (1.32 ± 0.07s) so that the peak of each sympathetic burst coincided with the diastolic period that initiated it (Usselman et al. 2015). Burst amplitude data were normalized by assigning a value of 100 to the largest burst observed during baseline and calibrating all other bursts against this value. Resting MSNA was quantified as burst frequency (burst min−1), burst incidence (burst 100 HB−1), mean burst amplitude (a.u.) and total activity [mean burst amplitude × burst frequency (a.u. min−1)].
Vascular sympathetic baroreflex gain was estimated from the relationship between DBP and MSNA burst probability during a modified Oxford test. DBP was used because MSNA correlates more closely with DBP than SBP (Sundlof & Wallin, 1978). All DBP values were assigned to a 3 mmHg bin to reduce the statistical impact of respiratory related oscillations (Eckberg & Eckberg, 1982). The percentage of cardiac cycles associated with a burst of MSNA (ranging from 0% to 100%) was calculated for each DBP bin to give values of burst probability (Usselman et al. 2015). Non‐linear saturation and threshold regions, if present, were excluded through visual inspection of data points by agreement of two observers (LLS and JPM). The slope of the linear relationship was determined by weighted linear regression analysis and this value provided an index of vascular sympathetic baroreflex gain. Only slopes with (i) at least five data points and (ii) r ≥ 0.5 were included in the group mean data (Hart et al. 2011; Taylor et al. 2015). Vascular sympathetic baroreflex gain for rising and falling pressures was not determined independently. The vascular sympathetic baroreflex operating point was taken as the average values for DBP and MSNA burst incidence during the resting period immediately prior to the modified Oxford test.
Cardiovagal baroreflex gain was estimated from the relationship between SBP and RRI during each modified Oxford test. SBP was used because it correlates more closely with RRI than DBP (Sundlof & Wallin, 1978). Values were averaged over 3 mmHg SBP bins. Baroreflex delays were accounted for by associating SBP values with either the concurrent heartbeat (when resting RRI > 800 ms) or subsequent heartbeat (when resting RRI < 800 ms) (Eckberg & Eckberg, 1982). Saturation and threshold regions were excluded through visual inspection of data (LLS and JPM). Slopes were determined by weighted linear regression analysis and only slopes with at least five data points and r ≥ 0.8 were included in the group mean data. To minimize the potential effects of hysteresis, we restricted data analysis to the rising arm of SBP during the modified Oxford test (Hunt & Farquhar, 2005). The cardiovagal baroreflex operating point was taken as the average value for SBP and RRI during the resting period immediately prior to the modified Oxford test.
Statistical analysis
The effects of HA acclimatization in Lowlanders were assessed using paired t tests, whereas differences between Lowlanders and Sherpa at HA and Sherpa at HA and Lowlanders at LA were assessed using independent t tests. The effects of manipulating peripheral chemoreceptor drive on baroreflex function at LA (AH, LA + 100% O2) and HA (HA + 100% O2) were assessed using paired t tests. Multiple t tests were chosen to maximize the number of subjects included in statistical analyses. A priori alpha was adjusted, using the experiment‐wise error rate, to correct for multiple comparisons (Busch et al. 2017). All statistical analyses were performed using Prism, version 7.03 (GraphPad Software Inc., San Diego, CA, USA). P < 0.05 was considered statistically significant. Group data are reported as the mean ± SD.
Results
Resting haemodynamics, basal sympathetic neural activity and arterial baroreflex function
Examples of MSNA and haemodynamic data recorded in one Lowlander and one Sherpa, under each of the experimental conditions, are presented in Fig. 1. In Lowlanders (n = 14), S pO2 was decreased and HR was increased with HA acclimatization, whereas CO, TPR and MAP were similar compared to LA. All parameters of MSNA were greater in Lowlanders at HA compared to LA (Table 2).
Figure 1. Example Recordings of MSNA and BP.
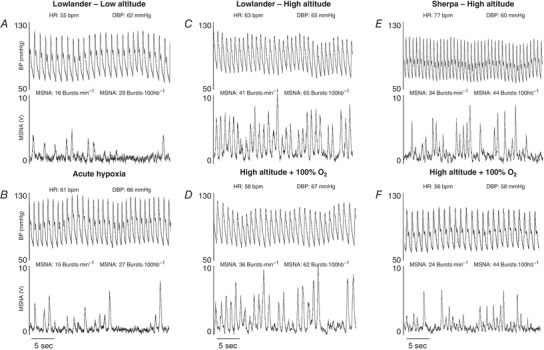
One representative Lowlander (aged 29 years) at LA (A), during acute hypoxia (B), following 8 days at HA (C), and during 100% oxygen breathing at HA (D). One representative Sherpa (aged 26 years) following 3 days at HA (E) and during 100% oxygen breathing at HA (F).
Table 2.
Haemodynamic and basal MSNA variables at 344 m (LA), 1400 m (KT) and 5050 m (HA)
| Lowlanders | Sherpa | ||||||
|---|---|---|---|---|---|---|---|
| P value | |||||||
| LA (n = 14) | HA (n = 14) | P value | KT (n = 4) | HA (n = 8) | HA vs. Lowlanders HA | HA vs. Lowlanders LA | |
| Haemodynamic variables | |||||||
| S pO2 (%) | 98 ± 1 | 82 ± 3 | 0.001 | 96 ± 1 | 81 ± 4 | 0.6 | 0.001 |
| Heart rate (beats min−1) | 54 ± 10 | 64 ± 13 | 0.006 | 63 ± 7 | 73 ± 7 | 0.16 | 0.002 |
| MAP (mmHg) | 84 ± 8 | 85 ± 10 | 0.84 | 92 ± 3 | 84 ± 9 | 0.84 | 0.9 |
| Cardiac output (L min−1) | 5.2 ± 1.0* | 5.2 ± 1.2* | 0.46 | 4.9 ± 1.2 | 5.8 ± 1.7 | 0.42 | 0.41 |
| Total peripheral resistance (mmHg L−1 min−1) | 16.7 ± 3.3* | 17.5 ± 3.9* | 0.94 | 19.8 ± 4.9 | 16.3 ± 6.8 | 0.73 | 0.9 |
| MSNA | |||||||
| Burst frequency (bursts min−1) | 11 ± 5 | 30 ± 9 | 0.001 | 11 ± 2 | 22 ± 11 | 0.05 | 0.003 |
| Burst incidence (bursts 100 HB−1) | 22 ± 12 | 48 ± 16 | 0.001 | 18 ± 6 | 30 ± 13 | 0.02 | 0.16 |
| Mean burst amplitude (a.u.) | 43 ± 8 | 50 ± 5 | 0.02 | 49 ± 7 | 53 ± 4 | 0.2 | 0.003 |
| Total activity (a.u. min−1) | 461 ± 194 | 1508 ± 548 | 0.001 | 521 ± 140 | 1168 ± 540 | 0.2 | 0.002 |
Data are presented as the mean ± SD. *Cardiac output and total peripheral resistance for Lowlanders (n = 10). Note: no intragroup comparison for Sherpa, HA vs. KT because only three were tested at both altitudes.
As a result of technically challenging conditions during pre‐expedition testing at KT, resting sympathetic neural activity could only be obtained in four out of nine Sherpa, and one of these four Sherpa was not re‐tested at HA (Table 2). At HA, Sherpa (n = 8) and Lowlanders had a similar S pO2 and resting haemodynamics; however, Sherpa exhibited significantly lower MSNA burst frequency than Lowlanders, with no difference in mean burst amplitude. Compared to Lowlanders at LA, Sherpa had lower S pO2 and higher HR but similar CO, TPR and MAP. Sherpa exhibited significantly greater MSNA burst frequency, as well as mean burst amplitude, vs. Lowlanders at LA.
Data from 10 Lowlanders were included in the comparisons of sympathetic and cardiovagal baroreflex function because baroreflex slopes for four participants did not fulfil the inclusion criteria. HA acclimatization had no effect on the baroreflex diastolic operating pressure for Lowlanders, although the MSNA operating point (burst incidence) was increased compared to LA. Vascular sympathetic baroreflex gain (i.e. slope) was not different at LA and HA (Fig. 2). HA acclimatization resulted in a downward shift of the cardiovagal baroreflex, as reflected by a reduction in RRI, with no change in prevailing SBP; this was accompanied by a reduction in reflex gain (Fig. 3).
Figure 2. Vascular sympathetic baroreflex function.
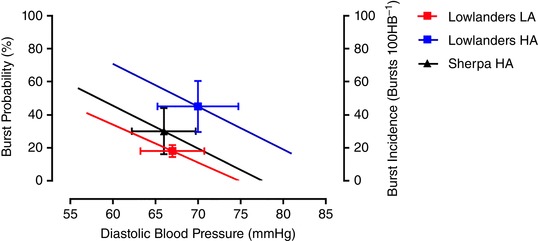
Group average regressions between MSNA burst probability and DBP in Lowlanders (n = 10) at 344 m (LA) and 5050 m (HA) and in Sherpa at HA (n = 7). The operating points are indicated by symbols and error bars (mean ± SD). The MSNA operating point was significantly elevated in Lowlanders at HA, relative to Lowlanders at LA. The MSNA operating point was lower in Sherpa relative to Lowlanders at HA, and similar to Lowlanders at LA. Operating DBP were similar. This indicated an upward resetting of the vascular sympathetic baroreflex following ascent to HA in Lowlanders. The slopes of the relationships were similar in Lowlanders at LA and HA (–2.3 ± 0.7 vs. –2.6 ± 1.2% mmHg−1; P = 0.33) and similar in Sherpa at HA (–2.6 ± 0.9% mmHg−1) compared to Lowlanders at both HA (P = 0.98) and LA (P = 0.99). This indicated that there were no differences in vascular sympathetic baroreflex gain. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 3. Cardiovagal baroreflex function.
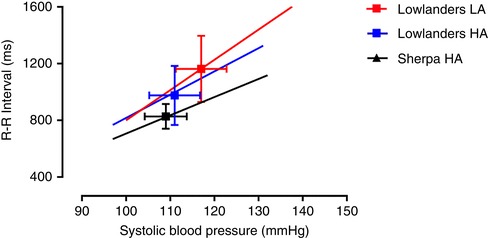
Group average regressions between RRI and SBP in Lowlanders (n = 10) at 344 m (LA) and 5050 m (HA) and in native Sherpa at HA (n = 7). The operating points are indicated by symbols and error bars (mean ± SD). RRI significantly decreased in Lowlanders at HA, relative to Lowlanders at LA, although it was similar in Sherpa relative to Lowlanders at HA. Operating SBP were similar. This indicated a downward (RRI) resetting of the cardiovagal baroreflex in Lowlanders following ascent to HA. The slope of the relationship was less steep in Lowlanders at HA (16.2 ± 8.2 ms mmHg−1) vs. LA (20.6 ± 5.0 ms mmHg−1; P = 0.007), indicating a reduction in cardiovagal baroreflex gain following ascent to HA in Lowlanders. The slope of the relationship between SBP and RRI was similar in Sherpa at HA (12.9 ± 5.4 ms mmHg−1; P = 0.60) relative to Lowlanders at HA, indicating no differences in reflex gain. Compared to Lowlanders at LA, operating SBP was similar but RRI was significantly smaller in Sherpa at HA, and the slope of the relationship was less steep (P = 0.01) [Color figure can be viewed at wileyonlinelibrary.com]
Modified Oxford tests were performed successfully in seven out of eight Sherpa investigated at HA; no baroreceptor tests were performed in KT. At HA, the diastolic operating pressure for Sherpa was similar to that of Lowlanders, although the MSNA operating point was lower for Sherpa. There was no difference in vascular sympathetic baroreflex gain (Fig. 2). Furthermore, compared to Lowlanders at LA, operating pressure, operating point and vascular sympathetic baroreflex gain for Sherpa were similar. The cardiovagal baroreflex gain in Sherpa was similar to that for Lowlanders at HA, although less than that of Lowlanders at LA. The cardiovagal baroreflex operating SBP was similar for Sherpa compared to Lowlanders at both HA and LA, whereas RRI was similar to Lowlanders at HA but lower than that at LA (Fig. 3). In Sherpa, cardiovagal baroreflex gain was similar to that of Lowlanders at HA, but less than that of Lowlanders at LA.
Arterial baroreflex–peripheral chemoreflex interaction at LA
As a result of the loss of MSNA signals in three participants, the data analyses for arterial baroreflex–peripheral chemoreflex interactions at LA are for 11 Lowlanders (Table 3). AH reduced S pO2, increased HR, MAP and CO, and decreased TPR. MSNA burst frequency was unchanged, although total MSNA was increased as a result of an augmented burst amplitude. Vascular sympathetic baroreflex gain was reduced during AH, with no change in baroreflex diastolic operating pressure or the MSNA operating point. Administration of 100% oxygen at LA had no effect on baseline haemodynamics, MSNA burst frequency, burst amplitude or indices of vascular sympathetic and cardiovagal baroreflex function.
Table 3.
Manipulation of peripheral chemoreceptor drive in Lowlanders at 344 m (LA)
| Lowlanders | |||||
|---|---|---|---|---|---|
| LA (n = 11) | AH (n = 11) | P value | LA + 100% O2 (n = 9) | P value† | |
| Haemodynamic variables | |||||
| S pO2 (%) | 98 ± 1 | 84 ± 4 | 0.001 | ||
| Heart rate (beats min−1) | 53 ± 10 | 68 ± 15 | 0.001 | 56 ± 11 | 0.55 |
| MAP (mmHg) | 84 ± 7 | 89 ± 5 | 0.05 | 88 ± 5 | 0.09 |
| Cardiac output (L min−1) | 5.1 ± 1.0* | 6.7 ± 1.8* | 0.002 | 5.7 ± 0.9 | 0.27 |
| Total peripheral resistance (mmHg L−1 min−1) | 16.8 ± 3.5* | 14.2 ± 3.7* | 0.01 | 15.9 ± 2.7 | 0.67 |
| MSNA | |||||
| Burst frequency (bursts min−1) | 11 ± 4 | 13 ± 6 | 0.43 | 11 ± 6 | 0.9 |
| Mean burst amplitude (a.u.) | 40 ± 7 | 52 ± 13 | 0.01 | 46 ± 14 | 0.06 |
| Total activity (a.u. min−1) | 502 ± 183 | 789 ± 489 | 0.06 | 549 ± 305 | 0.49 |
| Baroreflex function | |||||
| Vascular sympathetic baroreflex gain (% mmHg−1) | –2.7 ± 1.0 | –1.9 ± 0.6 | 0.02 | –2.3 ± 0.9 | 0.35 |
| Diastolic operating pressure (mmHg) | 67 ± 7 | 70 ± 4 | 0.09 | 70 ± 9 | 0.14 |
| Burst incidence operating point (bursts 100 HB−1) | 20 ± 6 | 19 ± 8 | 0.68 | 20 ± 9 | 0.77 |
| Cardiovagal baroreflex gain (ms mmHg−1) | 21.2 ± 8.4 | 23.5 ± 16.2 | 0.51 | 27.2 ± 17.4 | 0.24 |
| Systolic operating pressure (mmHg) | 117 ± 10 | 123 ± 7 | 0.12 | 121 ± 10 | 0.32 |
| RRI operating point (ms) | 1164 ± 226 | 933 ± 248 | 0.001 | 1177 ± 245 | 0.78 |
Data are presented as the mean ± SD. *Cardiac output and total peripheral resistance (n = 10). †Intragroup comparison for nine participants, LA vs. LA + 100% O2.
Arterial baroreflex–peripheral chemoreflex interaction at HA
As a result of MSNA signal losses, these comparisons were performed for nine Lowlanders and four Sherpa (Table 4). For Lowlanders exposed to 100% O2, S pO2 and MAP increased, and HR decreased, with no effect on any other baseline haemodynamics or MSNA. Vascular sympathetic baroreflex function gain was also unchanged. There was no change in cardiovagal baroreflex gain. For Sherpa breathing 100% oxygen, S pO2 increased and HR decreased, with no effect on other baseline haemodynamics. MSNA burst frequency, burst incidence, total activity and total MSNA were unchanged; however, mean burst amplitude decreased. Vascular sympathetic baroreflex gain was unchanged. Breathing 100% oxygen reduced RRI, with no change in cardiovagal baroreflex gain.
Table 4.
Manipulation of peripheral chemoreceptor drive in Lowlanders and Sherpa at 5050 m (HA)
| Lowlanders | Sherpa | |||||
|---|---|---|---|---|---|---|
| HA (n = 9) | HA + 100% O2 (n = 9) | P value | HA (n = 4) | HA + 100% O2 (n = 4) | P value | |
| Haemodynamic variables | ||||||
| S pO2 (%) | 82 ± 4 | 97 ± 2 | 0.001 | 82 ± 5 | 99 ± 1 | 0.008 |
| Heart rate (beats min−1) | 70 ± 12 | 61 ± 8 | 0.01 | 74 ± 6 | 62 ± 5 | 0.02 |
| MAP (mmHg) | 88 ± 9 | 93 ± 9 | 0.008 | 78 ± 8 | 79 ± 11 | 0.79 |
| Cardiac output (L min−1) | 5.4 ± 1.0 | 5.2 ± 1.0 | 0.60 | 6.2 ± 1.7 | 5.7 ± 1.8 | 0.34 |
| Total peripheral resistance (mmHg L−1 min−1) | 17.1 ± 3.7 | 18.3 ± 4.1 | 0.26 | 14.4 ± 7.4 | 16.5 ± 10.7 | 0.26 |
| MSNA | ||||||
| Burst frequency (bursts min−1) | 30 ± 10 | 27 ± 11 | 0.35 | 22 ± 8 | 17 ± 6 | 0.14 |
| Mean burst amplitude (a.u.) | 50 ± 5 | 46 ± 13 | 0.36 | 53 ± 5 | 46 ± 6 | 0.01 |
| Total activity (a.u. min−1) | 1495 ± 614 | 1289 ± 729 | 0.18 | 1158 ± 330 | 786 ± 250 | 0.08 |
| Baroreflex function | ||||||
| Vascular sympathetic baroreflex gain (% mmHg−1) | −2.6 ± 1.2 | −2.5 ± 1.0 | 0.16 | −2.8 ± 1.2 | −3.0 ± 1.3 | 0.69 |
| Diastolic operating pressure (mmHg) | 72 ± 9 | 74 ± 10 | 0.06 | 63 ± 8 | 65 ± 12 | 0.34 |
| Burst incidence operating point (bursts 100 HB−1) | 44 ± 16 | 45 ± 16 | 0.62 | 29 ± 10 | 29 ± 12 | 0.93 |
| Cardiovagal baroreflex gain (ms mmHg−1) | 21.5 ± 5.5 | 21.2 ± 11.4 | 0.92 | 13.2 ± 3.5 | 18.4 ± 9.1 | 0.20 |
| Systolic operating pressure (mmHg) | 113 ± 11 | 119 ± 9 | 0.01 | 103 ± 7 | 111 ± 12 | 0.10 |
| RRI operating point (ms) | 882 ± 129 | 994 ± 139 | 0.006 | 790 ± 64 | 960 ± 80 | 0.04 |
Data are presented as the mean ± SD.
Discussion
The principal novel findings of the present study are that: (i) baroreflex control of MSNA is preserved in Lowlanders following 10–20 days at HA; (ii) the operating point of the vascular sympathetic baroreflex is upwardly reset with no change in operating DBP for Lowlanders at HA; (iii) Sherpa have lower basal MSNA burst frequency compared to Lowlanders at HA but similar resting BP; (iv) Sherpa have similar vascular sympathetic baroreflex gain but a lower operating point compared to Lowlanders at HA and, finally, (v) eliminating peripheral chemoreceptor drive at HA did not influence the vascular sympathetic baroreflex operating point or gain for both Lowlanders and Sherpa. Taken together, these findings provide important new insight into reflex control of the vasoconstrictor drive and BP at HA, and highlight a novel adaptation in Sherpa.
Sympathoexcitation at HA
Following 10–20 days of HA exposure, we observed an almost three‐fold increase in MSNA burst frequency for Lowlanders at 5050 m; this is consistent with previous microneurographic studies at HA (Hansen & Sander, 2003; Lundby et al. 2017). Furthermore, for the first time, we demonstrate that the basal MSNA burst frequency of Sherpa is lower than that of Lowlanders at this altitude, despite similar peripheral oxygen saturation in both groups. Our observation for Sherpa contrasts with that of the only previous study of highlanders (Lundby et al. 2017), which found that Bolivian Aymara had a basal MSNA comparable to that of Lowlanders after 10 and 50 days of HA exposure. Although many factors may influence basal sympathetic outflow, the present study raises the importance of ethnicity. The divergent pathways of physiological adaptation observed in geographically distinct HA populations might extend to sympathetic nervous system activation, where adaptation in Sherpa appears to favour lower basal sympathetic activity. However, we also found that basal MSNA for Sherpa at HA was higher than that for Lowlanders at LA. Furthermore, for three Sherpa studied 4 days following descent to 1440 m, basal MSNA burst frequencies were ∼30% lower than those observed when they were re‐tested at 5050 m. Taken together, these findings suggest that hypoxia remains a significant physiological stressor for Sherpa despite generations of adaptation and lifelong exposure.
Remarkably, resting MAP for Lowlanders was similar at LA and HA, despite the significantly elevated basal MSNA at 5050 m. Moreover, Sherpa and Lowlanders exhibited similar MAP at 5050 m, even though Sherpa had markedly less basal MSNA. This may reflect differences in the release of vasoactive substances and vascular sensitivity to these factors. It is possible that α adrenergic receptor sensitivity is reduced in Lowlanders during prolonged HA hypoxia, meaning that they require more MSNA to produce the same vascular response. That the same dose of PE administered during the modified Oxford test elicited a smaller pressor response for lowlanders at HA than at LA supports this notion. Furthermore, Sherpa may possess a greater vascular responsiveness to sympathetic vasoconstrictor drive, meaning that the vascular effect of a burst of neural activity is greater. However, characterization of a dose–response relationship to vasoactive substances would be required to confirm these possibilities.
Arterial baroreflex function at HA
The data obtained in the present study indicate an upward resetting of the vascular sympathetic baroreflex in Lowlanders at 5050 m. This occurred without a change in the ability of the reflex to increase or decrease MSNA in response to a baroreceptor challenge (i.e. the gain was unchanged). Furthermore, the ability of the baroreflex to regulate MSNA in Sherpa and Lowlanders is similar, although the likelihood of a burst of MSNA at a given diastolic pressure is lower for Sherpa. Vascular sympathetic baroreflex function at HA had not been assessed prior to the present study. Previous reports of heightened MSNA burst incidence (Hansen & Sander, 2003; Lundby et al. 2017; Fisher et al. 2018) indirectly support an upward resetting of the vascular sympathetic baroreflex operating point for Lowlanders exposed to chronic HA hypoxia. However, in contrast to the present study, previous studies found an increase in resting MAP accompanied higher MSNA burst incidence (Hansen & Sander, 2003; Lundby et al. 2017; Fisher et al. 2018). This may be a result of methodological differences across studies in relation to the ascent profile, physical activity levels when at altitude and the final elevation achieved. In addition, a temporal relationship may exist between elevated sympathetic vasomotor activity and MAP in Lowlanders. Arterial baroreflex resetting and heightened sympathetic outflow initially may be homeostatic during early acclimatization; however, over time, other cardiovascular changes and alterations in constricting and dilating factors acting on the vasculature (Calbet et al. 2014; Bruno et al. 2016) could contribute to elevated MAP at HA. We suggest that future studies at HA should incorporate serial measurements of arterial baroreflex control of MSNA and other factors that modulate arterial pressure.
The secondary effects of increased ventilation at HA may complicate the effects of hypoxia on baroreflex control of the heart (Angell James & De Burgh Daly, 1969; Eckberg et al. 1980). Nevertheless, we determined how the cardiovagal component of the arterial baroreflex was affected in the present study. At HA, cardiovagal baroreflex gain was similar for Lowlanders and Sherpa, although we observed that the gain for Lowlanders was reduced compared to at LA. Interestingly, acute hyperoxia at 5050 m did not reverse this reduction in gain for Lowlanders. Taken together, our data suggest that altitude acclimatization has differential effects on the responsiveness of the vascular sympathetic and cardiovagal limbs of the arterial baroreflex.
Vascular sympathetic baroreflex–peripheral chemoreflex interactions
For Lowlanders exposed to AH, there was no change in basal MSNA burst frequency, although there was a modest increase in mean burst amplitude and thus a modest increase in total activity. This implies that MSNA burst frequency and amplitude can be regulated independently of each other, as suggested previously (Kienbaum et al. 2001; Salmanpour et al. 2011; Steinback & Shoemaker, 2012). Furthermore, the operating point of the vascular sympathetic baroreflex was not significantly different during AH, whereas the gain was reduced. These findings for AH are in contrast to those for HA and suggest that different mechanisms contribute to activation of central sympathetic outflow during acute and chronic hypoxic exposure. Vascular sympathetic baroreflex resetting in Lowlanders at 5050 m was not reversed during acute administration of 100% oxygen. Furthermore, MSNA burst frequency was not reduced, which is is consistent with previous studies that attempted to reduce peripheral chemoreflex drive at HA (Hansen & Sander, 2003; Fisher et al. 2018). Therefore, mechanisms other than the peripheral chemoreflex probably play a role in vascular sympathetic baroreflex resetting at HA. Interestingly, the peripheral chemoreflex may be more important in mediating HA sympathoexcitation in Sherpa. Although the vascular sympathetic baroreflex operating point was not changed during 100% oxygen administration, we observed a reduction in mean burst amplitude and total activity. This possibility, however, requires further investigation.
Experimental considerations
The present study is the first to record sympathetic neural discharges from Sherpa at HA. However, technically challenging conditions in Kathmandu limited the study to only four participants at a lower elevation. Furthermore, around half of Sherpa were light to moderate smokers and it is reported that tobacco smoking leads to increased basal MSNA and attenuates vascular sympathetic baroreflex sensitivity. However, smoking status was not a significant covariate for any indices in the present study. Compared with Lowlanders, Sherpa were naive to the microneurographic technique and may have experienced some anxiety during testing. Thus, we cannot rule out an overestimation of resting MSNA for Sherpa. Ascent to 5050 m was gradual to facilitate acclimatization. However, it was not possible for groups of participants to arrive on separate days to minimize any confounding effects of the varying time course of acclimatization once at 5050 m. Although we acknowledge that a difference between days 10 and 20 may have influenced our results, our analysis indicates that test day was not a significant covariate. We did not assess vascular sympathetic baroreflex gain to rising and falling pressure independently and we acknowledge that this fails to take baroreflex hysteresis into account (Rudas et al. 1999). A change in baseline MSNA may have influenced the responsiveness of the vascular sympathetic baroreflex to both rising and falling pressures in an equal but opposite manner (Hart et al. 2011).
The mechanisms by which sustained HA acclimatization produce baroreflex resetting and chronic sympathoexcitation require elucidation. Our findings suggest factors other than the peripheral chemoreflex play a role. We acknowledge that relative hypovolaemia (Ryan et al. 2014), systemic inflammation and oxidative stress (Lewis et al. 2014), erythropoietin production (Oshima et al. 2018) and changes in intracranial pressure (Schmidt et al. 2018) might all influence sympathetic outflow in HA hypoxia. Furthermore, sympathetic activation in response to elevated pulmonary artery pressure has been shown in experimental animals (Moore et al. 2011).
Conclusions
We demonstrate the highly effective arterial baroreflex control of sympathetic vasomotor activity in healthy humans during sustained hypoxia. Chronic resetting of the vascular sympathetic baroreflex supports elevated vasoconstrictor drive in Lowlanders during early acclimatization to HA, although without an increase in resting arterial pressure. Sherpa, by comparison, have a lower vascular sympathetic baroreflex operating point and lower vasoconstrictor drive but a similar vascular resistance and arterial pressure. For Lowlanders, vascular sympathetic baroreflex resetting and heightened sympathetic activity may protect against orthostatic hypotension at HA. By contrast, Sherpa may have adapted to HA to require lower sympathetic outflow for homeostatic control of BP. Such a difference may represent another example of a beneficial hypoxic adaptation in this highland population.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
Testing was conducted at the Centre for Heart, Lung and Vascular Health, University of British Columbia, Kelowna, Canada, and the Ev‐K2‐CNR Research Facility, Khumbu Valley, Nepal. JPM, CDS, MS and PNA contributed to conception and design of the work. LLS, JPM, CDS, MS and SAB contributed to the acquisition and analysis of data. LLS, JPM, MS, CDS, PNA and SJO contributed to the interpretation of data and the writing and critical revision of the manuscript. All authors approved the final version of the manuscript submitted for publication and agree to be accountable for all aspects of the work. All persons included as an author qualify for authorship, and all those who qualify for authorship are listed.
Funding
The study was supported by the Natural Sciences and Engineering Research Council of Canada (CDS, PNA), a Canada Research Chair in Cerebrovascular Physiology grant (PNA), and a University of Alberta, Presidents Grant for the Creative and Performance Arts – Human Performance Scholarship (CDS)
Translational perspective.
Sustained activation of the sympathetic nervous system and reduced vascular sympathetic baroreflex responsiveness are features of several disease states involving chronic systemic hypoxaemia. Together, chronic sympathetic nervous system activation and reduced vascular sympathetic baroreflex responsiveness facilitate elevated arterial pressure and haemodynamic instability in these populations. Our data demonstrate that sustained high altitude (HA) hypoxia, a model of chronic systemic hypoxaemia independent of co‐morbidity, is accompanied by sustained chronic sympathoexcitation. Notably, however, vascular sympathetic baroreflex responsiveness is preserved. Chronic resetting of the vascular sympathetic baroreflex, and hence sympathoexcitation, at HA are important for blood pressure homeostasis in acclimatized lowlanders and well‐adapted Sherpa. Furthermore, mechanisms acting independently of the peripheral chemoreflex appear to be involved in HA sympathoexcitation. This raises an intriguing possibility that these mechanisms could overlap with those that activate the sympathetic nervous system in disease states.
Acknowledgements
This article is dedicated to the memory of our colleague Dr Chris Willie. We are grateful to all those who participated in this study, as well as to Dr Prajan Subedi and Dr Silash Niroula for their Nepali translation, and also the staff of the EV‐K2‐CNR Research Station for their hospitality. Furthermore, we thank Frances Sobierajaski and Laurel Riske for their assistance with the data analysis.
Biography
Lydia L Simpson completed her undergraduate BSc degree in Sport and Exercise Sciences at the University of Birmingham in 2014 and was awarded an MSc in Human and Applied Physiology from Kings College London in 2016. Lydia joined Bangor University in 2016 as a PhD researcher, under the supervision of Dr Jonathan Moore. Her research investigates the adaptation of the sympathetic nervous system to chronic high altitude hypoxia, with a specific focus on the regulation of blood pressure by the sympathetic nervous system.

Edited by: Kim Barrett & Frank Powell
References
- Angell JE, De Burgh Daly M (1969). Cardiovascular responses in apnoeic asphyxia: role of arterial chemoreceptors and the modification of their effects by a pulmonary vagal inflation reflex. J Physiol 201, 87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM (2006). Andean, Tibetan, and Ethiopian patterns of adaptation to high‐altitude hypoxia. Integr Comp Biol 46, 18–24. [DOI] [PubMed] [Google Scholar]
- Beall CM (2007). Two routes to functional adaptation: Tibetan and Andean high‐altitude natives. Proc Natl Acad Sci U S A 104, 8655–8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, Ghiadoni L & Pratali L (2016). Vascular adaptation to extreme conditions: the role of hypoxia. Artery Res 14, 15–21. [Google Scholar]
- Busch SA, Davies HE, Van Diepen S, Simpson LL, Sobierajski F, Riske L, Stembridge M, Ainslie PN, Willie CK, Hoiland RL, Moore JP & Steinback CD (2017). Chemoreflex mediated arrhythmia during apnea at 5050m in low but not high altitude natives. J Appl Physiol 124, 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JAL, Boushel R, Robach P, Hellsten Y, Saltin B & Lundby C (2014). Chronic hypoxia increases arterial blood pressure and reduces adenosine and ATP induced vasodilatation in skeletal muscle in healthy humans. Acta Physiol 211, 574–584. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A & Wallin BG (1972a). Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand 84, 177–186. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A & Wallin BG (1972b). General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 85, 65–81. [DOI] [PubMed] [Google Scholar]
- Duplain H, Vollenweider L, Delabays A, Nicod P, Bärtsch P & Scherrer U (1999). Augmented sympathetic activation during short‐term hypoxia and high‐altitude exposure in subjects susceptible to high‐altitude pulmonary edema. Circulation 99, 1713–1718. [DOI] [PubMed] [Google Scholar]
- Eckberg DL & Eckberg MJ (1982). Human sinus node responses to repetitive, ramped carotid baroreceptor stimuli. Am J Physiol Heart Circ Physiol 242, H638–H644. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Kifle YT & Roberts VL (1980). Phase relationship between normal human respiration and baroreflex responsiveness. J Physiol 304, 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurum SC, Ghosh S, Janocha AJ, Xu W, Bauer S, Bryan NS, Tejero J, Hemann C, Hille R, Stuehr DJ, Feelisch M & Beall CM (2007). Higher blood flow and circulating NO products offset high‐altitude hypoxia among Tibetans. Proc Natl Acad Sci U S A 104, 17593–17598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JP, Flück D, Hilty MP & Lundby C (2018). Carotid chemoreceptor control of muscle sympathetic nerve activity in hypobaric hypoxia. Exp Physiol 103, 77–89. [DOI] [PubMed] [Google Scholar]
- Gilbert‐Kawai ET, Milledge JS, Grocott MPW & Martin DS (2014). King of the mountains: Tibetan and Sherpa physiological adaptations for life at high altitude. Physiology 29, 388–402. [DOI] [PubMed] [Google Scholar]
- Guyenet PG (2006). The sympathetic control of blood pressure. Nat Rev Neurosci 7, 335–346. [DOI] [PubMed] [Google Scholar]
- Halliwill JR & Minson CT (2002). Effect of hypoxia on arterial baroreflex control of heart rate and muscle sympathetic nerve activity in humans. J Appl Physiol 93, 857–864. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Morgan BJ & Charkoudian N (2003). Peripheral chemoreflex and baroreflex interactions in cardiovascular regulation in humans. J Physiol 552, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J & Sander M (2003). Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J Physiol 546, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EC, Wallin BG, Curry TB, Joyner MJ, Karlsson T & Charkoudian N (2011). Hysteresis in the sympathetic baroreflex: role of baseline nerve activity. J Physiol 589, 3395–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horscroft JA, Kotwica AO, Laner V, West JA, Hennis PJ, Levett DZH, Howard DJ, Fernandez BO, Burgess SL, Ament Z, Gilbert‐Kawai ET, Vercueli A, Landis BD, Mitchell K, Mythen MG, Branco C, Johnson RS, Feelisch M, Montgomery HE, Griffin JL, Grocott MPW, Gnaiger E, Martin DS, Murray AJ. (2017). Metabolic basis to Sherpa altitude adaptation. Proc Natl Acad Sci U S A 114, 6382–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt BE & Farquhar WB (2005). Nonlinearities and asymmetries of the human cardiovagal baroreflex. Am J Physiol Regul Integr Comp Physiol 288, R1339–R1346. [DOI] [PubMed] [Google Scholar]
- Kienbaum P, Karlsson T, Sverrisdottir YB, Elam M & Gunnar Wallin B (2001). Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol 531, 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis NCS, Bailey DM, duManoir GR, Messinger L, Lucas SJE, Cotter JD, Donnelly J, McEneny J, Young IS, Stembridge M, Burgess KR, Basnet AS & Ainslie PN (2014). Conduit artery structure and function in lowlanders and native highlanders: relationships with oxidative stress and role of sympathoexcitation. J Physiol 592, 1009–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby C, Calbet J, van Hall G, Saltin B & Sander M (2017). Sustained sympathetic activity in altitude acclimatizing lowlanders and high‐altitude natives. Scand J Med Sci Sports 1–8. [DOI] [PubMed] [Google Scholar]
- Mitchell KM, Bradbury KE, Posch AM, Beidleman BA, Fulco CS, Muza SR & Charkoudian N (2018). Influence of recent altitude exposure on sea level sympathetic neural & hemodynamic responses to orthostasis. Auton Neurosci Basic Clin 210, 18–23. [DOI] [PubMed] [Google Scholar]
- Moore JP, Hainsworth R & Drinkhill MJ (2011). Reflexes from pulmonary arterial baroreceptors in dogs: interaction with carotid sinus baroreceptors. J Physiol 589, 4041–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima N, Onimaru H, Yamagata A, Itoh S, Matsubara H, Imakiire T, Nishida Y, Kumagai H (2018). Erythropoietin, a putative neurotransmitter during hypoxia, is produced in RVLM neurons and activates them in neonatal Wistar rats. Am J Physiol Regul Integr Comp Physiol 314, R700–R708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querido JS, Wehrwein EA, Hart EC, Charkoudian N, Henderson WR & Sheel AW (2011). Baroreflex control of muscle sympathetic nerve activity as a mechanism for persistent sympathoexcitation following acute hypoxia in humans. Am J Physiol Regul Integr Comp Physiol 301, R1779–R1785. [DOI] [PubMed] [Google Scholar]
- Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA & Eckberg DL (1999). Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol Heart Circ Physiol 276, H1691–H1698. [DOI] [PubMed] [Google Scholar]
- Ryan BJ, Wachsmuth NB, Schmidt WF, Byrnes WC, Julian CG, Lovering AT, Subudhi AW & Roach RC (2014). Altitudeomics: rapid hemoglobin mass alterations with early acclimatization to and de‐acclimatization from 5260 m in healthy humans. PLoS ONE, 9, e108788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmanpour A, Brown LJ, Steinback CD, Usselman CW, Goswami R & Shoemaker JK (2011). Relationship between size and latency of action potentials in human muscle sympathetic nerve activity. J Neurophysiol 105, 2830–2842. [DOI] [PubMed] [Google Scholar]
- Schmidt EA, Despas F, Pavy‐Le Traon A, Czosnyka Z, Pickard JD, Rahmouni K, Pathak A & Senard JM (2018). Intracranial pressure is a determinant of sympathetic activity. Front Physiol 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback CD & Shoemaker KJ (2012). Differential regulation of sympathetic burst frequency and amplitude following acute hypoxia in humans. AJP Regul Integr Comp Physiol 303, R633–R638. [DOI] [PubMed] [Google Scholar]
- Steinback CD, Salzer D, Medeiros PJ, Kowalchuk J & Shoemaker JK (2009). Hypercapnic vs. hypoxic control of cardiovascular, cardiovagal, and sympathetic function. Am J Physiol Regul Integr Comp Physiol 296, R402–R410. [DOI] [PubMed] [Google Scholar]
- Sundlof G, Wallin BG (1978). Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol, 274, 621–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CE, Witter T, El Sayed K, Hissen SL, Johnson AW & Macefield VG (2015). Relationship between spontaneous sympathetic baroreflex sensitivity and cardiac baroreflex sensitivity in healthy young individuals. Physiol Rep, 3, e12536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usselman CW, Skow RJ, Matenchuk BA, Chari RS, Julian CG, Stickland MK, Davenport MH & Steinback CD (2015). Sympathetic baroreflex gain in normotensive pregnant women. J Appl Physiol 119, 468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie CK, Stembridge M, Hoiland RL, Tymo MM, Tremblay JC, Patrician A, Steinback C, MooreJ, Anholm J , Subedi P, Niroula S, Mcneil CJ, Mcmanus A, MacLeod DB & Ainslie PN (2018). UBC‐Nepal Expedition: an experimental overview of the 2016 University of British Columbia Scientific Expedition to Nepal Himalaya. PLoS ONE, 13, e0204660. [DOI] [PMC free article] [PubMed] [Google Scholar]


