Summary
Background
Evidence of the comparative effectiveness of biological therapies for psoriasis on health‐related quality of life (HRQoL) in routine clinical practice is limited.
Objectives
To examine the comparative effectiveness of adalimumab, etanercept and ustekinumab on HRQoL in patients with psoriasis, and to identify potential predictors for improved HRQoL.
Methods
This was a prospective cohort study in which changes in HRQoL were assessed using the Dermatology Life Quality Index (DLQI) and EuroQoL‐5D (EQ‐5D) at 6 and 12 months. Multivariable regression models were developed to identify factors associated with achieving a DLQI of 0/1 and improvements in the EQ‐5D utility score.
Results
In total, 2152 patients with psoriasis were included, with 1239 patients on adalimumab, 517 on etanercept and 396 on ustekinumab; 81% were biologic naïve. For the entire cohort, the median (interquartile range) DLQI and EQ‐5D improved from 18 (13–24) and 0·73 (0·69–0·80) at baseline to 2 (0–7) and 0·85 (0·69–1·00) at 6 months, respectively (P < 0·001). Similar improvements were achieved at 12 months. At 12 months, multivariable regression modelling showed that female sex, multiple comorbidities, smoking and a higher DLQI or a lower EQ‐5D utility score at baseline predicted a lower likelihood of achieving a DLQI of 0/1 or improvement in the EQ‐5D. Compared with adalimumab, patients receiving etanercept, but not ustekinumab, were less likely to achieve a DLQI of 0/1. There was no significant difference between the biological therapies in EQ‐5D improvement.
Conclusions
In routine clinical practice biological therapies produce marked improvement in HRQoL, which is influenced by the choice of biological therapy, baseline impairment in HRQoL, lifestyle characteristics and comorbidities. These findings should help inform selection of optimal biological therapy for patients related to improvements in HRQoL.
Short abstract
What's already known about this topic?
Evidence of the comparative effectiveness of biological therapies for psoriasis on health‐related quality of life (HRQoL) in routine clinical practice is limited.
Earlier observational studies were either cross‐sectional, thereby limiting the ability to compare changes in HRQoL, or cohort studies that have not taken into account important clinical factors that could influence treatment response, such as alterations in dosing regimens of biological therapies and the concomitant use of conventional systemic treatments for psoriasis.
What does this study add?
This large prospective cohort study found that in routine clinical practice, the use of biological therapies for psoriasis was associated with marked improvements in HRQoL over 12 months.
These improvements were influenced by the choice of biological therapy, baseline impairment in HRQoL, lifestyle characteristics and comorbidities.
Compared with adalimumab, patients receiving etanercept were less likely to achieve a DLQI of 0/1, but there was no significant difference between ustekinumab and adalimumab in the proportion of patients achieving a DLQI of 0/1.
There was no significant difference between the three biological therapies in level of improvement in the EQ‐5D.
Linked Comment: Finlay. Br J Dermatol 2017; 177:1164–1165.
Psoriasis is a chronic immune‐mediated inflammatory skin disorder, affecting approximately 0·9–8·5% of the population worldwide.1 Many patients with psoriasis have moderate‐to‐severe disease that profoundly impacts their emotional wellbeing and health‐related quality of life (HRQoL),2, 3 with levels of physical and mental disability comparable with those reported for other major medical disorders such as cancer, diabetes and cardiovascular disease.4, 5 Furthermore, patients with psoriasis have an increased risk of developing comorbid conditions such as psoriatic arthritis (PsA), which can also adversely affect their HRQoL.6
Biological therapies have revolutionized the treatment of moderate‐to‐severe psoriasis. The impact of these therapies on HRQoL has been reported in large randomized controlled trials (RCTs).7, 8, 9, 10, 11, 12, 13 However, there is a lack of head‐to‐head comparative RCTs assessing the longer‐term impact of these therapies on improvements in HRQoL.12, 14 Several meta‐analyses have compared the clinical efficacy of different biological therapies for psoriasis, but the results pertain largely to short‐term outcomes and do not always reflect findings in clinical practice.15, 16, 17, 18, 19, 20, 21
The effectiveness of biological therapies on disease activity in routine clinical practice has been demonstrated in several prospective observational cohort studies, with up to 80% of patients achieving at least a 75% improvement in the Psoriasis Area and Severity Index (PASI 75).22, 23, 24, 25, 26, 27, 28, 29 However, evidence of the effectiveness of biological therapies on HRQoL in routine clinical practice is limited to a few observational studies that were either cross‐sectional, thereby limiting the ability to compare changes in HRQoL,30, 31 or cohort studies that did not take into account important clinical factors that could influence treatment response.32, 33 Such factors include alterations in dosing regimens of biological therapies over time and the concomitant use of conventional systemic therapies for psoriasis.
The British Association of Dermatologists Biologic Interventions Register (BADBIR) is a U.K. and Republic of Ireland prospective, longitudinal pharmacovigilance register of patients with psoriasis receiving either biological or conventional systemic therapies. Due to its large size, rigorous data collection process, detailed collection of patient demographic characteristics and treatment regimens, and high external validity through participation of 153 dermatology centres,34 the register represents an ideal resource to assess the impact of biological therapies on HRQoL in patients with psoriasis in routine clinical practice. In this longitudinal observational study, we examined the comparative effectiveness of adalimumab, etanercept and ustekinumab on improvements in HRQoL in patients with psoriasis, and identified factors associated with these improvements.
Materials and methods
The BADBIR, established in September 2007, compares a cohort of patients with psoriasis on biological therapies to a similar cohort on conventional systemic therapies. Full details on the design of the BADBIR and the disease characteristics of its participants have been published previously.34, 35
Baseline assessment
Baseline data were collected with patient consent and included patients’ demographic characteristics and comorbidities, year of disease onset, standardized measures of health status using self‐reported outcome measures [Dermatology Life Quality Index (DLQI) and EuroQoL‐5D (EQ‐5D)], and detailed information about the patients’ current and previous treatment for psoriasis. Details of the comorbidities were classified using the Medical Dictionary for Regulatory Activities system.36
Follow‐up assessments
Data from patients were collected 6 monthly during the study period. Details of the biological therapies, including any change in the dose or therapy, and start and stop dates, were recorded. Information on any new concomitant systemic therapies for psoriasis and their start and stop dates were also captured. Patient questionnaires also recorded DLQI and EQ‐5D at 6‐ and 12‐month follow‐up.
Study population
Subjects in this study were selected from the August 2015 data cut‐off. Hence the study time‐frame was from September 2007 to August 2015. Adult patients with chronic plaque psoriasis, receiving adalimumab, etanercept or ustekinumab with follow‐up data of ≥ 6 months were included. The start of observation time was the start date of the index biological therapy (therapy received at enrolment). Only the first biological therapy started during registry participation was analysed. Patients were classified as either biologic naïve or non‐naïve based on their previous exposure to biological therapies prior to registration into the BADBIR. Evaluations were limited to patients who had a valid baseline DLQI (no more than one question left unanswered) and/or EQ‐5D questionnaire (fully completed) recorded within 6 months prior to the start of the index biological therapy and who had another completed questionnaire recorded within 4–8 months and/or 10–14 months (representing the 6‐ and 12‐month follow‐ups, respectively) after the start of the index biological therapy (Fig. S1; see Supporting Information).
Outcome measures
The DLQI consists of 10 questions evaluating the impact of skin disease on six aspects of HRQoL: symptoms and feelings, daily activities, leisure, work or school performance, personal relationships and treatment.37, 38 The total score ranges from 0 to 30, with a score of 0–1 indicating no impairment in HRQoL and higher scores indicating greater impairment.39 A decrease of ≥ 4 points is considered clinically meaningful.40
The EQ‐5D consists of five dimensions that define health: mobility, self‐care, activities, pain/discomfort and anxiety/depression.41 Responses to questions yield a utility score that ranges from –0·59–1·00, where 0 represents death, 1 represents full health, and negative values represent health states that are valued as worse than death,42 with a change of 0·05 points considered clinically meaningful.43
Statistical analyses
The primary outcome measures were the change in (i) the DLQI total and individual domain scores and (ii) the EQ‐5D profile and utility score from baseline to 6 and 12 months. The proportion of patients who achieved a DLQI of 0/1 at each time point was also assessed. Secondary outcomes included the proportion of patients who achieved an improvement of ≥ 4 and ≥ 0·05 points in the DLQI and EQ‐5D utility scores, respectively, at 6 and 12 months.
Patients were assigned to one of three unique biological cohorts based on their index biological therapy, and recorded as either biologic naïve or non‐naïve. The Wilcoxon signed‐rank test was performed to examine differences in the DLQI total and domain scores and EQ‐5D utility score between baseline and follow‐up results. The McNemar χ2‐test was used to examine differences in the proportion of patients reporting any problems in EQ‐5D dimensions between baseline and follow‐up results.
Predictors of change in the EQ‐5D utility score and likelihood of achieving a DLQI of 0/1 were identified at 6 and 12 months using linear and logistic regression models, respectively. An a priori list of covariates was determined to examine potential predictors of response (as presented in Table 5 ). Adalimumab (the most commonly prescribed biological therapy in the BADBIR) was used as the reference biological therapy to which the others were compared.44 Concurrent use of methotrexate, ciclosporin and/or other conventional systemic therapies was analysed as a binary variable (ever exposed/never exposed) throughout the study. Dosing patterns of biological therapy were examined using the time‐trend method, which compares the annual cumulative dose patients received to the annual recommended cumulative dose according to product prescribing information.45
Table 5.
Multivariable regression analyses of potential factors associated with achieving a Dermatology Life Quality Index (DLQI) of 0/1 and changes in the EuroQol‐5D (EQ‐5D) utility score at 6 and 12 months (Intention‐to‐treat analysis)
| Achieving a DLQI of 0 or 1a | Change in the EQ‐5D utility scoreb | |||
|---|---|---|---|---|
| 6 months | 12 months | 6 months | 12 months | |
| Demographics | ||||
| Age (years)c | 0·97 (0·87–1·09) | 1·04 (0·92–1·19) | −0·016 (−0·029 to −0·004)* | –0·015 (–0·027 to –0·002)* |
| Female | 0·91 (0·72–1·15) | 0·71 (0·54–0·93)* | −0·019 (−0·046 to 0·008) | 0·005 (−0·025 to 0·035) |
| Obesity statusd | ||||
| Obese (BMI ≥ 30 kg m−2) | 0·76 (0·60 to 0·96)* | 0·78 (0·60 to 1·02) | −0·036 (−0·062 to −0·010)* | −0·012 (−0·041 to 0·018) |
| Missing | 1·00 (0·59 to 1·71) | 0·99 (0·58 to 1·69) | 0·012 (−0·048 to 0·072) | −0·004 (−0·075 to 0·067) |
| Smoking statuse | ||||
| Ex‐smoker | 0·94 (0·70 to 1·26) | 0·77 (0·55 to 1·07) | −0·019 (−0·050 to 0·012) | 0·006 (−0·029 to 0·041) |
| Current smoker | 0·83 (0·61 to 1·13) | 0·61 (0·43 to 0·87)* | −0·047 (−0·081 to −0·012)* | −0·021 (−0·061 to 0·019) |
| Missing | 0·80 (0·56 to 1·13) | 0·66 (0·45 to 0·98)* | −0·014 (−0·053 to 0·025) | 0·016 (−0·029 to 0·061) |
| Comorbiditiesf | ||||
| Psoriatic arthritis | 1·15 (0·86 to 1·52) | 1·09 (0·79 to 1·49) | −0·049 (−0·083 to −0·014)* | −0·077 (−0·120 to −0·034)* |
| 1–2 comorbidities | 0·84 (0·64 to 1·10) | 0·51 (0·37 to 0·70)* | −0·005 (−0·023 to 0·034) | −0·019 (−0·051 to 0·013) |
| 3–4 comorbidities | 0·66 (0·46 to 0·95)* | 0·49 (0·32 to 0·75)* | −0·055 (−0·100 to −0·011)* | −0·057 (−0·107 to −0·007)* |
| ≥ 5 comorbidities | 0·61 (0·34 to 1·11) | 0·39 (0·20 to 0·75)* | −0·158 (−0·232 to −0·084)* | −0·147 (−0·217 to −0·076)* |
| Disease | ||||
| Disease duration (years)c | 1·14 (1·02 to 1·27)* | 1·12 (0·99 to 1·26) | −0·002 (−0·014 to 0·010) | −0·010 (−0·024 to 0·003) |
| Baseline DLQI | 0·98 (0·96 to 0·99)* | 0·96 (0·95 to 0·98)* | – | – |
| Baseline EQ–5Dg | – | – | 0·037 (0·031 to 0·043)* | 0·040 (0·034 to 0·046)* |
| Biologic naïveh | 1·23 (0·91 to 1·67) | 1·17 (0·83 to 1·64) | 0·055 (0·016 to 0·094)* | 0·014 (−0·025 to 0·053) |
| Concomitant methotrexatei | 0·64 (0·46 to 0·88)* | 0·53 (0·38 to 0·75)* | −0·036 (−0·073 to 0·002) | −0·009 (−0·046 to 0·029) |
| Concomitant ciclosporini | 0·58 (0·36 to 0·94)* | 0·70 (0·42 to 1·15) | 0·002 (−0·049 to 0·054) | 0·004 (−0·053 to 0·061) |
| Concomitant other systemicsi,j | 0·64 (0·35 to 1·18) | 0·60 (0·32 to 1·10) | −0·006 (−0·070 to 0·058) | −0·007 (−0·076 to 0·062) |
| Dosing patternk | ||||
| CD > RCD | 1·03 (0·63 to 1·69) | 0·74 (0·43 to 1·29) | 0·026 (−0·024 to 0·075) | −0·035 (−0·092 to 0·022) |
| CD < RCD | 0·65 (0·38 to 1·12) | 0·76 (0·46 to 1·26) | −0·070 (−0·138 to −0·001)* | 0·003 (−0·055 to 0·061) |
| Missing | 0·92 (0·57 to 1·48) | 0·87 (0·50 to 1·52) | 0·048 (−0·004 to 0·099) | 0·028 (−0·034 to 0·090) |
| Biological therapyl | ||||
| Etanercept | 0·37 (0·28 to 0·50)* | 0·39 (0·28 to 0·54)* | −0·031 (−0·062 to 0·001) | 0·0003 (−0·034 to 0·035) |
| Ustekinumab | 0·86 (0·59 to 1·25) | 0·89 (0·57 to 1·37) | −0·026 (−0·069 to 0·016) | −0·030 (−0·078 to 0·019) |
| Stopped index biological therapym | 0·16 (0·08 to 0·32)* | 0·35 (0·20 to 0·60)* | −0·077 (−0·151 to −0·002)* | −0·059 (−0·120 to 0·002) |
BMI, body mass index; CD, cumulative dose; RCD, annual recommended cumulative dose. aData are presented as odds ratio (95% confidence interval). bData are presented as regression coefficient (95% confidence interval). cTo evaluate odds ratios and regression coefficients for every 10‐year increase in age and disease duration at enrolment into the register, baseline continuous variables of age and disease duration were transformed to age and disease duration divided by 10. At 6 and 12 months, older age at enrolment (by 10 years) was associated with lower improvement in EQ‐5D values, and longer disease duration (by 10 years) was associated with higher odds of achieving a DLQI of 0/1. dReference category: nonobese (BMI < 30 kg m−2). eReference category: never smoker. fReference category: no comorbidities (excluding psoriatic arthritis). gTo evaluate regression coefficients for every 0·1 point increase in the EQ‐5D utility score, the baseline continuous variable of EQ‐5D utility score was transformed to EQ‐5D multiplied by 10. At 6 and 12 months, higher baseline EQ‐5D utility score (by 0·1 points) was associated with higher EQ‐5D values. hReference category: biologic non‐naïve patients. iIncluded as a yes/no variable, where ‘yes’ = ‘ever used the systemic therapy concomitantly with the biological therapy during the specified time period’ and ‘no’ = ‘never used systemic therapies concomitantly with the biological therapy during the specified time period’. jIncludes any of acitretin, fumaric acid esters and hydroxycarbamide. kReference category CD equal to the RCD; the RCDs according to National Institute for Health and Care Excellence guidelines were:1300 mg (50 mg × 26 weeks) for etanercept; 600 mg [80 mg + (40 mg × 13 weeks)] for adalimumab; and 180 mg (45 mg × 4 doses), or 360 mg (90 mg × 4 doses) if > 100 kg, for ustekinumab at 6 months and 2600 mg (50 mg × 52 weeks) for etanercept; 1120 mg [80 mg + (40 mg × 26 weeks)] for adalimumab; and 270 mg (45 mg × 6 doses), or 540 mg (90 mg × 6 doses) if > 100 kg, for ustekinumab at 12 months. The CD a patient received over the first 6 and 12 months of therapy was calculated as a time‐varying variable taking into consideration any gaps in treatment. lReference category: adalimumab. mReference category: continuous users of the index biological therapy. *P < 0·05.
The DLQI and EQ‐5D analyses were conducted primarily on an intention‐to‐treat basis, using any questionnaire recorded at the appropriate time points after the start of the index biological therapy whether or not the patient was still taking the same biological therapy. Sensitivity analyses in which patients who remained on their index biological therapy when the questionnaires were recorded were also conducted (treatment completers only). Given the large cohort studied and multiple statistical tests, a threshold of P ≤ 0·01 was considered to be statistically significant. All calculations were performed using Stata v.14·0 (StataCorp, College Station, TX, U.S.A.).
Results
In total, 2152 patients with psoriasis (adalimumab 1239, etanercept 517 and ustekinumab 396) were included (Fig. S1; see Supporting Information). The mean (± SD) age of patients, and disease duration were 45·2 ± 12·4 years and 22·4 ± 12·1 years, respectively; 39·4% were female. Mean body mass index (BMI) was 31·1 ± 7·3 kg m−2, with 46·9% having a BMI ≥ 30 kg m−2. Overall, 73·4% of patients had one or more comorbidities. Baseline demographic and disease characteristics are summarized in Table 1.
Table 1.
Patient demographic and disease characteristics
| All patientsa | Etanercept | Adalimumab | Ustekinumab | |
|---|---|---|---|---|
| n (%) | 2152 | 517 (24·0) | 1239 (57·6) | 396 (18·4) |
| Demographic characteristics | ||||
| Age (years), mean ± SD | 45·2 ± 12·4 | 45·1 ± 12·1 | 44·8 ± 12·4 | 46·7 ± 12·3 |
| Female | 847 (39·4) | 217 (42·0) | 485 (39·1) | 145 (36·6) |
| BMI category, kg m−2 | ||||
| Nonobese (BMI < 30) | 1011 (47·0) | 261 (50·5) | 582 (47·0) | 168 (42·4) |
| Obese (BMI ≥ 30) | 1009 (46·9) | 226 (43·7) | 590 (47·6) | 193 (48·7) |
| Missing | 132 (6·1) | 30 (5·8) | 67 (5·4) | 35 (8·8) |
| Smoking status | ||||
| Never smoked | 599 (27·8) | 132 (25·5) | 358 (28·9) | 109 (27·5) |
| Ex‐smoker | 648 (30·1) | 131 (25·3) | 395 (31·9) | 122 (30·8) |
| Current smoker | 532 (24·7) | 130 (25·2) | 293 (23·7) | 109 (27·5) |
| Missing | 373 (17·3) | 124 (24·0) | 193 (15·6) | 56 (14·1) |
| Psoriatic arthritis/comorbidities | ||||
| Psoriatic arthritis | 527 (24·5) | 129 (25·0) | 314 (25·3) | 84 (21·2) |
| No comorbidities | 572 (26·6) | 140 (27·1) | 328 (26·5) | 104 (26·3) |
| 1–2 comorbidities | 1042 (48·4) | 259 (50·1) | 623 (50·3) | 160 (40·4) |
| 3–4 comorbidities | 405 (18·8) | 96 (18·6) | 222 (17·9) | 87 (22·0) |
| ≥ 5 comorbidities | 133 (6·2) | 22 (4·3) | 66 (5·3) | 45 (11·4) |
| Disease | ||||
| Disease duration, (years) mean ± SD | 22·4 ± 12·1 | 22·9 ± 12·1 | 22·3 ± 12·1 | 22·0 ± 12·1 |
| Age of onset, (years) mean ± SD | 22·9 ± 12·9 | 22·2 ± 12·4 | 22·6 ± 12·5 | 24·8 ± 14·5 |
| Baseline DLQI, median (IQR) (n = 1804) | 18 (13–24) | 18 (13–24) | 18 (13–23) | 19 (13–24) |
| Baseline EQ‐5D, median (IQR) (n = 1618) | 0·73 (0·59–0·80) | 0·73 (0·52–0·80) | 0·73 (0·62–0·80) | 0·73 (0·59–0·80) |
| Unstable psoriasis | 268 (12·5) | 72 (13·9) | 146 (11·8) | 50 (12·6) |
| Medication history | ||||
| Biologic naïve | 1736 (80·7) | 481 (93·0) | 1029 (83·1) | 226 (57·1) |
| Concomitant methotrexateb | 358 (16·6) | 82 (15·9) | 210 (17·0) | 66 (16·7) |
| Concomitant ciclosporinb | 149 (6·9) | 42 (8·1) | 80 (6·5) | 27 (6·8) |
| Concomitant other systemicsb , c | 95 (4·4) | 23 (4·5) | 45 (3·6) | 27 (6·8) |
Data are presented as n (%) unless otherwise stated. BMI, body mass index; IQR, interquartile range; DLQI, Dermatology Life Quality Index; EQ‐5D, EuroQol‐5D. aHad a complete DLQI (only one question left unanswered) and/or EQ‐5D (no question left unanswered) questionnaire recorded within 6 months prior to the start of the index biological therapy, as well as had another complete DLQI and/or EQ‐5D questionnaire recorded within 4–8 months (representing the 6‐month follow‐up) and/or 10–14 months (representing the 12‐month follow‐up) after the start of the index biological therapy. bEver used conventional systemic therapies concomitantly with a biological therapy throughout the study period of 12 months. cIncludes any of acitretin, fumaric acid esters and hydroxycarbamide.
Improvements in the Dermatology Life Quality Index
For the entire cohort, the median [interquartile range (IQR)] DLQI improved from 18 (13–24) at baseline to 2 (0–7) at 6 months [median change –13 (–19 to –6); P < 0·001] (Table 2). Similar changes were also observed at 12 months. Moreover, the proportion of patients reporting a DLQI of 0/1 increased throughout the study for the whole cohort, from 1·7% at baseline to 45·7% and 48·5% at 6 and 12 months (P < 0·001), respectively (Table 3). In addition, 83·6% and 85·9% of the whole cohort achieved an improvement of ≥ 4 points in the total DLQI from baseline at 6 and 12 months, respectively (Table 3). Although 54·3% and 51·5% of the entire cohort did not achieve a DLQI of 0/1 at 6 and 12 months, respectively, 75% of these patients achieved an improvement of ≥ 4 points in their total DLQI from baseline to 6 months; the corresponding figure at 12 months was 81%.
Table 2.
Values of the Dermatology Life Quality Index (DLQI) total and individual domain scores in patients with psoriasis at different follow‐up times (Intention‐to‐treat analysis)a
| All patients | Etanercept | Adalimumab | Ustekinumab | |
|---|---|---|---|---|
| DLQI total score (scale: 0–30) | ||||
| Baseline | 18 [13–24] (1804)b | 18 [13–24] (431) | 18 [13–23] (1060) | 19 [13–24] (313) |
| 6 months | 2 [0–7] (1454)c* | 4 [1–9] (342)* | 1 [0–6] (860)* | 2 [0–7] (252)* |
| Change from baseline to 6 months | −13 [−19 to −6] (1454) | −11 [−17 to −6] (342) | −14 [−20 to −7] (860) | −14 [−19 to −7] (252) |
| 12 months | 2 [0–7] (1187)d* | 3 [1–9] (293)* | 1 [0–6] (689)* | 1 [0–6] (205)* |
| Change from baseline to 12 months | −13 [−19 to −7] (1187) | −11 [−19 to −6] (293) | −14 [−19 to −8] (689) | −14 [−20 to −7] (205) |
| Symptoms and feelings (scale: 0–6) | ||||
| Baseline | 5 [4–6] (1757) | 5 [4–6] (417) | 5 [4–6] (1031) | 5 [4–6] (309) |
| 6 months | 1 [0–2] (1387)* | 2 [1–3] (321)* | 1 [0–2] (820)* | 1 [0–2] (246)* |
| 12 months | 1 [0–2] (1123)* | 1 [0–3] (273)* | 1 [0–2] (651)* | 1 [0–2] (199)* |
| Daily activities (scale: 0–6) | ||||
| Baseline | 4 [3–5] (1757) | 4 [3–5] (417) | 4 [3–5] (1031) | 4 [3–5] (309) |
| 6 months | 0 [0–2] (1387)* | 1 [0–2] (321)* | 0 [0–1] (820)* | 0 [0–1] (246)* |
| 12 months | 0 [0–1] (1123)* | 1 [0–2] (273)* | 0 [0–1] (651)* | 0 [0–1] (199)* |
| Leisure (scale: 0–6) | ||||
| Baseline | 4 [2–6] (1757) | 4 [2–6] (417) | 3 [2–5] (1031) | 4 [2–6] (309) |
| 6 months | 0 [0–1] (1387)* | 0 [0–2] (321)* | 0 [0–1] (820)* | 0 [0–1] (246)* |
| 12 months | 0 [0–1] (1123)* | 0 [0–2] (273)* | 0 [0–1] (651)* | 0 [0–1] (199)* |
| Work or school (scale: 0–3) | ||||
| Baseline | 1 [0–2] (1756) | 1 [0–2] (416) | 1 [0–2] (1031) | 1 [0–2] (309) |
| 6 months | 0 [0–0] (1386)* | 0 [0–1] (321)* | 0 [0–0] (820)* | 0 [0–0] (245)* |
| 12 months | 0 [0–0] (1121)* | 0 [0–0] (271)* | 0 [0–0] (651)* | 0 [0–0] (199)* |
| Personal relationships (scale: 0–6) | ||||
| Baseline | 2 [1–4] (1757) | 2 [1–4] (417) | 2 [1–4] (1031) | 3 [1–5] (309) |
| 6 months | 0 [0–1] (1387)* | 0 [0–2] (321)* | 0 [0–0] (820)* | 0 [0–1] (246)* |
| 12 months | 0 [0–1] (1123)* | 0 [0–1] (273)* | 0 [0–0] (651)* | 0 [0–1] (199)* |
| Treatment problem (scale: 0–3) | ||||
| Baseline | 2 [1–3] (1733) | 2 [1–3] (414) | 2 [1–3] (1013) | 2 [1–3] (306) |
| 6 months | 0 [0–1] (1355)* | 0 [0–1] (317)* | 0 [0–1] (797)* | 0 [0–1] (241)* |
| 12 months | 0 [0–1] (1098)* | 0 [0–1] (270)* | 0 [0–1] (631)* | 0 [0–1] (197)* |
aValues are presented as median [interquartile range] (number of patients). b47 (2·6%), c67 (4·6%) and d64 (5·4%) patients were included in the analysis of the total DLQI, but were not included in the DLQI individual domain analyses because they only had a total DLQI recorded by the research nurse. *P < 0·001 (calculated for each follow‐up vs. baseline within the same cohort).
Table 3.
Proportion of patients achieving a Dermatology Life Quality Index (DLQI) of 0/1 and a clinically meaningful improvement of ≥ 4 points from baseline at different follow‐up times (Intention‐to‐treat analysis)
| All patients | Etanercept | Adalimumab | Ustekinumab | |
|---|---|---|---|---|
| Proportion of patients achieving a DLQI of 0/1 | ||||
| Baseline | 31 (1·7) [1804] | 7 (1·6) [431] | 18 (1·7) [1060] | 6 (1·9) [313] |
| 6 months | 665 (45·7) [1454]* | 101 (29·5) [342]* | 445 (51·9) [860]* | 118 (46·8) [252]* |
| 12 months | 576 (48·5) [1187]* | 97 (33·1) [293]* | 376 (54·6) [689]* | 103 (50·2) [205]* |
| Proportion of patients achieving a clinically meaningful improvement of ≥ 4 points from baseline | ||||
| 6 months | 1215 (83·6) [1454] | 286 (83·6) [342] | 716 (83·3) [860] | 213 (84·5) [252] |
| 12 months | 1020 (85·9) [1187] | 248 (84·6) [293] | 596 (86·5) [689] | 176 (85·9) [205] |
Data are presented as n (% of patients included in the analysis at that time point) [number of patients included in the analysis]. *P < 0·001 (calculated for each follow‐up vs. baseline within the same cohort).
Significant improvements were also achieved within 6 months of treatment in all of the six DLQI domains (Fig. 1). Similar response rates were observed at 12 months. The median values of the DLQI total and individual domain scores for each biological cohort, over the 12 month follow‐up period, are presented in Table 2.
Figure 1.
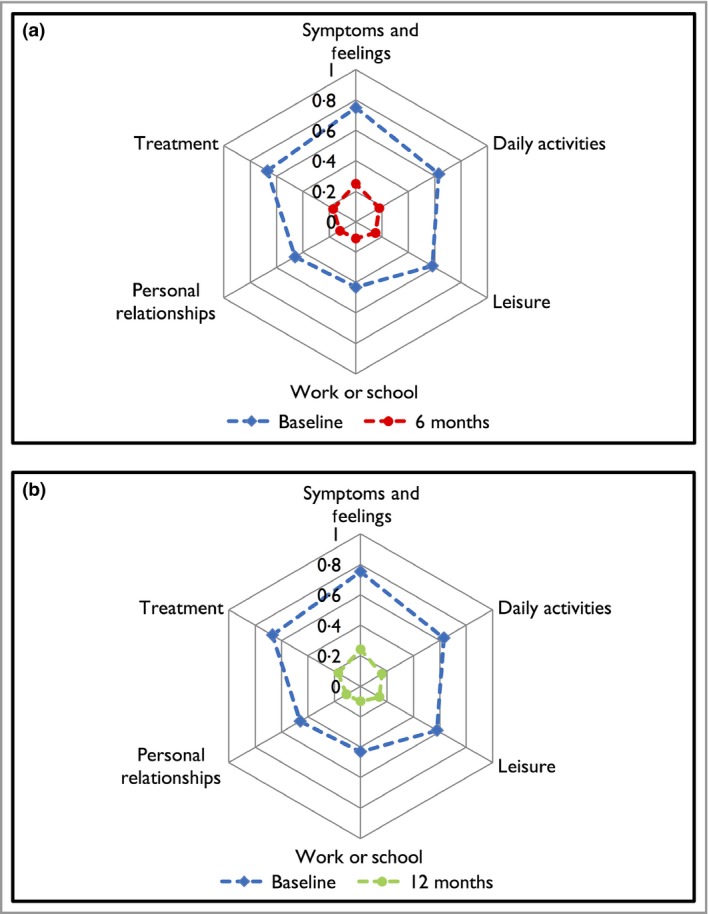
(a) Spider plot of the mean scores at baseline and 6 months in the Dermatology Life Quality Index (DLQI) domains for patients with psoriasis. (b) Spider plot of the mean scores at baseline and 12 months in the DLQI domains for patients with psoriasis. To facilitate direct comparison across the six DLQI domains, the scale was unified to 0–1. To alter the scale for each of the symptoms and feelings, daily activities, leisure and personal relationships domains, the score was divided by 6 (the maximum possible score), and for each of the work or school performance and treatment domains, the score was divided by 3 (the maximum possible score) (Intention‐to‐treat analysis).
Improvements in the EuroQol‐5D
The median (IQR) EQ‐5D utility score for the entire cohort improved from 0·73 (0·59–0·80) at baseline to 0·85 (0·69–1·00) at 6 months [median change 0·07 (0–0·273); P < 0·001], with 54·2% of patients achieving a clinically meaningful change of ≥ 0·05 points. Similar response rates were found at 12 months (Table 4). The proportion of patients reporting any problems in the EQ‐5D dimensions was significantly reduced from baseline at 6 months. The greatest decrease for the entire cohort was in the pain/discomfort dimension (from 74·5% to 44·6%; P < 0·001), whereas the smallest was found in the self‐care dimension (from 18·0% to 12·5%; P < 0·001). Similar decreases in dimension scores were also found at 12 months (Fig. 2). The median values of the EQ‐5D utility scores and the proportions of patients reporting any problems in the EQ‐5D dimensions for each biological cohort over the 12‐month follow‐up period are shown in Table 4.
Table 4.
Values of the EuroQol‐5D (EQ‐5D) utility scores and proportions of patients reporting any problem in the EQ‐5D dimensions in patients with psoriasis at different follow‐up times (Intention‐to‐treat analysis)
| All patients | Etanercept | Adalimumab | Ustekinumab | |
|---|---|---|---|---|
| EQ‐5D utility score, median [IQR] (n) | ||||
| Baseline | 0·73 [0·59–0·80] (1618) | 0·73 [0·52–0·80] (391) | 0·73 [0·62–0·80] (907) | 0·73 [0·59–0·80] (320) |
| 6 months | 0·85 [0·69–1·00] (1358)** | 0·80 [0·69–1·00] (316)** | 0·85 [0·73–1·00] (774)** | 0·85 [0·67–1·00] (268)** |
| Change from baseline to 6 months | 0·07 [0·00–0·27] (1358) | 0·07 [0·00–0·24] (316) | 0·11 [0·00–0·27] (774) | 0·07 [0·00–0·24] (268) |
| 12 months | 0·85 [0·69–1·00] (1108)** | 0·80 [0·69–1·00] (277)** | 0·85 [0·71–1·00] (604)** | 0·85 [0·66–1·00] (227)** |
| Change from baseline to 12 months | 0·10 [0·00–0·28] (1108) | 0·12 [0·00–0·28] (277) | 0·11 [0·00–0·27] (604) | 0·07 [0·00–0·28] (227) |
| EQ‐5D dimensions, n (%) | ||||
| Mobility | ||||
| Baseline | 555 (34·3) | 140 (35·8) | 299 (33·0) | 116 (36·3) |
| 6 months | 365 (26·9)** | 88 (27·9)* | 190 (24·8)** | 85 (31·7)* |
| 12 months | 327 (29·5)** | 85 (30·7)* | 163 (27·0)* | 79 (34·8) |
| Self‐care | ||||
| Baseline | 291 (18·0) | 77 (19·7) | 147 (16·2) | 67 (20·9) |
| 6 months | 170 (12·5)** | 41 (13·0)* | 81 (10·5)** | 48 (17·9) |
| 12 months | 149 (13·5)** | 32 (11·6)** | 74 (12·3)* | 43 (18·9) |
| Usual activities | ||||
| Baseline | 700 (43·3) | 181 (46·3) | 381 (42·0) | 138 (43·1) |
| 6 months | 339 (25·0)** | 78 (24·7)** | 182 (23·5)** | 79 (29·5)** |
| 12 months | 279 (25·2)** | 70 (25·3)** | 141 (23·3)** | 68 (30·0)** |
| Pain/discomfort | ||||
| Baseline | 1206 (74·5) | 296 (75·7) | 672 (74·1) | 238 (74·4) |
| 6 months | 606 (44·6)** | 158 (50·0)** | 329 (42·5)** | 119 (44·4)** |
| 12 months | 501 (45·2)** | 139 (50·2)** | 255 (42·2)** | 107 (47·1)** |
| Anxiety/depression | ||||
| Baseline | 826 (51·1) | 215 (55·0) | 453 (49·9) | 158 (49·4) |
| 6 months | 461 (34·0)** | 123 (38·9)** | 252 (32·6)** | 86 (32·1)** |
| 12 months | 361 (32·6)** | 101 (36·5)** | 187 (31·0)** | 73 (32·2)** |
IQR, interquartile range. *P < 0·05 (calculated for each follow‐up vs. baseline within the same cohort). **P < 0·001 (calculated for each follow‐up vs. baseline within the same cohort).
Figure 2.
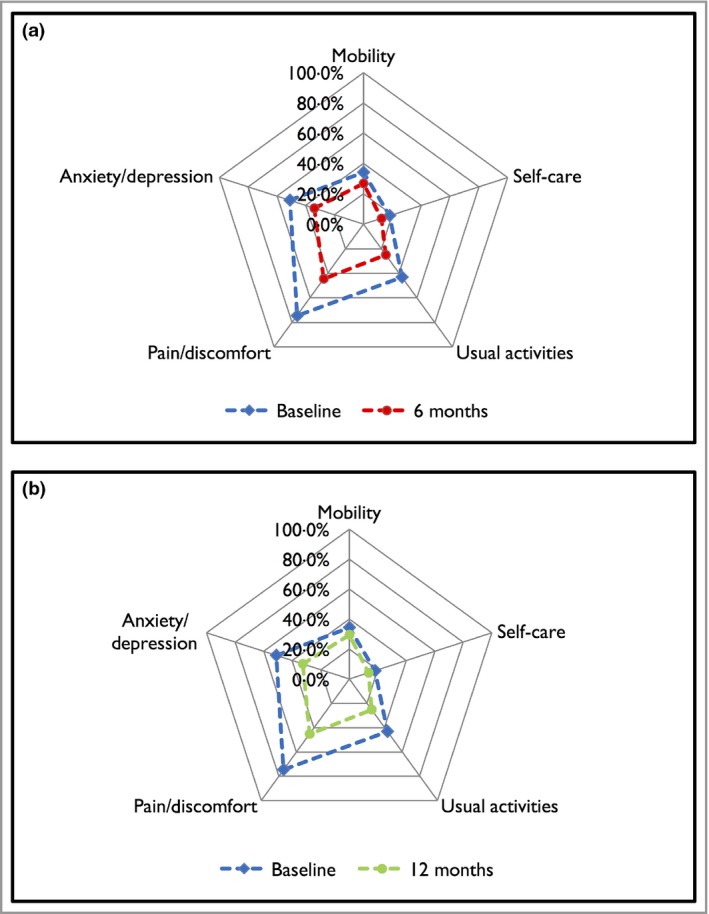
(a) Spider plot of the proportion of patients reporting any problem at baseline and 6 months in the EuroQol‐5D (EQ‐5D) dimensions for patients with psoriasis. (b) Spider plot of the proportion of patients reporting any problem at baseline and 12 months in the EQ‐5D dimensions for patients with psoriasis (Intention‐to‐treat analysis).
Factors associated with HRQoL improvements
Predictors of being less likely to achieve a DLQI of 0/1 at 12 months included: female sex [odds ratio (OR) 0·71, 95% confidence interval (CI) 0·54–0·93], current smoker vs. never smoked (OR 0·61, CI 0·43–0·87), having any comorbidity vs. having no comorbidities (1–2 comorbidities: OR 0·51, CI 0·37–0·70; 3–4 comorbidities: OR 0·49, CI 0·32–0·75; and ≥ 5 comorbidities: OR 0·39, CI 0·20–0·75); a higher baseline DLQI (for every 1 point increase in the DLQI; OR 0·96, CI 0·95–0·98); concomitant use of methotrexate (OR 0·53, CI 0·38–0·75); receiving etanercept vs. receiving adalimumab (OR 0·39, CI 0·28–0·54); and stopping the index biological therapy (OR 0·35, CI 0·20–0·60) (Table 5).
For the change in the EQ‐5D, the multivariable model suggested that with each 10‐year increase in a patient's age there were significantly lower EQ‐5D utility scores at 12 months (regression coefficient –0·015, CI –0·027 to –0·002). Moreover, presence of PsA (regression coefficient –0·077, CI –0·120 to –0·034) and multiple other comorbidities, compared with absence of comorbidities (3–4 comorbidities: regression coefficient –0·057, CI –0·107 to –0·007; or ≥ 5 comorbidities: regression coefficient –0·147, CI –0·217 to –0·076) was significantly associated with lower EQ‐5D utility scores, whereas having a higher baseline EQ‐5D (for every 0·1 point increase in the EQ‐5D utility score; regression coefficient 0·040, CI 0·034–0·046) was significantly associated with higher EQ‐5D response (Table 5).
Sensitivity analyses
Sensitivity analyses were performed to investigate improvements in the DLQI and EQ‐5D among patients who remained on their index biological therapy at the time the DLQI and/or EQ‐5D questionnaires were recorded. In total, 1294 and 942 patients were included in the DLQI sensitivity analyses at 6 and 12 months, respectively; 1222 and 887 patients were included in the EQ‐5D analyses. Compared with the intention‐to‐treat analyses, a total of 160 and 245 patients were excluded from the DLQI sensitivity analyses at 6 and 12 months, respectively; 136 and 221 patients were excluded from the EQ‐5D analyses because they discontinued their index biological therapy at the time the questionnaires were recorded. Results from the sensitivity analyses did not change the main findings as the magnitude of the improvements observed in the DLQI (Table S1 andS2; see Supporting Information) and EQ‐5D (Table S3; see Supporting Information) were consistent with the main analyses. Likewise, results from the multivariable regression models yielded similar predictors to the main findings (Table S4; see Supporting Information).
Discussion
This large prospective cohort study found that in routine clinical practice, the use of biological therapies for psoriasis is associated with marked improvements in HRQoL over 12 months. Improvements were influenced by several factors including the choice of biological therapy, baseline impairment in HRQoL, smoking and presence of comorbidities. Compared with adalimumab, patients receiving etanercept, but not ustekinumab, were less likely to achieve a DLQI of 0/1, but there was no significant difference between the three biological therapies in improvement in the EQ‐5D. For the DLQI and the EQ‐5D, a change of 4 and 0·05 points, respectively, correlates with a minimum clinically important difference (MCID).40, 43 The median differences observed in this cohort study were greater than the MCID at both 6 and 12 months’ follow‐up.
Interestingly, we found that the effectiveness of biological therapies in patients in the BADBIR was less than their reported efficacy in RCTs. For example, the proportion of patients achieving a DLQI of 0/1 in RCTs was 54·4% for etanercept and 57·4% for ustekinumab,7, 46 compared with 29·5% and 46·8% of patients on etanercept and ustekinumab at 6 months in this cohort study. Furthermore, results from RCTs indicated that EQ‐5D change was between 0·12 and 0·21,7, 47, 48 compared with a change between 0·07 and 0.11 for EQ‐5D at 6 months in the present study. This is likely to be due to differences in demographic and disease characteristics of patients with psoriasis commencing biological therapies in routine clinical practice compared with those enrolled into a clinical trial.35
Our findings are in line with those reported by Norlin et al.,32 who did not find significant differences in change in the EQ‐5D between different biological therapies. However, by comparison, our study has important strengths: the sample size was much larger and we accounted for important clinical factors including smoking and the presence of comorbidities other than PsA. In contrast to our study, Gelfand et al.30 and Takeshita et al.31 reported that absolute differences in the DLQI were small and not statistically significant across adalimumab, etanercept and ustekinumab. However, the cross‐sectional design of these studies limits the ability to assess changes in response to therapy. Strober et al.33 reported that improvements in the DLQI from baseline to 6 and 12 months were significantly better in the ustekinumab group than that in the adalimumab and etanercept groups. However, this study did not adjust for important clinical factors that could influence treatment response, such as dosing adjustments and the concomitant use of conventional systemic therapies with biological therapies.
We have shown that patients on etanercept, but not ustekinumab, were less likely to achieve a DLQI of 0/1 compared with those on adalimumab. This finding aligns with other studies reporting that patients are more likely to discontinue etanercept due to ineffectiveness compared with adalimumab or ustekinumab.44 Nevertheless, we found no significant difference between the three biological therapies in improvement in the EQ‐5D. Compared with the EQ‐5D, the DLQI is a dermatology‐specific measure that is more relevant to psoriasis. Hence, the DLQI may have a greater ability to measure specific impairments resulting from the disease and detect smaller changes in health relative to the EQ‐5D.49 However, the use of a generic utility instrument (EQ‐5D) allows comparison across different diseases and calculation of quality‐adjusted life years, which will provide valuable data to support cost‐effectiveness analysis.50 To our knowledge, this is the first study that has reported on the impact of biological therapies on HRQoL assessed using both a dermatology‐specific measure and a generic utility instrument.
We found that patients who discontinued their biological therapy were less likely to show improvements in HRQoL compared with those who continued therapy. This observation suggests that drug survival is an important proxy marker of effectiveness and real‐world utility.44, 51
Our study also reports that patients with lower HRQoL (higher DLQI/lower EQ‐5D) at baseline were significantly less likely to achieve a DLQI of 0/1 or show improvement in the EQ‐5D. This finding acknowledges that the ‘cumulative life course impairment’ from living with psoriasis may be a self‐perpetuating social disconnection and failure to achieve ‘full life potential’ in some patients, despite receiving effective therapy.5, 52 Hence, the devastating impact psoriasis can have on self‐esteem and identity underscores the availability of patient support and psychological treatment as part of routine care.53
Consistent with previous studies,54 we found that being a current smoker was a predictor of poor improvement in HRQoL, whereas being an ex‐smoker did not predict change in HRQoL, suggesting that smoking could influence response to biological therapies.
As the BADBIR was established primarily as a pharmacovigilance register, there are some limitations to studying the impact of biological therapy on HRQoL that should be considered in interpreting our findings. Firstly, information on patients’ adherence to treatment was not available. Furthermore, as data were collected on a 6‐monthly basis, the study design prevents a more detailed analysis of the time to initial improvement in HRQoL. It has been suggested, in a Swedish study of antitumour necrosis factor use in PsA, that utility improvements occur rapidly (within 2 weeks) and are maintained thereafter.55 An inherent limitation in an observational study is nonrandomization that may introduce selection bias, and although this is partially negated by adjustment for clinically relevant covariates, the presence of unmeasured confounders cannot be discounted.
Our results reflect current use of biological therapies for patients with psoriasis in the U.K. and Republic of Ireland, which should be considered in the context of guidelines published by the BAD56 and the National Institute for Health and Care Excellence for the management of psoriasis.57 Guidelines for the management of psoriasis are also similar in Scotland58 and the Republic of Ireland.59 Our findings also provide a more solid basis for health economic modelling compared with RCT data due to the greater external validity of the BADBIR.60
Further work is required to investigate whether subsequent switching of biological therapies will predict HRQoL changes. Data from patients with PsA in Sweden suggest that improvements in HRQoL during the first and second courses of biological therapies are similar.55 Equally important is the need to investigate whether improvements in HRQoL were associated with improvements in disease activity. An earlier study of the PsA cohort within the British Society for Rheumatology Biologics Register found that improvements in HRQoL were significantly associated with improvements in disease activity.61
In summary, this large prospective cohort study provides novel insights into the extent of improvement in HRQoL in patients with psoriasis receiving treatment with biological therapies in routine clinical practice, and key determinants of treatment response, which are also of particular importance as they support the concept that lifestyle modifications, including smoking cessation, may enhance the effectiveness of biological therapies. These findings should be considered, along with the other known benefits and risks of biological therapies, when choosing the most appropriate treatment for patients with psoriasis.
Supporting information
Fig S1. Patient selection.
Table S1. Values of the Dermatology Life Quality Index total and individual domain scores in patients with psoriasis at different follow‐up times [Treatment completers analysis].
Table S2. Proportion of patients achieving a Dermatology Life Quality Index of 0/1 and a clinically meaningful improvement of ≥ 4 points from baseline at different follow‐up times [Treatment completers analysis].
Table S3. Values of the EuroQol‐5D (EQ‐5D) utility scores and proportions of patients reporting any problem in the EQ‐5D dimensions in patients with psoriasis at different follow‐up times [Treatment completers analysis].
Table S4. Multivariable regression analyses of potential factors associated with achieving a Dermatology Life Quality Index of 0/1 and changes in the EuroQol‐5D utility score at 6 and 12 months [Treatment completers analysis].
Video S1. Author video.
Acknowledgments
The authors acknowledge the substantial contribution of the British Association of Dermatologists Biologic Interventions Register (BADBIR) team to the administration of the project, in particular the database manager, Mr Hassan Ali, for his advice and support. The BADBIR acknowledges the support of the National Institute for Health Research (NIHR) through the clinical research networks and its contribution in facilitating recruitment into the registry. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the BADBIR, NIHR, NHS or the Department of Health.
The authors are grateful to the members of the Data Monitoring Committee: Dr Robert Chalmers, Dr Carsten Flohr (Chair), Dr Karen Watson and David Prieto‐Merino and the BADBIR Steering Committee (in alphabetical order): Prof Jonathan Barker, Ms Marilyn Benham (CEO of BAD), Prof David Burden (Chair), Mr Ian Evans, Prof Christopher Griffiths, Dr Sagair Hussain, Prof Brian Kirby, Ms Linda Lawson, Dr Kayleigh Mason, Dr Kathleen McElhone, Dr Ruth Murphy, Prof Anthony Ormerod, Dr Caroline Owen, Prof Nick Reynolds, Prof Catherine Smith and Prof Richard Warren. Prof Christopher Griffiths is a NIHR Senior Investigator. Finally, we acknowledge the enthusiastic collaboration of all of the dermatologists and specialist nurses in the U.K. and the Republic of Ireland who provided the data. The principal investigators at the participating sites at the time of data cut‐off are listed at www.badbir.org.
Funding sources The British Association of Dermatologists Biologic Interventions Register (BADBIR) is coordinated by The University of Manchester. The BADBIR is funded by the British Association of Dermatologists (BAD). The BAD receives income from Pfizer, Janssen Cilag, AbbVie, Novartis, Samsung Bioepis and Eli Lilly for providing pharmacovigilance services. This income finances a separate contract between the BAD and The University of Manchester who coordinate the BADBIR. All decisions concerning analysis, interpretation and publication are made independently of any industrial contribution. C.E.M.G. and R.B.W. are funded in part by the Medical Research Council (MR/L011808/1). N.J.R.'s laboratory/research is supported by the NIHR‐Newcastle Biomedical Research Centre.
Conflicts of interest D.M.A. has received grant funding from AbbVie and served on advisory boards for Pfizer and GSK. R.B.W. has acted as a consultant and/or speaker and/or received research grants for AbbVie, Amgen, Almirall, Celgene, Eli Lilly, Pfizer, LEO Pharma, Novartis, Janssen, Medac and Xenoport. C.H.S.'s department has received funding for research support from AbbVie, Janssen, Novartis, Wyeth and Pfizer. N.J.R. has received honoraria, travel support and/or research grants (Newcastle University) from AbbVie, Amgen, AstraZeneca, Bristol‐Myers Squibb, Celgene, Genentech, Janssen, LEO Pharma Research Foundation, Novartis, Pfizer and Stiefel GSK. C.E.M.G. has received honoraria and/or research grants from AbbVie, Actelion, Amgen, Celgene, LEO Pharma, Eli Lilly, GSK‐Stiefel, Janssen, MSD, Novartis, Pfizer, Sandoz and UCB. The remaining authors declare no conflicts of interest.
References
- 1. Parisi R, Symmons DPM, Griffiths CEM et al Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133:377–85. [DOI] [PubMed] [Google Scholar]
- 2. Kimball AB, Jacobson C, Weiss S et al The psychosocial burden of psoriasis. Am J Clin Dermatol 2005; 6:383–92. [DOI] [PubMed] [Google Scholar]
- 3. Krueger G, Koo J, Lebwohl M et al The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient‐membership survey. Arch Dermatol 2001; 137:280–4. [PubMed] [Google Scholar]
- 4. Rapp SR, Feldman SR, Exum ML et al Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999; 41:401–7. [DOI] [PubMed] [Google Scholar]
- 5. Kimball AB, Gieler U, Linder D et al Psoriasis: is the impairment to a patient's life cumulative? J Eur Acad Dermatol Venereol 2010; 24:989–1004. [DOI] [PubMed] [Google Scholar]
- 6. Wakkee M, Nijsten T. Comorbidities in dermatology. Dermatol Clin 2009; 27:137–47. [DOI] [PubMed] [Google Scholar]
- 7. Reich K, Segaert S, Van de Kerkhof P et al Once‐weekly administration of etanercept 50 mg improves patient‐reported outcomes in patients with moderate‐to‐severe plaque psoriasis. Dermatology 2009; 219:239–49. [DOI] [PubMed] [Google Scholar]
- 8. Reich K, Nestle FO, Papp K et al Improvement in quality of life with infliximab induction and maintenance therapy in patients with moderate‐to‐severe psoriasis: a randomized controlled trial. Br J Dermatol 2006; 154:1161–8. [DOI] [PubMed] [Google Scholar]
- 9. Revicki D, Willian MK, Saurat JH et al Impact of adalimumab treatment on health‐related quality of life and other patient‐reported outcomes: results from a 16‐week randomized controlled trial in patients with moderate to severe plaque psoriasis. Br J Dermatol 2008; 158:549–57. [DOI] [PubMed] [Google Scholar]
- 10. Langley RG, Elewski BE, Lebwohl M et al Secukinumab in plaque psoriasis – results of two phase 3 Trials. N Engl J Med 2014; 371:326–38. [DOI] [PubMed] [Google Scholar]
- 11. Lebwohl M, Papp K, Han C et al Ustekinumab improves health‐related quality of life in patients with moderate‐to‐severe psoriasis: results from the PHOENIX 1 trial. Br J Dermatol 2010; 162:137–46. [DOI] [PubMed] [Google Scholar]
- 12. Ali FM, Cueva AC, Vyas J et al A systematic review of the use of quality‐of‐life instruments in randomized controlled trials of psoriasis. Br J Dermatol 2016; 176:577–93. [DOI] [PubMed] [Google Scholar]
- 13. Gooderham M, Gavino‐Velasco J, Clifford C et al A review of psoriasis, therapies, and suicide. J Cutan Med Surg 2016; 20:293–303. [DOI] [PubMed] [Google Scholar]
- 14. Naldi L, Svensson A, Zenoni D et al Comparators, study duration, outcome measures and sponsorship in therapeutic trials of psoriasis: update of the EDEN Psoriasis Survey 2001–2006. Br J Dermatol 2010; 162:384–9. [DOI] [PubMed] [Google Scholar]
- 15. Reich K, Burden AD, Eaton JN, Hawkins NS. Efficacy of biologics in the treatment of moderate to severe psoriasis: a network meta‐analysis of randomized controlled trials. Br J Dermatol 2012; 166:179–88. [DOI] [PubMed] [Google Scholar]
- 16. Schmitt J, Rosumeck S, Thomaschewski G et al Efficacy and safety of systemic treatments for moderate‐to‐severe psoriasis: meta‐analysis of randomized controlled trials. Br J Dermatol 2014; 170:274–303. [DOI] [PubMed] [Google Scholar]
- 17. Galván‐Banqueri M, Marin Gil R, Santos Ramos B, Bautista Paloma FJ. Biological treatments for moderate‐to‐severe psoriasis: indirect comparison. J Clin Pharm Ther 2013; 38:121–30. [DOI] [PubMed] [Google Scholar]
- 18. Lin VW, Ringold S, Devine EB. Comparison of ustekinumab with other biological agents for the treatment of moderate to severe plaque psoriasis: a bayesian network meta‐analysis. Arch Dermatol 2012; 148:1403–10. [DOI] [PubMed] [Google Scholar]
- 19. Lucka TC, Pathirana D, Sammain A et al Efficacy of systemic therapies for moderate‐to‐severe psoriasis: a systematic review and meta‐analysis of long‐term treatment. J Eur Acad Dermatol Venereol 2012; 26:1331–44. [DOI] [PubMed] [Google Scholar]
- 20. Baker EL, Coleman CI, Reinhart KM et al Effect of biologic agents on non‐PASI outcomes in moderate‐to‐severe plaque psoriasis: systematic review and meta‐analyses. Dermatol Ther (Heidelb) 2012; 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katugampola RP, Lewis VJ, Finlay AY. The Dermatology Life Quality Index: assessing the efficacy of biological therapies for psoriasis. Br J Dermatol 2007; 156:945–50. [DOI] [PubMed] [Google Scholar]
- 22. van Lümig PP, van de Kerkhof PC, Boezeman JB et al Adalimumab therapy for psoriasis in real‐world practice: efficacy, safety and results in biologic‐naïve vs. non‐naïve patients. J Eur Acad Dermatol Venereol 2013; 27:593–600. [DOI] [PubMed] [Google Scholar]
- 23. Menting SP, Sitaram AS, Bonnerjee‐van der Stok HM et al Drug survival is not significantly different between biologics in patients with psoriasis vulgaris: a single‐centre database analysis. Br J Dermatol 2014; 171:875–83. [DOI] [PubMed] [Google Scholar]
- 24. Mazzotta A, Esposito M, Costanzo A, Chimenti S. Efficacy and safety of etanercept in psoriasis after switching from other treatments: an observational study. Am J Clin Dermatol 2009; 10:319–24. [DOI] [PubMed] [Google Scholar]
- 25. Van Lümig PP, Driessen RJ, Boezeman JB et al Long‐term efficacy of etanercept for psoriasis in daily practice. Br J Dermatol 2012; 166:445–7. [DOI] [PubMed] [Google Scholar]
- 26. Puig L, Camacho Martínez FM, Gimeno Carpio E et al Efficacy and safety of clinical use of etanercept for the treatment of moderate‐to‐severe psoriasis in Spain: results of a multicentric prospective study at 12 months follow‐up. Dermatology 2012; 225:220–30. [DOI] [PubMed] [Google Scholar]
- 27. Gisondi P, Conti A, Galdo G et al Ustekinumab does not increase body mass index in patients with chronic plaque psoriasis: a prospective cohort study. Br J Dermatol 2013; 168:1124–7. [DOI] [PubMed] [Google Scholar]
- 28. Clemmensen A, Spon M, Skov L et al Responses to ustekinumab in the anti‐TNF agent‐naïve vs. anti‐TNF agent‐exposed patients with psoriasis vulgaris. J Eur Acad Dermatol Venereol 2011; 25:1037–40. [DOI] [PubMed] [Google Scholar]
- 29. Zweegers J, Otero ME, van den Reek JM et al Effectiveness of biologic and conventional systemic therapies in adults with chronic plaque psoriasis in daily practice: a systematic review. Acta Derm Venereol 2016; 96:453–8. [DOI] [PubMed] [Google Scholar]
- 30. Gelfand JM, Wan J, Callis Duffin K et al Comparative effectiveness of commonly used systemic treatments or phototherapy for moderate to severe plaque psoriasis in the clinical practice setting. Arch Dermatol 2012; 148:487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takeshita J, Wang S, Shin DB et al Comparative effectiveness of less commonly used systemic monotherapies and common combination therapies for moderate to severe psoriasis in the clinical setting. J Am Acad Dermatol 2014; 71:1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Norlin JM, Steen Carlsson K, Persson U, Schmitt‐Egenolf M. Switch to biological agent in psoriasis significantly improved clinical and patient‐reported outcomes in real‐world practice. Dermatology 2012; 225:326–32. [DOI] [PubMed] [Google Scholar]
- 33. Strober BE, Bissonnette R, Fiorentino D et al Comparative effectiveness of biologic agents for the treatment of psoriasis in a real‐world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol 2016; 74:e4. [DOI] [PubMed] [Google Scholar]
- 34. Burden AD, Warren RB, Kleyn CE et al The British Association of Dermatologists’ Biologic Interventions Register (BADBIR): design, methodology and objectives. Br J Dermatol 2012; 166:545–54. [DOI] [PubMed] [Google Scholar]
- 35. Iskandar IYK, Ashcroft DM, Warren RB et al Demographics and disease characteristics of patients with psoriasis enrolled in the British Association of Dermatologists Biologic Interventions Register. Br J Dermatol 2015; 173:510–18. [DOI] [PubMed] [Google Scholar]
- 36. Bousquet C, Lagier G, Lillo‐Le Louët A et al Appraisal of the MedDRA conceptual structure for describing and grouping adverse drug reactions. Drug Saf 2005; 28:19–34. [DOI] [PubMed] [Google Scholar]
- 37. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19:210–16. [DOI] [PubMed] [Google Scholar]
- 38. Badia X, Mascaro JM, Lozano R. Measuring health‐related quality of life in patients with mild to moderate eczema and psoriasis: clinical validity, reliability and sensitivity to change of the DLQI. Br J Dermatol 1999; 141: 698–702. [DOI] [PubMed] [Google Scholar]
- 39. Hongbo Y, Thomas CL, Harrison MA et al Translating the science of quality of life into practice: what do Dermatology Life Quality Index scores mean? Br J Dermatol 2005; 125:659–64. [DOI] [PubMed] [Google Scholar]
- 40. Basra MK, Salek MS, Camilleri L et al Determining the Minimal Clinically Important Difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology 2015; 230:27–33. [DOI] [PubMed] [Google Scholar]
- 41. EuroQol Group . EuroQol – a new facility for the measurement of health‐related quality of life. Health Policy 1990; 16:199–208. [DOI] [PubMed] [Google Scholar]
- 42. Dolan P, Gudex C, Kind P et al A social tariff for EuroQol: results from a UK general population survey. Discussion paper 138. York: Centre for Health Economics, The University of York. September 1995. Available at: www.york.ac.uk/che/pdf/DP138.pdf (last accessed 10 August 2017).
- 43. Dolan P. Modeling Valuations for EuroQol Health States. Med Care 1997; 35:1095–108. [DOI] [PubMed] [Google Scholar]
- 44. Warren RB, Smith CH, Yiu ZZ et al Differential drug survival of biological therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). J Invest Dermatol 2015; 135:2632–40. [DOI] [PubMed] [Google Scholar]
- 45. Iskandar IYK, Ashcroft DM, Warren RB et al Patterns of biologic therapy use in the management of psoriasis: cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR). Br J Dermatol 2017; 176:1297–1307. [DOI] [PubMed] [Google Scholar]
- 46. Thaçi D, Blauvelt A, Reich K et al Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol 2015; 73:400–9. [DOI] [PubMed] [Google Scholar]
- 47. Daudén E, Griffiths CE, Ortonne JP et al Improvements in patient‐reported outcomes in moderate‐to‐severe patients with psoriasis receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol 2009; 23:1374–82. [DOI] [PubMed] [Google Scholar]
- 48. Shikiar R, Bresnahan BW, Stone SP et al Validity and reliability of patient reported outcomes used in psoriasis: results from two randomized clinical trials. Health Qual Life Outcomes 2003; 1:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pietersma S, van den Akker‐van Marle ME, de Vries M. Generic quality of life utility measures in health‐care research: conceptual issues highlighted for the most commonly used utility measures. IJW 2013; 3:173–81. [Google Scholar]
- 50. Marra CA, Woolcott JC, Kopec JA et al A comparison of generic, indirect utility measures (the HUI2, HUI3, SF‐6D, and the EQ‐5D) and disease‐specific instruments (the RAQoL and the HAQ) in rheumatoid arthritis. Soc Sci Med 2005; 60:1571–82. [DOI] [PubMed] [Google Scholar]
- 51. Saad AA, Ashcroft DM, Watson KD et al Persistence with anti‐tumour necrosis factor therapies in patients with psoriatic arthritis: observational study from the British Society of Rheumatology Biologics Register. Arthritis Res Ther 2009; 11:R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mattei PL, Corey KC, Kimball AB. Cumulative life course impairment: evidence for psoriasis. Curr Probl Dermatol 2013; 44:82–90. [DOI] [PubMed] [Google Scholar]
- 53. Bundy C, Borthwick M, McAteer H et al Psoriasis: snapshots of the unspoken: using novel methods to explore patients’ personal models of psoriasis and the impact on well‐being. Br J Dermatol 2014; 171:825–31. [DOI] [PubMed] [Google Scholar]
- 54. Di Lernia V, Ricci C, Lallas A, Ficarelli E. Clinical predictors of non‐response to any tumor necrosis factor (TNF) blockers: a retrospective study. J Dermatolog Treat 2014; 25:73–4. [DOI] [PubMed] [Google Scholar]
- 55. Gülfe A, Kristensen LE, Saxne T et al Rapid and sustained health utility gain in anti‐tumour necrosis factor‐treated inflammatory arthritis: observational data during 7 years in southern Sweden. Ann Rheum Dis 2010; 69:352–7. [DOI] [PubMed] [Google Scholar]
- 56. Smith CH, Anstey AV, Barker JNWN et al British Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2009. Br J Dermatol 2009; 161:987–1019. [DOI] [PubMed] [Google Scholar]
- 57. National Institute for Health and Care Excellence . Psoriasis: assessment and management. Clinical Guideline [CG153]. 2012. Available at: www.nice.org.uk/guidance/cg153 (last accessed 10 August 2017). [PubMed]
- 58. Burden AD, Boon MH, Leman J et al Diagnosis and management of psoriasis and psoriatic arthritis in adults: summary of SIGN guidance. BMJ 2010; 341:c5623. [DOI] [PubMed] [Google Scholar]
- 59. Nast A, Gisondi P, Ormerod AD et al European S3‐Guidelines on the systemic treatment of psoriasis vulgaris – update 2015 – short version – EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol 2015; 29:2277–94. [DOI] [PubMed] [Google Scholar]
- 60. Baltussen R, Leidl R, Ament A. Real world designs in economic evaluation. Bridging the gap between clinical research and policy‐making. Pharmacoeconomics 1999; 16:449–58. [DOI] [PubMed] [Google Scholar]
- 61. Saad AA, Ashcroft DM, Watson KD et al Improvements in quality of life and functional status in patients with psoriatic arthritis receiving anti‐tumor necrosis factor therapies. Arthritis Care Res (Hoboken) 2010; 62:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Patient selection.
Table S1. Values of the Dermatology Life Quality Index total and individual domain scores in patients with psoriasis at different follow‐up times [Treatment completers analysis].
Table S2. Proportion of patients achieving a Dermatology Life Quality Index of 0/1 and a clinically meaningful improvement of ≥ 4 points from baseline at different follow‐up times [Treatment completers analysis].
Table S3. Values of the EuroQol‐5D (EQ‐5D) utility scores and proportions of patients reporting any problem in the EQ‐5D dimensions in patients with psoriasis at different follow‐up times [Treatment completers analysis].
Table S4. Multivariable regression analyses of potential factors associated with achieving a Dermatology Life Quality Index of 0/1 and changes in the EuroQol‐5D utility score at 6 and 12 months [Treatment completers analysis].
Video S1. Author video.


