Abstract
Background
Dyssynergic defecation (DD) is present in approximately 30% of patients with idiopathic chronic constipation (CC). Diagnostic criteria for DD require objective testing such as anorectal manometry (ARM); yet, ARM remains a limited resource in Canada. The aim of this study is to determine the predictability of DD in patients with CC using a standardized self-reported symptom questionnaire.
Method
In this study, 166 consecutive English-speaking patients with CC who were referred for ARM completed a symptom questionnaire. DD was diagnosed if pelvic floor dyssynergy was demonstrated by ARM and balloon expulsion time was more than one minute. Likelihood ratios (LRs) were calculated for individual symptoms and prespecified symptom combinations. Likelihood ratios greater than five or less than 0.2 were considered significant. A recursive partitioning tree was used to find the symptoms best able to predict DD.
Results
No single constipation symptom was sufficient to predict a diagnosis of DD. Patients who reported sometimes feeling an urge to defecate and a prolonged straining duration of greater than five minutes were more likely to have DD (LR = 7.74). In patients who reported straining often or always and had a short straining duration of less than two minutes, the diagnosis of DD was less likely (LR = 0.04). The recursive partitioning tree analysis similarly identified a sense of urge with a prolonged straining duration as predictor for DD, as well as an incomplete evacuation as another potential predictor.
Conclusion
Questions regarding need to strain, duration of straining, urge to defecate, and incomplete evacuation are useful to predict the presence of DD in patients with CC. These questions will enable clinicians to make a clinical diagnosis of DD to guide treatment.
Keywords: Anorectal manometry, Chronic constipation, Dyssynergic defecation
INTRODUCTION
Idiopathic chronic constipation (CC) is a common digestive health problem affecting between 16% and 33.5% of adults in North America (1). The American Gastroenterological Association (AGA) recognizes four clinical subgroups of CC including normal transit, slow transit, dyssynergic defecation (DD) and a combination disorder (1, 2). DD can be found in up to 30% of patients with CC and occurs more often in female patients (3, 4). DD is a disorder of ano-rectal function and occurs when there is impaired relaxation or inappropriate contraction of pelvic floor muscles with attempted defecation (5). It is important to distinguish those patients with DD from those with other forms of CC, as those with DD have been shown to benefit from biofeedback therapy as compared with sham biofeedback (6) and conventional therapies such as polyethylene glycol–based laxatives (7), diet modification (7) and diazepam (8).
DD can be diagnosed in patients who satisfy the Rome diagnostic criteria for functional constipation or constipation predominant irritable bowel syndrome and who demonstrate impaired evacuation on balloon expulsion test (BET) or defecography, contraction of the anal sphincter inappropriately, or <20% relaxation of sphincter pressure on anorectal manometry (ARM), imaging or electromyography, and/or evidence of inadequate propulsion on ARM or imaging (9). Using these criteria, the diagnosis of DD is typically made using ARM with BET (10). However, the accessibility of this test remains limited to specialized tertiary centers in Canada. A recent Canadian national survey indicates that even though ARM is accessible to 83.9% of academic gastroenterologists and 35.0% of community gastroenterologists; BET is only available to 35.5% and 23.3%, respectively (11). Due to this limited accessibility, it is important to develop strategies to clinically determine which patients with CC would benefit most from further investigation with ARM or an early referral for biofeedback therapy—or both. This would allow for optimizing referral of patients with CC to specialized centers where these diagnostic and treatment programs are offered.
Digital rectal exam (DRE) has been shown to be accurate to positively identify patients with DD. In these studies, evidence of dyssynergia on DRE was determined by having two of the following: paradoxical anal sphincter contraction, inability to contract the abdominal muscles, ineffective anal sphincter relaxation, or absence of perineal descent. Two studies demonstrated a positive predictive value over 90% (12, 13). However, these studies were performed in a tertiary referral center. It is unclear whether these results can be generalized to other health care practitioners in other settings.
Though patients with DD often complain of excess straining and need for digital manipulation to allow defecation, there is limited data illustrating that patient symptom history alone is helpful in distinguishing DD from the other subtypes of CC (10). Several studies have demonstrated that no particular symptom was useful in identifying patients with DD and that there was often significant symptom overlap between DD and other CC subtypes (14–18). One study attempted to use a constipation scoring system to diagnose constipation and to distinguish among the various CC subtypes. It was found that this particular system was effective in diagnosing constipation but not in distinguishing DD from other forms of CC (19).
The purpose of this study is to determine if a set of standardized questions regarding constipation symptoms can be used to predict which patients with CC are more likely to have DD, thus aiding in appropriate referral to specialized centers for further investigation or therapy.
METHODS
Patient Population and Selection
This study was approved by the research ethics board at the University Health Network in Toronto, Ontario. In this prospective observational cohort study, consecutive English-speaking patients with CC who were referred to the motility laboratory in our center for ARM and BET between January 1, 2012, and January 1, 2014, were included in the study. Prior to the ARM and BET, all patients were asked to independently complete a 17-question questionnaire (Appendix 1) regarding a variety of CC symptoms based on the Rome III diagnostic criteria, such as frequency of bowel movements, sensation of the urge to have a bowel movement, the need to strain, the feeling of incomplete evacuation, feeling of obstruction during defecation and the need for digital manipulation to have a bowel movement. Additional data, including patient age, duration of constipation symptoms and stool form using the Bristol Stool chart, were collected.
Anorectal Manometry and Balloon Expulsion Testing
All patients underwent ARM (Sierra Scientific Instrument, ManoScan 360, Model A100) and BET according to department standard protocol. A single operator performed the ARM and BET. The anorectal manometric pressure profile was measured using a 10-cm solid state catheter with 12 sensors (Given Imaging, Duluth, GA, USA). The average of three attempts at bearing down was used to determine residual anal sphincter pressures, percent anal sphincter relaxation, intra-rectal pressures and the recto-anal pressure gradient during attempted defecation. Subsequently, the BET was performed where a non-latex balloon was inserted in the rectum and subsequently filled with 50 mL of water. The patient was then instructed to bear down as if defecating while sitting on a commode. The time required to expel the balloon was recorded. All data were collected and analyzed using the Manoview ARTM software V2.0 (Given Imaging). The presence of DD was defined by diagnostic standard (i.e., the presence of paradoxical anal sphincter contraction or impaired increase in intra-rectal pressure during staining, as well as a prolonged balloon expulsion time greater than one minute).
Statistical Analysis
Sensitivity, specificity, positive and negative predictive values and likelihood ratios (LRs), along with their 95% confidence intervals (CIs), were calculated for individual symptoms of constipation. LRs and CIs were also calculated for prespecified combinations of clinically relevant symptoms as determined by a local expert. LRs greater than five or less than 0.2 were considered clinically relevant. The preselected combinations of symptoms that were chosen included the following symptoms: the urge to have a bowel movement, the need to strain with a bowel movement, the frequency of a sensation of urge and need to strain, the strain duration, the use of digital manipulation and the sense of incomplete evacuation.
Subsequently, a recursive binary partitioning tree analysis was performed using the rpart library in R version 3.0.3. This approach automatically finds combinations of questionnaire responses that identify groups of patients with a similar diagnosis; it is of particular interest to determine whether this automatic procedure identified any of the prespecified combinations. Thirteen questions (question 5–17 of the questionnaire, Appendix 1) were entered as potential predictors, and model performance was evaluated with a 10-fold cross-validation. The final tree presented is the one whose size gave the smallest cross-validation error.
RESULTS
A total of 166 consecutive patients were included in the study. Three patients were excluded due to incomplete questionnaires. Among the 163 patient records used for the analysis, 138 (79.8%) were female, and the average age was 50.1 ± 18.1 years. There were 87 patients (53.4%) diagnosed with DD based on the results of ARM and BET.
When the individual constipation symptoms were analyzed independently, there was not one single symptom that was strong enough to predict the presence or absence of DD (Table 1). When the combinations of symptoms were examined, the combination of frequently having the urge to defecate and requiring a prolonged strain duration of greater than five minutes was found to be useful in predicting the presence of DD, with an LR of 7.74 (Table 2). On the other hand, having a need to strain during defecation and a short strain duration of less than two minutes (LR of 0.04, Table 3) was useful in ruling out the presence of DD. The other preselected combinations of symptoms of urge to have a bowel movement, need to strain with a bowel movement, digital manipulation, the frequency of a sensation of urge and need to strain, strain duration, and sense of incomplete evacuation were less useful in predicting the presence of DD, having LRs that were between 0.2 and five (data not shown).
Table 1.
The use of single constipation symptoms to predict the presence of DD
| Reported Symptom | With DD | Without DD | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | +LR (95% CI) | -LR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Urge to defecate | 76/87 | 68/76 | 87.4 (78.8–92.8) | 10.5 (5.4–19.4) | 52.8 (44.7–60.8) | 42.1 (23.1–63.7) | 0.98 (0.87–1.09) | 1.2 (0.51–2.83) |
| Need to strain | 71/87 | 56/76 | 81.6 (72.2–88.4) | 26.3 (17.7–37.2) | 55.9 (47.2–64.2) | 55.6 (39.6–79.5) | 1.11 (0.94–1.31) | 0.7 (0.39–1.25) |
| Need to strain when urge | 56/86 | 41/74 | 65.1 (54.6–74.3) | 44.6 (33.8–55.9) | 57.7 (47.8–67.1) | 52.4 (40.3–64.2) | 1.18 (0.91–1.52) | 0.78 (0.53–1.15) |
| Need to strain with soft stool | 41/85 | 23/75 | 48.2 (37.9–58.7) | 69.3 (58.2–78.6) | 64.1 (51.8–74.7) | 54.2 (44.2–63.8) | 1.57 (1.05–2.36) | 0.75 (0.58–0.96) |
| Sense of incomplete evacuation | 79/87 | 61/75 | 90.8 (82.9–95.3) | 18.7 (11.5–28.9) | 56.4 (48.2–64.4) | 63.6 (43–80.3) | 1.12 (0.98–1.27) | 0.49 (0.22–1.11) |
| Sense of blockage | 72/86 | 55/76 | 83.7 (74.5–90) | 27.6 (18.8–38.6) | 56.7 (48–65) | 60 (43.6–74.4) | 1.16 (0.98–1.37) | 0.59 (0.32–1.08) |
| Need to use digital manipulation for defecation | 37/87 | 33/76 | 42.5 (32.7–53) | 56.6 (45.4–67.1) | 52.9 (41.3–64.1) | 46.2 (36.5–56.3) | 0.98 (0.69–1.4) | 1.02 (0.78–1.33) |
Table 2.
The use of frequency of sense of urge to defecate combined with patient reported strain duration to predict DD; LR >5 was deemed to be useful in predicting CC patients with DD
| Frequency of sense of urge to defecate | Duration of strain | LR (95% CI) |
|---|---|---|
| Sometimes | <5 minutes | 0.38 (0.12–1.19) |
| Always | <5 minutes | 0.65 (0.41–1.03) |
| Never | <5 minutes | 0.74 (0.26–2.10) |
| Never | >5 minutes | 1.29 (0.48–3.46) |
| Always | >5 minutes | 2.15 (0.88–5.26) |
| Sometimes | >5 minutes | 7.74 (1.00–59.3) |
Table 3.
The use of the frequency of need to strain combined with patient reported strain duration to predict DD. LR < 0.2 was deemed to be useful in predicting CC patients without DD
| Frequency of need to strain (often/always) | Duration of strain | LR (95% CI) |
|---|---|---|
| Yes | >2 minutes | 1.85 (1.33–2.78) |
| No | <2 minutes | 0.99 (0.59–1.73) |
| No | >2 minutes | 0.86 (0.24–3.34) |
| Yes | <2 minutes | 0.04 (0.01–0.15) |
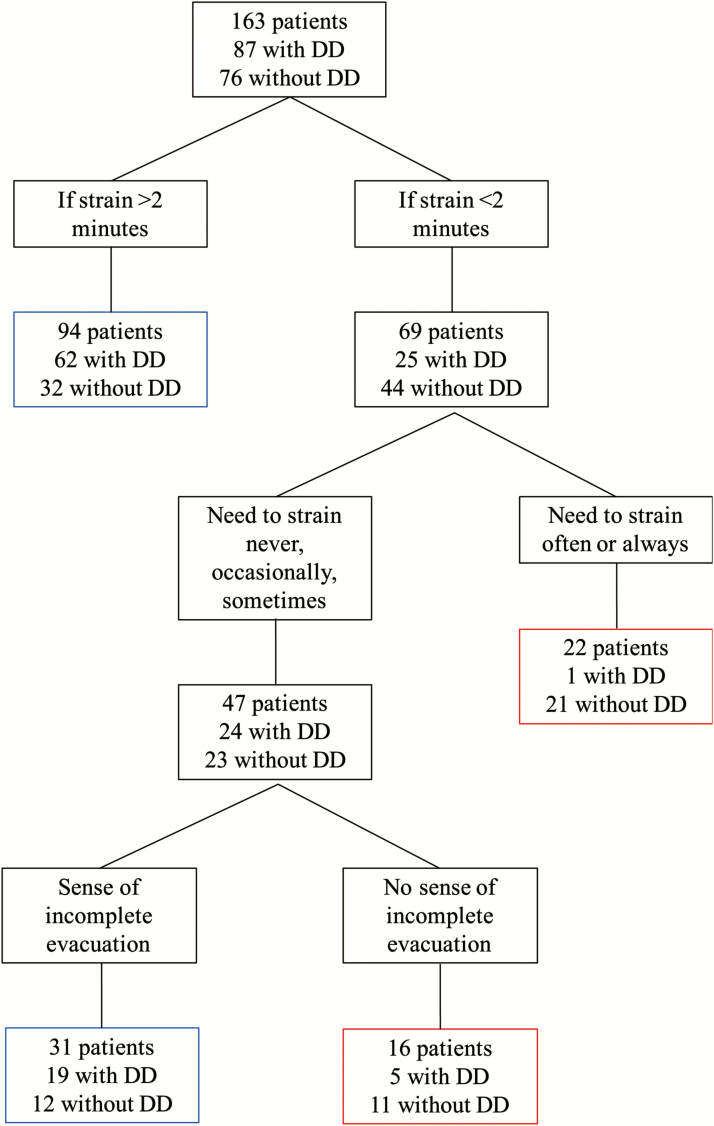
The recursive partitioning tree analysis (Figure 1) identified that the need to strain, strain duration and a sense of incomplete evacuation were useful to predict the absence of DD. This approach found that patients who reported having a strain duration of less than two minutes despite reporting the need to strain often or always with bowel movements were unlikely to have DD (4.5%, one out of 22). In patients who strained infrequently (never, occasional, sometimes) with bowel movements and a strain duration of less than two minutes, the addition of the lack of a sensation of incomplete evacuation probably helped to identify those less likely to have DD (31.3%, five out of 16); whereas the presence of a sense of incomplete evacuation helped to identify those more likely to have DD (61.3%, 19 out of 31). Patients who self-reported a prolonged strain duration of greater than two minutes were more likely to have a diagnosis of DD (66.0%, 62 out of 94).
Figure 1.
Recursive Partitioning Tree.
Blue boxes represent the groups most likely to have DD. Red boxes represent the groups less likely to have DD.
DISCUSSION
This study demonstrates that although no single constipation symptom alone is sufficient in ruling in or ruling out the presence of DD in patients with chronic constipation, the combination of the presence of a sense of urge and a prolonged straining duration is predictive of the diagnosis of DD. Using the recursive partitioning tree analysis, it identified that patients who reported to strain frequently with defecation and having to strain with a duration of less than two minutes are less likely to have DD (4.5%). This was similarly illustrated when the combinations of constipation symptoms were analyzed; the LR of having DD was found to be 0.04 when a patient reported a need to strain during defecation with a strain duration of less than two minutes. Having two different independent analyses reaching the same conclusion increases confidence in this prediction rule. In addition, these observations are consistent with our clinical experience in managing patients with chronic constipation. To identify patients with DD, we found that the sense of urge to defecate with a prolonged strain duration during defecation is predictive of a diagnosis of DD. The LR of DD was 7.74 when patients reported a sense of urge to defecate and a strain duration of more than five minutes. This is in parallel with the concept of the current diagnostic criteria for DD when a prolonged BET is used for making the diagnosis. Furthermore, the automated recursive tree-based analysis model also identified that a sense of incomplete evacuation may also be a helpful question to identify those patients with CC who have DD. This is borne out clinically where patients who have a diagnosis of DD often complain of unsatisfactory bowel movements with a sense of incomplete bowel movements or a sensation of retained stool after defecation.
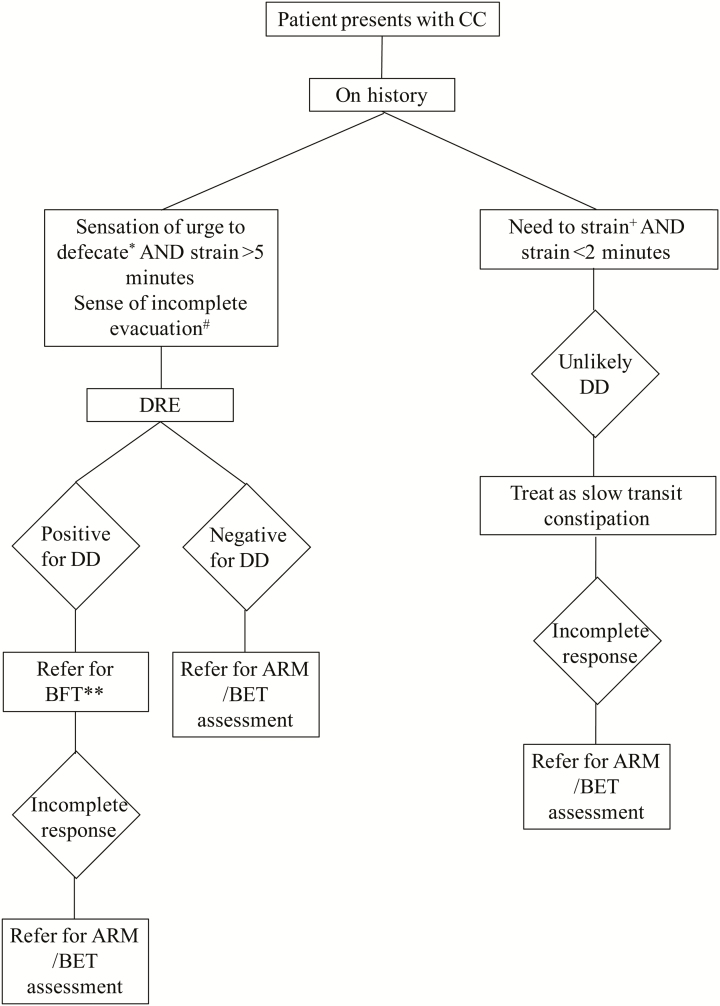
Previous studies have shown that DRE has a very high positive predictive value for DD (12, 13). We believe that a combination of DRE with the clinical history of a prolonged strain duration with a sense of urge and a sense of incomplete evacuation will promote a more timely and accurate diagnosis of DD (Figure 2). We suggest that a patient presenting with CC who describes a sense of urge with defecation and a straining duration of less than two minutes is unlikely to have DD, and hence, the treatment algorithm for slow transit CC(20) should be used once adequate fiber intake is assured. If the patient does not respond satisfactorily with an adequate trial of medical therapy, objective testing to determine if the patient has pelvic floor dyssynergy should be pursued. In patients with a prolonged strain duration of greater than five minutes with a sense of urge to defecate and DRE revealing pelvic floor dyssynergy, the likelihood of having a diagnosis of DD is high. Thus, ARM and BET are not required to confirm the diagnosis before referring patients for biofeedback therapy in addition to standard therapy for the management of chronic constipation (20). In addition, the presence of a sensation of incomplete evacuation on history may further help determine the likelihood of the diagnosis of DD in patients with CC. In those that do not respond to biofeedback therapy in this setting, further pelvic floor assessment with ARM and BET should be pursued to guide management. If a patient has a history suggestive of DD but a negative DRE for DD, further investigation with ARM and BET to ascertain the diagnosis of DD is suggested.
Figure 2.
Suggested Algorithm for Diagnosis of DD in Patients Presenting with CC.
*Sensation of urge to defecate sometimes. +Frequency of need to strain often or always. #Additional question of a sense of incomplete evacuation is probably helpful to increase the likelihood of DD. **Patients with DD referred for BFT would still benefit from optimizing bowel function.
Although this study was performed in a single tertiary referral centre that specializes in motility and functional gastrointestinal disorders, direct referrals from community physicians are accepted, and thus, the study results are likely generalizable to a broader patient population with CC. Nevertheless, some of the patients in this study were treatment refractory CC patients. Further study with larger number of patients will be worth undertaking to validate these questions in a more general population of patients with CC, such as those seen in family practice or in a general gastroenterology clinic. Furthermore, this was a relatively small sample size and confidence intervals, as a result, were wide. A larger sample size may have allowed us to determine if the sense of incomplete evacuation is truly of predictive value in determining a diagnosis of DD as suggested by the recursive binary partitioning tree analysis.
CONCLUSION
Our study presents novel data illustrating that patients self-reporting constipation symptoms using a standardized questionnaire is useful in predicting DD in patients with CC. The reported combination of a prolonged strain duration of greater than five minutes with a sense of urge to defecate helps to rule in the diagnosis of DD; whereas, having an need to strain but a shorter strain duration of less than two minutes helps to rule out the diagnosis of DD. We recommend that clinical history elucidating the need and duration of straining in combining with the sense of urge during a bowel movement should be determined in all patients presenting with CC as a way to clinically decide if DD is a cause of their CC. This would allow clinicians to make a timely diagnosis to implement an appropriate treatment program, including biofeedback therapy or a referral for further investigation with ARM and BET.
Author Contributions
CP collected the data, interpreted the data and drafted the manuscript. GT analyzed the data and critically revised the manuscript. AC designed the study and critically revised the manuscript. LL designed the study, interpreted the data and critically revised the manuscript.
Conflicts of interest
CHP, Allergan (educational support). AC, Janssen (advisory board), Abbvie (advisory board), Takeda (advisory board). LWCL, Abbvie (advisory board, speaker’s bureau), Allergen (advisory board, educational support, speaker’s bureau), Medtronic (educational support, speaker’s bureau).
REFERENCES
- 1. Bharucha AE, Pemberton JH, Locke GR. American Gastroenterological Association technical review on constipation. Gastroenterology 2013;144(1):218–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bharucha AE, Dorn SD, Lembo A, et al. American Gastroenterological Association medical position statement on constipation. Gastroenterology 2013;144(1):211–7. [DOI] [PubMed] [Google Scholar]
- 3. Pare P, Bridge R, Champion MC, et al. Recommendations on chronic constipation (including associated with irritable bowel syndrome) treatment. Can J Gastroenterol 2007;21(Supplement B):3B–22B. [PMC free article] [PubMed] [Google Scholar]
- 4. Rao SSC, Tuteja AK, Vellema T, et al. Dyssyndergic defecation: demographics, symptoms, stool patterns, and quality of life. J Clin Gastroenterol 2004;38(8):680–5. [DOI] [PubMed] [Google Scholar]
- 5. Bharucha AE, Wald A, Enck P, Rao S. Functional anorectal disorders. Gastroenterology 2006;130(5):1510–8. [DOI] [PubMed] [Google Scholar]
- 6. Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol 2007;5(3):331–8. [DOI] [PubMed] [Google Scholar]
- 7. Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology 2006;130(3):657–64. [DOI] [PubMed] [Google Scholar]
- 8. Heymen S, Scarlett Y, Jones K, et al. Randomized, controlled trial shows biofeedback to be superior to alternative treatments for patients with pelvic floor dyssynergia-type constipation. Dis Colon Rectum 2007;50(4):428–41. [DOI] [PubMed] [Google Scholar]
- 9. Rao SS, Patcharatrakul T. Diagnosis and treatment of dyssynergic defecation. J Neurogastroenterol Motil 2016;22(3):423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu LWC. Chronic constipation: Current treatment options. Can J Gastroenterol 2011;25(Supplement B):22B– 8B. [PMC free article] [PubMed] [Google Scholar]
- 11. Parker CH, Tomlinson GA, Correia AJ, et al. Predictability of functional defecation disorder in patients with chronic constipation using a standardized constipation symptom questionnaire. Gastroenterology 146(5):S–717. [Google Scholar]
- 12. Tantiphlachiva K, Rao P, Attaluri A, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol 2010;8(11):955–60. [DOI] [PubMed] [Google Scholar]
- 13. Soh JS, Lee HJ, Jung KW, et al. The diagnostic value of a digital rectal examination compared with high-resolution anorectal manometry in patients with chronic constipation and fecal incontinence. Am J Gastroenterol 2015;110(8):1197–204. [DOI] [PubMed] [Google Scholar]
- 14. Dinning PG, Jones M, Hunt L, et al. Factor analysis identifies subgroups of constipation. World J Gastroenterol 2011;17(11):1468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eltringham MT, Khan U, Bain IM, et al. Functional defecation disorder as a clinical subgroup of chronic constipation: analysis of symptoms and physiological parameters. Scand J Gastroenterol 2008;43(3):262–9. [DOI] [PubMed] [Google Scholar]
- 16. Glia A, Lindber G, Nilsson LH, et al. Clinical value of symptom assessment in patients with constipation. Dis Colon Rectum 1999;42:1401–10. [DOI] [PubMed] [Google Scholar]
- 17. Grotz RL, Pemberton JH, Talley NJ, et al. Discriminant value of psychological distress, symptom profiles, and segmental colonic dysfunction in outpatients with severe idiopathic constipation. Gut 1995;35:798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koch A, Voderholzer WA, Klauser AG, et al. Symptoms in chronic constipation. Dis Colon Rectum 1997;40:902–6. [DOI] [PubMed] [Google Scholar]
- 19. Knowles CH, Eccersley AJ, Scott SM, et al. Linear discriminant analysis of symptoms in patients with chronic constipation. Dis Colon Rectum 2000;43:1419–26. [DOI] [PubMed] [Google Scholar]
- 20. Tse Y, Armstrong D, Andrews CN, et al. Treatment algorithm for chronic idiopathic constipation and constipation-predominant irritable bowel syndrome derived from a canadian national survey and needs assessment on choices of therapeutic agents. Can J Gastroenterol & Hepatol 2017;2017:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]