Abstract
Aims
Residual beta‐cell secretion in type 1 diabetes is commonly assessed by area‐under‐curve of plasma C‐peptide concentration (AUCCpep) following mixed‐meal tolerance test (MMTT). We aimed to investigate alternative measures of beta‐cell responsiveness.
Methods
We analyzed data from 32 youth (age 7 to 17 years) undergoing MMTT within 6 months of type 1 diabetes diagnosis. We related AUCCpep with (a) validated mechanistic index of postprandial beta‐cell responsiveness M I accounting for glucose level during MMTT, and (b) pragmatic marker calculated as baseline plasma C‐peptide concentration corrected for baseline plasma glucose concentration.
Results
Postprandial responsiveness M I was correlated with age and BMI SDS (R s = 0.66 and 0.44, P < 0.01 and P < 0.05) and was more correlated with glycated hemoglobin than AUCCpep (R s = 0.79, P = 0.04). The pragmatic marker was highly correlated with AUCCpep (R s = 0.94, P < 0.01).
Conclusions
Postprandial responsiveness M I may be more relevant to glucose control than AUCCpep. Baseline C‐peptide corrected for baseline glucose appears to be a suitable surrogate of AUCCpep if MMTT is not performed.
Keywords: beta‐cell secretion, C‐peptide, mixed‐meal tolerance test, type 1 diabetes
1. INTRODUCTION
The area‐under‐curve of sequential C‐peptide concentrations (AUCCpep) during the mixed‐meal tolerance test (MMTT) is the gold‐standard method to assess residual beta‐cell (ie, insulin) secretion in type 1 diabetes.1 Traditionally, glucose excursions during the MMTT are not taken into account, although these impact on the magnitude of C‐peptide response.2
In this work, we re‐analyzed MMTT data obtained in newly diagnosed children and adolescents with type 1 diabetes aged 7 to 17 years2 (a) to identify surrogate mechanistic and pragmatic markers of AUCCpep and (b) to explore the relationships among demographic and clinical factors, and AUCCpep and its surrogate markers.
2. METHODS
We analyzed data obtained from 32 participants with newly diagnosed type 1 diabetes (age 12.4[2.9] years, 12 males, HbA1c 6.8%[1.1], BMI SDS 0.62[1.02], total daily dose of insulin 0.57[0.23] U/kg; mean [SD]) who underwent MMTT within 6 months of diagnosis (mean time since diagnosis 142[38] days).2 The National Research Ethics Committee East of England‐Cambridge South approved the study.
All participants (aged ≥16 years) gave informed consent, and children <16 years gave assent and their parents gave informed consent to the study procedures.
The MMTT was performed following an overnight fast, with no food or drink other than water from midnight, and at baseline glucose levels between 4 and 11.1 mmol/L. Long‐acting insulin and basal rates for insulin pump users were continued as normal. The use of rapid‐acting insulin bolus was acceptable up to 2 hours before the MMTT and the use of short‐acting insulin bolus up to 6 hours before the MMTT. Participants ingested 6 mL/kg of Boost meal solution (maximum 360 mL), within 10 minutes. Blood samples for the measurement of C‐peptide and glucose were collected 10 minutes prior to the meal (−10 minutes), at the time of ingestion (0 minutes), and at 15, 30, 60, 90 and 120 minutes.
Plasma C‐peptide was assayed in singleton on a Diasorin Liaison XL automated immunoassay analyzer using a one‐step chemiluminescence immunoassay (Diasorin S.p.A, 13040 Saluggia [VC], Italy). Glucose levels were analyzed via an adaption of the hexokinase‐glucose‐6‐phosphate dehydrogenase method.3 HbA1c was analyzed on the Tosoh G8 High Performance Liquid Chromatography Analyzer using the gold standard ion‐exchange method with <2% between‐batch imprecision (Tosoh Bioscience, Inc., South San Francisco, CA).
AUCCpep and incremental IAUCCpep was calculated using the trapezoidal method. A compartment model validated in normal subjects and those with newly diagnosed type 2 diabetes4 of C‐peptide kinetics was used to estimate two mechanistic indices of pancreatic beta‐cell responsiveness: (a) postprandial responsiveness M I (ability of postprandial glucose to stimulate beta‐cell insulin secretion; a change in plasma glucose by 1 mmoL/L results in a change in the C‐peptide secretion by M I pmol/L/min) and (b) basal responsiveness M 0 (ability of fasting glucose to stimulate beta‐cell insulin secretion; M 0 approximates fasting C‐peptide divided by the fasting plasma glucose concentration) (see Supporting Information, Appendix S1 ).4 Baseline plasma C‐peptide concentration divided by the baseline plasma glucose concentration was calculated as a pragmatic marker of beta‐cell function.
The Spearman rank correlation coefficient was used to explore relationships between age, BMI SDS (body mass index SD score), HbA1c, total daily dose of insulin, baseline plasma C‐peptide, AUCC pep, baseline plasma C‐peptide divided by baseline plasma glucose, IAUCCpep, insulin dose‐adjusted A1C (IDAA1C) defined as A1C (percent) + [4 × insulin dose (units per kilogram per 24 hours)],5 M 0 and M I. Fisher's r‐to‐z transformation was applied for testing the difference between two Spearman rank correlation coefficients.6 P values less than 0.05 were considered statistically significant. As these are post hoc evaluations, all observations are considered exploratory. Statistical analyses were performed using SPSS, version 21 (IBM Software, Hampshire, UK). Data are reported as mean (SD) or median [interquartile range], unless stated otherwise.
3. RESULTS
The postprandial and fasting beta‐cell responsiveness M I and M 0 were estimated at 3.3[1.6‐5.4] and at 3.1[2.0‐4.5] 10−9/minutes, respectively. Figure S1 shows a sample model fit to measured plasma C‐peptide including measurements of plasma glucose (the forcing function). Figure S2 depicts weighted residuals across all participants demonstrating acceptable fit of the model to plasma C‐peptide measurements.
Table 1 reports the Spearman rank correlation among demographic and clinical factors, AUCCpep and its surrogate markers. The strongest correlations found for age and BMI SDS were with M I (RS = 0.66 and 0.44, respectively, P < 0.01 and P < 0.05, respectively). The total daily dose of insulin was inversely correlated with M 0 (R S = −0.42, P < 0.05) and baseline C‐peptide over baseline glucose (R S = −0.38, P < 0.05).
Table 1.
Spearman rank correlation between demographic/clinical factors and markers of beta‐cell responsiveness (N = 32)
| Age (y) | HbA1c (%) | BMI SDS | TDD (U/kg) | AUCCpep (pmol/L/min) | Baseline C‐peptide (pmol/L) | Baseline C‐peptide over baseline glucose | M 0 (/min) | M I (/min) | IDAA1c | IAUCCpep (pmol/L/min) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y) | 1.00 | −0.34 | 0.14 | −0.08 | 0.46 ** | 0.27 | 0.31 | 0.27 | 0.66 ** | −0.31 | 0.50 |
| HbA1c (%) | 1.00 | 0.01 | −0.01 | −0.19 | −0.08 | −0.13 | −0.15 | −0.36 * | 0.72 ** | −0.14 | |
| BMI SDS | 1.00 | −0.01 | 0.41 * | 0.38 * | 0.41 * | 0.36 * | 0.44 * | −0.18 | 0.69 ** | ||
| TDD (U/kg) | 1.00 | −0.32 | −0.27 | −0.38 * | −0.42 * | −0.28 | 0.64 ** | −0.22 | |||
| AUCCpep (pmol/L/min) | 1.00 | 0.88 ** | 0.94 ** | 0.92 ** | 0.79 ** | −0.36 | 0.99 ** | ||||
| Baseline C‐peptide (pmol/L) | 1.00 | 0.95 ** | 0.89 ** | 0.54 ** | −0.24 | 0.87 ** | |||||
| Baseline C‐peptide over baseline glucose | 1.00 | 0.94 ** | 0.67 ** | −0.35 | 0.91 ** | ||||||
| M 0 (/min) | 1.00 | 0.63 ** | −0.37 | 0.89 ** | |||||||
| M I (/min) | 1.00 | −0.48 | 0.79 ** | ||||||||
| IDAA1c | 1.00 | −0.28 | |||||||||
| IAUCCpep (pmol/L/min) | 1.00 |
P < 0.05,
P < 0.01. Significant correlations are shown in boldface.
Abbreviations: BMI SDS, body mass index SD score; IDAA1c, insulin‐dose adjusted HbA1c; IAUCCpep, incremental area‐under‐curve of C‐peptide; TDD, total daily dose of insulin.
Figure 1 demonstrates that AUCCpep has a stronger correlation with baseline C‐peptide corrected for baseline glucose (R S = 0.94) than baseline C‐peptide per se (R S = 0.88). Figure S3 relates baseline HbA1c vs AUCCpep and log‐transformed M I. AUCCpep was not correlated with HbA1c (R S = −0.19, P = NS) whereas M I is (R S = −0.36, P < 0.05); the difference between the two correlation coefficients is statistically significant (P = 0.04).
Figure 1.
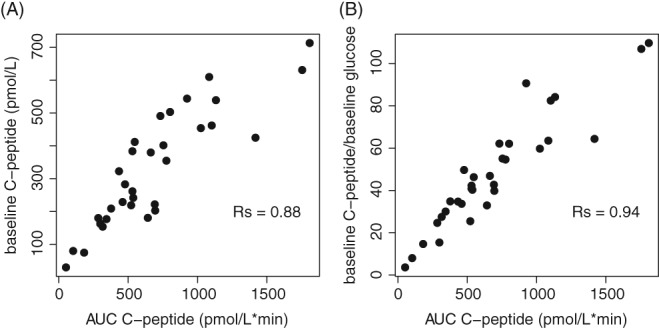
The scatter plot of AUCCpep vs baseline C‐peptide (A) and AUCCpep vs baseline C‐peptide corrected for baseline glucose level (B)
4. DISCUSSION
The present analysis demonstrates the feasibility of using a model of C‐peptide kinetics to assess residual beta‐cell function during MMTT in newly‐diagnosed type 1 diabetes. Traditionally, the AUCCpep during a MMTT has not been corrected for glucose excursions, which are likely to affect the amplitude of the C‐peptide response.2 Our data show that using more advanced measures of beta‐cell function, such as M I and M 0, can identify meaningful correlations with clinical parameters such as TDD and HbA1c, which are not identified using uncorrected AUCCpep. In addition, we show baseline C‐peptide corrected for baseline glucose to be a surrogate marker of AUCCpep.
The basal responsiveness M 0 and the postprandial responsiveness M I were estimated at median 3.3 and median 3.1 10−9/min, respectively. These values are considerably smaller than those estimated in normal subjects where M 0 were estimated at a mean of 10.3 and M I at 90.0 10−9/min.7 In two subjects, M I was estimated at zero and in one subject M 0 at zero due to the lack of increased C‐peptide levels post‐meal and undetectable C‐peptide level at baseline. These estimations are clinically meaningful as individuals with complete basal and postprandial insulin responsiveness can be identified.
The positive correlations between age and M I, and between BMI SDS and M I suggest that postprandial responsiveness is more preserved in older and heavier children and adolescents with newly‐diagnosed type 1 diabetes than the younger and lighter individuals.
Figure 1 demonstrates that baseline C‐peptide corrected for baseline glucose is highly correlated with AUCCpep and could be used as a surrogate marker of insulin secretion instead of AUCCpep. A previous study has shown the plausibility of using 90‐min‐stimulated C‐peptide concentration or baseline C‐peptide as a substitute for AUCCpep to represent insulin secretion with a similar correlation coefficient RS = 0.96 but in a larger population (N = 421).8 Data from the present analysis suggest that baseline C‐peptide corrected for baseline glucose may be a more appropriate marker than baseline C‐peptide and a more cost‐effective marker than the stimulated C‐peptide concentration sidestepping the need for MMTT and complexity of the assessment.
We show that M I was more tightly correlated with HbA1c than AUCCpep (P = 0.04) indicating that M I may be a more clinically relevant marker of C‐peptide secretion than AUCCpep. The study is limited by a relatively small sample size. Further analyses with larger datasets and longitudinal evaluations are warranted. We applied parameters of C‐peptide kinetics determined in healthy subjects. As C‐peptide is eliminated primarily by the kidney and assuming comparable kidney function among healthy individuals and those with recently diagnosed type 1 diabetes, we consider this limitation to be of little significance to our findings.
Alternative C‐peptide secretion models assume a more complex relationship between glucose concentration and insulin secretion compared to the model used in the present study.9, 10 These alternative models may provide additional information about C‐peptide secretory characteristics but require more frequent sampling. Our parameter M I represents the dominant relationship between plasma glucose and C‐peptide secretion and is accounted for in the alternative approaches.9, 10
In conclusion, baseline C‐peptide corrected for baseline glucose may be a suitable surrogate marker of residual beta‐cell in newly‐diagnosed type 1 diabetes. Postprandial pancreatic responsiveness estimated through a model of C‐peptide kinetics appears more relevant to glucose control than the conventional area‐under‐curve of plasma C‐peptide concentration following MMTT.
CONFLICTS OF INTEREST
R.H. reports having received speaker honoraria from Eli Lilly and Novo Nordisk, serving on advisory panel for Eli Lilly and Novo Nordisk, receiving license fees from BBraun and Medtronic; and having served as a consultant to BBraun. M.E.W. has received license fees from Becton Dickinson and has served as a consultant to Beckton Dickinson. Y.R., M.T., R.H.W. and D.B.D. declare no competing financial interests exist.
Supporting information
Appendix S1. Model of C‐peptide kinetics during mixed‐meal tolerance test.
Figure S1. A sample model fit from a participant undergoing MMTT (meal ingested at time 0 minutes). The green solid line with circles represents the measured plasma C‐peptide; the blue dashed line with triangles represents model fit; the red solid line with crosses represents the measured plasma glucose (the forcing function).
Figure S2. Normalized residuals (weighted by the measurement error with 6% coefficient of variation) of model fit to the plasma C‐peptide concentration (mean ± SD, N = 32).
Figure S3. Scatter plot of baseline HbA1c vs AUCCpep (left panel) and scatter plot of baseline HbA1c vs log‐transformed postprandial pancreatic responsiveness MI (right panel) (R S, Spearman correlation coefficient).
ACKNOWLEDGEMENTS
We thank all participants and parents for participating in the study. We greatly acknowledge Katrin Mooslehner, Radka Platte, Karen Whitehead and Di Wingate for their dedicated help with sample processing; Kayleigh Aston, Kimberley Dale, Andy Kempa, Clare Megson, Monica Mitchell and Criona O'Brien, research nurses, the staff at the National Institute for Health Research (NIHR)/Wellcome Trust Clinical Research Facility Cambridge and NIHR/Wellcome Trust Clinical Research Facility, Birmingham Children's Hospital, for recruitment of participants, assistance and help with data collection. This study was funded by National Institute for Health Research Cambridge Biomedical Research Centre, JDRF, Wellcome Trust Strategic Award (100574/Z/12/Z), Efficacy and Mechanism Evaluation Programme National Institute for Health Research (14/23/09), Horizon 2020 (SC1‐731560). Novo Nordisk UK Research Foundation, NIHR Cambridge Biomedical Research Centre, JDRF (9‐2011‐253/5‐SRA‐2015‐130‐A‐N), the Wellcome Trust (WT091157/107212 and WT083650/Z/07/Z) and the Innovative Medicines Initiative 2 Joint Undertaking (grant agreement No 115797 [INNODIA]). This Joint Undertaking receives support from the Union's Horizon 2020 research and innovation programme and “EFPIA”, “JDRF” and “The Leona M. and Harry B. Helmsley Charitable Trust”.
AUTHOR CONTRIBUTIONS
Y.R. and R.H. had complete access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Y.R. and R.H. carried out data analysis. Y.R. and R.H. wrote the manuscript. All authors critically reviewed the report.
Ruan Y, Willemsen RH, Wilinska ME, Tauschmann M, Dunger DB, Hovorka R. Mixed‐meal tolerance test to assess residual beta‐cell secretion: Beyond the area‐under‐curve of plasma C‐peptide concentration. Pediatr Diabetes. 2019;20:282–285. 10.1111/pedi.12816
Funding information Efficacy and Mechanism Evaluation Programme National Institute for Health Research, Grant/Award Number: 14/23/09; Horizon 2020 Framework Programme, Grant/Award Number: SC1‐731560; Wellcome Strategic Award , Grant/Award Number: 100574/Z/12/Z; The Leona M. and Harry B. Helmsley Charitable Trust; JDRF; EFPIA; Innovative Medicines Initiative 2 Joint Undertaking, Grant/Award Number: 115797; the Wellcome Trust, Grant/Award Number: WT083650/Z/07/Z, WT091157/107212; NIHR Cambridge Biomedical Research Centre; Novo Nordisk UK Research Foundation; Horizon 2020; JDRF, Grant/Award Number: 9‐2011‐253/5‐SRA‐2015‐130‐A‐N; National Institute for Health Research Cambridge Biomedical Research Centre
REFERENCES
- 1. Palmer JP, Fleming GA, Greenbaum CJ, et al. C‐peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta‐cell function: report of an ADA workshop, 21‐22 October 2001. Diabetes. 2004;53(1):250‐264. [DOI] [PubMed] [Google Scholar]
- 2. Willemsen RH, Burling K, Barker P, et al. Frequent monitoring of C‐peptide levels in newly diagnosed type 1 subjects using dried blood spots collected at home. J Clin Endocrinol Metab. 2018;103(9):3350‐3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kunst A, Drager B, Ziegenhorn J. UV methods with hexokinase and glucose‐6‐phosphate 456 dehydrogenase Methods of Enzymatic Analysis. Deerfield, FL: Verlag Chemie; 1983:163‐172. [Google Scholar]
- 4. Albarrak AI, Luzio SD, Chassin LJ, Playle RA, Owens DR, Hovorka R. Associations of glucose control with insulin sensitivity and pancreatic beta‐cell responsiveness in newly presenting type 2 diabetes. J Clin Endocrinol Metab. 2002;87(1):198‐203. [DOI] [PubMed] [Google Scholar]
- 5. Mortensen HB, Hougaard P, Swift P, et al. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care. 2009;32(8):1384‐1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Myers L, Sirois JM. Spearman Correlation Coefficients, Differences Between Encyclopedia of Statistical Sciences. Vol 12 Hoboken, N.J.: John Wiley and Sons, Inc; 2006. [Google Scholar]
- 7. Hovorka R, Chassin L, Luzio SD, Playle R, Owens DR. Pancreatic beta‐cell responsiveness during meal tolerance test: model assessment in normal subjects and subjects with newly diagnosed noninsulin‐dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83(3):744‐750. [DOI] [PubMed] [Google Scholar]
- 8. Besser RE, Shields BM, Casas R, Hattersley AT, Ludvigsson J. Lessons from the mixed‐meal tolerance test: use of 90‐minute and fasting C‐peptide in pediatric diabetes. Diabetes Care. 2013;36(2):195‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mari A, Schmitz O, Gastaldelli A, Oestergaard T, Nyholm B, Ferrannini E. Meal and oral glucose tests for assessment of beta‐cell function: modeling analysis in normal subjects. Am J Physiol Endocrinol Metab. 2002;283(6):E1159‐E1166. [DOI] [PubMed] [Google Scholar]
- 10. Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes. 2014;63(4):1203‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Model of C‐peptide kinetics during mixed‐meal tolerance test.
Figure S1. A sample model fit from a participant undergoing MMTT (meal ingested at time 0 minutes). The green solid line with circles represents the measured plasma C‐peptide; the blue dashed line with triangles represents model fit; the red solid line with crosses represents the measured plasma glucose (the forcing function).
Figure S2. Normalized residuals (weighted by the measurement error with 6% coefficient of variation) of model fit to the plasma C‐peptide concentration (mean ± SD, N = 32).
Figure S3. Scatter plot of baseline HbA1c vs AUCCpep (left panel) and scatter plot of baseline HbA1c vs log‐transformed postprandial pancreatic responsiveness MI (right panel) (R S, Spearman correlation coefficient).


