ABSTRACT
Cast syndrome, commonly known as superior mesenteric artery (SMA) syndrome is a rare cause of small bowel obstruction caused by compression of third part of duodenum from narrowing of the angle between superior mesenteric artery and abdominal aorta resulting in symptoms of duodenal outflow obstruction. A 46-year-old male presented with acute worsening of chronic abdominal pain, nausea and vomiting aggravated with eating. Computed tomography of abdomen and pelvis revealed the dilatation of gastric and proximal duodenum due to compression of third part of duodenum between superior mesenteric artery and aorta. Conservative management with total parental nutrition failed and patient underwent gastrojejunostomy with relief of his symptoms. Cast syndrome is a rare condition but should be kept in mind in patients with abdominal pain, vomiting, early satiety and weight loss. CT abdomen usually reveals the diagnosis but upper GI endoscopy helps to rule out other causes of duodenal obstruction and gastric dilatation.
INTRODUCTION
Superior mesenteric artery (SMA) syndrome is caused by compression of third part of duodenum due to narrowing of angle between abdominal aorta and superior mesenteric artery. It is also known as Wilkie’s syndrome, Cast syndrome or duodenal ileus. This was first described by Von Rokitansky in 1842 and later popularized by Wilkie. Incidence of SMA syndrome is around 0.1–0.3% [1] making it not only rare but also unfamiliar gastro-vascular condition to many internists. Most important risk factor is recent weight loss. This condition is more common in young female patients. Conservative management with nutritional support helps, failure to improve will need surgical intervention. Even though it is a rare disorder, mortality is around 33% [2].
CASE PRESENTATION
A 46-year-old Hispanic male with a known history of type 2 diabetes mellitus presented with 2 days history of acute epigastric abdominal pain with associated postprandial nausea and vomiting. His other medical conditions include hypertension, hyperlipidemia, diabetic neuropathy and seizure disorder. On examination, he was thin built with body mass index (BMI) of 20.1, and vital signs revealed mild tachycardia and hypotension. Remainder of the exam was unrevealing. Pertinent labs revealed creatinine of 1.4 mg/dl, hemoglobin 10.3 g/dl, albumin 1.5 g/dl, prealbumin 4.7 mg/dl and normal liver function tests. Patient had similar symptoms intermittently for the last 2 years. In the past, his symptoms were attributed to gastroparesis due to his long-standing diabetes mellitus. He had multiple CT scans of abdomen done during this period but most of them were without contrast. These scans were either normal or showed mild gastric distension at times which was thought to be secondary to gastroparesis. Of the several scans, he had one CT abdomen with IV contrast approximately one year prior to this presentation, this revealed mild gastric and proximal duodenal dilatation but there was no evidence of duodenal obstruction. He had an upper GI endoscopy recently and this revealed dilated stomach and duodenum along with pyloric and proximal duodenal circumferential ulceration and was treated with proton pump inhibitors. Due to his intermittent symptoms he had poor oral-intake and had a weight loss of about 15 lbs in the last few months (Figs 1–4).
Figure 1:
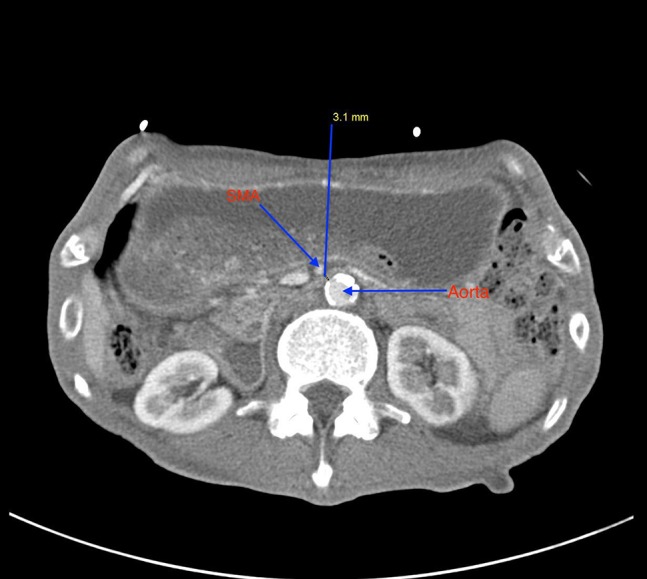
This axial section of CT abdomen and pelvis shows decrease in distance between abdominal aorta and superior mesenteric artery, measured at 3.1 mm.
Figure 4:

This section of CT shows gastric dilatation.
Figure 2:

This sagittal section of CT abdomen and pelvis shows narrow angle between abdominal aorta and superior mesenteric artery.
Figure 3:

This axial section of CT abdomen and pelvis shows dilated second part of duodenum.
Patient was admitted for possible gastroparesis exacerbation and peptic ulcer disease. He was treated with intravenous fluids and Pantoprazole. Supportive care with fluids normalized his renal functions but not his abdominal pain, hence CT abdomen with IV contrast was undertaken. This revealed dilatation of stomach and proximal duodenum and decompression of rest of the duodenum and small bowel. The aortomesenteric distance was measured at 3.1 mm and narrowed aortomesenteric angle of 14°. All these findings along with his symptoms were consistent with SMA syndrome.
Gastric decompression with nasogastric tube was done and Gastroenterology and General surgery were consulted. He underwent upper gastrointestinal endoscopy which revealed distended stomach and proximal duodenum with presence of food but no evidence of ulceration as seen few weeks ago. Endoscope could not be advanced into distal duodenum.
Patient was given total parental nutrition for about 3 weeks. His nutritional status improved as seen by improvement in his albumin (from 1.5 to 2.5 g/dl) and prealbumin (from 4.7 to 21.5 mg/dl). Patient felt better and gained weight but continued to have abdominal pain, nausea and vomiting when challenged with diet. Due to lack of improvement with conservative management, patient was taken to operation room by general surgeon and underwent open gastrojejunostomy. Intraoperatively, decreased amount of intrabdominal fat was seen. Patient did well postoperatively and was tolerating diet well prior to discharge.
DISCUSSION
The third part of duodenum passes between the aorta and SMA. It crosses the aorta anteriorly at L3 vertebrae and is suspended by the ligament of Treitz. SMA originates from aorta at L1 vertebrae anteriorly at an acute angle. Normal aortomesenteric angle is 25–60 degrees and normal aortomesenteric distance is 10 –28 mm. In SMA syndrome, the angle can be narrowed down to as low as 7 degrees and aortomesenteric distance to as low as 2 mm. This decrease in angle and distance causes compression of third part of duodenum [3].
In our patient the aortomesenteric distance on CT scan was measured at 3.1 mm. Common cause is loss of retroperitoneal fat or mesenteric fat cushion from weight loss leading to decrease aortomesenteric angle and distance. Other rare causes include abnormal high insertion or short ligament of Treitz and low origin of SMA [4].
SMA syndrome is more common in females. Diagnosis is challenging as many other conditions can present with symptoms of abdominal pain, nausea and vomiting. In our patient, due to history of long-standing diabetes, his symptoms were attributed in the past to gastroparesis and recently to peptic ulcer disease. Our patient had multiple CT scans of abdomen in the past but most of them were without contrast possibly due to acute renal failure on most of the presentations. We think that patient had SMA syndrome for some time but was not severe enough to cause severe duodenal compression and out flow obstruction. Due to his prolonged abdominal pain, nausea and vomiting, he had poor oral intake leading to weight loss and loss of retroperitoneal fat which in turn caused further decrease in aortomesenteric angle and distance leading to duodenal obstruction [5].
Initial treatment for SMA syndrome is usually conservative management with gastric decompression, electrolyte replacement and nutritional support with total parental nutrition. These measures cause weight gain and restoration of retroperitoneal fat and thereby restoring normal aortomesenteric angle and distance. Failure of conservative management as in our patient warrants surgical management. Duodenojejunostomy is the procedure of choice [6]. Other options include gastrojejunostomy as performed in our patient.
CONCLUSION
Cast syndrome or SMA syndrome is rare but potentially fatal cause of small intestinal obstruction. Clinicians should be cognizant of this catastrophic gastro-vascular condition when evaluating patients presenting with recurrent abdominal pain, nausea, vomiting and associated weight loss. Contrast enhanced CT scan helps with diagnosis but upper GI endoscopy may be needed to rule out other potential mechanical causes.
ACKNOWLEDGMENTS
Department of Radiology at Christus Santa Rosa Hospital Westover Hills for providing with imaging studies of the patient.
CONFLICT OF INTEREST STATEMENT
None.
FUNDING
None.
ETHICAL APPROVAL
Not needed.
CONSENT
Consent obtained from the patient.
GUARANTOR
Dr Madhu Badireddy, MD.
REFERENCES
- 1. Shiu JR, Chao HC, Luo CC, Lai MW, Kong MS, Chen SY, et al. Clinical and nutritional outcomes in children with idiopathic superior mesenteric artery syndrome. J Pediatr Gastroenterol Nutr 2010;51:177–82. [DOI] [PubMed] [Google Scholar]
- 2. Kothari TH, Machnicki S, Kurtz L. Superior mesenteric artery syndrome. Can J Gastroenterol 2011;25:599–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hines JR, Gore RM, Ballantyne GH. Superior mesenteric artery syndrome. Diagnostic criteria and therapeutic approaches. Am J Surg 1984;148:630–2. [DOI] [PubMed] [Google Scholar]
- 4. Sinagra E, Raimondo D, Albano D, Guarnotta V, Blasco M, Testai S, et al. Superior mesenteric artery syndrome: clinical, endoscopic, and radiological findings. Gastroenterol Res Pract 2018;2018:1937416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang ZA. Superior mesenteric artery syndrome: a vicious cycle. BMJ Case Rep 2018;2018:bcr-2018–226002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Catarino Santos S, Loureiro AR, Simão R, Pereira J, Pinheiro LF, Casimiro C. Wilkie’s syndrome: a case report of favourable minimally invasive surgery. J Surg Case Rep 2018;2018:rjy027. [DOI] [PMC free article] [PubMed] [Google Scholar]


